1. Introduction
Understanding species’ responses to climate change is a cornerstone of contemporary biogeographic research, especially regarding plant species’ distribution patterns in tropical ecosystems. The impact of climate change on species distribution has long been an essential focus in biogeographic research [
1]. Comprehensive assessment of species’ climatic niches and biogeographic distributions (both historical and contemporary) provides critical baseline data for modeling climate change impacts on ecosystem dynamics [
2]. Global climate fluctuations—including temperature shifts, precipitation variability, and extreme weather events—significantly alter these patterns, necessitating robust models to predict future impacts on species distribution and abundance [
3].
Recent advancements in species distribution models (SDMs)—including generalized linear models, generalized additive models, and random forests—have opened new avenues for evaluating climate change’s potential effects on biodiversity [
4] As predictive ecological tools, species distribution models (SDMs) statistically derive habitat suitability surfaces by modeling the relationship between species occurrence points and multivariate environmental predictors. Empirical studies confirm maximum entropy (MaxEnt) as a high-performing SDM approach, especially when modeling species distributions with limited occurrence records [
5]. SDMs have been widely applied in several fields, such as forecasting species’ potential distributions, conserving rare and endangered plants and animals, managing invasive species, and studying paleobiogeography. This methodological approach not only deepens our comprehension of species’ ecological niches but also shapes conservation strategies, supporting biodiversity preservation amid environmental changes and human impacts [
3,
4].
Symplocos cochinchinensis (Lour.) S. Moore, commonly referred to as Vietnamese alum, plays a significant role in the Symplocaceae family [
6]. Displaying a wide ecological amplitude, this species thrives in diverse tropical and subtropical habitats worldwide, with notable populations in China, Vietnam, Malaysia, India, and other countries [
7]. Considered an evergreen shrub or tree, it occupies specific ecological niches, primarily in humid tropical biomes, and shows a disjunctive distribution pattern in several areas.
The plant can grow up to 7 m tall, featuring gray-green to slightly gray bark. Its leaves are solitary, alternate, and thick, with yellow, aromatic flowers and spherical fruit. Leaves of
S. cochinchinensis are widely used in folk medicine to treat conditions such as inflammation, diarrhea, excessive menstruation, and uterine issues, among others [
8]. Owing to its bioactivity, extracts from the leaves are used to treat a variety of diseases, including diabetes, HIV, bacterial infections, inflammation, oxidation, and tumors. This species plays a crucial role in both the tree and shrub layers, serving as the principal and often pioneer species of subtropical evergreen broadleaf forests. Thus, it is vital for the regeneration of broad-leaved evergreen forests [
9].
S. cochinchinensis exhibits facultative asexual and sexual reproduction, with reproductive mode plasticity mediated by habitat conditions. Asexual reproduction dominates in habitats with ample moisture, fertility, and high canopy cover, whereas sexual reproduction prevails in areas with low moisture, fertility, and high light [
7]. The bottlenecks differ for the two reproductive types: from seed to seedling for sexual reproduction and from seedling to mature plant for asexual reproduction. New habitats are initially colonized through the establishment of adult plants, and then, facilitated by the ease of asexual reproduction, space is quickly filled through division [
6,
7]. By establishing itself in challenging conditions, it can help stabilize the soil and create conditions that allow other species to colonize, gradually contributing to the ecological succession process [
9].
S. cochinchinensis plays a remarkable ecological role, showing significant resistance to toxic crater gases and acidic soil conditions [
8]. The subspecies
S. cochinchinensis var.
laurina, a pioneer species in the Java caldera region, can dwarf to very small sizes while maintaining its flowering and fruiting capabilities [
7]. Additionally, it is resilient and frequently the initial species to colonize the shrub layer in shallow water forests. As a pioneer species, the plant is influenced by various factors, including frost, fire, wind, precipitation, and diurnal temperature differences. Furthermore, the species thrives in marginal areas, rendering it susceptible to anthropogenic harm through extraction for firewood and medicinal purposes. These factors significantly influence the plant’s establishment in its natural habitats [
6]. Biodiversity hotspots contain numerous underexplored plants. The Symplocaceae family is an example, with limited species studied to date. While the focus has primarily been on their medicinal properties, the ecological attributes of
S. cochinchinensis warrant deeper investigation [
10].
It also serves as a foundational species in Southeast Asian tropical ecosystems. As dominant habitat-forming trees, they provide critical shelter and microhabitats for numerous associated species while functioning as pioneer plants that colonize and stabilize disturbed areas. Their pivotal role in ecological succession makes them particularly significant in the context of climate change, as their distribution shifts will profoundly influence the survival and distribution patterns of dependent organisms within their ecological communities.
Despite the critical ecological roles of species such as
S. cochinchinensis, research directly addressing their responses to climate change is sparse [
11]. Very few studies have been focused on the reaction to global climate change of species with broad ecological niches; this study provides crucial theoretical foundations for understanding these cascading effects and developing appropriate conservation strategies. This project seeks to bridge this gap by simulating the geographical distribution and potential abundance of
S. cochinchinensis under climate change scenarios for 2050 and 2070. Using a comprehensive suite of statistical and model simulation analyses, this study aims to clarify the spatial distribution patterns of this dominant tropical tree species and evaluate its vulnerability or adaptability to forthcoming climatic scenarios. Focusing on
S. cochinchinensis, recognized for its medicinal and ecological value, this research contributes to the conservation of a crucial species and enriches our understanding of how tropical biodiversity responds to global climate change. It promises to provide predictive insights into the effects of climate change on tropical tree species, informing conservation priorities and management strategies in tropical ecosystems. Through detailed analysis and modeling, this project aims to make a significant contribution to global biodiversity conservation and climate change mitigation efforts.
S. cochinchinensis represents one of the most extensively documented plant species in Southeast Asia, with over 5000 georeferenced specimens recorded—making it an ideal model organism for studying climate change impacts on widespread tropical flora. This study specifically aims to:
(1) Establish baseline distribution models for this ecological generalist under current climate conditions, leveraging its unparalleled occurrence data density in the region.
(2) Identify the bioclimatic thresholds that define its broad ecological amplitude.
(3) Project how this “niche space occupier” may redistribute under extreme climate scenarios (SSP585 2050/2070), thereby serving as a bellwether for similar tropical species.
We hypothesize that:
(1) The species’ high dispersal capacity (evidenced by its pioneer characteristics) will facilitate faster range shifts than predicted for most tropical plants.
(2) Current biodiversity hotspots will retain higher habitat suitability than newly suitable areas, reflecting legacy effects of co-evolved biotic interactions.
(3) As a climatic generalist, S. cochinchinensis will exhibit distributional changes proportional to precipitation variability (Bio15) rather than temperature extremes—contrasting with patterns observed in niche specialists.
