Abstract
Soil viruses, ubiquitous and abundant biological entities that are integral to microbial communities, exert pivotal impacts on ecosystem functionality, particularly within carbon (C) and nitrogen (N) cycles, through intricate interactions with bacteria, archaea, fungi, and other microbial taxa. While their contributions to soil ecosystem dynamics are increasingly elucidated, the specific roles of soil viruses in karst forest soil remain largely underexplored. Karst ecosystems (covering 15% of the global terrestrial surface) are characterized by unique geological formations, thin and patchy soil layers, high pH and Ca2+, and rapid hydrological dynamics, collectively fostering unique environmental conditions that may shape viral ecology and modulate C and N cycling. This perspective synthesizes existing knowledge of soil viral functions with the distinctive characteristics of karst forest soil, proposing potential mechanisms by which soil viruses could influence C and N cycling in such fragile ecosystems. Soil viruses regulate C and N cycles both directly and indirectly via their interactions with microbial hosts, mainly including shaping the microbial community structure, mediating horizontal gene transfer and microbial metabolism, increasing C and N availability and alleviating nutrient limitations, promoting C and N sequestration, and mitigating climate change. This work aims to bridge soil viral ecology and karst biogeochemical cycles, providing insights into sustainable forest stewardship and climate resilience. We delineate critical knowledge gaps and propose future perspectives, advocating for targeted metagenomic and long-term experimental studies into viral diversity, virus–host-environment interactions, and temporal dynamics. Specifically, we advocate the following research priorities to advance our understanding of soil viruses in karst forest ecosystems in future studies: (I) soil viral diversity, abundance, and activity: characterizing the diversity, abundance, and activity of soil viruses in karst forests using metagenomics and complementary molecular approaches; (II) virus–host interactions: investigating the dynamics between the viruses and key microbial taxa involved in C and N cycling; (III) biogeochemical impacts: quantifying the contributions of viral lysis and horizontal gene transfer to C and N fluxes within karst forest soil; and (IV) modeling the viral impacts on C and N cycles: developing integrative models that incorporate soil virus-mediated processes into existing karst forest soil biogeochemical frameworks at different temporal and spatial scales. Such efforts are essential to validate the hypothesized viral roles and underlying mechanisms, offering a foundation for nature-based solutions to facilitate C and N cycling and support ecological restoration in vulnerable karst regions amid global climate change.
1. Introduction
Soil viruses, ubiquitous and enigmatic constituents of microbial communities, play pivotal roles in regulating ecosystem processes through their intricate interactions with bacteria, archaea, fungi, and other microbial taxa [1,2,3]. These interactions directly influence carbon (C) and nitrogen (N) biogeochemical cycles, shape the microbial community structure, and bolster ecosystem resilience [1,4,5]. Through mechanisms such as lysing microbial host cells (lysis) or integrating into their genomes as prophages (lysogeny), formatting microbial necromass, transferring horizontal genes, and shaping microbial diversity, soil viruses influence global C and N cycles—processes vital to climate mitigation and ecosystem productivity [5,6,7,8]. Nevertheless, the specific roles of soil viruses in C and N cycling processes are still not completely understood [9]. In particular, in nutrient-limited ecosystems, such as karst forest ecosystems, soil viruses may play key roles by driving microbial turnover and increasing C and N availability.
Karst ecosystems, covering approximately 15% of the Earth’s terrestrial surface, are characterized by their unique landscapes formed on soluble carbonate rocks (e.g., limestone and dolomite) [10,11,12]. These areas feature thin and patchy soil layers, a high pH due to calcium carbonate dissolution, and dual hydrological structures characterized by rapid ecohydrological dynamics [11,12,13]. Such conditions foster diverse microbial assemblages that drive essential biogeochemical transformation [14,15]. Moreover, the environmental heterogeneity inherent to karst landscapes may exert distinct impacts on the soil viral community [16], potentially amplifying or altering their roles in C and N cycles within karst forest soil. Furthermore, the unique physicochemical properties of karst soil—high porosity, carbonate content, and soil organic matter (SOM) dynamics—create a distinctive ecological niche where virus–host interactions could have profound effects on C and N fluxes.
Despite their ecological significance, the roles and functions of soil viruses infecting microorganisms within karst forest C and N cycles remain largely unknown, limiting our capacity to predict and manage degraded karst ecosystems [17,18]. Elucidating these viral roles holds promise for advancing sustainable forest management by enhancing soil health, plant productivity, and ecosystem resilience to climate change [3,6,7,19].
Therefore, this perspective aims to synthesize the existing knowledge of soil viral ecology and its biogeochemical implications, extending these insights into a karst-specific context to hypothesize the potential mechanisms by which viruses regulate C and N cycles in karst forest soil and advocate for targeted research to address critical knowledge gaps (Figure 1).
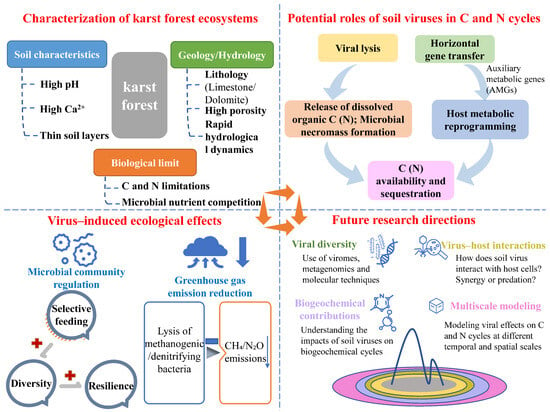
Figure 1.
A conceptual diagram illustrating the potential roles of soil viruses in karst forest soil carbon and nitrogen cycles, and their ecological implications and future research directions.
2. General Roles of Soil Viruses in C and N Cycles
Soil viruses, primarily bacteriophages and archaeal viruses, exhibit remarkable abundance and diversity in terrestrial ecosystems [1,3]. Their significance in microbial ecology and biogeochemistry is increasingly recognized, with profound implications for C and N cycling [5,7,20]. The impacts of soil viral infections on C and N cycling vary across ecosystems, depending on specific environmental contexts and conditions [21,22]. Generally, soil viruses modulate C and N cycles through several key mechanisms, primarily via interactions with microbial hosts [1,23,24].
