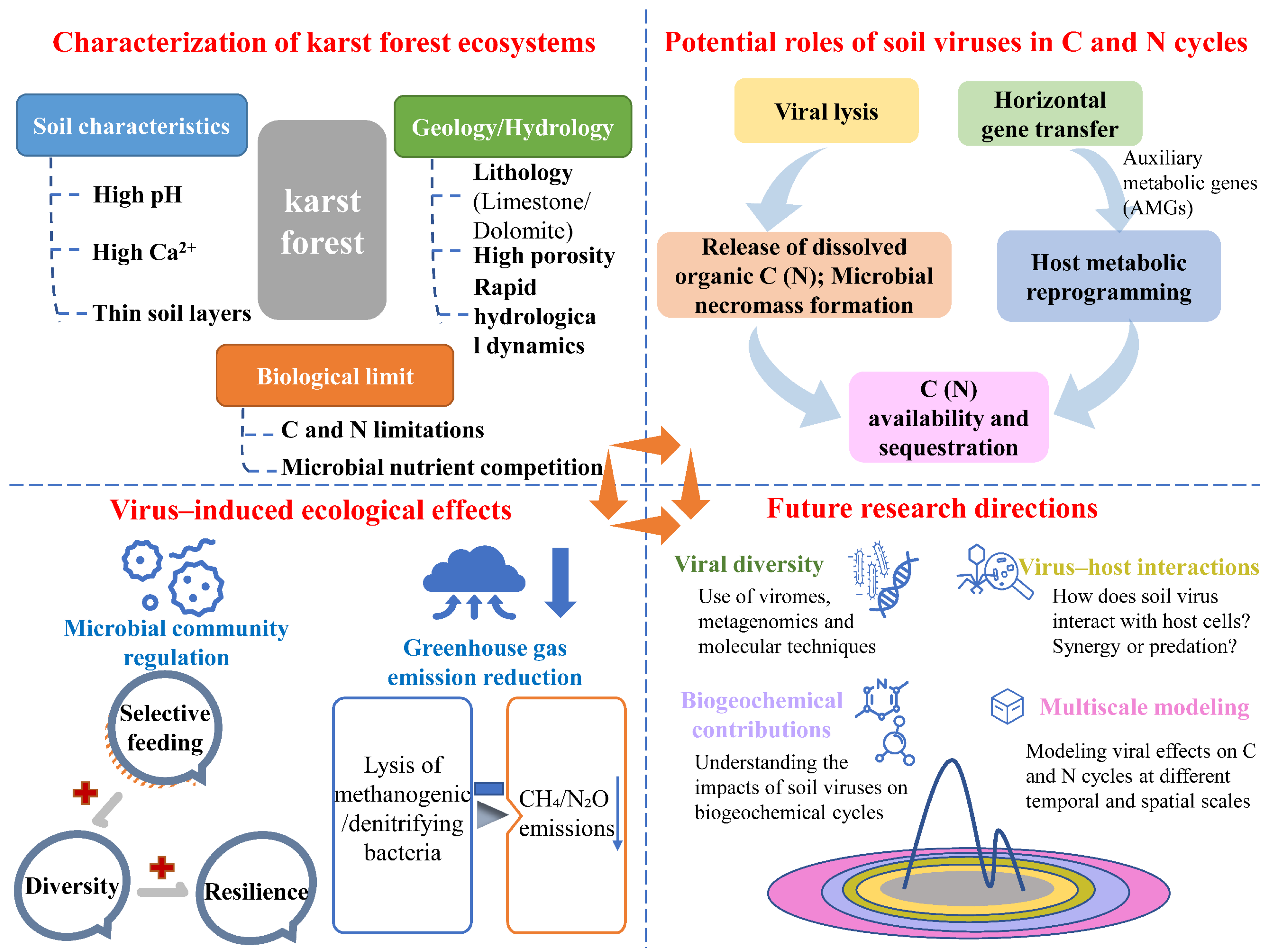
Potential Roles of Soil Viruses in Karst Forest Soil Carbon and Nitrogen Cycles
Abstract
1. Introduction
2. General Roles of Soil Viruses in C and N Cycles
2.1. Microbial Host Cell Lysis and Nutrient Release
2.2. Horizontal Gene Transfer and Metabolic Modulation
2.3. Microbial Community Dynamics
3. Unique Characteristics of Karst Forest Soil
3.1. Geology and Hydrology
3.2. Soil Chemistry with High Ca2+ and pH
3.3. Thin and Pathy Soil Layers and C and N Limitations
4. Hypothesized Roles of Soil Viruses in Karst Forest Soil C and N Cycles
4.1. Shaping Microbial Community Structure
4.2. Mediating Horizontal Gene Transfer and Microbial Metabolism
4.3. Increasing C and N Availability and Alleviating Nutrient Limitations
4.4. Promoting C Sequestration and Mitigating Climate Changes
5. Challenges and Innovations
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chevallereau, A.; Pons, B.J.; van Houte, S.; Westra, E.R. Interactions between bacterial and phage communities in natural environments. Nat. Rev. Microbiol. 2022, 20, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Gómez, P.; Buckling, A. Bacteria-phage antagonistic coevolution in soil. Science 2011, 332, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Wu, R. Soil viral diversity, ecology and climate change. Nat. Rev. Microbiol. 2023, 21, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P.; López-Mondéjar, R.; Kohout, P. Forest microbiome and global change. Nat. Rev. Microbiol. 2023, 21, 487–501. [Google Scholar] [CrossRef]
- Carreira, C.; Lønborg, C.; Acharya, B.; Aryal, L.; Buivydaite, Z.; Corrêa, F.B.; Chen, T.; Elberg, C.L.; Emerson, J.B.; Hillary, L.; et al. Integrating viruses into soil food web biogeochemistry. Nat. Microbiol. 2024, 9, 1918–1928. [Google Scholar] [CrossRef]
- Liang, X.; Radosevich, M.; DeBruyn, J.M.; Wilhelm, S.W.; McDearis, R.; Zhuang, J. Incorporating viruses into soil ecology: A new dimension to understand biogeochemical cycling. Crit. Rev. Environ. Sci. Technol. 2024, 54, 117–137. [Google Scholar] [CrossRef]
- Tong, D.; Xu, J. Element cycling by environmental viruses. Natl. Sci. Rev. 2025, 11, nwae459. [Google Scholar] [CrossRef]
- Zimmerman, A.E.; Graham, E.B.; McDermott, J.; Hofmockel, K.S. Estimating the importance of viral contributions to soil carbon dynamics. Glob. Change Biol. 2024, 30, e17524. [Google Scholar] [CrossRef]
- Osburn, E.D.; Baer, S.G.; Evans, S.E.; McBride, S.G.; Strickland, M.S. Effects of experimentally elevated virus abundance on soil carbon cycling across varying ecosystem types. Soil Biol. Biochem. 2024, 198, 109556. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, Y.; Li, H.; Zhu, S.; Sun, X.; Wu, K.; Shui, W. The characterization of microbial communities and associations in karst Tiankeng. Front. Microbiol. 2022, 13, 1002198. [Google Scholar] [CrossRef]
