Global Trajectories of Forest Soil Acidification: A Scientometric Synthesis of Drivers, Impacts and Sustainable Solutions
Abstract
1. Introduction
2. Historical Summary of Forest Soil Acidification
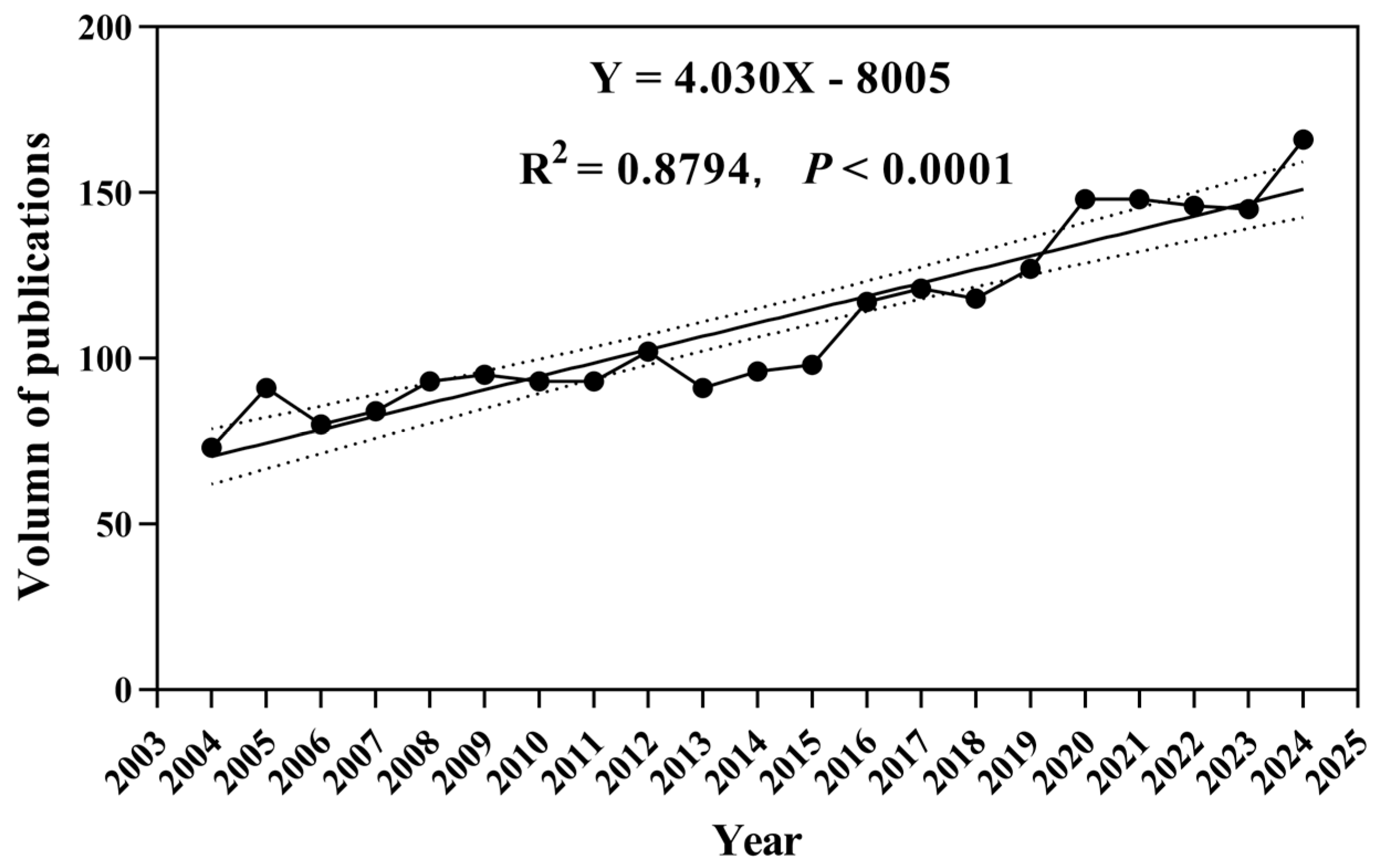
2.1. Data Sources
2.2. Research Methods
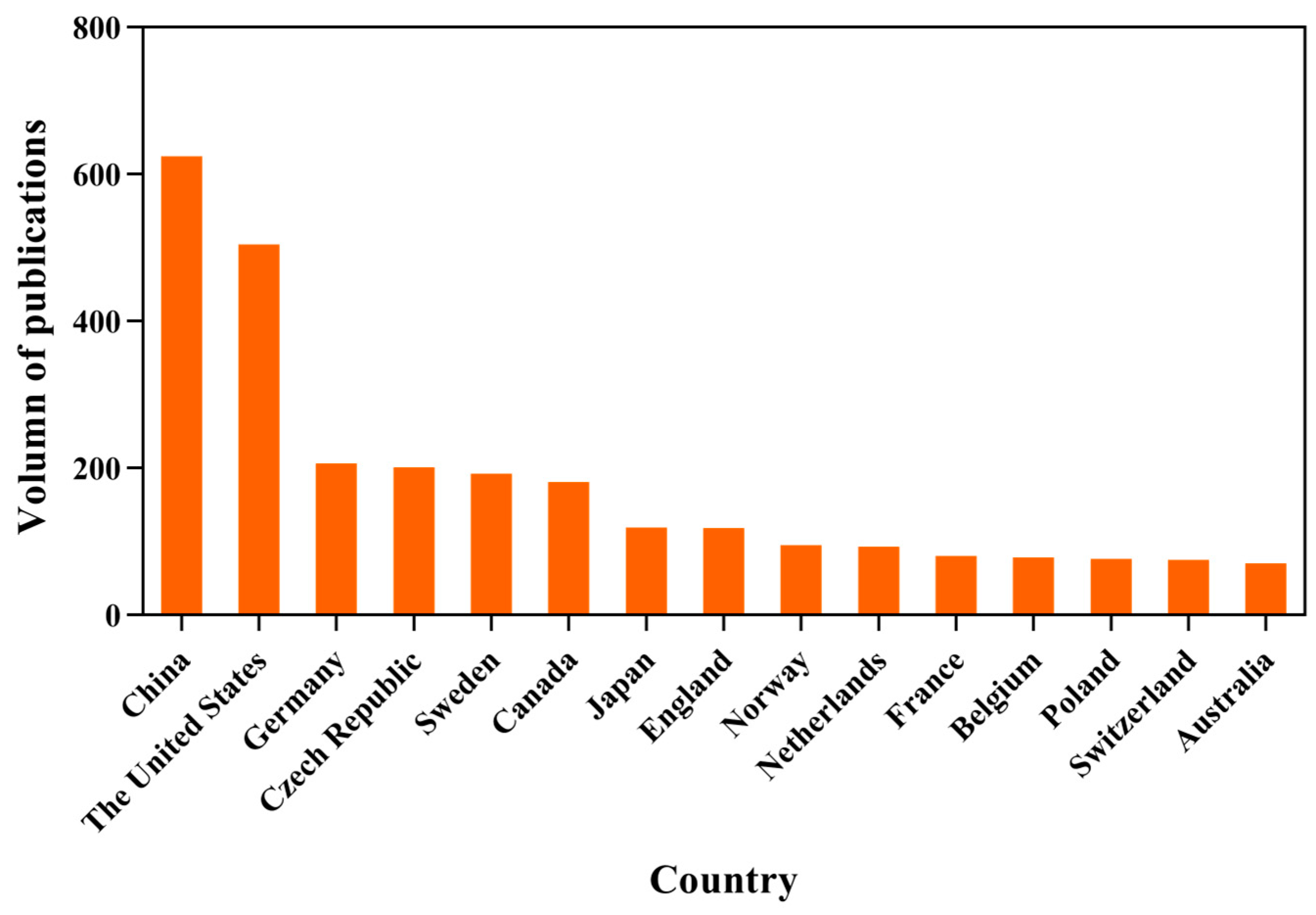
2.3. Contribution of the Countries
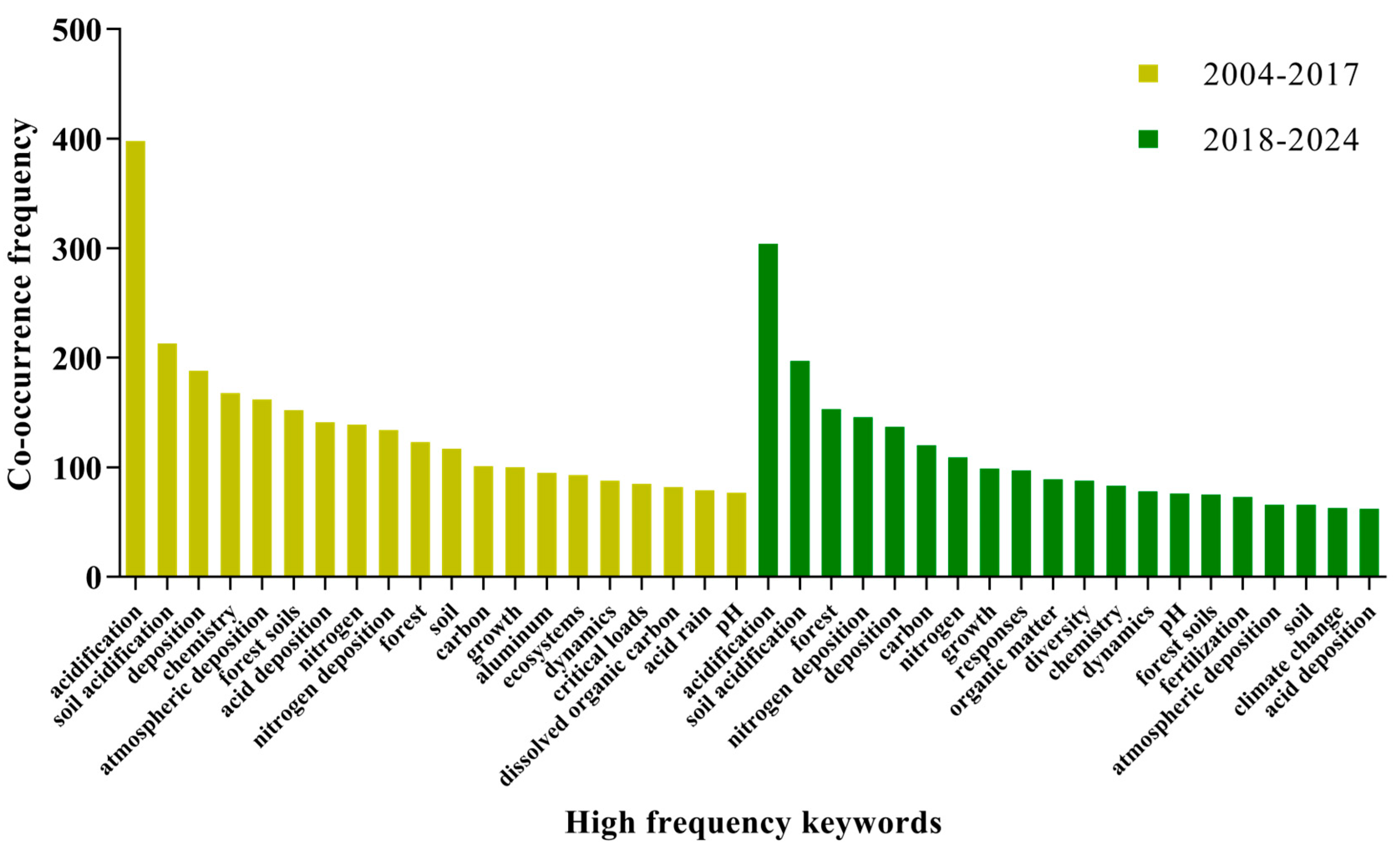
2.4. Research Focus
2.5. Scope and Level of Research
3. Overall Global Forest Soil Acidification
3.1. Overall Soil Acidification
3.2. Differences in Acidification in the Same Geographical Area
3.3. Differences in Acidification in Different Geographical Areas
4. Long Time-Scale Effects of Acidification and Forest Succession
4.1. Long Time-Scale Effects of Acidification
4.2. Impacts of Forest Succession and Weather Extremes on Acidification Processes
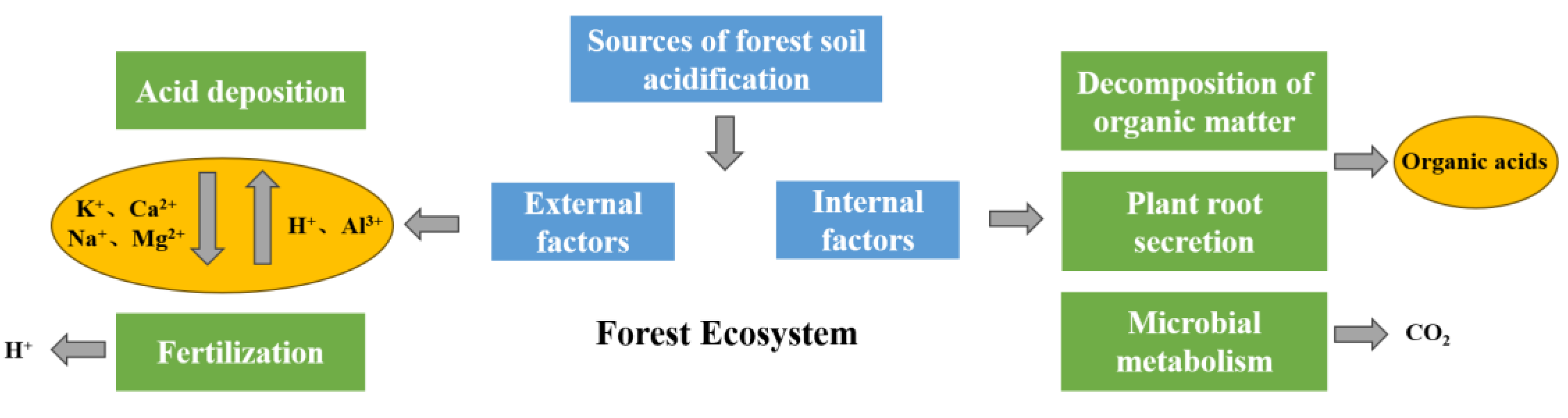
5. Eco-Environmental Effects of Forest Soil Acidification
5.1. Effects of Forest Soil Acidification on Soil Physicochemical Properties
5.2. Effects of Forest Soil Acidification on Plant Growth
5.3. Effects of Forest Soil Acidification on Soil Fauna