2.1. Microbial Host Cell Lysis and Nutrient Release
The virus-induced lysis of microbial host cells releases intracellular dissolved organic C and N compounds into the soil matrix—a process termed the “Viral Shunt”—enhancing C and N bioavailability for microbial and plant uptake and yielding a microbial necromass that promotes soil C and N retention, potentially accelerating C and N cycling [9,25,26,27,28]. It was estimated that the “Viral Shunt” contributed an average of 8.6‰ of the global C cycle, with a higher contribution in terrestrial ecosystems (6.7‰) compared to marine ecosystems (1.4‰) [29].
2.2. Horizontal Gene Transfer and Metabolic Modulation
Soil viruses encode and transfer auxiliary metabolic genes (AMGs) to their hosts, modifying host metabolic pathways and facilitating the spread and exchange of functional genes related to C and N cycling processes among microbial populations [30,31,32,33]. Metagenomic analyses have identified AMGs linked to C and N turnover in soil viral communities [33,34,35], which can alter host metabolism and redirect host cellular resources toward viral replication. Lysogenic viruses, in particular, can integrate into host genomes, exerting a sustained influence on microbial metabolism over longer timescales [36,37,38]. For instance, AMGs encoding glycoside hydrolases may enhance SOM decomposition, while nitrogenase genes could boost N fixation, suggesting the bottom-up control of soil viruses in C and N dynamics [26,32,34]. In addition, the viral reprogramming of host metabolism may alter microbial C and N use efficiency and turnover rates [5,25].
2.3. Microbial Community Dynamics
Through the selective predation of specific microbial taxa, soil viruses shape microbial community composition and diversity, with cascading effects on C and N cycling processes [22,39,40]. Experimental evidence demonstrates that introducing exogenous viruses into soil alters associations between bacterial and fungal diversity, dissolved organic C, and total N, impacting litter decomposition and C respiration rates in a community-specific manner [22]. Moreover, Braga et al. [39] also revealed that variations in soil viral stress modified bacterial community composition and diversity, with active phages elevating the soil ammonium concentration. Furthermore, soil viruses can influence N fixation, nitrification, and denitrification processes by lysing key microbial functional groups or transferring N-related functional genes, with phyla such as Proteobacteria, Acidobacteria, Actinobacteria, and Bacteroidetes dominating in virus-amended treatments [5,41,42].
These general findings provide a conceptual framework, yet their relevance to karst forest soil requires further consideration of unique environmental conditions.
3. Unique Characteristics of Karst Forest Soil
Karst forest soil is characterized by distinct geological and hydrological properties, soil chemistry, thin soil layers, and resource limitations [11,12]. This soil is developed from carbonate (e.g., limestone and dolomite) bedrock, where dissolution and weathering processes create a heterogeneous matrix of soil and rock. The unique physical and chemical properties of karst soil pose challenges to microbial communities, including limited C and N availability and fluctuating moisture conditions [43]. Consequently, karst forest ecosystems present several distinctive features that may influence soil viral ecology and their roles in C and N cycles.
3.1. Geology and Hydrology
Karst landscapes arise from the dissolution and weathering of soluble carbonate bedrock, resulting in features like caves, sinkholes, and underground rivers [10,11,12]. This geomorphology leads to rapid water infiltration through fractures and conduits, influencing nutrient transport and microbial distribution and causing high spatial variability in soil moisture and nutrient availability [44]. Such hydrological dynamics can create microhabitats with contrasting conditions for viral survival and proliferation, fostering spatial heterogeneity within viral communities in karst forest soil [16]. Wu et al. [45] demonstrated that fluctuations in soil moisture profoundly alter the composition, activity, and functional potential of both DNA and RNA soil viruses. Furthermore, Wu et al. [46] identified soil moisture as the dominant factor shaping viral reproductive strategies by altering nutrient availability and host population dynamics, highlighting a shift in viral life history strategies under water stress, which may affect virus–bacteria interactions and nutrient dynamics, enhancing host adaptability. Thus, hydrological dynamics can modulate soil viral activity by altering soil moisture and redox conditions and thereby influence C and N cycles in karst forest ecosystems. The Global Soil Virus Atlas also indicates high spatial turnover in viral communities [20], suggesting that karst areas may harbor distinct viral populations adapted to high pH and rapid hydrological fluxes.
3.2. Soil Chemistry with High Ca2+ and pH
Karst soil typically has a high pH (>8.0) and Ca2+ content due to calcium carbonate dissolution and weathering, selectively favoring specific microbial taxa (e.g., Acidobacteria and Verrucomicrobia) adapted to alkaline conditions [47,48]. Previous studies have documented that the soil viral community structure was significantly influenced by pH [33,49,50], suggesting that karst soil may support specialized viral populations adapted to alkaline conditions. Moreover, soil pH is a significant factor governing the bacterial community structure in karst soil, with profound implications for virus–bacteria interactions [51]. In addition, elevated Ca2+ can significantly promote hyphae-forming bacteria and mineral–microbial byproducts associations, increasing litter incorporation into microbial biomass and C use efficiency by 45% while reducing cumulative CO2 production by 4% [52]. Therefore, the distinct soil chemistry and pH characteristics of karst forest soil likely modulate viral community dynamics and virus–bacteria interactions, thereby impacting C and N cycling processes.
3.3. Thin and Pathy Soil Layers and C and N Limitations
Thin and patchy soil in karst forest ecosystems may lead to intense competition for resources, including C, nutrients, and water, shaping microbial diversity and potentially amplifying the viral impacts on community dynamics [11,53,54]. Soil organic C, derived primarily from plant litter and root exudates, is often limited in karst areas and serves as a key determinant of microbial abundance [53,54]. Moreover, the high carbonate bedrock content of this soil complicates C cycling, as the interplay between microbial C pump-mediated organic C and mineral C pump-mediated inorganic C pools influences soil respiration and C sequestration [55,56,57,58]. N, often a limiting nutrient in karst forest soil [59], relies heavily on microbial N fixation and mineralization to sustain primary productivity [14]. Despite these nutrient limitations, karst soil hosts diverse microbial communities, including bacteria, fungi, archaea, viruses, and protists, which play essential roles in C and N cycling [60]. Notably, soil viral lysing can alleviate microbial nutrient limitations and accumulate recalcitrant dissolved organic matter components in soil [61]. Hence, the unique soil layers and resource limitations of the karst environment may be alleviated by viral activity, subsequently affecting C and N cycles.