3. Results
Three pivotal insights emerge from studying this hyper-abundant species: (1) The stability of its core habitats despite climate shifts (
Figure 5) challenges the assumption that widespread species uniformly face range contractions; (2) its dependency on precipitation seasonality (Bio15) rather than absolute rainfall (Bio12) reveals a previously underestimated vulnerability in tropical generalists; and (3) the >20% expansion into novel habitats (
Table 3) demonstrates unexpected plasticity. Collectively, these findings redefine how we model climate impacts on ecological “winners” versus “losers”.
3.1. Distribution Area Under Current Climate
Our research showed despite their broad ecological niches, species like S. cochinchinensis face significant challenges under future climate conditions. Key findings include: (1) Niche shifts: The species’ suitable habitats are projected to shift toward more suitable microclimates, potentially involving migration to higher latitudes or elevations. This niche shift reflects the species’ ability to adapt to changing environmental conditions, but it also highlights the vulnerability of its current habitats to climate-induced stressors. (2) Contrasting trends: While S. cochinchinensis may expand into newly suitable areas, it will likely experience contractions in marginally suitable habitats. The reduction in barely suitable habitats and the increase in highly suitable areas suggest that S. cochinchinensis may undergo significant biogeographical changes, with some populations thriving in new regions while others decline in traditional ranges, revealing the complex responses of tropical species to climate change in Southeast Asia.
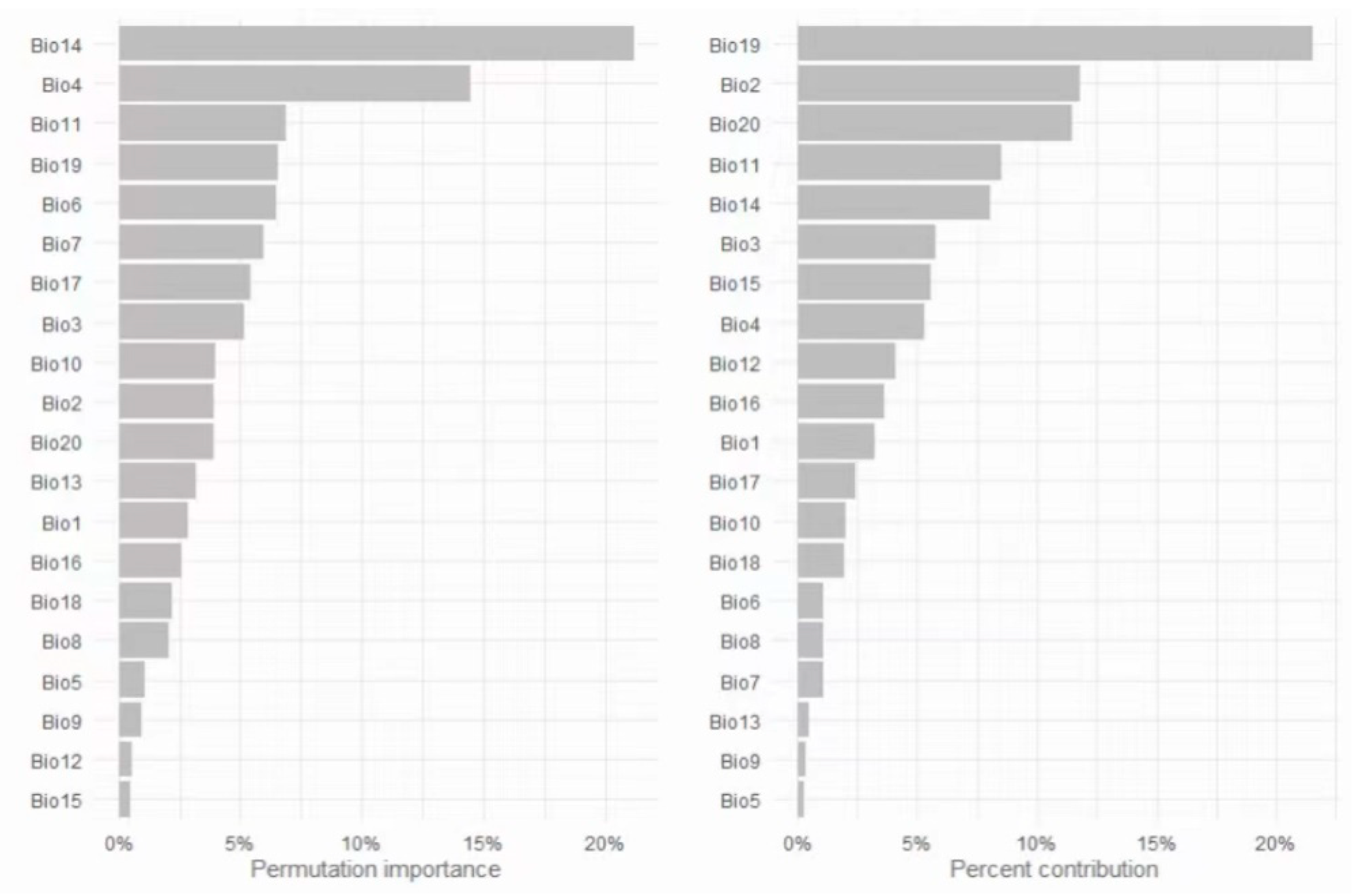
Precipitation of the Wettest Month (Bio 13) was the most influential variable, contributing 57.8% to the model (
Table 3), whereas Mean Temperature of the Wettest Quarter (Bio 8) exhibited the highest permutation importance (29.8%), indicating the model’s predictive accuracy is particularly sensitive to this variable. “Precipitation of Warmest Quarter” (Bio 18) follows as the second most contributory factor at 28.2%, despite a lower permutation importance of 4.9%.
Bio 13 (Precipitation of Wettest Month) emerged as the most influential variable, accounting for 57.8% of the model’s predictive power. This underscores the species’ strong dependence on humid environments, particularly in regions with high precipitation during the wettest month, which likely provides essential moisture for its growth and reproduction. Bio 18 highlights the importance of an adequate water supply during the warmest season for the species’ adaptation. In terms of permutation importance, Bio 13 scores 18.3%, while Bio 18 scores 4.9%. Despite the lower permutation importance of Bio 18, its high contribution rate suggests its significance in the model. The high permutation importance of Bio 13 further confirms the critical role of precipitation during the wettest month in shaping the distribution of S. cochinchinensis.