- Li, S.; Liu, C.; Chen, J.; Wang, S. Karst ecosystem and environment: Characteristics, evolution processes, and sustainable development. Agric. Ecosyst. Environ. 2021, 306, 107173. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, C.; Chen, H.; Yue, Y.; Zhang, W.; Zhang, M.; Qi, X.; Fu, Z. Karst landscapes of China: Patterns, ecosystem processes and services. Landsc. Ecol. 2019, 34, 2743–2763. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Nottingham, A.T.; Xiao, D.; Kuzyakov, Y.; Xu, L.; Chen, H.; Xiao, J.; Duan, P.; Tang, T.; et al. Lithological controls on soil aggregates and minerals regulate microbial carbon use efficiency and necromass stability. Environ. Sci. Technol. 2024, 58, 21186–21199. [Google Scholar] [CrossRef]
- Xiao, D.; He, X.; Zhang, W.; Cheng, M.; Hu, P.; Wang, K. Diazotroph and arbuscular mycorrhizal fungal diversity and community composition responses to karst and non-karst soils. Appl. Soil Ecol. 2022, 170, 104227. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Kuzyakov, Y.; Xiao, L.; Xiao, D.; Xu, L.; Chen, H.; Zhao, J.; Wang, K. Linking bacterial life strategies with soil organic matter accrual by karst vegetation restoration. Soil Biol. Biochem. 2023, 177, 108925. [Google Scholar] [CrossRef]
- Huang, D.; Xia, R.; Chen, C.; Liao, J.; Chen, L.; Wang, D.; Alvarez, P.J.J.; Yu, P. Adaptive strategies and ecological roles of phages in habitats under physicochemical stress. Trends Microbiol. 2024, 32, 902–916. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhao, Y.; Xiao, D.; Xu, Z.; Zhang, W.; Xiao, J.; Wang, K. Dynamics of soil nitrogen availability following vegetation restoration along a climatic gradient of a subtropical karst region in China. J. Soils Sediment. 2021, 21, 2167–2178. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Chen, H.; Xu, L.; Xiao, J.; Luo, Y.; Wang, K. Lithologic control of microbial-derived carbon in forest soils. Soil Biol. Biochem. 2022, 167, 108600. [Google Scholar] [CrossRef]
- Emerson, J.B. Soil viruses: A new hope. mSystems 2019, 4, e00120-19. [Google Scholar] [CrossRef]
- Graham, E.B.; Camargo, A.P.; Wu, R.; Neches, R.Y.; Nolan, M.; Paez-Espino, D.; Kyrpides, N.C.; Jansson, J.K.; McDermott, J.E.; Hofmockel, K.S.; et al. A global atlas of soil viruses reveals unexplored biodiversity and potential biogeochemical impacts. Nat. Microbiol. 2024, 9, 1873–1883. [Google Scholar] [CrossRef]
- Wu, H.; Cui, H.; Fu, C.; Li, R.; Qi, F.; Liu, Z.; Yang, G.; Xiao, K.; Qiao, M. Unveiling the crucial role of soil microorganisms in carbon cycling: A review. Sci. Total Environ. 2024, 909, 168627. [Google Scholar] [CrossRef] [PubMed]
- Albright, M.B.N.; Gallegos-Graves, L.V.; Feeser, K.L.; Montoya, K.; Emerson, J.B.; Shakya, M.; Dunbar, J. Experimental evidence for the impact of soil viruses on carbon cycling during surface plant litter decomposition. ISME Commun. 2022, 2, 24. [Google Scholar] [CrossRef]