5.4. Impact of Forest Soil Acidification on Microbial Diversity
5.5. Impact of Forest Soil Acidification on the Fluxes of Major Greenhouse Gases
6. Sustainable Solutions
6.1. Source Control: Reduce Acid Input
6.2. Soil Remediation and Improvement
6.3. Ecological Adaptive Management
6.4. Long-Term Monitoring and Early Warning Systems, Policy and Economic Incentives, Public Participation Education
6.5. Key Considerations
7. Conclusions
- (1)
- Scale of study and applicability of results: Existing studies often focus on relatively small areas within forests, limiting the broader applicability of their findings. To improve the scientific validity and generalizability of the conclusions of the studies, future research should expand to larger regional or global scales, both temporally and spatially. Integrating long-term observational data with predictive modeling, particularly in relation to forest succession and extreme weather events, will help improve the universality and scientific validity of the conclusions.
- (2)
- Complex interactions within forest ecosystems: Forest soil acidification is a complex process that occurs within forest ecosystems, and it is affected by both internal and external factors. Therefore, future research should more rigorously investigate the interactions among key factors—such as acid deposition, litter decomposition and root exudation—to better understand their combined effects. In particular, the impacts of acid deposition in driving forest soil acidification should be thoroughly explored.
- (3)
- Buffering mechanisms in forest ecosystems: The effects of acid deposition and anthropogenic factors, among others, can accelerate forest soil acidification. The mechanisms by which forest ecosystems buffer against acid deposition have been explored in studies focusing on topics such as ion-exchange reactions, the weathering of soil minerals and soil biology. Moreover, changes in the diversity of soil micro-organisms can be used as a sensitive indicator of ecosystem health. Changes in soil acidity can significantly affect micro-organisms in the soil, which may adapt to such changes through a series of internal biochemical reactions, which may then impact the acidification process in forest soils. Therefore, research on the mechanisms underlying acid-buffer deposition in forest ecosystems should be strengthened.