Conversely, “Mean Diurnal Range” (Bio 2) contributes 6.1% but has a significant permutation importance of 17.7%. “Max Temperature of Warmest Month” (Bio 5) has the minimal contribution at 0.2%, yet its permutation importance is 7%. This analysis unveils the complexity of factors deemed critical by MaxEnt for identifying the species’ suitable habitats. Precipitation of Driest Month (Bio14) showed a contribution of 3.5% and permutation importance of 5.1%. This variable likely affects the species’ resilience to drought conditions. Elevation (Bio20) contributed 2.1% with a permutation importance of 6.7%, suggesting that the species has a certain preference for altitude but is not as restricted by elevation as it is by other factors. Temperature Seasonality (Bio4), with a 1.4% contribution and 10.5% permutation importance, indicates some sensitivity to seasonal temperature variations.
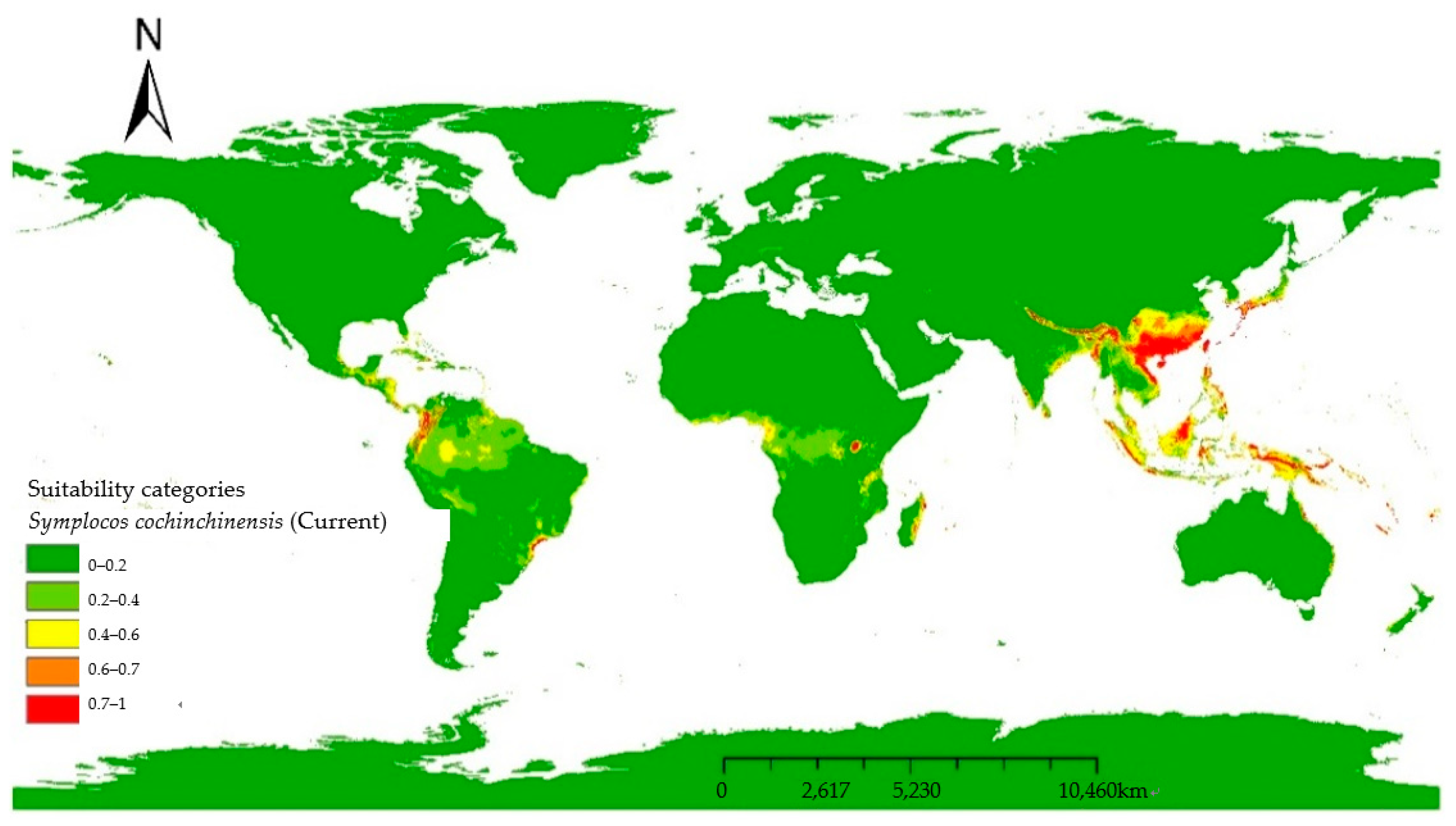
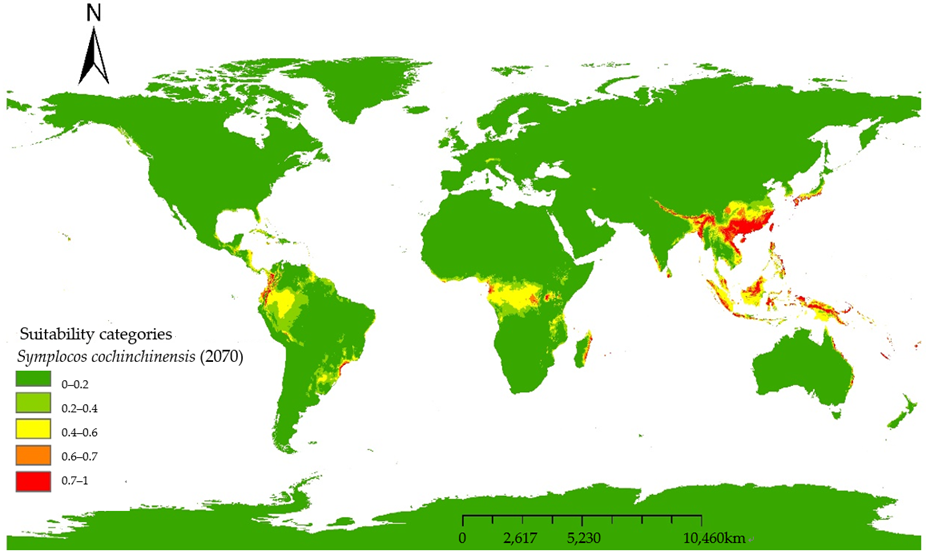
For clarity and detailed analysis, MaxEnt model predictions were mapped using ArcGIS 9.3 (
Figure 5). Adopting the classification method by [
17] we reclassified potential habitats into five categories: Very highly suitable (0.7–1.0); highly suitable habitat (0.6–0.7); suitable habitat (0.4–0.6); barely suitable habitat (0.2–0.4); and unsuitable habitat (0–0.2). For each modeled period, habitat areas were categorized as barely suitable or unsuitable (0–0.4), suitable (0.4–0.6), and highly or very highly suitable (0.6–1) (
Table 3). The model of the current period predicts an area of 305, 431, and 147.71 million square kilometers of highly or very suitable habitat, suitable habitat, and unsuitable or barely suitable habitat, respectively. Current predictions indicate that the suitable habitats of
S. cochinchinensis are primarily in tropical and subtropical regions (
Table 4), with dense aggregations in Southeast Asia, especially Vietnam and southern China, suggesting optimal conditions for growth and reproduction. Southern China has a fairly wide range of suitable habitat that can support
S. cochinchinensis but may not be optimal for its proliferation, and shifts to barely suitable habitat might be used by the species for expansion but could be subject to stressors that inhibit extensive growth with increasing latitude. Australia’s eastern coastal region is also dotted with a narrow zone of suitability, which is as far south as
S. cochinchinensis can reach. The peninsulas and archipelagos between Australia and China, as well as southern Japan and southern Korea and the Solomon Islands, are almost potentially suitable habitat for
S. cochinchinensis. Despite its native range, potentially suitable habitats for
S. cochinchinensis extend to South America and Africa, including Colombia, Costa Rica, southern Brazil, Madagascar’s southern forests, and Cameroon.