- Wu, R.; Davison, M.R.; Nelson, W.C.; Smith, M.L.; Lipton, M.S.; Jansson, J.K.; McClure, R.S.; McDermott, J.E.; Hofmockel, K.S. Hi-C metagenome sequencing reveals soil phage-host interactions. Nat. Commun. 2023, 14, 7666. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, L.; Zhang, C.; Ma, D.; Zhang, J. Bacteria–virus interactions are more crucial in soil organic carbon storage than iron protection in biochar-amended paddy soils. Environ. Sci. Technol. 2023, 57, 19713–19722. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Mason-Jones, K. Viruses in soil: Nano-scale undead drivers of microbial life, biogeochemical turnover and ecosystem functions. Soil Biol. Biochem. 2018, 127, 305–317. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, D.; Ge, T.; Wang, Y.; Zhang, Y.; Liang, C.; Liao, H.; Liang, X. Unveiling the top-down control of soil viruses over microbial communities and soil organic carbon cycling: A review. Clim. Smart Agric. 2024, 1, 100022. [Google Scholar] [CrossRef]
- Wu, H.; Wan, S.; Ruan, C.; Wan, W.; Han, M.; Chen, G.; Liu, Y.; Zhu, K.; Liang, C.; Wang, G. Soil microbial necromass: The state-of-the-art, knowledge gaps, and future perspectives. Eur. J. Soil Biol. 2023, 115, 103472. [Google Scholar] [CrossRef]
- Wu, H.; Wan, S.; Ruan, C.; Niu, X.; Chen, G.; Liu, Y.; Zhu, K.; Schulin, R.; Wang, G. Phage-bacterium interactions and nutrient availability can shape C and N retention in microbial biomass. Eur. J. Soil Biol. 2022, 73, e13296. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, Y.; Dungait, J.A.J.; Liu, J.; Lin, S.; Jia, J.; Yu, G. The “regulator” function of viruses on ecosystem carbon cycling in the Anthropocene. Front. Public Health 2022, 10, 858615. [Google Scholar] [CrossRef]
- Liu, C.; Liao, H.; Gao, T.; Ai, C.; Tang, X.; Friman, V.; Zhou, S. Deciphering the hidden role of soil viruses in nitrogen cycling revealed by metagenomic stable isotope probing. Innov. Geosci. 2024, 2, 100101. [Google Scholar] [CrossRef]
- Sun, M.; Yuan, S.; Xia, R.; Ye, M.; Balcázar, J.L. Underexplored viral auxiliary metabolic genes in soil: Diversity and eco-evolutionary significance. Environ. Microbiol. 2023, 25, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Trubl, G.; Jang, H.B.; Roux, S.; Emerson, J.B.; Solonenko, N.; Vik, D.R.; Solden, L.; Ellenbogen, J.; Runyon, A.T.; Bolduc, B.; et al. Soil viruses are underexplored players in ecosystem carbon processing. mSystems 2018, 3, e00076-18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Liu, S.; Sun, M.; Yi, X.; Duan, G.; Ye, M.; Gillings, M.R.; Zhu, Y. Adaptive expression of phage auxiliary metabolic genes in paddy soils and their contribution toward global carbon sequestration. Proc. Natl. Acad. Sci. USA 2024, 121, e2419798121. [Google Scholar] [CrossRef]
