1. Introduction
The Amazon Basin, renowned for its unparalleled biodiversity and vast carbon reserves, has experienced significant deforestation due to the conversion of forests into agricultural lands [
1]. Agriculture stands as the second leading cause of forest conversion in the Amazon, leading to substantial soil erosion, river siltation, and contamination from agrochemicals [
2]. In 2023, the Amazon rainforest experienced a deforestation rate of less than 4000 km
2, primarily in Brazil, Peru, and Bolivia. This extensive deforestation not only diminishes biodiversity but also disrupts essential ecosystem services, including carbon sequestration and climate regulation [
3].
Implementing agroecological practices, such as organic fertilization and biofertilization, within agroforestry systems and pastures has been shown to mitigate soil quality decline. Agroforestry systems, which integrate trees with crops or livestock, have been found to enhance soil fertility and physical properties [
4]. For instance, the establishment of agroforestry systems over areas previously occupied by extensive pasturelands has been shown to enhance soil fertility by 12.79% and improve soil physical indicators by 6.5% [
5]. These practices promote nutrient recycling, improve soil structure, and increase biodiversity, thereby countering the adverse effects of conventional agriculture [
6].
Seasonal variations in tropical regions, characterized by distinct dry and rainy periods, significantly influence aboveground biomass production, litter dynamics, soil fertility, soil physical properties, and microbial gene diversity [
7]. During the rainy season, increased moisture availability typically enhances plant growth, leading to higher biomass accumulation and litter deposition [
8]. Conversely, the dry season may reduce microbial activity and nutrient mineralization rates, affecting soil fertility and structure [
7]. These seasonal fluctuations necessitate adaptive management strategies to maintain ecosystem functionality and productivity throughout the year [
6].
Over time, agroecological practices can lead to substantial improvements in both provisioning and regulating ecosystem services [
9]. Provisioning services, such as crop yield and timber production, benefit from enhanced soil fertility and structure, while regulating services, including carbon sequestration, erosion control, and water regulation, are bolstered by increased vegetation cover and biodiversity [
4]. Long-term studies have demonstrated that agroforestry systems can significantly reduce soil erosion rates and improve soil health indicators compared to monocultures, thereby sustaining agricultural productivity and environmental quality [
10].
We aimed to investigate the impact of agroecological practices—control, biofertilization, and organic fertilization—on ecosystem services (provisioning and regulating services) in three distinct agroecosystems: an agroforestry system, a natural ecosystem (Amazon rainforest), and pasture. This study evaluated how these practices influence both provisioning services (e.g., aboveground dry biomass and litter deposition) and regulating services (e.g., soil carbon, nutrient cycling, and erosion control) in Tropical Oxisols within the Amazon Basin. We hypothesized that (a) the conversion of natural ecosystems to pasture significantly reduces both provisioning and regulating ecosystem services in the Amazon basin; (b) agroforestry systems that mimic the belowground root diversity of natural ecosystems can enhance regulating services, which in turn increases provisioning services compared to pasture; and (c) agroecological practices can mitigate the negative impacts on both regulating and provisioning services in simplified ecosystems such as pasture. These hypotheses are grounded in three key cause–effect pathways: (i) provisioning services are an outcome of effective regulating services [
11]; (ii) degradation of regulating services diminishes provisioning capacity [
12]; and (iii) overexploitation of provisioning services weakens regulating services [
5].
2. Materials and Methods
2.1. Experimental Sites, Soil Classification, and Climate Characterization
The experiment was carried out in three sites across the Amazon Basin (Cruzeiro do Sul, AC, 7°37′17″ S 72°42′43″ W, 192 m.a.s.l; Boca do Acre, AM, 8°45′12″ S 67°23′09″ W, 104 m.a.s.l; and Cerejeiras, RO, 13°10′07″ S 61°14′29″ W, 194 m.a.s.l.) from 2022 to 2024, with soil sampling during the dry and rainy seasons (e.g., following a semestral schedule of sampling). The soil in the three study sites was described as reddish Oxisol with a uniform sandy loam texture and fine granular structure [
13]. Details about the climate classification, average temperature, annual maximum temperature, annual precipitation, and relative humidity are provided in
Table 1.