- (4)
- Cross-ecosystem impacts of acid deposition: The acidification of forest soils can have harmful effects on both biotic and abiotic components of forest ecosystems. Moreover, under the influence of acid deposition, acidic substances originating in forest ecosystems may be transported to adjacent ecosystems, such as agricultural ecosystems, via surface runoff. These cross-ecosystem impacts remain poorly understood. Therefore, strengthening long-term field monitoring and sample plot studies will be essential to quantify these environmental hazards and support the sustainable development and management of interconnected ecosystems.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Wang, Q.; Zhu, J.; Xu, L.; Li, M.; Rengel, Z.; Xiao, J.; Hobbie, E.A.; Piao, S.; Luo, W. Higher soil acidification risk in southeastern Tibetan Plateau. Sci. Total Environ. 2021, 755, 143372. [Google Scholar] [CrossRef]
- Chen, D.; Lan, Z.; Bai, X.; Grace, J.B.; Bai, Y. Evidence that acidification-induced declines in plant diversity and productivity are mediated by changes in below-ground communities and soil properties in a semi-arid steppe. J. Ecol. 2013, 101, 1322–1334. [Google Scholar] [CrossRef]
- Li, Y.; Compson, Z.G.; Kuang, X.; Yu, L.; Song, Q.; Liu, J.; Huang, D.; Zhou, H.; Huang, S.; Li, T. Increased stability of a subtropic bamboo forest soil bacterial communities through integration of water and fertilizer management compared to conventional management. BMC Plant Biol. 2024, 24, 1072. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, X.; Song, X.; Xiao, Y.; Wang, A.; Li, D. Spatial variation in soil base saturation and exchangeable cations in tropical and subtropical China. Agronomy 2023, 13, 781. [Google Scholar] [CrossRef]
- Yao, X.; Hui, D.; Hou, E.; Xiong, J.; Xing, S.; Deng, Q. Differential responses and mechanistic controls of soil phosphorus transformation in Eucalyptus plantations with N fertilization and introduced N2-fixing tree species. New Phytol. 2023, 237, 2039–2053. [Google Scholar] [CrossRef]
- Ahrends, B.; Fortmann, H.; Meesenburg, H. The influence of tree species on the recovery of forest soils from acidification in Lower Saxony, Germany. Soil. Syst. 2022, 6, 40. [Google Scholar] [CrossRef]
- Wang, B.; Chen, J.; Huang, G.; Zhao, S.; Dong, F.; Zhang, Y.; He, W.; Wang, P.; Yan, Z. Growth and nutrient stoichiometry responses to N and P fertilization of 8-year old Masson pines (Pinus massoniana) in subtropical China. Plant Soil. 2022, 477, 343–356. [Google Scholar] [CrossRef]
- Mak, S.; Tiva, L.K.; Phearun, P.; Gohet, E.; Lacote, R.; Gay, F. Impact of mineral fertilization on the growth of immature rubber trees: New insights from a field trial in Cambodia. J. Rubber Res. 2022, 25, 141–149. [Google Scholar] [CrossRef]
- Gallo, J.; Vacek, Z.; Vacek, S. Quarter of a century of forest fertilization and liming research at the Department of Silviculture in Prague, Czech Republic. Cent. Eur. For. J. 2021, 67, 123–134. [Google Scholar] [CrossRef]
- Okorkov, V.; Okorkova, L.; Fenova, O. Changes in the physicochemical properties of gray forest soils in the Opol’e region under the long-term application of fertilizers. Russ. Agric. Sci. 2015, 41, 241–246. [Google Scholar] [CrossRef]
- Zhu, Q.; de Vries, W.; Liu, X.; Hao, T.; Zeng, M.; Shen, J.; Zhang, F. Enhanced acidification in Chinese croplands as derived from element budgets in the period 1980–2010. Sci. Total Environ. 2018, 618, 1497–1505. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, L.; Li, C.; Chen, M.; Zhou, Y.; Meng, M.; Li, Z.; Zhang, J.; Liu, X. Effects of short-term simulated acid rain and nitrogen deposition on soil nutrients and enzyme activities in Cunninghamia lanceolata plantation. Front. Ecol. Evol. 2024, 12, 1365954. [Google Scholar] [CrossRef]
- Wu, T.; Ullah, S.; Zhong, L.; Xu, Y.; Wei, G.; Yang, M. Impact of Simulated Acid Rain on Soil Base Cations Dissolution between Eucalyptus Pure Plantations and Eucalyptus–Castanopsis fissa Mixed Plantations. Forests 2023, 14, 2159. [Google Scholar] [CrossRef]
- Fujii, K.; Hayakawa, C.; Panitkasate, T.; Maskhao, I.; Funakawa, S.; Kosaki, T.; Nawata, E. Acidification and buffering mechanisms of tropical sandy soil in northeast Thailand. Soil. Tillage Res. 2017, 165, 80–87. [Google Scholar] [CrossRef]
- Fujii, K.; Shibata, M.; Kitajima, K.; Ichie, T.; Kitayama, K.; Turner, B.L. Plant–soil interactions maintain biodiversity and functions of tropical forest ecosystems. Ecol. Res. 2018, 33, 149–160. [Google Scholar] [CrossRef]
- Hirano, Y.; Tanikawa, T.; Makita, N. Biomass and morphology of fine roots in eight Cryptomeria japonica stands in soils with different acid-buffering capacities. For. Ecol. Manag. 2017, 384, 122–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Lei, Z.; Ren, H.; Chen, H. Effect of Acid Production from Forest Litter on the Availability of Heavy Metals in Soil. Forests 2024, 15, 2097. [Google Scholar] [CrossRef]
- Ilek, A.; Gąsecka, M.; Magdziak, Z.; Saitanis, C.; Siegert, C.M. Seasonality Affects Low-Molecular-Weight Organic Acids and Phenolic Compounds’ Composition in Scots Pine Litterfall. Plants 2024, 13, 1293. [Google Scholar] [CrossRef]
- Dannenmann, M.; Simon, J.; Gasche, R.; Holst, J.; Naumann, P.S.; Kögel-Knabner, I.; Knicker, H.; Mayer, H.; Schloter, M.; Pena, R. Tree girdling provides insight on the role of labile carbon in nitrogen partitioning between soil microorganisms and adult European beech. Soil. Biol. Biochem. 2009, 41, 1622–1631. [Google Scholar] [CrossRef]