- Bi, L.; Yu, D.T.; Du, S.; Zhang, L.M.; Zhang, L.Y.; Wu, C.F.; Xiong, C.; Han, L.L.; He, J.Z. Diversity and potential biogeochemical impacts of viruses in bulk and rhizosphere soils. Environ. Microbiol. 2021, 23, 588–599. [Google Scholar] [CrossRef]
- Wang, L.; Lin, D.; Xiao, K.; Ma, L.; Fu, Y.; Huo, Y.; Liu, Y.; Ye, M.; Sun, M.; Zhu, D.; et al. Soil viral–host interactions regulate microplastic-dependent carbon storage. Proc. Natl. Acad. Sci. USA 2024, 121, e2413245121. [Google Scholar] [CrossRef] [PubMed]
- Feiner, R.; Argov, T.; Rabinovich, L.; Sigal, N.; Borovok, I.; Herskovits, A.A. A new perspective on lysogeny: Prophages as active regulatory switches of bacteria. Nat. Rev. Microbiol. 2015, 13, 641–650. [Google Scholar] [CrossRef]
- Huang, D.; Yu, P.; Ye, M.; Schwarz, C.; Jiang, X.; Alvarez, P.J.J. Enhanced mutualistic symbiosis between soil phages and bacteria with elevated chromium-induced environmental stress. Microbiome 2021, 9, 150. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, Z.; Liu, H.; Li, Y.; Ge, T.; Tang, X.; He, Y.; Ma, B.; Xu, J.; Anantharaman, K.; et al. Soil nutrient conditions alter viral lifestyle strategy and potential function in phosphorous and nitrogen metabolisms. Soil Biol. Biochem. 2024, 189, 109279. [Google Scholar] [CrossRef]
- Braga, L.P.P.; Spor, A.; Kot, W.; Breuil, M.; Hansen, L.H.; Setubal, J.C.; Philippot, L. Impact of phages on soil bacterial communities and nitrogen availability under different assembly scenarios. Microbiome 2020, 8, 52. [Google Scholar] [CrossRef]
- Castledine, M.; Buckling, A. Critically evaluating the relative importance of phage in shaping microbial community composition. Trends Microbiol. 2024, 32, 957–969. [Google Scholar] [CrossRef]
- Huang, X.; Braga, L.P.P.; Ding, C.; Yang, B.; Ge, T.; Di, H.; He, Y.; Xu, J.; Philippot, L.; Li, Y. Impact of viruses on prokaryotic communities and greenhouse gas emissions in agricultural soils. Adv. Sci. 2024, 11, 2407223. [Google Scholar] [CrossRef]
- Li, H.; Zhao, S.; Gao, M.; Zhou, Y.; Xu, B.; Yang, L.; Yang, X.; Su, J. Experimental evidence for viral impact on microbial community, nitrification, and denitrification in an agriculture soil. J. Hazard. Mater. 2025, 489, 137532. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth-Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Hartmann, A.; Goldscheider, N.; Wagener, T.; Lange, J.; Weiler, M. Karst water resources in a changing world: Review of hydrological modeling approaches. Rev. Geophys. 2014, 52, 218–242. [Google Scholar] [CrossRef]