3.2. Future Potential Distribution
This paradox—where a species simultaneously shows resilience (colonizing new areas) and vulnerability (precipitation dependence)—mirrors the “generalist’s dilemma” theorized by Brown et al. [
18]. While its seed dispersal traits facilitate migration (supporting H
2), the required precipitation synchrony (Bio13 + Bio15) creates ecological bottlenecks unseen in rare species studies. Notably, the 31% habitat turnover rate suggests that even common species may undergo distributional upheavals comparable to endangered taxa.
Direct comparison of habitat suitability changes between time periods is challenging when solely examining the projections (
Figure 6 and
Figure 7).
Table 4 details habitat areas across different suitability levels over time, summarizing values for barely suitable and unsuitable habitats, as well as those for highly and very highly suitable habitats. As of the current period, the vast majority of the species’ habitat is categorized as unsuitable, occupying an area of 13,955 million square kilometers. Comparatively, barely suitable habitats cover 816 million square kilometers, while suitable habitats are significantly smaller at 431 million square kilometers. Areas of high suitability, combining the highly suitable and very highly suitable categories, collectively span 305 million square kilometers, indicating limited but vital regions for the species’ sustenance.
The 2025 model projections indicate “Precipitation of Wettest Month” (Bio13) and “Precipitation of Warmest Quarter” (Bio18) lead in contribution at 46.8% and 41.6%, respectively, with their importance rankings increasing to 20.8 and 6.9 (
Table 5), a trend that slightly intensifies by 2027. This trend underscores the ongoing and potentially escalating impact of precipitation patterns on species habitats amid changing climatic conditions.
Variables like “Mean Diurnal Range” (Bio2), “Mean Temperature of Wettest Quarter” (Bio8), and “Temperature Seasonality” (Bio4), despite lower contributions, exhibit notable ranked importance. In both future scenarios, “Elevation” (Bio20) and “Max Temperature of Warmest Month” (Bio5) were minor variables, indicating they were not the main drivers of habitat changes for the species within the studied timeframe. This also suggests that extreme dry and hot climates, resultant of climate change, may have a lesser impact on the species.
By 2050, unsuitable habitats marginally increase to 13,956 million square kilometers. Notably, barely suitable habitats decrease to 710 million square kilometers, while suitable habitats expand to 508 million square kilometers, and high suitability areas increase to 333 million square kilometers. This suggests a modest expansion of more optimal living conditions for the species, despite the general trend of habitat degradation.
By 2070, unsuitable habitats significantly rise to 14,026 million square kilometers, reflecting increased unsuitability for the species. Barely suitable habitats reduce further to 595 million square kilometers. However, high suitability habitats grow to 350 million square kilometers, signaling a climatic shift possibly favoring S. cochinchinensis’s habitability with higher “Precipitation of Wettest Month” (Bio 13) and higher “Mean Temperature of Wettest Quarter” (Bio 8).
The SSP585 scenario, a high-emission pathway, projects significant climatic changes for the SE Asia region, including a temperature increase of 2.5–4.5 °C by 2070, more erratic precipitation patterns (intensified wet seasons and prolonged dry periods), increased frequency of extreme weather events, and sea level rise. These climatic alterations are projected to drive niche shifts in S. cochinchinensis populations, characterized by a significant reduction in less suitable habitats alongside a subtle expansion in more suitable regions. Furthermore, it emphasizes the critical role of ecological resilience and the potential for new refugia, which may be essential for the species’ long-term survival.
4. Discussion
4.1. Spatial Distribution Pattern
In MaxEnt models, percent contribution elucidates the factors leading to a robust model, whereas permutation importance reveals what maintains the model’s strength [
19]. Consider each environmental variable as a team member, with percent contribution measuring their influence on the model’s development process. It is a bit like saying, “Variable A did 57.8% of the work in getting us to a good model”. Conversely, permutation importance assesses each variable’s unique impact by evaluating the model’s performance in their absence. If the project struggles without their input, it shows that their contribution was crucial, even if it was not the most prominent throughout the process. It tells us, “When we ignored the work of Variable A, our project success dropped by 18.3%”. Discrepancies between percent contribution and permutation importance may stem from factors like correlation and unique information. In the current habitat suitability model, “Precipitation of Wettest Month” (Bio13) leads with a 57.8% contribution and an 18.3% permutation importance, underscoring its critical role in water availability during the wet season, essential for species survival and reproduction. However, its permutation importance is lower compared to Bio 8, which implies that while Bio 13 is a significant driver in the model-building phase, the model does not rely on it exclusively. “Precipitation of Warmest Quarter” (Bio 18) significantly contributes during model training, yet its lower permutation importance suggests the model’s performance is not solely dependent on it, likely due to compensation from other variables. Bio 8 (Mean Temperature of Wettest Quarter) and Bio 2 (Mean Diurnal Range) have a lower percent contribution but a high permutation importance, indicating that even though it did not seem as critical while building the model, it actually holds unique information that, when altered, significantly reduces the model’s accuracy. It reveals that this variable’s influence on the species distribution is significant when considered independently, possibly affecting the species during peak growth periods. Interestingly, “Mean Temperature of Wettest Quarter” (Bio 8) and “Temperature Seasonality” (Bio 4) show a dramatic increase in permutation importance by 2070. This shift indicates that temperature during this period may become a more critical limiting factor for the species’ survival and distribution, possibly due to alterations in the seasonal climate patterns that affect the species’ growth and reproductive cycles. with the habitat suitability of the species increasingly affected by temperature fluctuations, particularly in the wetter seasons, and are likely to play an important role in the movement of its ecological niche. The variables “Precipitation of Wettest Month” (Bio 13) and “Precipitation of Warmest Quarter” (Bio 18) remain highly influential in the future scenarios, with their percent contributions remaining substantial. This suggests that water availability during key periods continues to be a critical determinant of habitat suitability for the species. Predicted shifts in variable importance indicate
S. cochinchinensis may undergo distributional changes as it adapts to modified precipitation and temperature regimes. The model implies the species must adapt to varying moisture and temperature conditions, potentially leading to migration, local adaptation, or population decline in shrinking habitats.