- Gruba, P.; Mulder, J. Tree species affect cation exchange capacity (CEC) and cation binding properties of organic matter in acid forest soils. Sci. Total Environ. 2015, 511, 655–662. [Google Scholar] [CrossRef]
- Clarholm, M.; Skyllberg, U. Translocation of metals by trees and fungi regulates pH, soil organic matter turnover and nitrogen availability in acidic forest soils. Soil Biol. Biochem. 2013, 63, 142–153. [Google Scholar] [CrossRef]
- Yu, H.; He, N.; Wang, Q.; Zhu, J.; Gao, Y.; Zhang, Y.; Jia, Y.; Yu, G. Development of atmospheric acid deposition in China from the 1990s to the 2010s. Environ. Pollut. 2017, 231, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Grider, A.; Ponette-González, A.; Heindel, R. Calcium and ammonium now control the pH of wet and bulk deposition in Ohio, US. Atmos. Environ. 2023, 310, 119986. [Google Scholar] [CrossRef]
- Zhigacheva, E.S.; Sase, H.; Nakata, M.; Ohizumi, T.; Gromov, S.A.; Takahashi, M. Stream water acidification in the Far East of Russia under changing atmospheric deposition and precipitation patterns. Limnology 2022, 23, 415–428. [Google Scholar] [CrossRef]
- Bradová, M.; Tejnecký, V.; Borůvka, L.; Němeček, K.; Ash, C.; Šebek, O.; Svoboda, M.; Zenáhlíková, J.; Drábek, O. The variations of aluminium species in mountainous forest soils and its implications to soil acidification. Environ. Sci. Pollut. Res. 2015, 22, 16676–16687. [Google Scholar] [CrossRef] [PubMed]
- Pavlů, L.; Borůvka, L.; Drábek, O.; Nikodem, A. Effect of natural and anthropogenic acidification on aluminium distribution in forest soils of two regions in the Czech Republic. J. For. Res. 2021, 32, 363–370. [Google Scholar] [CrossRef]
- Wuyts, K.; De Schrijver, A.; Staelens, J.; Verheyen, K. Edge effects on soil acidification in forests on sandy soils under high deposition load. Water Air Soil Pollut. 2013, 224, 1545. [Google Scholar] [CrossRef]
- Zhang, F.; Jin, Q.; Peng, H.; Zhu, T. Soil acidification in moso bamboo (Phyllostachys edulis) forests and changes of soil metal ions (Cu, Pb) concentration. Arch. Agron. Soil Sci. 2021, 67, 1799–1808. [Google Scholar] [CrossRef]
- Tejnecký, V.; Drábek, O.; Borůvka, L.; Nikodem, A.; Kopáč, J.; Vokurková, P.; Šebek, O. Seasonal variation of water extractable aluminium forms in acidified forest organic soils under different vegetation cover. Biogeochemistry 2010, 101, 151–163. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Xu, C.; Zhu, Q.; Huang, D. Effects of soil acidification and liming on the phytoavailability of cadmium in paddy soils of central subtropical China. Environ. Pollut. 2016, 219, 99–106. [Google Scholar] [CrossRef]
- Achilles, F.; Tischer, A.; Bernhardt-Römermann, M.; Chmara, I.; Achilles, M.; Michalzik, B. Effects of moderate nitrate and low sulphate depositions on the status of soil base cation pools and recent mineral soil acidification at forest conversion sites with European beech (“Green Eyes”) embedded in Norway spruce and Scots pine stands. Forests 2021, 12, 573. [Google Scholar] [CrossRef]
- Kwon, T.-S.; Kim, Y.S.; Lee, S.W.; Park, Y.-S. Changes of soil arthropod communities in temperate forests over 10 years (1998–2007). J. Asia-Pac. Entomol. 2016, 19, 181–189. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Zhang, G.; Jiang, L.; Han, X. Mechanisms of soil acidification reducing bacterial diversity. Soil Biol. Biochem. 2015, 81, 275–281. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, K.; Zhang, W.; Liu, J.; Ding, X.; Cai, X.a.; Mo, J. Sulfur deposition still contributes to forest soil acidification in the Pearl River Delta, South China, despite the control of sulfur dioxide emission since 2001. Environ. Sci. Pollut. Res. 2019, 26, 12928–12939. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Lu, X.; Zhou, K.; Chen, H.; Zhu, X.; Mori, T.; Mo, J. Effects of long-term nitrogen and phosphorus additions on soil acidification in an N-rich tropical forest. Geoderma 2017, 285, 57–63. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Chen, R.; Ni, X.; Cao, J. Effects of forest type on nutrient fluxes in throughfall, stemflow, and litter leachate within acid-polluted locations in Southwest China. Int. J. Environ. Res. Public Health 2022, 19, 2810. [Google Scholar] [CrossRef]
- Park, S.-C.; Han, B.-H.; Kwak, J.-I.; Kim, J.-Y. Ecological Characteristics and Changes to the Forest in the Rear Garden at Changdeokgung, a World Cultural Heritage Site. Forests 2021, 12, 774. [Google Scholar] [CrossRef]
- Hopf, S.-E.; Tresch, S.; Belyazid, S.; Sverdrup, H.; Augustin, S.; Kurz, D.; Rihm, B.; Braun, S. Dendrochemical indicators of tree rings reveal historical soil acidification in Swiss forest stands. Dendrochronologia 2023, 81, 126099. [Google Scholar] [CrossRef]
- De Schrijver, A.; Mertens, J.; Geudens, G.; Staelens, J.; Campforts, E.; Luyssaert, S.; De Temmerman, L.; De Keersmaeker, L.; De Neve, S.; Verheyen, K. Acidification of forested podzols in North Belgium during the period 1950–2000. Sci. Total Environ. 2006, 361, 189–195. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, J.; Wang, Q.; Xu, L.; Li, M.; Dai, G.; Mulder, J.; Xi, Y.; He, N. Soil acidification in China’s forests due to atmospheric acid deposition from 1980 to 2050. Sci. Bull. 2022, 67, 914–917. [Google Scholar] [CrossRef]