2.2. Studied Ecosystems—Classification and Characterization
In each studied site, we analyzed the effects of three agroecological practices in two agroecosystems (Agroforestry system and pasture) and an additional treatment by considering a natural ecosystem. The basal soil physicochemical properties of the studied ecosystems in the three studied sites are provided in
Table 2.
The agroforestry system consisted of integrating
Coffea arabica L. (coffee),
Hevea brasiliensis L. (rubber tree),
Zea mays L. (maize), and
Phaseolus vulgaris L. (common bean). This system is classified as a diversified land use approach that optimizes both economic and ecological functions. In this system,
C. arabica was grown under partial shade provided by
H. brasiliensis, which not only supports coffee production by moderating microclimate conditions but also offers latex as an additional income source.
P. vulgaris was intercropped between the rows, contributing to nitrogen fixation through its symbiosis with
Rhizobium bacteria, improving soil fertility for the other crops. We expected that this agroforestry system would enhance biodiversity, promote nutrient cycling, and minimize soil erosion, while also providing multiple products, such as coffee beans, rubber, maize, and legumes. The shade-tolerant nature of coffee in this context helps improve soil structure and water retention, enhancing ecosystem resilience [
5].
For the pasture ecosystem, we studied well-conserved pastures of
Urochloa brizantha (Hochst. ex A.Rich.) R.Webster in the Amazon Basin.
U. brizantha is a robust and drought-tolerant grass species, commonly used due to its capacity to produce high-quality forage while maintaining soil structure and preventing erosion. In well-managed systems, rotational grazing practices help sustain the pasture’s productivity by allowing time for the grass to recover, reducing the risk of overgrazing and land degradation.
U. brizantha also contributes to regulating services, such as soil carbon sequestration, by storing carbon in its root systems and improving soil organic matter content. These pastures, when well-maintained, can be resilient to both drought and heavy rainfall, offering a sustainable forage solution while reducing negative environmental impacts typical of poorly managed pastures [
14].
Finally, we considered in each study site a primary forest fragment of the Amazon tropical rainforest as a reference treatment. The primary forest fragments considered in this study were characterized by rich biodiversity and endemic tree species occurrence, such as
Handroanthus serratifolius (Vahl) S.Grose,
Buchenavia tetraphylla (Aubl.) R.A.Howard,
Hura crepitans L.,
Albizia niopoides (Benth.) Burkart,
Apuleia leiocarpa (Vogel) J.F.Macbr.,
Barnebydendron riedelli (Tul.) J.H.Kirkbr.,
Copaifera multijuga L.,
Hymenaeae courbaril L.,
Parkia paraensis Ducke,
Schizolobium parahyba (Vell.) Blake,
Eschweilera grandiflora (Aubl.) Sandwith,
Ceiba pentandra Gaertn.,
Ceiba samauma (Mart.) K.Schum.,
Sterculia apetala (Jacq.) H.Karst.,
Cedrela odorata L.,
Castilla ulei Warb.,
Ficus insipida Willd.,
Astronium lecointei Ducke,
Eschweilera bracteosa (Poepp. ex O.Berg) Miers.,
Bertholletia excelsa Humb. & Bonpl.,
Euterpe precatoria Mart., and
Dipteryx odorata Willd. These endemic species play key roles in the provision of both provisioning services (e.g., fruits, nuts, and timber) and regulating services (e.g., climate regulation, water cycling, and carbon storage). The complex stratification of the rainforest, with its canopy, understory, and ground layers, supports a highly diverse community of flora and fauna, contributing to nutrient cycling and the maintenance of soil fertility. This ecosystem is essential for stabilizing the regional climate and sustaining hydrological cycles, while its dense vegetation cover helps prevent soil erosion and promote biodiversity conservation. The ecological interactions within this system are fundamental for the long-term resilience and functioning of the Amazon Basin, making it a critical focus for conservation efforts [
15].