The spatial distribution of
S. cochinchinensis, a widely dispersed tropical tree, forms a complex pattern influenced by biotic and abiotic factors throughout its extensive range. Predominantly located in the understory of humid tropical forests,
S. cochinchinensis demonstrates a heterogeneous distribution, indicating a preference for varied ecological niches, from lowland rainforests to montane areas [
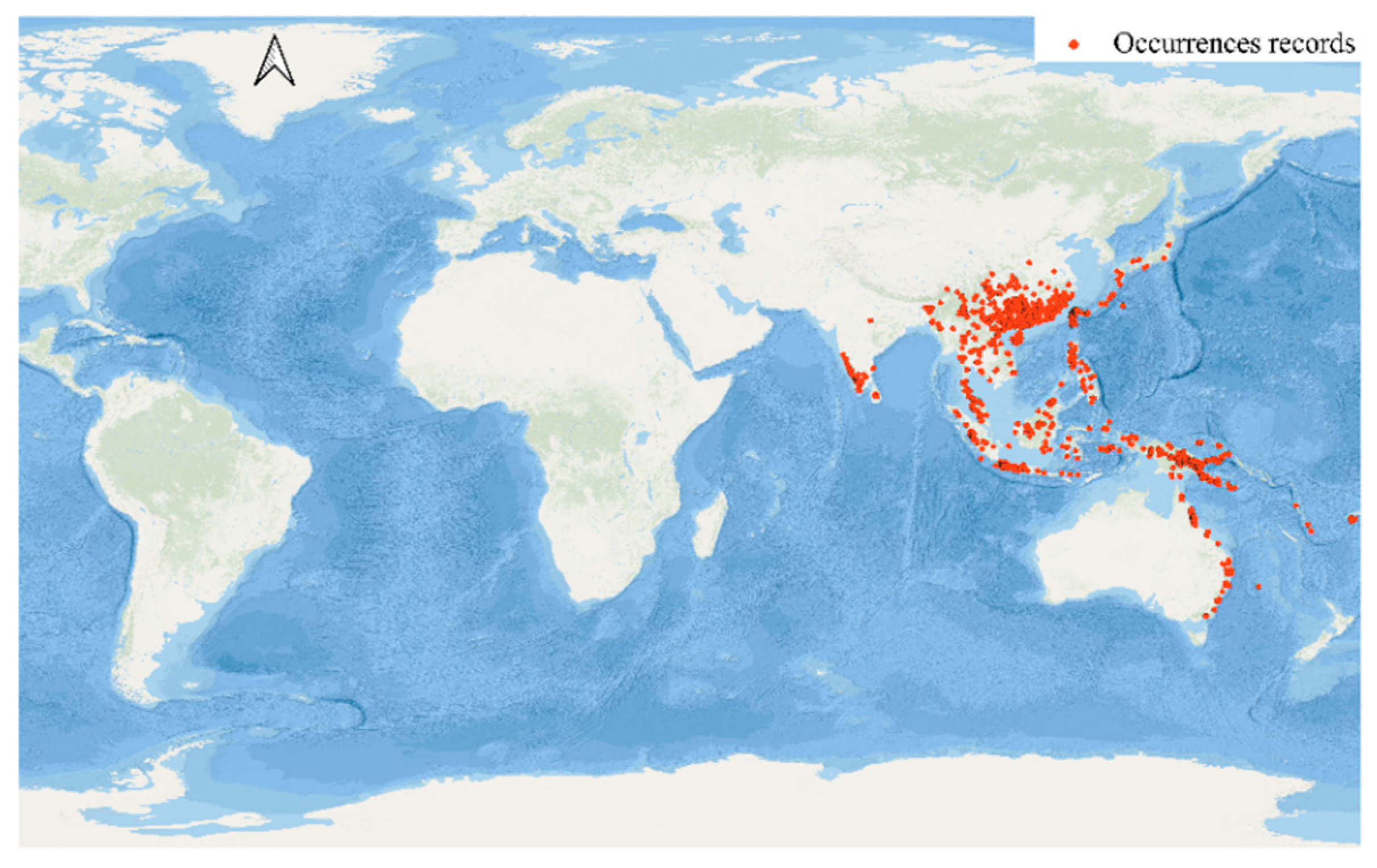
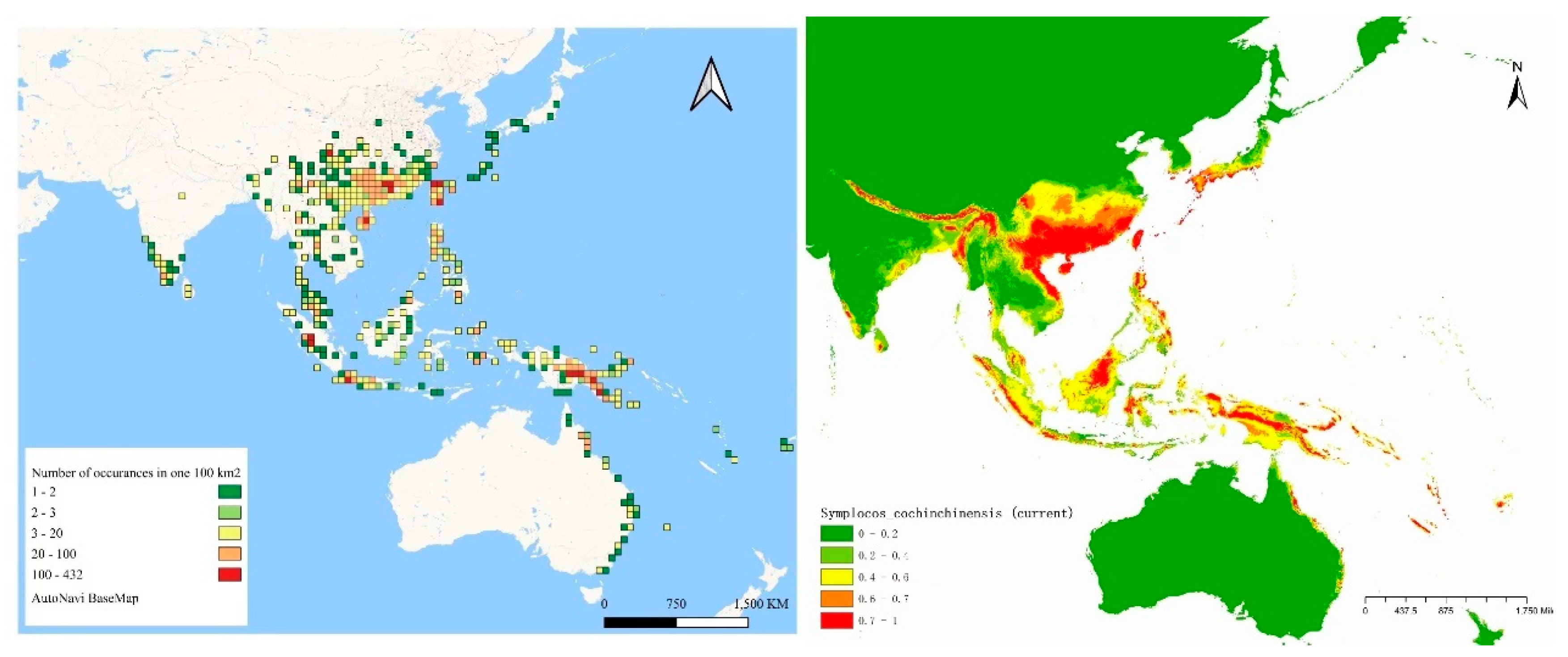
20]. To examine the current distribution and possible discrepancies in observed richness, we compiled diverse data, including species occurrence records (
Figure 8), MaxEnt-based fitness zone predictions (
Figure 8), and the native range of
S. cochinchinensis (
Figure 9), as outlined by Plants of the World Online (POWO).