Collectively, these characteristics suggest that karst soil harbors unique microbial host and viral communities, with significant implications for regulating C and N cycling processes.
4. Hypothesized Roles of Soil Viruses in Karst Forest Soil C and N Cycles
Based on the general viral ecology and distinctive conditions of karst ecosystems, we proposed four hypotheses delineating the potential roles and mechanisms by which soil viruses influence C and N cycling in karst forest soil.
4.1. Shaping Microbial Community Structure
Soil viruses infect the key microorganisms involved in C and N cycling, thereby altering the fate of soil C and N, which can result in either an increase or decrease in respiratory C loss, reflecting the context-dependent nature of viral pressures on microbial communities [9,26]. In karst forest ecosystems, the thin soil layers and high pH may select specific microbial taxa vulnerable to viral predation, thereby influencing virus–host interactions and C and N turnover dynamics [40]. For example, taxa such as Proteobacteria, Actinobacteria, Acidobacteria, Bacteroidetes, Chloroflexi, and Verrucomicrobia, which are prevalent in karst soil [51,62], may serve as viral hosts. The lysis of dominant decomposers or N-fixing microorganisms can restructure microbial communities and influence subsequent C sequestration and N fixation processes [63,64,65]. Virus-induced mortality generates microbial necromass, a critical constituent of soil organic C and N pools [5,19,27,28,66]. Moreover, the soil viral predation of methanogens or denitrifiers may regulate methane (CH4) and nitrous oxide (N2O) emissions, influencing greenhouse gas fluxes in karst ecosystems. Zhao et al. [67] demonstrated that soil viruses enhance dissolved organic matter mineralization by shifting microbial communities and enriching some taxa (such as Pseudomonas, Anaerocolumna, and Caulobacter) involved in degrading complex compounds, consequently amplifying C cycling functions. Additionally, temperate viruses that enter lysogeny maintain long-term associations with their hosts, and mutually beneficial interactions can evolve that support the efficient reproduction of both viruses and bacteria [36]. Notably, virus–host coevolution, referring to the reciprocal evolution between hosts and the viruses that infect them, emerges as a key regulator of ecological and evolutionary processes within microbial communities [68,69].
4.2. Mediating Horizontal Gene Transfer and Microbial Metabolism
While acting as agents of microbial mortality, soil viruses simultaneously foster microbial diversity and evolution through horizontal gene transfer (HGT) and community restructuring [19,26]. Soil viruses facilitate HGT among microbial populations, bolstering microbial adaptability and evolution, particularly in SOM turnover and nutrient cycling [19]. By encoding and transferring AMGs, soil viruses reprogram microbial C and N metabolism, exerting a pivotal influence on ecosystem processes [19,20,70]. Moreover, the discovery of a variety of virus-encoded AMGs in soil underscores the complexity and indispensability of viral involvement in biogeochemical cycles, including C, N, sulfur, and phosphorus [30,71,72]. Notably, the porous structure and rapid ecohydrological processes of karst forest soil may facilitate viral-mediated HGT and microbial metabolism, promoting the spread of functional genes involved in C and N cycling.
4.3. Increasing C and N Availability and Alleviating Nutrient Limitations
In nutrient-limited karst forest soil, viral lysis may disproportionately increase labile C and N pools by releasing intracellular dissolved organic C and N, which could improve the substrate and N availability for the growth of plants and microbes [25,39]. These processes potentially accelerate C and N cycles, especially in karst areas with rapid hydrological fluxes. Experimental viral additions to non-karst soil have been shown to boost respiration rates and CO2 emissions [22], suggesting similar effects in karst ecosystems. Additionally, rapid water movement may further promote microbial motility and nutrient transport, potentially sustaining plant productivity in otherwise oligotrophic conditions [73,74].
4.4. Promoting C Sequestration and Mitigating Climate Changes
Soil viruses influence microbial death and necromass formation, which is critical to C sequestration and could benefit mitigate climate change and optimize forest management strategies aiming to increase SOM inputs and soil C stocks [25,66,75,76]. Moreover, karst forest ecosystems, highly sensitive to climate change, experience shifts in soil moisture driven by precipitation patterns [11,12]. Viruses may enhance microbial community resilience by promoting microbial diversity and environmental adaptability through virus–host interplays [16,77]. The adaptability of viral communities to environmental perturbations, surpassing that of prokaryotic communities, underscores their unique potential to regulate microbial mortality with nutrient turnover and food web dynamics, potentially offering resilience to ecosystems under climate change [16,21,22,38]. As research continues, it is anticipated that the roles of soil viruses in food webs, SOM decomposition, and greenhouse gas emissions will become clearer, further highlighting their significance in biogeochemical cycling [6,22]. Thus, investigating soil viruses in karst forest ecosystems is crucial not only for scientific advancement but also for informing ecosystem management strategies and combating climate change.
Collectively, these hypotheses provide a conceptual framework for exploring the interplay between soil viruses, microbial communities, and biogeochemical cycles in karst forest soil. Despite the importance of soil viruses being increasingly recognized, the lack of relevant research limits our comprehensive understanding of C and N cycles in karst forest soil. Future investigations should prioritize soil viral diversity, virus–host interactions, and their spatiotemporal dynamics in karst regions to validate these proposed mechanisms.