- Lie, Z.; Huang, W.; Zhou, G.; Zhang, D.; Yan, J.; Jiang, J.; Neilson, R.; Zhou, S.; Zhang, W.; Ramos Aguila, L.C. Acidity of Soil and Water Decreases in Acid-Sensitive Forests of Tropical China. Environ. Sci. Technol. 2023, 57, 11075–11083. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Kazuyuki, I. Soil acidification stimulates the emission of ethylene from temperate forest soils. Adv. Atmos. Sci. 2009, 26, 1253–1261. [Google Scholar] [CrossRef]
- Verstraeten, G.; Baeten, L.; De Frenne, P.; Vanhellemont, M.; Thomaes, A.; Boonen, W.; Muys, B.; Verheyen, K. Understorey vegetation shifts following the conversion of temperate deciduous forest to spruce plantation. For. Ecol. Manag. 2013, 289, 363–370. [Google Scholar] [CrossRef]
- Clesse, M.; Legout, A.; Ranger, J.; Zeller, B.; van Der Heijden, G. Soil chemical fertility change over four decades in the Morvan Mountains and influence of tree species (Burgundy, France). For. Ecosyst. 2022, 9, 100043. [Google Scholar] [CrossRef]
- Hédl, R.; Petřík, P.; Boublík, K. Long-term patterns in soil acidification due to pollution in forests of the Eastern Sudetes Mountains. Environ. Pollut. 2011, 159, 2586–2593. [Google Scholar] [CrossRef]
- Zhu, Q.; De Vries, W.; Liu, X.; Zeng, M.; Hao, T.; Du, E.; Zhang, F.; Shen, J. The contribution of atmospheric deposition and forest harvesting to forest soil acidification in China since 1980. Atmos. Environ. 2016, 146, 215–222. [Google Scholar] [CrossRef]
- Moffat, A.; Kvaalen, H.; Solberg, S.; Clarke, N. Temporal trends in throughfall and soil water chemistry at three Norwegian forests, 1986–1997. For. Ecol. Manag. 2002, 168, 15–28. [Google Scholar] [CrossRef]
- Tian, D.; Jiang, L.; Ma, S.; Fang, W.; Schmid, B.; Xu, L.; Zhu, J.; Li, P.; Losapio, G.; Jing, X. Effects of nitrogen deposition on soil microbial communities in temperate and subtropical forests in China. Sci. Total Environ. 2017, 607, 1367–1375. [Google Scholar] [CrossRef]
- Mo, J.; Brown, S.; Peng, S.; Kong, G. Nitrogen availability in disturbed, rehabilitated and mature forests of tropical China. For. Ecol. Manag. 2003, 175, 573–583. [Google Scholar] [CrossRef]
- Fang, K.; Kou, D.; Wang, G.; Chen, L.; Ding, J.; Li, F.; Yang, G.; Qin, S.; Liu, L.; Zhang, Q. Decreased soil cation exchange capacity across northern China’s grasslands over the last three decades. J. Geophys. Res. Biogeosci. 2017, 122, 3088–3097. [Google Scholar] [CrossRef]
- Huang, J.; Mo, J.; Zhang, W.; Lu, X. Research on acidification in forest soil driven by atmospheric nitrogen deposition. Acta Ecol. Sin. 2014, 34, 302–310. [Google Scholar] [CrossRef]
- Fujii, K.; Kanetani, S.; Tetsuka, K. Effects of volcanic parent materials on the acid buffering capacity of forest soils on Yakushima Island, Japan. Soil Sci. Plant Nutr. 2020, 66, 680–692. [Google Scholar] [CrossRef]
- Cecchini, G.; Andreetta, A.; Marchetto, A.; Carnicelli, S. Soil solution fluxes and composition trends reveal risks of nitrate leaching from forest soils of Italy. Catena 2021, 200, 105175. [Google Scholar] [CrossRef]
- Li, B.; Wang, Z.; Zhang, X. Assessment of the maximum allowed acid deposition load at current stage in China. J. Environ. Sci. 2017, 56, 140–144. [Google Scholar] [CrossRef]
- Jang, J.-H.; Han, B.-H.; Lee, K.-J.; Choi, J.-W.; Noh, T.-H. A study on the characteristics and changes of vegetation structure of the plant community in Mt. Kwanak. Korean J. Environ. Ecol. 2013, 27, 344–356. [Google Scholar]
- Xing, G.; Wang, X.; Jiang, Y.; Yang, H.; Mai, S.; Xu, W.; Hou, E.; Huang, X.; Yang, Q.; Liu, W. Variations and influencing factors of soil organic carbon during the tropical forest succession from plantation to secondary and old–growth forest. Front. Ecol. Evol. 2023, 10, 1104369. [Google Scholar] [CrossRef]
- Yu, L.; Belyazid, S.; Akselsson, C.; Van Der Heijden, G.; Zanchi, G. Storm disturbances in a Swedish forest—A case study comparing monitoring and modelling. Ecol. Model. 2016, 320, 102–113. [Google Scholar] [CrossRef]
- Schlyter, P.; Stjernquist, I.; Bärring, L.; Jönsson, A.M.; Nilsson, C. Assessment of the impacts of climate change and weather extremes on boreal forests in northern Europe, focusing on Norway spruce. Clim. Res. 2006, 31, 75–84. [Google Scholar] [CrossRef]
- Křeček, J.; Palán, L.; Stuchlík, E. Impacts of land use policy on the recovery of mountain catchments from acidification. Land Use Policy 2019, 80, 439–448. [Google Scholar] [CrossRef]
- Shen, W.; Ren, H.; Jenerette, G.D.; Hui, D.; Ren, H. Atmospheric deposition and canopy exchange of anions and cations in two plantation forests under acid rain influence. Atmos. Environ. 2013, 64, 242–250. [Google Scholar] [CrossRef]
- Yamada, T.; Takenaka, C.; Yoshinaga, S.; Hirai, K. Long-term changes in the chemical properties of Japanese cedar (Cryptomeria japonica) forest soils under high precipitation in southwest Japan. J. For. Res. 2013, 18, 466–474. [Google Scholar] [CrossRef]
- Sanderson, T.M.; Barton, C.; Cotton, C.; Karathanasis, T. Long-term evaluation of acidic atmospheric deposition on soils and soil solution chemistry in the Daniel Boone National Forest, USA. Water Air Soil Pollut. 2017, 228, 403. [Google Scholar] [CrossRef]