2.3. Agroecological Practices—Brief Characterization
As agroecological practices, we considered three treatments: foliar biofertilization, organic fertilization with cow manure, and a control treatment (non-fertilization). Foliar biofertilization (a mixture of fresh cattle manure, unrefined sugar, fresh milk, and yeast in a 20:1:1:1 × 10
−3 ratio) in the Amazon Basin is a common agroecological practice aimed at enhancing plant nutrition by applying nutrient-rich solutions directly to the leaves. In our study, farmers applied foliar biofertilizers at doses of 2 L ha
−1. The application rate was once every two weeks during the growing season, with a focus on critical growth stages, such as flowering and fruiting. The main objective of foliar biofertilization was to improve nutrient uptake efficiency, increase plant resistance to stress (e.g., drought, pests), and boost crop yields without relying on synthetic fertilizers [
16]. Organic fertilization with cow manure is a widely adopted agroecological practice in the Amazon Basin, aimed at improving soil fertility and structure. In the experiment, farmers applied cow manure at doses of 25 tons per hectare. The application was performed once a year by incorporating it into the soil during land preparation. The primary objectives of using cow manure are to replenish soil nutrients, increase soil organic matter, and promote sustainable crop production, particularly in tropical soils that are prone to nutrient depletion [
17]. Finally, the control treatment provided a baseline comparison, against which the effects of biofertilization and organic fertilization can be measured. All agroecological practices were applied and monitored from 2022. The chemical composition of foliar biofertilizer and organic fertilizer are shown in
Table 3.
2.4. Experimental Design
A field experiment was conducted at three independent sites from 2022 to 2024, with soil sampling considering the seasonal variation between the dry and rainy seasons. We analyzed the effects of agroecological practices in an agroforestry system, and a pasture ecosystem on both provisioning and regulating services. The experiment in each site followed a randomized block design using a factorial scheme of 3 × 2 × 2 + 1, with agroecological practices (control, biofertilization, and organic fertilization), agroecosystems (agroforestry system and pasture), seasons (dry and rainy), and an additional reference treatment (natural ecosystem) as the main factors, replicated in five blocks. The studied plots measured 50 × 60 m. Within each plot designated for ecosystem service measurements, we conducted sampling during both the dry and rainy seasons from 2022 to 2024. Sampling activities included the following:
(i) Plant material collection for estimating aboveground dry biomass and yield: In the natural ecosystem and for perennial plants (e.g., C. arabica and H. brasiliensis) in the agroforestry system, aboveground biomass was estimated using species-specific allometric equations. In the pasture system, aboveground fresh biomass was measured by clipping plant material within a 1.5 × 1.5 m area and later drying it to estimate the dry biomass and productivity.
(ii) Soil sampling for physical and biochemical properties: For physical properties, we collected undisturbed soil samples using volumetric cylinders (5.57 cm diameter × 4.1 cm height). For biochemical analyses, disturbed soil samples were taken from the top 20 cm using a soil auger.
(iii) Litter sampling was conducted using a metallic square frame (1.5 × 1.5 m) placed on the soil surface. Collected litter was used to estimate litter deposition and litter quality, including lignin, carbon (C), nitrogen (N), and phosphorus (P) contents. In pasture areas, litter consisted of dead leaves, stems, and other plant debris accumulated on the surface within the same 1.5 × 1.5 m area.
(iv) Soil monoliths (20 × 20 × 20 cm) were collected at the end of the dry and rainy seasons each year, in a randomly selected location within each plot. These samples were used to assess root density and the abundance of soil bacteria, fungi, and archaea via qPCR.