5. Challenges and Innovations
Investigating the roles of soil viruses in C and N cycles, particularly within the karst forest ecosystem, remains an emergent field fraught with challenges and limitations. One primary challenge is the intricate complexity of the bedrock–plant–soil–microbe continuum in karst forest ecosystems, which differ significantly from those in non-karst environments, confounding efforts to delineate viral impacts on these systems [78,79]. Moreover, the context-dependent nature of viral influence further complicates research endeavors. While some studies suggest that increased viral loads can alter C and nutrient cycling, the specific outcomes vary across ecosystems, indicating that universal generalizations may be untenable [9,22]. This variability demands a refined understanding of how viruses interact with microbial communities and influence C and N processes across diverse ecological settings. Additionally, the inherent fragility and sensitivity of karst ecosystems to environmental changes pose additional challenges [11]. The intricate relationships among viruses, microbial communities, bedrock, and soil properties in karst regions require meticulous scrutiny to preserve the delicate ecological balance that sustains these ecosystems [80].
Concurrently, the rapid development of high-throughput, multi-omics technology, and metagenomics (as well as metatranscriptomics, metaproteomics, metabolomics, etc.) has made it possible to conduct in-depth research on soil viromics [81,82]. Metagenomics and metavirome sequencing have become cornerstone tools for studying soil viral diversity [83,84], yet their application in karst systems faces unique hurdles. The high Ca2+ content of karst forest soil enhances viral adsorption to mineral surfaces [13,18], potentially diminishing the efficiency of extracellular DNA or RNA recovery. Additionally, spatial heterogeneity in karst fissure networks complicates representative sampling, as viral communities at rock–soil interfaces exhibit higher diversity than bulk soil. Recent innovations, such as microfluidic-enabled single-virus genomics, hold promise for resolving virus–host interactions [85,86]. Nanoscale Secondary Ion Mass Spectrometry (NanoSIMS) can quantify virus-mediated C transfers at the single-cell level [87,88], while synchrotron-based X-ray microscopy provides a detailed visualization of virus-mineral associations within porous microenvironment [89].
6. Conclusions and Future Perspectives
Soil viruses are emerging as critical regulators in karst forest soil C and N cycles, shaped by the ecosystem’s unique environmental conditions [6,90]. Previous relevant studies have indicated that the energy and nutrients stored in viruses, as well as their biogeochemical significance in soil, may have been overlooked, especially in places where resources are scarce and microbial movement may be physically restricted. Although some progress has been made in understanding the role of viruses in soil biogeochemistry, their specific functions and impacts remain poorly resolved. Broadening our understanding of these intricate interactions is crucial for fully comprehending the ecological impacts of viruses in soil ecosystems [5]. By shaping virus–host interactions, soil viruses contribute to microbial mortality, C and N cycling, and micro-food web dynamics [5,21], particularly in nutrient-limited karst forest ecosystems. Moreover, recent studies have shown that soil viruses are abundant and affected by climate change. Since soil viral communities are closely related to their host communities, the changes in viruses caused by climate change may be an indirect consequence of changes in their hosts. With climate change, the complex interactions between the physical environment of soil habitats and hosts determine the ultimate fate and functions of soil viruses. Interestingly, soil viruses can carry AMGs, which may contribute to carbon and nutrient cycling as well as other yet-to-be-understood processes in soil [3]. The potential roles of soil viruses in C and N cycles suggest nature-based solutions for enhancing soil C storage and mitigating climate change [3]. Integrating viral ecology into karst soil research offers exciting opportunities to uncover novel mechanisms of C and N cycling and improve our understanding of ecosystem functioning in karst forest ecosystems. Understanding virus–microbe–environment interactions can aid in developing strategies that not only improve soil health but also enhance the resilience of karst forest ecosystems to environmental changes [19,26]. Notably, incorporating insights from the roles of soil viruses in karst forest soil C and N cycles into sustainable forest management could lead to innovative practices that improve soil health, plant productivity, and ecological restoration in degraded karst ecosystems [6,85].
Despite their recognized importance, more research is needed to fully understand the specific regulatory mechanisms employed by viruses in karst forest soil C and N cycles. To guide this effort, we propose three critical scientific questions: (1) How do soil viruses modulate microbial C and N cycling in calcium-rich, heterogeneous karst forest soil? (2) What environmental drivers govern virus–host interactions in karst forest soil? (3) Can viral-informed strategies enhance karst forest ecosystem resilience to climate change? Meanwhile, we advocate for the following research priorities to advance our understanding of soil viruses in karst forest ecosystems in future studies:
(I) Soil viral diversity, abundance, and activity: Characterizing the diversity, abundance, and activity of soil viruses in karst forests using metagenomics and complementary molecular approaches;
(II) Virus–host interactions: Investigating the dynamics between viruses and key microbial taxa involved in C and N cycling;
(III) Biogeochemical impacts: Quantifying the contributions of viral lysis and horizontal gene transfer to C and N fluxes within karst forest soil;
(IV) Modeling viral impacts on C and N cycles: Developing integrative models that incorporate soil virus-mediated processes into existing karst forest soil biogeochemical frameworks on different temporal and spatial scales.
Author Contributions
Conceptualization, W.Z. and H.W.; writing—original draft preparation, H.W. and N.W.; validation, Q.L., T.T., P.H., P.D., Q.Z., J.X., J.Z., W.Z., H.C. and K.W.; writing—review and editing, H.W., N.W. and W.Z.; supervision, W.Z. and H.W.; project administration, W.Z. and H.W.; funding acquisition, W.Z. and H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Natural Science Foundation of Guangxi Zhuang Autonomous Region (2023GXNSFBA026286; 2025GXNSFAA069812); the Natural Science Foundation of China (42407045); the Natural Science Foundation of Hunan Province (2023JJ40652); the Natural Science Foundation of Changsha (kq2208243); the China Postdoctoral Science Foundation (2022M723365).
Data Availability Statement
Data will be made available upon request.