- Wu, J.; Liang, G.; Hui, D.; Deng, Q.; Xiong, X.; Qiu, Q.; Liu, J.; Chu, G.; Zhou, G.; Zhang, D. Prolonged acid rain facilitates soil organic carbon accumulation in a mature forest in Southern China. Sci. Total Environ. 2016, 544, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Chuman, T.; Oulehle, F.; Zajícová, K.; Hruška, J. The legacy of acidic deposition controls soil organic carbon pools in temperate forests across the C zech R epublic. Eur. J. Soil Sci. 2021, 72, 1780–1801. [Google Scholar] [CrossRef]
- Liao, B.; Guo, Z.; Probst, A.; Probst, J.-L. Soil heavy metal contamination and acid deposition: Experimental approach on two forest soils in Hunan, Southern China. Geoderma 2005, 127, 91–103. [Google Scholar] [CrossRef]
- Richardson, J.; Friedland, A.; Kaste, J.; Jackson, B. Forest floor lead changes from 1980 to 2011 and subsequent accumulation in the mineral soil across the northeastern United States. J. Environ. Qual. 2014, 43, 926–935. [Google Scholar] [CrossRef]
- Li, W.; Johnson, C.E. Relationships among pH, aluminum solubility and aluminum complexation with organic matter in acid forest soils of the Northeastern United States. Geoderma 2016, 271, 234–242. [Google Scholar] [CrossRef]
- Pourpoint, F.; Templier, J.; Anquetil, C.; Vezin, H.; Trébosc, J.; Trivelli, X.; Chabaux, F.; Pokrovsky, O.S.; Prokushkin, A.S.; Amoureux, J.-P. Probing the aluminum complexation by Siberian riverine organic matter using solid-state DNP-NMR. Chem. Geol. 2017, 452, 1–8. [Google Scholar] [CrossRef]
- Zhang, J.; Lyu, Z.; Shao, S.; Li, F.; Yang, S.; Song, W.; Li, W.; Li, S. Effects of aluminum toxicity induced by acid deposition on pine forest ecosystem in Longli of Guizhou Province, Southwestern China. Chin. Geogr. Sci. 2016, 26, 495–507. [Google Scholar] [CrossRef]
- Izuta, T.; Yamaoka, T.; Nakaji, T.; Yonekura, T.; Yokoyama, M.; Funada, R.; Koike, T.; Totsuka, T. Growth, net photosynthesis and leaf nutrient status of Fagus crenata seedlings grown in brown forest soil acidified with H2SO4 or HNO3 solution. Trees 2004, 18, 677–685. [Google Scholar] [CrossRef]
- Li, Z.; Dai, P.; Wang, Y.; Li, T.; Webb, A.A.; Wang, Y.; Li, Z.; Kou, T.; Shi, G.; Zhang, B. Effects of liming on health and growth of young Schima superba trees under canopy of a Pinus massoniana stand damaged by soil acidification in Chongqing, China. New For. 2016, 47, 801–813. [Google Scholar] [CrossRef]
- Wang, Y.; Solberg, S.; Yu, P.; Myking, T.; Vogt, R.D.; Du, S. Assessments of tree crown condition of two Masson pine forests in the acid rain region in south China. For. Ecol. Manag. 2007, 242, 530–540. [Google Scholar] [CrossRef]
- Kuang, Y.W.; Li, J.; Sun, F.F.; Hou, E.Q.; Zhou, G.Y.; Zhang, D.Q.; Huang, L.-B. Homogeneity of δ15N in needles of Masson pine (Pinus massoniana L.) was altered by air pollution. Environ. Pollut. 2010, 158, 1963–1967. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liao, L.; Liu, Y.; Wang, Q.; Murray, P.J.; Jiang, X.; Zou, G.; Cai, J.; Zhao, X. Effects of Phyllostachys pubescens expansion on underground soil fauna community and soil food web in a Cryptomeria japonica plantation, Lushan Mountain, subtropical China. J. Soils Sediments 2021, 21, 2212–2227. [Google Scholar] [CrossRef]
- Li, X.; Yin, X.; Wang, Z.; Fan, W. Interaction between decomposing litter and soil fauna of the Betula ermanii forest floor of the Changbai Mountains, China. Can. J. For. Res. 2014, 44, 1507–1514. [Google Scholar] [CrossRef]
- Xue, H.; Wang, Q.; Mao, K.; Liu, Y.; Jiang, X.; Murray, P.J.; Zhang, L.; Liu, W. Positive Effects of Reforestation on the Diversity and Abundance of Soil Fauna in a Landscape Degraded Red Soil Area in Subtropical China. Forests 2022, 13, 1596. [Google Scholar] [CrossRef]
- Kudrin, A.; Perminova, E.; Taskaeva, A.; Ditts, A.; Konakova, T. A Meta-Analysis of the Effects of Harvesting on the Abundance and Richness of Soil Fauna in Boreal and Temperate Forests. Forests 2023, 14, 923. [Google Scholar] [CrossRef]
- Dimitrie, D.A.; Burke, D.J.; Benard, M.F. Response of American Toads and Their Invertebrate Prey to Experimentally Elevated Soil pH. Ichthyol. Herpetol. 2023, 111, 128–137. [Google Scholar] [CrossRef]
- Šalamún, P.; Hanzelová, V.; Miklisová, D.; Šestinová, O.; Findoráková, L.; Kováčik, P. The effects of vegetation cover on soil nematode communities in various biotopes disturbed by industrial emissions. Sci. Total Environ. 2017, 592, 106–114. [Google Scholar] [CrossRef]
- Fu, Q.; Shao, Y.; Wang, S.; Liu, F.; Tian, G.; Chen, Y.; Yuan, Z.; Ye, Y. Soil Microbial Distribution Depends on Different Types of Landscape Vegetation in Temperate Urban Forest Ecosystems. Front. Ecol. Evol. 2022, 10, 858254. [Google Scholar] [CrossRef]
- Kang, H.; Yu, W.; Dutta, S.; Gao, H. Soil microbial community composition and function are closely associated with soil organic matter chemistry along a latitudinal gradient. Geoderma 2021, 383, 114744. [Google Scholar] [CrossRef]
- Maltz, M.R.; Chen, Z.; Cao, J.; Arogyaswamy, K.; Shulman, H.; Aronson, E.L. Inoculation with Pisolithus tinctorius may ameliorate acid rain impacts on soil microbial communities associated with Pinus massoniana seedlings. Fungal Ecol. 2019, 40, 50–61. [Google Scholar] [CrossRef]
- Ni, Y.; Yang, T.; Ma, Y.; Zhang, K.; Soltis, P.S.; Soltis, D.E.; Gilbert, J.A.; Zhao, Y.; Fu, C.; Chu, H. Soil pH determines bacterial distribution and assembly processes in natural mountain forests of eastern China. Glob. Ecol. Biogeogr. 2021, 30, 2164–2177. [Google Scholar] [CrossRef]