Specimen occurrences offer a crucial yet partial view of
S. cochinchinensis’s current distribution. These records, illustrated with dense clusters of occurrence points, are most abundant in Southeast Asia, notably in the Greater Sunda Islands (Borneo, Sumatra, Java) and the Philippines. The high density of occurrence points in these regions may signify areas of ecological richness where the species is both prevalent and well-documented due to suitable habitat conditions or heightened research interest [
21]. Fishnet maps reveal widespread but uneven distribution of occurrence sites, indicating variable local abundance across its extensive habitat range. However, interpreting these data requires caution due to potential biases. Accessibility, research funding, and local taxonomic expertise may disproportionately influence where specimens are collected, thus distorting our perception of species richness [
22]. For instance, the occurrence data for Northeast India and parts of the Himalayas are sparse despite these regions falling within the species’ native range. This gap may indicate either actual lower species density or, more likely, less extensive research efforts. In the Central South Peninsula region, Vietnam is a very suitable habitat, but the remaining areas, such as Burma and Laos, are unsuitable habitats. The reason for this is likely to be a lack of sample points due to sampling effects by scientists.
MaxEnt model predictions provide a complementary view by using environmental variables to estimate potentially suitable habitats for
S. cochinchinensis. Predictions indicate a wider habitat range than what occurrence records suggest, pointing to areas where the species may exist but is yet undocumented [
23]. The MaxEnt map shows significant potential in regions with current low documented occurrences, such as Northeast India and Tibet, and a high probability of suitable habitats that align with the regions of dense occurrence records in Southeast Asia. The forecasts underscore the species’ adaptability and resilience, suggesting
S. cochinchinensis may thrive beyond its currently documented locations [
24].
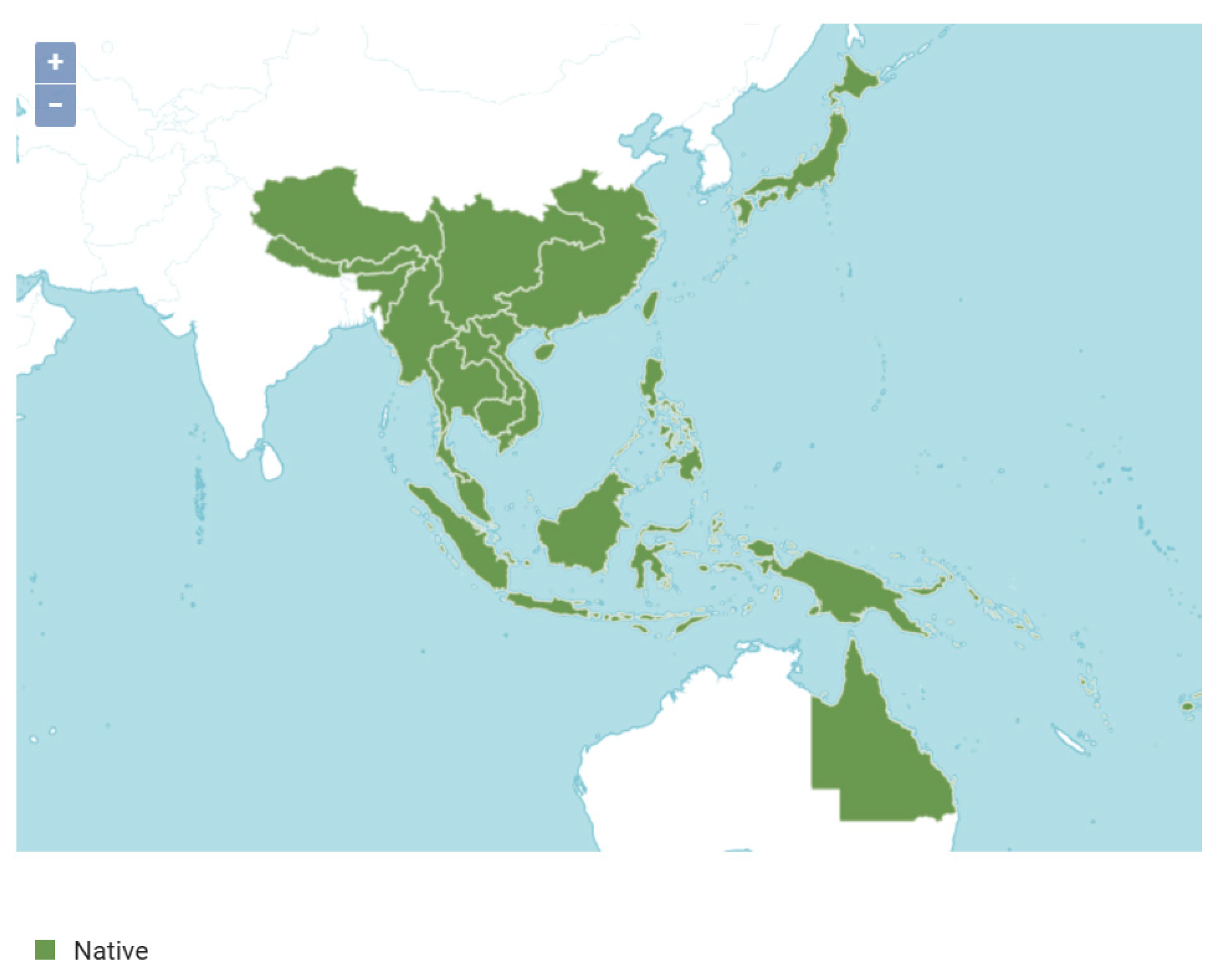
The native range of
S. cochinchinensis, informed by comprehensive literature and historical records, offers a wider perspective on the species’ indigenous distribution. These records encompass large areas of tropical Asia and the Pacific, indicating the species’ historically broad occurrence [
25]. This distribution highlights the species’ ability to thrive in varied ecological zones, from the Himalayan foothills to Queensland’s rainforests. Comparisons with occurrence and MaxEnt data reveal agreement in areas such as Southeast Asia, where the species is notably established. However, it exposes discrepancies in regions such as southern China and the eastern Himalayas, where native occurrence data are sparse despite the species’ presence.