2.5. Aboveground Dry Biomass and Yield
To estimate total aboveground dry biomass (tADB) at the plot level, we tailored our approach to the traits of each agroecosystem (agroforestry system, natural ecosystem, and pasture). We began by selecting fifty plants from the middle of each plot. Annual plant species were harvested at the aboveground level, while perennial plant species had their biomass estimated using allometric equations. Fresh biomass of annual plants was air-dried, then oven-dried at 65 °C for 24 h. For yield calculations, grains were dried and standardized to 14% moisture. tADB estimates were converted from kg ha−1 to t ha−1. Below, we detail the methods used for estimating the total aboveground dry biomass across the studied agroecosystems:
In the agroforestry system, aboveground dry biomass and yield were measured at the physiological maturity of P. vulgaris and Z. mays. Biomass was estimated using plant material, while the grain data were used for yield estimates. For H. brasiliensis and C. arabica, aboveground biomass was calculated with allometric equations: ADB(H. brasiliensis) = exp(−3.31 + 0.95(lnD2H)) × 1.02 and ADB(C. arabica) = 0.117 × D1.732 × H0.760. Here, ADB is aboveground dry biomass (kg/plant), D is diameter at breast height (m), and H is plant height (m). Total aboveground dry biomass (t ha−1) was calculated as follows: tADB = [(PD × ADB(H. brasiliensis)) + (PD × ADB(C. arabica)) + (PD × (ADB + yield(P. vugaris)) + (PD × (ADB + yield(Z. mays))] × 0.00334, where PD is plant density (plants/plot), and 0.00334 converts kg/plot to t/ha.
In the natural ecosystem, aboveground dry biomass (t ha
−1) was estimated using the following equation: ADB = −8.261 × (
D × 1.737) × (
L × 0.891) × (
P × 0.969), where ADB is aboveground dry biomass,
D is diameter at breast height (cm),
L is commercial stem length (m), and
P is basic wood density (g cm
−3) [
18].
In the pasture ecosystem, aboveground dry biomass (t ha
−1) was estimated by collecting fresh biomass using metallic squares (1 × 1 m), air drying the fresh biomass until a constant weight, estimating the dry biomass, and applying the dry biomass results in the following equation: ADB = PDB × 10, where ADB is aboveground dry biomass (t ha
−1),
PDB is the plant dry biomass (kg m
−2), and 10 converts kg/m
2 to t/ha [
19].
2.6. Litter Characterization
To determine the litter deposition, litter was collected in the rainy and dry seasons from 2022 to 2024. Three metallic squares (1 × 1 m) were placed on the soil surface in each plot, with sampling points randomly selected using digital maps and geographic coordinates. Litter material within the squares was gathered into plastic bags, then air-dried at 60 °C for 48 h until a constant dry biomass was achieved [
20]. Nutrient contents (C, N, and P) were analyzed following [
21], and lignin content was determined using Klason’s method [
22].
2.7. Soil Physical and Biochemical Characterization
To physically characterize soil samples, we determined the bulk density, soil moisture, soil porosity, soil resistance, geometric mean diameter (GMD), and weighted mean diameter (WMD). Bulk density was estimated using the soil dry weight and cylinder volume, as described by [
23]. To determine soil moisture, and soil porosity, we followed the protocols described by [
24]. Soil resistance was estimated using an electronic bench pen [
24]. The GMD and WMD were estimated following the protocol described by [
25].
For the soil biochemical properties, we analyzed soil pH, soil organic carbon, total nitrogen, available P, microbial respiration, microbial carbon, and microbial nitrogen. In each plot, we collected five samples, and these samples collected at each sampling point were mixed. However, the samples for each plot were measured separately. The soil pH was measured in a suspension of soil and distilled water (1:2.5,
v:
v, soil: water suspension), as described by [
26]. The soil organic carbon was determined by the rapid dichromate oxidation method [
27]. The total nitrogen was determined using sulfuric acid and potassium sulphate digestion, as described by Kjeldahl [
26]. Soil P was estimated colorimetrically using a spectrophotometer at 882 nm by extraction with Mehlich-1. The microbial respiration was determined by the incubation method, while for the microbial carbon and nitrogen, we used the fumigation-extraction method [
28].