- Wu, R.; Davison, M.R.; Gao, Y.; Nicora, C.D.; Mcdermott, J.E.; Burnum-Johnson, K.E.; Hofmockel, K.S.; Jansson, J.K. Moisture modulates soil reservoirs of active DNA and RNA viruses. Commun. Biol. 2021, 4, 992. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ma, Z.; Ruan, C.; Hu, W.; Han, M.; Wan, W.; Wang, Y.; Zvomuya, F.; Liang, C.; Liu, Y.; et al. Effects of soil moisture on soil viral reproductive strategies in an agricultural soil. Eur. J. Soil Sci. 2024, 75, e13531. [Google Scholar] [CrossRef]
- He, T.; Li, J.; Du, X.; Pei, G.; Wang, A.; Hu, B.; Zhang, W.; Zhang, W.; Sun, J. Changes in SOC, pH, and Ca associated with microorganism mediated SOC mineralization and temperature sensitivity following vegetation restoration in karst regions. Plant Soil 2025. [Google Scholar] [CrossRef]
- Tang, J.; Tang, X.; Qin, Y.; He, Q.; Yi, Y.; Ji, Z. Karst rocky desertification progress: Soil calcium as a possible driving force. Sci. Total Environ. 2019, 649, 1250–1259. [Google Scholar] [CrossRef]
- Lee, S.; Sorensen, J.W.; Walker, R.L.; Emerson, J.B.; Nicol, G.W.; Hazard, C. Soil pH influences the structure of virus communities at local and global scales. Soil Biol. Biochem. 2022, 166, 108569. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Wang, L.; Zhu, Z.; Liu, Y.; Wang, J.; Chen, J.; Ge, T. Contrasting viral diversity and potential biogeochemical impacts in paddy and upland soils. Appl. Soil Ecol. 2024, 199, 105399. [Google Scholar] [CrossRef]
- Cheng, X.; Yun, Y.; Wang, H.; Ma, L.; Tian, W.; Man, B.; Liu, C. Contrasting bacterial communities and their assembly processes in karst soils under different land use. Sci. Total Environ. 2021, 751, 142263. [Google Scholar] [CrossRef] [PubMed]
- Shabtai, I.A.; Wilhelm, R.C.; Schweizer, S.A.; Höschen, C.; Buckley, D.H.; Lehmann, J. Calcium promotes persistent soil organic matter by altering microbial transformation of plant litter. Nat. Commun. 2023, 14, 6609. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, D.; Xiao, K.; Wang, K. Soil microbial processes and resource limitation in karst and non-karst forests. Funct. Ecol. 2018, 32, 1400–1409. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Mao, Q.; Xiao, K.; Wang, K. Resource limitation of soil microbes in karst ecosystems. Sci. Total Environ. 2019, 650, 241–248. [Google Scholar] [CrossRef]
- Huang, Y.; Song, X.; Wang, Y.; Canadell, J.G.; Luo, Y.; Ciais, P.; Chen, A.; Hong, S.; Wang, Y.; Tao, F.; et al. Size, distribution, and vulnerability of the global soil inorganic carbon. Science 2024, 384, 233–239. [Google Scholar] [CrossRef]
- Qin, C.; Li, S.; Yu, G.; Bass, A.M.; Yue, F.; Xu, S. Vertical variations of soil carbon under different land uses in a karst critical zone observatory (CZO), SW China. Geoderma 2022, 412, 115741. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Xiao, K.; Zhao, Y.; Liang, C.; Zhao, M.; Moore, O.W.; Otero-Fariña, A.; Zhu, Y.; Johnson, K.; Peacock, C.L. Introducing the soil mineral carbon pump. Nat. Rev. Earth Environ. 2023, 4, 135–136. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Pan, F.; Li, D.; Chen, H.; Wang, K. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Long, X.; Li, J.; Liao, X.; Wang, J.; Zhang, W.; Wang, K.; Zhao, J. Stable soil biota network enhances soil multifunctionality in agroecosystems. Glob. Change Biol. 2025, 31, e70041. [Google Scholar] [CrossRef]