Overlaying the three data types (
Figure 8 and
Figure 9) uncovers zones of convergence and discrepancy. Areas overlapping in all datasets are crucial, signaling zones of high-confidence presence and significant ecological importance for
S. cochinchinensis. Significant data convergence in Southeast Asia, notably Indonesia (Sumatra, Borneo, and Java), Malaysia, the Philippines, and Thailand, marks these as high-confidence regions for the species. These areas should be conservation priorities, maintaining both biodiversity and genetic reservoirs of the species [
26]. Thus, these regions might not be immediate priorities for additional sampling given the relative abundance of existing data, unless the goal is to assess changes over time or the impact of specific environmental factors. Areas without overlap suggest the need for additional field investigations. Occurrence records and native range maps may represent historical distributions now modified by human activity or environmental shifts. Conversely, MaxEnt predictions may highlight potential yet unrealized ranges, offering opportunities for the species’ expansion or re-establishment. Less-studied regions of the species’ range include midland and Tibet in China, northern Japan, Northeast India (specifically Assam), Nepal, Myanmar, Laos, Vietnam, and northeastern Australia.
Occurrence records and predicted habitats indicate that
S. cochinchinensis flourishes in moist environments, such as stream banks and valleys, enhancing its foliage and fruit production, which is essential for attracting seed-dispersing frugivores [
27,
28].
S. cochinchinensis occupies nearly all subtropical forests in Asia, notably Southeast Asia’s tropical rainforests. Southern China’s ten provinces with the highest forest coverage—Hong Kong, Fujian, Jiangxi, Guangxi, Zhejiang, Hainan, Taiwan, Yunnan, Guangdong, and Guizhou—are identified as highly suitable habitats for
S. cochinchinensis [
29]. Northeastern Australia’s temperate rainforests, unlike its central deserts, also provide highly suitable habitats. The species’ widespread occurrence across Southeast Asian islands and mainland, parts of East Asia, and the Pacific underscores its ecological plasticity, adapting to various light conditions and canopy structures [
30]. The species exhibits clumped distributions, suggesting localized optimal conditions or historical human impacts leading to habitat fragmentation [
22,
28].
In summary,
S. cochinchinensis is particularly abundant in regions with high habitat suitability. For detailed quantitative analyses of species richness and distribution, more specific data, like population density estimates or biomass measurements, are essential [
30]. While the provided maps offer a macro-level overview, they cannot substitute for the detailed field studies required to accurately evaluate species richness and distribution patterns.
4.2. Influence Factors of Simulation Accuracy
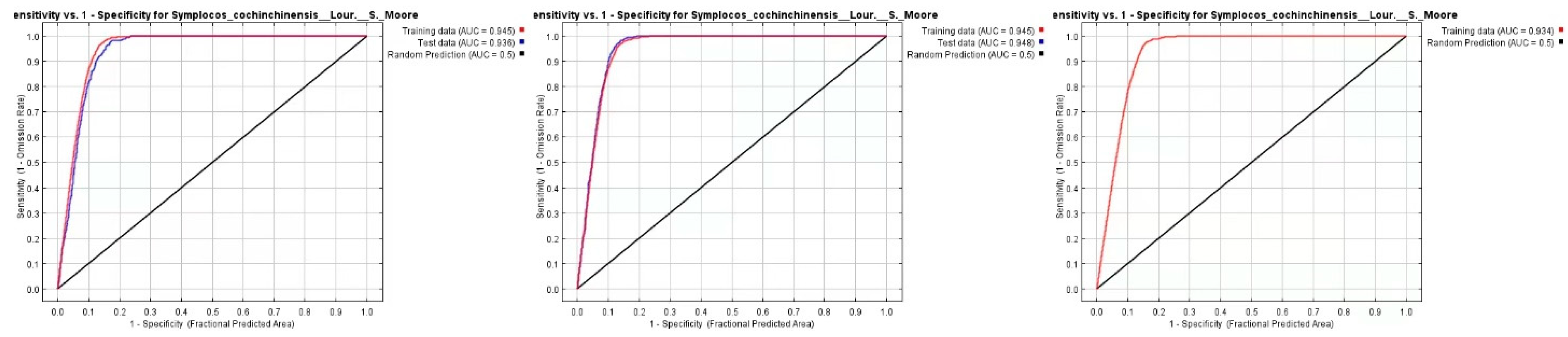
ROC curves (see
Figure 10) for current and future projections (2050 and 2070) for
S. cochinchinensis exhibit high AUC values, demonstrating the model’s robust predictive capability [
31]. Consistently high AUC values across projections underscore the selected environmental variables as likely crucial determinants of the species’ distribution. It is crucial to note that AUC scores do not reflect the actual prevalence or potential abundance of the species within the predicted habitats [
32,
33]. The models presuppose migration to new habitats, an assumption challenged by physical barriers, rapid climate shifts, and other constraints. Ongoing monitoring is vital to validate predictions and adjust conservation strategies as the species’ responses evolve. Although high AUC values indicate robust model performance, they should be interpreted cautiously, as models inherently simplify ecological complexity and may not account for all uncertainties under climate change scenarios [
30,
32].
MaxEnt modeling’s prediction of potential habitats for
S. cochinchinensis in South America and Africa prompts a deeper investigation into the species’ ecological range and the precision of niche modeling. The native range of
S. cochinchinensis is confined to the tropical and subtropical regions of Asia and the Pacific, where it has co-evolved with the local biota and abiotic conditions [
6]. However, the MaxEnt model’s prediction of suitable habitats extending to ecologically analogous regions in the tropics of South America and Africa may underscore the species’ potential adaptability to a broader set of climatic and edaphic conditions than previously documented within its native distribution.
These predictions suggest a fundamental niche for
S. cochinchinensis broader than its realized niche within its native habitat. Climatic similarities, particularly in terms of temperature regimes, precipitation patterns, and humidity levels, between these non-native regions and the species’ indigenous habitats may allow for the theoretical support of its life history traits. This supposition relies on the assumption that the species could potentially establish in these novel environments in the absence of dispersal limitations or biotic resistance factors such as competition, predation, and disease—factors not typically accounted for in correlative niche models [
18].