2.8. Density of Fine Roots and Abundance of Soil Bacteria, Fungi, and Archaea via qPCR
To characterize the root density of each agroecosystem, we collected three soil monoliths (20 × 20 × 20 cm) at each plot. The monoliths were wrapped in plastic film and transported with minimal disturbance until analysis. To estimate the density of fine roots, we just collected fine roots (diameter less than 2 mm) from the soil monoliths. These roots were washed using a 0.5 mm nylon mesh bag. Root dry biomass (g) was determined after drying the samples for 48 h at 65 °C. Root density was calculated by dividing the root dry biomass by the monolith volume.
To characterize the abundance of soil microorganisms via qPCR, we consider the abundance of Archaea, Bacteria, and Fungi. Archaea indicate microorganisms that play a crucial role in nutrient cycling, particularly in extreme or anaerobic environments. Bacteria signify a diverse group of microorganisms essential for decomposing organic matter, fixing nitrogen, and promoting plant health. Fungi represent organisms that decompose complex organic materials, form symbiotic relationships with plants (mycorrhizae), and contribute to soil structure and fertility. We separated bulk from rhizospheric soil using a standardized protocol from [
29]. The dsDNA was extracted from frozen rhizosphere soil samples using the protocol described by [
30]. The dsDNA was quantified with the QuantiFluor
TM system using 485 nm excitation and 520 nm emission in the FLUOstar Omega microplate reader (BMG Labtech, Ortenberg, Germany). To account for DNA loss during extraction, dsDNA content and marker gene abundances were corrected by dividing the data by the extraction efficiency, as described in [
31]. Archaea, Bacteria, and Fungi were quantified via quantitative real-time PCR (qPCR), targeting ITS1 for fungi and the 16S rRNA gene for archaea and bacteria, using the LightCycler 480 SYBR Green I Master and Probes Master in the LightCycler 480 Instrument II. Primers used were the following: (i) NSI1 and 58A2R for Fungi [
32,
33]; (ii) BAC338F and BAC805R with probe BAC516F for Bacteria; and (iii) ARC787F and ARC1059R with probe ARC915F for Archaea [
32]. Reaction mixtures, cycling conditions, and cloning fragments for qPCR standards were used as proposed by [
30]. Standard preparation was performed as described by [
33].
2.9. Models for Provisioning and Regulating Services
We developed two primary artificial models: (i) Provisioning Services (PS), representing the direct products obtained from the studied plots, including above- and belowground biomass and litter; and (ii) Regulating Services (RS), which encompass processes that maintain environmental balance and soil health, such as nutrient cycling, erosion control, and soil quality. In constructing these models, we considered the following: (i) PS is a function of aboveground dry biomass, litter deposition, and fine root density; and (ii) RS is a function of litter quality and soil physicochemical properties. The models were developed using the “stepwise” procedure. Microbial gene diversity and dsDNA content were excluded from these models, as they are associated with supporting services, which fall outside the aim of this manuscript. Additionally, we identified three key factors influencing the artificial models for provisioning (PS) and regulating services (RS): seasonal dependency, where PS and RS are influenced by seasonal variation (SV); ecosystem dependency, where PS and RS are shaped by the ecosystem type (ECO); and management dependency, where PS and RS are affected by agroecological practices (AP). To deepen our understanding, we also analyzed interactions between these factors, considering PS and RS as functions of SV and ECO; SV and AP; AP and ECO; and the combined influence of SV, AP, and ECO.
2.10. Statistical Analysis
First, all data about aboveground dry biomass, litter deposition, litter quality, soil physical properties, soil chemical properties, root density, and microbial gene diversity were tested for normality and homoscedasticity using the “shapiro.test” and “bartlett” functions in the “stats” and “dplyr” packages, respectively. Using the “decostand” function provided in the “vegan” package, we log- transformed all data to meet the required criteria. To address the complexity of our experimental design, we initially performed a comprehensive ANOVA that accounted for all relevant sources of variation, including study sites (df = 2), years (df = 2), seasons (df = 1), agroecological practices (df = 2), ecosystems (df = 2), and their interactions. This full model was applied to evaluate treatment effects on aboveground dry biomass, litter deposition, litter quality, soil physical and chemical properties, root density, and microbial gene abundance.