- Liu, C.; Jin, Y.; Hu, Y.; Tang, J.; Xiong, Q.; Xu, M.; Bibi, F.; Beng, K.C. Drivers of soil bacterial community structure and diversity in tropical agroforestry systems. Agric. Ecosyst. Environ. 2019, 278, 24–34. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, Y.; Qu, Z.; Zhu, T.; Yu, L. Responses of Soil N2O Emission and CH4 Uptake to N Input in Chinese Forests across Climatic Zones: A Meta-Study. Atmosphere 2022, 13, 1145. [Google Scholar] [CrossRef]
- Hassan, M.U.; Batool, M.; Farooq, T.H.; Arif, M.S.; Aamer, M.; Waqas, M.A.; Albasher, G.; Sajjad, M.; Shakoor, A. How does dolomite application affect the greenhouse gases emissions from the terrestrial environment?—A global synthesis. Fuel 2023, 332, 126048. [Google Scholar] [CrossRef]
- Gatica, G.; Fernández, M.E.; Juliarena, M.P.; Gyenge, J. Environmental and anthropogenic drivers of soil methane fluxes in forests: Global patterns and among-biomes differences. Glob. Change Biol. 2020, 26, 6604–6615. [Google Scholar] [CrossRef]
- Xie, D.; Si, G.; Zhang, T.; Mulder, J.; Duan, L. Nitrogen deposition increases N2O emission from an N-saturated subtropical forest in southwest China. Environ. Pollut. 2018, 243, 1818–1824. [Google Scholar] [CrossRef]
- Skidmore, A.; Abdullah, H.; Siegenthaler, A.; Adiningrat, D.P.; Rousseau, M.; Duan, Y.; Torres-Rodriguez, A.; Neinavaz, E. Forest soils further acidify in core Natura 2000 areas amongst unaware government policy. Ecol. Indic. 2024, 159, 111621. [Google Scholar] [CrossRef]
- Van Straaten, O.; Kulp, L.; Martinson, G.O.; Zederer, D.P.; Talkner, U. Forest liming in the face of climate change: The implications of restorative liming for soil organic carbon in mature German forests. Soil 2023, 9, 39–54. [Google Scholar] [CrossRef]
- Greve, M.; Block, J.; Schüler, G.; Werner, W. Long term effects of forest liming on the acid-base budget. Appl. Sci. 2021, 11, 955. [Google Scholar] [CrossRef]
- Brunner, I.; Zimmermann, S.; Zingg, A.; Blaser, P. Wood-ash recycling affects forest soil and tree fine-root chemistry and reverses soil acidification. Plant Soil 2004, 267, 61–71. [Google Scholar] [CrossRef]
- Zhang, J.-K.; Zhang, S.-Y.; Niu, C.-H.; Jiang, J.; Sun, H.-J. Positive effects of biochar on the degraded forest soil and tree growth in China: A systematic review. Phyton-Int. J. Exp. Bot. 2022, 91, 1601–1616. [Google Scholar] [CrossRef]
- Tarin, M.W.K.; Fan, L.; Shen, L.; Lai, J.; Li, J.; Deng, Z.; Chen, L.; He, T.; Rong, J.; Zheng, Y. Rice straw biochar impact on physiological and biochemical attributes of Fokienia hodginsii in acidic soil. Scand. J. For. Res. 2020, 35, 59–68. [Google Scholar] [CrossRef]
- Wang, B.; Gao, Y.; Lai, X.; Luo, L.; Zhang, X.; Hu, D.; Shen, Z.; Hu, S.; Zhang, L. The effects of biochar derived from feedstock with different Si and Al concentration on soil N2O and CO2 emissions. Environ. Pollut. 2023, 317, 120731. [Google Scholar] [CrossRef]
- Rousseau, M.; Siegenthaler, A.; Skidmore, A.K.; de Groot, G.A.; Laros, I. Further reduction in soil bacterial diversity under severe acidification in European temperate forests. Eur. J. Soil Sci. 2024, 75, e70005. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Lin, G.; Zhu, F.; Wu, Z.; Gundersen, P.; Zeng, D.-H.; Hobbie, E.A.; Zhu, W.; Fang, Y. Higher resistance of larch-broadleaf mixed forests than larch forests against soil acidification under experimental nitrogen addition. Plant Soil 2024, 505, 335–349. [Google Scholar] [CrossRef]
- Lin, N.; Deng, N.; Lu, D.; Xie, H.; Feng, M.; Chen, S. Short-term effects of thinning on tree growth and soil nutrients in the middle-aged Chinese fir (Cunninghamia lanceolata (Lamb.) hook.) plantations. Forests 2022, 14, 74. [Google Scholar] [CrossRef]
- Prietzel, J.; Falk, W.; Reger, B.; Uhl, E.; Pretzsch, H.; Zimmermann, L. Half a century of Scots pine forest ecosystem monitoring reveals long-term effects of atmospheric deposition and climate change. Glob. Change Biol. 2020, 26, 5796–5815. [Google Scholar] [CrossRef]
- Schlutow, A.; Schröder, W.; Jenssen, M.; Nickel, S. Modelling of soil characteristics as basis for projections of potential future forest ecosystem development under climate change and atmospheric nitrogen deposition. Environ. Sci. Eur. 2021, 33, 87. [Google Scholar] [CrossRef]
- Wilpert, K.V. Forest soils—What’s their peculiarity? Soil Syst. 2022, 6, 5. [Google Scholar] [CrossRef]
- Zetterberg, T.; Olsson, B.A.; Löfgren, S.; von Brömssen, C.; Brandtberg, P.-O. The effect of harvest intensity on long-term calcium dynamics in soil and soil solution at three coniferous sites in Sweden. For. Ecol. Manag. 2013, 302, 280–294. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhou, J.; Chen, H. Global Trajectories of Forest Soil Acidification: A Scientometric Synthesis of Drivers, Impacts and Sustainable Solutions. Forests 2025, 16, 733. https://doi.org/10.3390/f16050733
Zhang Y, Zhou J, Chen H. Global Trajectories of Forest Soil Acidification: A Scientometric Synthesis of Drivers, Impacts and Sustainable Solutions. Forests. 2025; 16(5):733. https://doi.org/10.3390/f16050733
Chicago/Turabian StyleZhang, Yujie, Jiangmin Zhou, and Hualin Chen. 2025. "Global Trajectories of Forest Soil Acidification: A Scientometric Synthesis of Drivers, Impacts and Sustainable Solutions" Forests 16, no. 5: 733. https://doi.org/10.3390/f16050733
APA StyleZhang, Y., Zhou, J., & Chen, H. (2025). Global Trajectories of Forest Soil Acidification: A Scientometric Synthesis of Drivers, Impacts and Sustainable Solutions. Forests, 16(5), 733. https://doi.org/10.3390/f16050733