- Tong, D.; Wang, Y.; Yu, H.; Shen, H.; Dahlgren, R.A.; Xu, J. Viral lysing can alleviate microbial nutrient limitations and accumulate recalcitrant dissolved organic matter components in soil. ISME J. 2023, 17, 1247–1256. [Google Scholar] [CrossRef]
- Xue, Y.; Tian, J.; Quine, T.A.; Powlson, D.; Xing, K.; Yang, L.; Kuzyakov, Y.; Dungait, J.A.J. The persistence of bacterial diversity and ecosystem multifunctionality along a disturbance intensity gradient in karst soil. Sci. Total Environ. 2020, 748, 142381. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Radosevich, M.; Zhuang, J.; DeBruyn, J.M.; Staton, M.; Schaeffer, S.M. Identification of novel viruses and their microbial hosts from soils with long-term nitrogen fertilization and cover cropping management. mSystems 2022, 7, e00571-22. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Shi, W.; Wu, N.; Liu, W.; Francis, F.; Wang, X. Enterobacter-infecting phages in nitrogen-deficient paddy soil impact nitrogen-fixation capacity and rice growth by shaping the soil microbiome. Sci. Total Environ. 2024, 956, 177382. [Google Scholar] [CrossRef]
- Zhou, Z.; Liang, X.; Zhang, N.; Xie, N.; Huang, Y.; Zhou, Y.; Li, B. The impact of soil viruses on organic carbon mineralization and microbial biomass turnover. Appl. Soil Ecol. 2024, 202, 105554. [Google Scholar] [CrossRef]
- Nannipieri, P.; Angst, G.; Mueller, C.; Pietramellara, G. The role of death and lysis of microbial and plant cells in the formation of soil organic matter. Soil Biol. Biochem. 2025, 204, 109750. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, X.; Zhu, Z.; Yuan, Z.; Yu, S.; Liu, Y.; Wang, J.; Mason-Jones, K.; Kuzyakov, Y.; Chen, J.; et al. Phages affect soil dissolved organic matter mineralization by shaping bacterial communities. Environ. Sci. Technol. 2025, 59, 2070–2081. [Google Scholar] [CrossRef]
- Koskella, B.; Brockhurst, M.A. Bacteria–phage coevolution as a driver of ecological and evolutionary processes in microbial communities. FEMS Microbiol. Rev. 2014, 38, 916–931. [Google Scholar] [CrossRef]
- Safari, F.; Sharifi, M.; Farajnia, S.; Akbari, B.; Karimi Baba Ahmadi, M.; Negahdaripour, M.; Ghasemi, Y. The interaction of phages and bacteria: The co-evolutionary arms race. Crit. Rev. Biotechnol. 2020, 40, 119–137. [Google Scholar] [CrossRef]
- Ji, M.; Fan, X.; Cornell, C.R.; Zhang, Y.; Yuan, M.M.; Tian, Z.; Sun, K.; Gao, R.; Liu, Y.; Zhou, J. Tundra soil viruses mediate responses of microbial communities to climate warming. mBio 2023, 14, e0300922. [Google Scholar] [CrossRef]