Acknowledgments
We thank the Institutional Center for Shared Technologies and Facilities of the Institute of Subtropical Agriculture, Chinese Academy of Sciences.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chevallereau, A.; Pons, B.J.; van Houte, S.; Westra, E.R. Interactions between bacterial and phage communities in natural environments. Nat. Rev. Microbiol. 2022, 20, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Gómez, P.; Buckling, A. Bacteria-phage antagonistic coevolution in soil. Science 2011, 332, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Wu, R. Soil viral diversity, ecology and climate change. Nat. Rev. Microbiol. 2023, 21, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P.; López-Mondéjar, R.; Kohout, P. Forest microbiome and global change. Nat. Rev. Microbiol. 2023, 21, 487–501. [Google Scholar] [CrossRef]
- Carreira, C.; Lønborg, C.; Acharya, B.; Aryal, L.; Buivydaite, Z.; Corrêa, F.B.; Chen, T.; Elberg, C.L.; Emerson, J.B.; Hillary, L.; et al. Integrating viruses into soil food web biogeochemistry. Nat. Microbiol. 2024, 9, 1918–1928. [Google Scholar] [CrossRef]
- Liang, X.; Radosevich, M.; DeBruyn, J.M.; Wilhelm, S.W.; McDearis, R.; Zhuang, J. Incorporating viruses into soil ecology: A new dimension to understand biogeochemical cycling. Crit. Rev. Environ. Sci. Technol. 2024, 54, 117–137. [Google Scholar] [CrossRef]
- Tong, D.; Xu, J. Element cycling by environmental viruses. Natl. Sci. Rev. 2025, 11, nwae459. [Google Scholar] [CrossRef]
- Zimmerman, A.E.; Graham, E.B.; McDermott, J.; Hofmockel, K.S. Estimating the importance of viral contributions to soil carbon dynamics. Glob. Change Biol. 2024, 30, e17524. [Google Scholar] [CrossRef]
- Osburn, E.D.; Baer, S.G.; Evans, S.E.; McBride, S.G.; Strickland, M.S. Effects of experimentally elevated virus abundance on soil carbon cycling across varying ecosystem types. Soil Biol. Biochem. 2024, 198, 109556. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, Y.; Li, H.; Zhu, S.; Sun, X.; Wu, K.; Shui, W. The characterization of microbial communities and associations in karst Tiankeng. Front. Microbiol. 2022, 13, 1002198. [Google Scholar] [CrossRef]
- Li, S.; Liu, C.; Chen, J.; Wang, S. Karst ecosystem and environment: Characteristics, evolution processes, and sustainable development. Agric. Ecosyst. Environ. 2021, 306, 107173. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, C.; Chen, H.; Yue, Y.; Zhang, W.; Zhang, M.; Qi, X.; Fu, Z. Karst landscapes of China: Patterns, ecosystem processes and services. Landsc. Ecol. 2019, 34, 2743–2763. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Nottingham, A.T.; Xiao, D.; Kuzyakov, Y.; Xu, L.; Chen, H.; Xiao, J.; Duan, P.; Tang, T.; et al. Lithological controls on soil aggregates and minerals regulate microbial carbon use efficiency and necromass stability. Environ. Sci. Technol. 2024, 58, 21186–21199. [Google Scholar] [CrossRef]
- Xiao, D.; He, X.; Zhang, W.; Cheng, M.; Hu, P.; Wang, K. Diazotroph and arbuscular mycorrhizal fungal diversity and community composition responses to karst and non-karst soils. Appl. Soil Ecol. 2022, 170, 104227. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Kuzyakov, Y.; Xiao, L.; Xiao, D.; Xu, L.; Chen, H.; Zhao, J.; Wang, K. Linking bacterial life strategies with soil organic matter accrual by karst vegetation restoration. Soil Biol. Biochem. 2023, 177, 108925. [Google Scholar] [CrossRef]
- Huang, D.; Xia, R.; Chen, C.; Liao, J.; Chen, L.; Wang, D.; Alvarez, P.J.J.; Yu, P. Adaptive strategies and ecological roles of phages in habitats under physicochemical stress. Trends Microbiol. 2024, 32, 902–916. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhao, Y.; Xiao, D.; Xu, Z.; Zhang, W.; Xiao, J.; Wang, K. Dynamics of soil nitrogen availability following vegetation restoration along a climatic gradient of a subtropical karst region in China. J. Soils Sediment. 2021, 21, 2167–2178. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Chen, H.; Xu, L.; Xiao, J.; Luo, Y.; Wang, K. Lithologic control of microbial-derived carbon in forest soils. Soil Biol. Biochem. 2022, 167, 108600. [Google Scholar] [CrossRef]
- Emerson, J.B. Soil viruses: A new hope. mSystems 2019, 4, e00120-19. [Google Scholar] [CrossRef]
- Graham, E.B.; Camargo, A.P.; Wu, R.; Neches, R.Y.; Nolan, M.; Paez-Espino, D.; Kyrpides, N.C.; Jansson, J.K.; McDermott, J.E.; Hofmockel, K.S.; et al. A global atlas of soil viruses reveals unexplored biodiversity and potential biogeochemical impacts. Nat. Microbiol. 2024, 9, 1873–1883. [Google Scholar] [CrossRef]
- Wu, H.; Cui, H.; Fu, C.; Li, R.; Qi, F.; Liu, Z.; Yang, G.; Xiao, K.; Qiao, M. Unveiling the crucial role of soil microorganisms in carbon cycling: A review. Sci. Total Environ. 2024, 909, 168627. [Google Scholar] [CrossRef] [PubMed]
- Albright, M.B.N.; Gallegos-Graves, L.V.; Feeser, K.L.; Montoya, K.; Emerson, J.B.; Shakya, M.; Dunbar, J. Experimental evidence for the impact of soil viruses on carbon cycling during surface plant litter decomposition. ISME Commun. 2022, 2, 24. [Google Scholar] [CrossRef]