Based on the significant effects identified in the full model, we then conducted three focused explanatory ANOVAs to better represent the variability in specific variables:
Applied to aboveground dry biomass; litter deposition; litter lignin; litter C, N, and P contents; root density; bacteria abundance; fungi abundance; archaea abundance; and ds DNA. Seasons and ecosystems were used as replicates, as they did not show significant effects on these variables.
- 2.
Seasons × Agroecological practice × Ecosystem:
Applied to soil pH, SOC, total N, P, microbial C, and microbial N. Years were used as replicates, as they were not statistically significant for these variables.
- 3.
Season × ecosystem:
Applied to litter lignin and litter nutrients (N, P, and C). Years and agroecological practices were used as replicates.
- 4.
Ecosystem × Agroecological practice:
Applied to bulk density, soil moisture, soil porosity, soil resistance, GMD, and WMD. Years and seasons were used as replicates.
In all cases, study sites were treated as replicates, as there were no significant differences among them. Bonferroni’s test was used as the post hoc test (
p < 0.05). To construct the predictive models for provisioning and regulating services, we used multiple linear regression analyses. The step() function from the stats package was used for stepwise model selection based on the Akaike Information Criterion (AIC), allowing automatic inclusion or exclusion of predictors to identify the most parsimonious models. Prior to model fitting, all predictor variables were checked for multicollinearity using the vif() function from the car package, and variables with VIF > 5 were either excluded or replaced by alternative predictors to reduce collinearity. Model assumptions (normality of residuals, homoscedasticity, and independence) were validated using diagnostic plots from the ggplot2 and performance packages. Adjusted R
2 and F-values were calculated to assess model fit and explanatory power. Interaction terms for ecosystem, seasonal, and management dependency were added using manually defined categorical variables, and their effects were incorporated into separate models. All visualizations were produced using ggplot2, and statistical outputs were formatted using broom and dplyr packages. All statistical analyses were conducted using R software, 3.4.0 [