- Jansson, J.K. Soil viruses: Understudied agents of soil ecology. Environ. Microbiol. 2023, 25, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, T.; Chen, Q.; Chen, T.; Hu, J.; Sun, L.; Wang, B.; Li, W.; Ni, J. Unveiling the unknown viral world in groundwater. Nat. Commun. 2024, 15, 6788. [Google Scholar] [CrossRef]
- Wang, G.; Or, D. Aqueous films limit bacterial cell motility and colony expansion on partially saturated rough surfaces. Environ. Microbiol. 2010, 12, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Or, D. Hydration dynamics promote bacterial coexistence on rough surfaces. ISME J. 2013, 7, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Trubl, G.; Kimbrel, J.A.; Liquet-Gonzalez, J.; Nuccio, E.E.; Weber, P.K.; Pett-Ridge, J.; Jansson, J.K.; Waldrop, M.P.; Blazewicz, S.J. Active virus-host interactions at sub-freezing temperatures in Arctic peat soil. Microbiome 2021, 9, 208. [Google Scholar] [CrossRef]
- Umair, M.; Sun, N.; Du, H.; Hui, N.; Altaf, M.; Du, B.; Yin, S.; Liu, C. Bacterial communities are more sensitive to water addition than fungal communities due to higher soil K and Na in a degraded karst ecosystem of Southwestern China. Front. Microbiol. 2020, 11, 562546. [Google Scholar] [CrossRef] [PubMed]
- Buivydaitė, Ž.; Aryal, L.; Corrêa, F.B.; Chen, T.; Langlois, V.; Elberg, C.L.; Netherway, T.; Wang, R.; Zhao, T.; Acharya, B.; et al. Meeting report: The first soil viral workshop 2022. Virus Res. 2023, 331, 199121. [Google Scholar] [CrossRef]
- Santillán, J.; López-Martínez, R.; Aguilar-Rangel, E.J.; Hernández-García, K.; Vásquez-Murrieta, M.S.; Cram, S.; Alcántara-Hernández, R.J. Microbial diversity and physicochemical characteristics of tropical karst soils in the northeastern Yucatan peninsula, Mexico. Appl. Soil Ecol. 2021, 165, 103969. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, L.; Brandt, M.; Zhang, X.; Wang, K. A social-ecological framework to enhance sustainable reforestation under geological constraints. Earth’s Future 2024, 12, e2023EF004335. [Google Scholar] [CrossRef]
- Hultman, J.; Waldrop, M.P.; Mackelprang, R.; David, M.M.; McFarland, J.; Blazewicz, S.J.; Harden, J.; Turetsky, M.R.; McGuire, A.D.; Shah, M.B.; et al. Multi-omics of permafrost, active layer and thermokarst bog soil microbiomes. Nature. 2015, 521, 208–212. [Google Scholar] [CrossRef]
- Chen, Y.; Sadiq, S.; Tian, J.; Chen, X.; Lin, X.; Shen, J.; Chen, H.; Hao, Z.; Wille, M.; Zhou, Z.; et al. RNA viromes from terrestrial sites across China expand environmental viral diversity. Nat. Microbiol. 2022, 7, 1312–1323. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.M.; Kramer, R.; Van Goethem, M.W.; Makhalanyane, T.P.; Hogg, I.; Cowan, D.A. Environmental drivers of viral community composition in Antarctic soils identified by viromics. Microbiome 2017, 5, 83. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Li, H.; Duan, C.; Zhou, X.; Luo, Q.; An, X.; Zhu, Y.; Su, J. Response of soil viral communities to land use changes. Nat. Commun. 2022, 13, 6027. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; He, H.; Zheng, S. Microfluidics in single-cell virology: Technologies and applications. Trends. Biotechnol. 2020, 38, 1360–1372. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Liu, D.; Zhang, Z. Advances in virus-host interaction research based on microfluidic platforms. Chin. Chem. Lett. 2024, 35, 110302. [Google Scholar] [CrossRef]
- Herrmann, A.M.; Ritz, K.; Nunan, N.; Clode, P.L.; Pett-Ridge, J.; Kilburn, M.R.; Murphy, D.V.; O Donnell, A.G.; Stockdale, E.A. Nano-scale secondary ion mass spectrometry—A new analytical tool in biogeochemistry and soil ecology: A review article. Soil Biol. Biochem. 2007, 39, 1835–1850. [Google Scholar] [CrossRef]
- Kaiser, C.; Kilburn, M.R.; Clode, P.L.; Fuchslueger, L.; Koranda, M.; Cliff, J.B.; Solaiman, Z.M.; Murphy, D.V. Exploring the transfer of recent plant photosynthates to soil microbes: Mycorrhizal pathway vs direct root exudation. New Phytol. 2015, 205, 1537–1551. [Google Scholar] [CrossRef]
- Peth, S.; Chenu, C.; Leblond, N.; Mordhorst, A.; Garnier, P.; Nunan, N.; Pot, V.; Ogurreck, M.; Beckmann, F. Localization of soil organic matter in soil aggregates using synchrotron-based X-ray microtomography. Soil Biol. Biochem. 2014, 78, 189–194. [Google Scholar] [CrossRef]
- Coclet, C.; Sorensen, P.O.; Karaoz, U.; Wang, S.; Brodie, E.L.; Eloe-Fadrosh, E.A.; Roux, S. Virus diversity and activity is driven by snowmelt and host dynamics in a high-altitude watershed soil ecosystem. Microbiome 2023, 11, 237. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Wu, N.; Ling, Q.; Tang, T.; Hu, P.; Duan, P.; Zhang, Q.; Xiao, J.; Zhao, J.; Zhang, W.; et al. Potential Roles of Soil Viruses in Karst Forest Soil Carbon and Nitrogen Cycles. Forests 2025, 16, 735. https://doi.org/10.3390/f16050735
Wu H, Wu N, Ling Q, Tang T, Hu P, Duan P, Zhang Q, Xiao J, Zhao J, Zhang W, et al. Potential Roles of Soil Viruses in Karst Forest Soil Carbon and Nitrogen Cycles. Forests. 2025; 16(5):735. https://doi.org/10.3390/f16050735
Chicago/Turabian StyleWu, Hanqing, Nan Wu, Qiumei Ling, Tiangang Tang, Peilei Hu, Pengpeng Duan, Qian Zhang, Jun Xiao, Jie Zhao, Wei Zhang, and et al. 2025. "Potential Roles of Soil Viruses in Karst Forest Soil Carbon and Nitrogen Cycles" Forests 16, no. 5: 735. https://doi.org/10.3390/f16050735
APA StyleWu, H., Wu, N., Ling, Q., Tang, T., Hu, P., Duan, P., Zhang, Q., Xiao, J., Zhao, J., Zhang, W., Chen, H., & Wang, K. (2025). Potential Roles of Soil Viruses in Karst Forest Soil Carbon and Nitrogen Cycles. Forests, 16(5), 735. https://doi.org/10.3390/f16050735