- Wu, R.; Davison, M.R.; Nelson, W.C.; Smith, M.L.; Lipton, M.S.; Jansson, J.K.; McClure, R.S.; McDermott, J.E.; Hofmockel, K.S. Hi-C metagenome sequencing reveals soil phage-host interactions. Nat. Commun. 2023, 14, 7666. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, L.; Zhang, C.; Ma, D.; Zhang, J. Bacteria–virus interactions are more crucial in soil organic carbon storage than iron protection in biochar-amended paddy soils. Environ. Sci. Technol. 2023, 57, 19713–19722. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Mason-Jones, K. Viruses in soil: Nano-scale undead drivers of microbial life, biogeochemical turnover and ecosystem functions. Soil Biol. Biochem. 2018, 127, 305–317. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, D.; Ge, T.; Wang, Y.; Zhang, Y.; Liang, C.; Liao, H.; Liang, X. Unveiling the top-down control of soil viruses over microbial communities and soil organic carbon cycling: A review. Clim. Smart Agric. 2024, 1, 100022. [Google Scholar] [CrossRef]
- Wu, H.; Wan, S.; Ruan, C.; Wan, W.; Han, M.; Chen, G.; Liu, Y.; Zhu, K.; Liang, C.; Wang, G. Soil microbial necromass: The state-of-the-art, knowledge gaps, and future perspectives. Eur. J. Soil Biol. 2023, 115, 103472. [Google Scholar] [CrossRef]
- Wu, H.; Wan, S.; Ruan, C.; Niu, X.; Chen, G.; Liu, Y.; Zhu, K.; Schulin, R.; Wang, G. Phage-bacterium interactions and nutrient availability can shape C and N retention in microbial biomass. Eur. J. Soil Biol. 2022, 73, e13296. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, Y.; Dungait, J.A.J.; Liu, J.; Lin, S.; Jia, J.; Yu, G. The “regulator” function of viruses on ecosystem carbon cycling in the Anthropocene. Front. Public Health 2022, 10, 858615. [Google Scholar] [CrossRef]
- Liu, C.; Liao, H.; Gao, T.; Ai, C.; Tang, X.; Friman, V.; Zhou, S. Deciphering the hidden role of soil viruses in nitrogen cycling revealed by metagenomic stable isotope probing. Innov. Geosci. 2024, 2, 100101. [Google Scholar] [CrossRef]
- Sun, M.; Yuan, S.; Xia, R.; Ye, M.; Balcázar, J.L. Underexplored viral auxiliary metabolic genes in soil: Diversity and eco-evolutionary significance. Environ. Microbiol. 2023, 25, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Trubl, G.; Jang, H.B.; Roux, S.; Emerson, J.B.; Solonenko, N.; Vik, D.R.; Solden, L.; Ellenbogen, J.; Runyon, A.T.; Bolduc, B.; et al. Soil viruses are underexplored players in ecosystem carbon processing. mSystems 2018, 3, e00076-18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Liu, S.; Sun, M.; Yi, X.; Duan, G.; Ye, M.; Gillings, M.R.; Zhu, Y. Adaptive expression of phage auxiliary metabolic genes in paddy soils and their contribution toward global carbon sequestration. Proc. Natl. Acad. Sci. USA 2024, 121, e2419798121. [Google Scholar] [CrossRef]
- Bi, L.; Yu, D.T.; Du, S.; Zhang, L.M.; Zhang, L.Y.; Wu, C.F.; Xiong, C.; Han, L.L.; He, J.Z. Diversity and potential biogeochemical impacts of viruses in bulk and rhizosphere soils. Environ. Microbiol. 2021, 23, 588–599. [Google Scholar] [CrossRef]
- Wang, L.; Lin, D.; Xiao, K.; Ma, L.; Fu, Y.; Huo, Y.; Liu, Y.; Ye, M.; Sun, M.; Zhu, D.; et al. Soil viral–host interactions regulate microplastic-dependent carbon storage. Proc. Natl. Acad. Sci. USA 2024, 121, e2413245121. [Google Scholar] [CrossRef] [PubMed]
- Feiner, R.; Argov, T.; Rabinovich, L.; Sigal, N.; Borovok, I.; Herskovits, A.A. A new perspective on lysogeny: Prophages as active regulatory switches of bacteria. Nat. Rev. Microbiol. 2015, 13, 641–650. [Google Scholar] [CrossRef]
- Huang, D.; Yu, P.; Ye, M.; Schwarz, C.; Jiang, X.; Alvarez, P.J.J. Enhanced mutualistic symbiosis between soil phages and bacteria with elevated chromium-induced environmental stress. Microbiome 2021, 9, 150. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, Z.; Liu, H.; Li, Y.; Ge, T.; Tang, X.; He, Y.; Ma, B.; Xu, J.; Anantharaman, K.; et al. Soil nutrient conditions alter viral lifestyle strategy and potential function in phosphorous and nitrogen metabolisms. Soil Biol. Biochem. 2024, 189, 109279. [Google Scholar] [CrossRef]
- Braga, L.P.P.; Spor, A.; Kot, W.; Breuil, M.; Hansen, L.H.; Setubal, J.C.; Philippot, L. Impact of phages on soil bacterial communities and nitrogen availability under different assembly scenarios. Microbiome 2020, 8, 52. [Google Scholar] [CrossRef]
- Castledine, M.; Buckling, A. Critically evaluating the relative importance of phage in shaping microbial community composition. Trends Microbiol. 2024, 32, 957–969. [Google Scholar] [CrossRef]
- Huang, X.; Braga, L.P.P.; Ding, C.; Yang, B.; Ge, T.; Di, H.; He, Y.; Xu, J.; Philippot, L.; Li, Y. Impact of viruses on prokaryotic communities and greenhouse gas emissions in agricultural soils. Adv. Sci. 2024, 11, 2407223. [Google Scholar] [CrossRef]
- Li, H.; Zhao, S.; Gao, M.; Zhou, Y.; Xu, B.; Yang, L.; Yang, X.; Su, J. Experimental evidence for viral impact on microbial community, nitrification, and denitrification in an agriculture soil. J. Hazard. Mater. 2025, 489, 137532. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth-Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Hartmann, A.; Goldscheider, N.; Wagener, T.; Lange, J.; Weiler, M. Karst water resources in a changing world: Review of hydrological modeling approaches. Rev. Geophys. 2014, 52, 218–242. [Google Scholar] [CrossRef]