34].
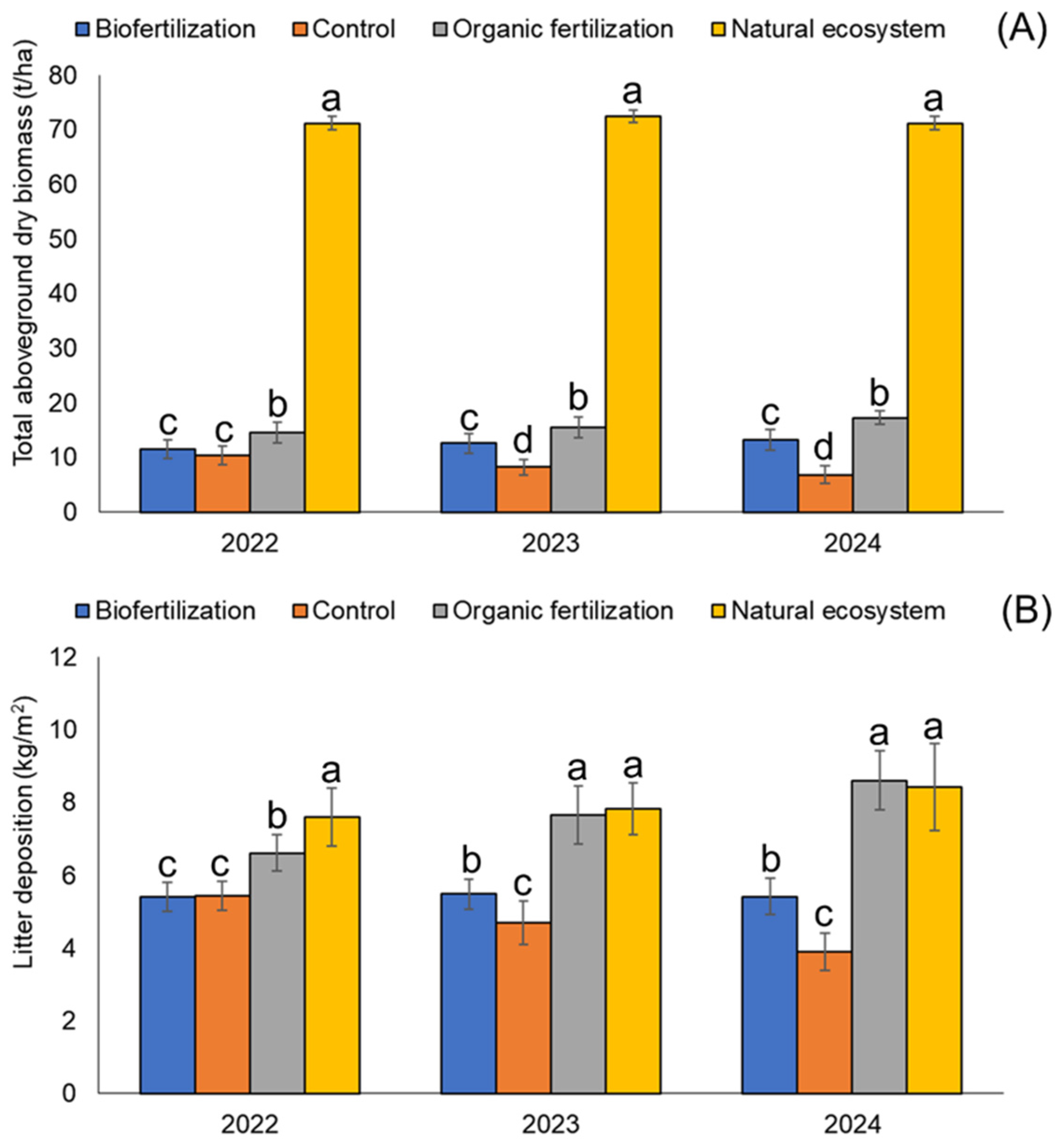
5. Conclusions
Our study demonstrates that agroecological practices significantly influence key ecosystem services in agroforestry systems and pastures, though their effects remain distinct from those observed in the natural ecosystem. Organic fertilization consistently enhanced total aboveground dry biomass and litter deposition over time, with seasonal variations favoring nutrient cycling and habitat provision, whereas the control exhibited a marked decline in these parameters. Soil chemical properties, including soil organic carbon, phosphorus, and microbial biomass, showed an exponential increase under organic fertilization, while the control plots suffered depletion, reinforcing the role of organic amendments in mitigating soil degradation. In contrast, biofertilization yielded moderate improvements, with selective effects on microbial communities and root density. Soil physical properties were strongly influenced by agroecological practices, with organic fertilization reducing bulk density and soil resistance while enhancing porosity and aggregate stability, thus mitigating compaction associated with plant diversity reduction. Litter quality varied seasonally and across ecosystems, with higher lignin and carbon content in the dry season and increased nitrogen and phosphorus during the rainy season, further emphasizing the role of plant diversity and organic inputs in sustaining soil fertility. Rhizobiome analyses revealed complex microbial responses, with organic fertilization favoring fungal proliferation and biofertilization selectively enhancing archaeal abundance, suggesting differential microbial dynamics in response to agroecological management. Despite improvements under organic fertilization and biofertilization, agroforestry systems and pastures still exhibited lower biological and physicochemical resilience than the natural ecosystem, underscoring the importance of maintaining plant diversity and organic matter inputs to sustain long-term soil health and ecosystem functionality. These findings highlight the critical role of agroecological practices in enhancing provisioning and regulating services, offering insights into sustainable land use strategies that balance productivity and ecological integrity in the Amazon basin.