- Wu, R.; Davison, M.R.; Gao, Y.; Nicora, C.D.; Mcdermott, J.E.; Burnum-Johnson, K.E.; Hofmockel, K.S.; Jansson, J.K. Moisture modulates soil reservoirs of active DNA and RNA viruses. Commun. Biol. 2021, 4, 992. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ma, Z.; Ruan, C.; Hu, W.; Han, M.; Wan, W.; Wang, Y.; Zvomuya, F.; Liang, C.; Liu, Y.; et al. Effects of soil moisture on soil viral reproductive strategies in an agricultural soil. Eur. J. Soil Sci. 2024, 75, e13531. [Google Scholar] [CrossRef]
- He, T.; Li, J.; Du, X.; Pei, G.; Wang, A.; Hu, B.; Zhang, W.; Zhang, W.; Sun, J. Changes in SOC, pH, and Ca associated with microorganism mediated SOC mineralization and temperature sensitivity following vegetation restoration in karst regions. Plant Soil 2025. [Google Scholar] [CrossRef]
- Tang, J.; Tang, X.; Qin, Y.; He, Q.; Yi, Y.; Ji, Z. Karst rocky desertification progress: Soil calcium as a possible driving force. Sci. Total Environ. 2019, 649, 1250–1259. [Google Scholar] [CrossRef]
- Lee, S.; Sorensen, J.W.; Walker, R.L.; Emerson, J.B.; Nicol, G.W.; Hazard, C. Soil pH influences the structure of virus communities at local and global scales. Soil Biol. Biochem. 2022, 166, 108569. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Wang, L.; Zhu, Z.; Liu, Y.; Wang, J.; Chen, J.; Ge, T. Contrasting viral diversity and potential biogeochemical impacts in paddy and upland soils. Appl. Soil Ecol. 2024, 199, 105399. [Google Scholar] [CrossRef]
- Cheng, X.; Yun, Y.; Wang, H.; Ma, L.; Tian, W.; Man, B.; Liu, C. Contrasting bacterial communities and their assembly processes in karst soils under different land use. Sci. Total Environ. 2021, 751, 142263. [Google Scholar] [CrossRef] [PubMed]
- Shabtai, I.A.; Wilhelm, R.C.; Schweizer, S.A.; Höschen, C.; Buckley, D.H.; Lehmann, J. Calcium promotes persistent soil organic matter by altering microbial transformation of plant litter. Nat. Commun. 2023, 14, 6609. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, D.; Xiao, K.; Wang, K. Soil microbial processes and resource limitation in karst and non-karst forests. Funct. Ecol. 2018, 32, 1400–1409. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Mao, Q.; Xiao, K.; Wang, K. Resource limitation of soil microbes in karst ecosystems. Sci. Total Environ. 2019, 650, 241–248. [Google Scholar] [CrossRef]
- Huang, Y.; Song, X.; Wang, Y.; Canadell, J.G.; Luo, Y.; Ciais, P.; Chen, A.; Hong, S.; Wang, Y.; Tao, F.; et al. Size, distribution, and vulnerability of the global soil inorganic carbon. Science 2024, 384, 233–239. [Google Scholar] [CrossRef]
- Qin, C.; Li, S.; Yu, G.; Bass, A.M.; Yue, F.; Xu, S. Vertical variations of soil carbon under different land uses in a karst critical zone observatory (CZO), SW China. Geoderma 2022, 412, 115741. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Xiao, K.; Zhao, Y.; Liang, C.; Zhao, M.; Moore, O.W.; Otero-Fariña, A.; Zhu, Y.; Johnson, K.; Peacock, C.L. Introducing the soil mineral carbon pump. Nat. Rev. Earth Environ. 2023, 4, 135–136. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Pan, F.; Li, D.; Chen, H.; Wang, K. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Long, X.; Li, J.; Liao, X.; Wang, J.; Zhang, W.; Wang, K.; Zhao, J. Stable soil biota network enhances soil multifunctionality in agroecosystems. Glob. Change Biol. 2025, 31, e70041. [Google Scholar] [CrossRef]
- Tong, D.; Wang, Y.; Yu, H.; Shen, H.; Dahlgren, R.A.; Xu, J. Viral lysing can alleviate microbial nutrient limitations and accumulate recalcitrant dissolved organic matter components in soil. ISME J. 2023, 17, 1247–1256. [Google Scholar] [CrossRef]
- Xue, Y.; Tian, J.; Quine, T.A.; Powlson, D.; Xing, K.; Yang, L.; Kuzyakov, Y.; Dungait, J.A.J. The persistence of bacterial diversity and ecosystem multifunctionality along a disturbance intensity gradient in karst soil. Sci. Total Environ. 2020, 748, 142381. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Radosevich, M.; Zhuang, J.; DeBruyn, J.M.; Staton, M.; Schaeffer, S.M. Identification of novel viruses and their microbial hosts from soils with long-term nitrogen fertilization and cover cropping management. mSystems 2022, 7, e00571-22. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Shi, W.; Wu, N.; Liu, W.; Francis, F.; Wang, X. Enterobacter-infecting phages in nitrogen-deficient paddy soil impact nitrogen-fixation capacity and rice growth by shaping the soil microbiome. Sci. Total Environ. 2024, 956, 177382. [Google Scholar] [CrossRef]
- Zhou, Z.; Liang, X.; Zhang, N.; Xie, N.; Huang, Y.; Zhou, Y.; Li, B. The impact of soil viruses on organic carbon mineralization and microbial biomass turnover. Appl. Soil Ecol. 2024, 202, 105554. [Google Scholar] [CrossRef]
- Nannipieri, P.; Angst, G.; Mueller, C.; Pietramellara, G. The role of death and lysis of microbial and plant cells in the formation of soil organic matter. Soil Biol. Biochem. 2025, 204, 109750. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, X.; Zhu, Z.; Yuan, Z.; Yu, S.; Liu, Y.; Wang, J.; Mason-Jones, K.; Kuzyakov, Y.; Chen, J.; et al. Phages affect soil dissolved organic matter mineralization by shaping bacterial communities. Environ. Sci. Technol. 2025, 59, 2070–2081. [Google Scholar] [CrossRef]
- Koskella, B.; Brockhurst, M.A. Bacteria–phage coevolution as a driver of ecological and evolutionary processes in microbial communities. FEMS Microbiol. Rev. 2014, 38, 916–931. [Google Scholar] [CrossRef]
- Safari, F.; Sharifi, M.; Farajnia, S.; Akbari, B.; Karimi Baba Ahmadi, M.; Negahdaripour, M.; Ghasemi, Y. The interaction of phages and bacteria: The co-evolutionary arms race. Crit. Rev. Biotechnol. 2020, 40, 119–137. [Google Scholar] [CrossRef]
- Ji, M.; Fan, X.; Cornell, C.R.; Zhang, Y.; Yuan, M.M.; Tian, Z.; Sun, K.; Gao, R.; Liu, Y.; Zhou, J. Tundra soil viruses mediate responses of microbial communities to climate warming. mBio 2023, 14, e0300922. [Google Scholar] [CrossRef]
- Jansson, J.K. Soil viruses: Understudied agents of soil ecology. Environ. Microbiol. 2023, 25, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, T.; Chen, Q.; Chen, T.; Hu, J.; Sun, L.; Wang, B.; Li, W.; Ni, J. Unveiling the unknown viral world in groundwater. Nat. Commun. 2024, 15, 6788. [Google Scholar] [CrossRef]
- Wang, G.; Or, D. Aqueous films limit bacterial cell motility and colony expansion on partially saturated rough surfaces. Environ. Microbiol. 2010, 12, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Or, D. Hydration dynamics promote bacterial coexistence on rough surfaces. ISME J. 2013, 7, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Trubl, G.; Kimbrel, J.A.; Liquet-Gonzalez, J.; Nuccio, E.E.; Weber, P.K.; Pett-Ridge, J.; Jansson, J.K.; Waldrop, M.P.; Blazewicz, S.J. Active virus-host interactions at sub-freezing temperatures in Arctic peat soil. Microbiome 2021, 9, 208. [Google Scholar] [CrossRef]
- Umair, M.; Sun, N.; Du, H.; Hui, N.; Altaf, M.; Du, B.; Yin, S.; Liu, C. Bacterial communities are more sensitive to water addition than fungal communities due to higher soil K and Na in a degraded karst ecosystem of Southwestern China. Front. Microbiol. 2020, 11, 562546. [Google Scholar] [CrossRef] [PubMed]
- Buivydaitė, Ž.; Aryal, L.; Corrêa, F.B.; Chen, T.; Langlois, V.; Elberg, C.L.; Netherway, T.; Wang, R.; Zhao, T.; Acharya, B.; et al. Meeting report: The first soil viral workshop 2022. Virus Res. 2023, 331, 199121. [Google Scholar] [CrossRef]
- Santillán, J.; López-Martínez, R.; Aguilar-Rangel, E.J.; Hernández-García, K.; Vásquez-Murrieta, M.S.; Cram, S.; Alcántara-Hernández, R.J. Microbial diversity and physicochemical characteristics of tropical karst soils in the northeastern Yucatan peninsula, Mexico. Appl. Soil Ecol. 2021, 165, 103969. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, L.; Brandt, M.; Zhang, X.; Wang, K. A social-ecological framework to enhance sustainable reforestation under geological constraints. Earth’s Future 2024, 12, e2023EF004335. [Google Scholar] [CrossRef]
- Hultman, J.; Waldrop, M.P.; Mackelprang, R.; David, M.M.; McFarland, J.; Blazewicz, S.J.; Harden, J.; Turetsky, M.R.; McGuire, A.D.; Shah, M.B.; et al. Multi-omics of permafrost, active layer and thermokarst bog soil microbiomes. Nature. 2015, 521, 208–212. [Google Scholar] [CrossRef]
- Chen, Y.; Sadiq, S.; Tian, J.; Chen, X.; Lin, X.; Shen, J.; Chen, H.; Hao, Z.; Wille, M.; Zhou, Z.; et al. RNA viromes from terrestrial sites across China expand environmental viral diversity. Nat. Microbiol. 2022, 7, 1312–1323. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.M.; Kramer, R.; Van Goethem, M.W.; Makhalanyane, T.P.; Hogg, I.; Cowan, D.A. Environmental drivers of viral community composition in Antarctic soils identified by viromics. Microbiome 2017, 5, 83. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Li, H.; Duan, C.; Zhou, X.; Luo, Q.; An, X.; Zhu, Y.; Su, J. Response of soil viral communities to land use changes. Nat. Commun. 2022, 13, 6027. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; He, H.; Zheng, S. Microfluidics in single-cell virology: Technologies and applications. Trends. Biotechnol. 2020, 38, 1360–1372. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Liu, D.; Zhang, Z. Advances in virus-host interaction research based on microfluidic platforms. Chin. Chem. Lett. 2024, 35, 110302. [Google Scholar] [CrossRef]
- Herrmann, A.M.; Ritz, K.; Nunan, N.; Clode, P.L.; Pett-Ridge, J.; Kilburn, M.R.; Murphy, D.V.; O Donnell, A.G.; Stockdale, E.A. Nano-scale secondary ion mass spectrometry—A new analytical tool in biogeochemistry and soil ecology: A review article. Soil Biol. Biochem. 2007, 39, 1835–1850. [Google Scholar] [CrossRef]
- Kaiser, C.; Kilburn, M.R.; Clode, P.L.; Fuchslueger, L.; Koranda, M.; Cliff, J.B.; Solaiman, Z.M.; Murphy, D.V. Exploring the transfer of recent plant photosynthates to soil microbes: Mycorrhizal pathway vs direct root exudation. New Phytol. 2015, 205, 1537–1551. [Google Scholar] [CrossRef]
- Peth, S.; Chenu, C.; Leblond, N.; Mordhorst, A.; Garnier, P.; Nunan, N.; Pot, V.; Ogurreck, M.; Beckmann, F. Localization of soil organic matter in soil aggregates using synchrotron-based X-ray microtomography. Soil Biol. Biochem. 2014, 78, 189–194. [Google Scholar] [CrossRef]
- Coclet, C.; Sorensen, P.O.; Karaoz, U.; Wang, S.; Brodie, E.L.; Eloe-Fadrosh, E.A.; Roux, S. Virus diversity and activity is driven by snowmelt and host dynamics in a high-altitude watershed soil ecosystem. Microbiome 2023, 11, 237. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).