A critical appraisal of MaxEnt model outputs is crucial, as they rely on the premise that current and historical distributions can predict future occurrences using only environmental variables. Such predictions are purely hypothetical and do not account for the complexities of long-distance dispersal mechanisms, ecological interactions, or the potential for invasive behavior if the species were introduced. Moreover, MaxEnt and similar ecological niche models can overestimate the habitable range of species by not fully integrating the intricate web of biotic interactions and the species’ specific requirements [
34].
Projecting S. cochinchinensis into non-native regions raises discussions on biogeographical dispersal and the impact of human activities on distribution patterns. However, these theoretical habitats should be approached with caution, particularly in the context of biodiversity conservation and the risks associated with biological invasions.
Sampling bias significantly impacts our understanding of distribution and ecological needs, potentially skewing conservation priorities. Typically, specimen collection and ecological surveys are more frequently conducted in areas that are easily accessible, such as those near research institutions, along transportation routes, or within protected regions. Consequently, these areas become overrepresented in occurrence databases, creating an illusion of ecological preference or abundance for the species in these locales [
21,
34]. Conversely, regions that are remote, politically unstable, or lack funding for scientific research are less likely to be sampled thoroughly. This leads to significant gaps in occurrence data, which hampers the ability to form a complete picture of the species’ true distribution and can misinform conservation efforts.
Since models like MaxEnt depend on occurrence records for species distribution predictions, input data significantly influences outcomes. If the initial data is biased towards certain regions or environments, the model’s predictions will inherently reflect these biases, potentially overestimating the species’ presence in well-sampled areas while under predicting it in areas that have been neglected. This phenomenon is particularly problematic for widespread species such as
S. cochinchinensis, where the actual range is extensive and the habitat preferences are diverse. The resultant models may fail to identify critical habitats or areas of ecological significance, leading to misguided conservation actions and the oversight of areas that may be crucial for the species’ resilience and long-term survival [
17,
20,
28].
Mitigating sampling bias requires targeted efforts to gather data from underrepresented areas. This could include deploying remote sensing technologies, encouraging citizen science in remote areas, and establishing international collaborations to facilitate research in less-accessible regions. Equitable distribution of research efforts, both geographically and across different habitat types, will result in more accurate models of species distribution, which are crucial for formulating effective conservation strategies for
S. cochinchinensis and maintaining the ecological integrity of tropical forest ecosystems [
23].
4.3. Response to Global Climate Change
The SSP585 scenario, termed the “high-emission scenario,” represents one pathway within the Shared Socioeconomic Pathways (SSPs), delineating various climate and development futures. SSP585 envisions a future marked by unbridled economic growth and surging energy demand, primarily satisfied through fossil fuels. In SSP585, there is an assumption of limited adoption of environmentally friendly technologies and a lack of global cooperation towards sustainability goals, leading to high greenhouse gas (GHG) emissions. Under SSP585, the projections are alarming, forecasting global warming, sea-level rise, extreme weather, species extinction, and biodiversity loss. There will be profound impacts on agriculture, water availability, health, and livelihoods, with the potential for increased conflict over scarce resources (Ru et al., 2024).
At first glance, a one million square kilometer increase in unsuitable habitats by 2050 may seem minor. However, the reduction in barely suitable habitats suggests a shift away from marginal living conditions. Simultaneously, the increase in highly suitable and very highly suitable habitats indicates a propensity for the species to expand into areas that may become climatically suitable due to warming trends. Our models suggest range shifts toward higher elevations and latitudes, consistent with expected climate-driven migration patterns in tropical species, where cooler conditions persist despite global temperature increases.
By 2070, a significant increase in unsuitable habitat underscores the intensified impact of climate change on traditional ranges. The continued decrease in barely suitable habitats underlines a notable trend of ecological strain. However, the growth in highly suitable habitat areas suggests that certain regions may emerge as new ecological strongholds. These shifts may reflect the species’ movement towards regions that offer climatic respite—cooler or wetter microclimates within a generally warming landscape.
While specific expansion locations and directions are not detailed, they can be deduced from broader climatic trends. Typically, plant species like
S. cochinchinensis would move poleward or to higher elevations in search of cooler climates. Thus, it is plausible that the species is migrating towards higher altitudes within its current range or spreading into higher latitude areas that were previously too cold. However, the species’ ability to establish itself in new areas will depend on various factors, including the presence of suitable soil types, dispersal mechanisms, and the absence of barriers to seed and pollen flow [
35,
36].
Widespread species like
S. cochinchinensis respond to climate change distinctly compared to rare species. Widespread species often possess more genetic diversity, which can facilitate adaptation to changing conditions. Moreover, their presence across a variety of habitats may allow for more substantial ecological plasticity, enabling them to persist and potentially thrive even as conditions change. In contrast, rare species typically have smaller population sizes, less genetic variation, and often inhabit specialized niches, making them more vulnerable to climate-induced alterations. Their ability to migrate or adapt is generally more constrained, thus increasing their risk of extinction under rapid climate change [
36].
As a widespread species, S. cochinchinensis has shown potential to maintain and even expand its habitat range amidst climate change. The species’ success will hinge on its reproductive biology, dispersal mechanisms, and the availability of corridors that facilitate migration to suitable habitats.
4.4. Ecological and Biodiversity Implications
Furthermore, the potential decline or redistribution of S. cochinchinensis due to climate change could disrupt many ecological processes, leading to reduced habitat quality and biodiversity loss. This underscores the interconnectedness of species within ecosystems and highlights the importance of conserving widespread species not only for their intrinsic value but also for their role in maintaining ecosystem stability and supporting rare species. These distributional changes could trigger cascading ecological effects: (1) Ecosystem disruption: declines or redistribution of S. cochinchinensis may degrade habitat quality and reduce biodiversity, highlighting the role of widespread species in maintaining ecosystem stability (e.g., supporting rare species). (2) Broader warning: such patterns may foreshadow similar challenges for other tropical tree species, particularly under high-emission scenarios where current habitats become unsuitable.
4.5. Conservation and Restoration Implications
The findings of this study highlight the urgent need for conservation and restoration efforts to protect S. cochinchinensis and its habitats. As a species with significant ecological and economic value, S. cochinchinensis plays a crucial role in maintaining ecosystem stability, particularly in tropical and subtropical forests. Notably, it is currently the most extensively sampled plant species in Southeast Asia, with an average of over 5000 georeferenced specimens, making it one of the best-documented species in the region. Its widespread occurrence across most areas, as evidenced by its extensive specimen records, underscores its ecological importance and its interactions with other species in forest ecosystems. The projected shifts in its distribution under future climate scenarios emphasize the vulnerability of its current habitats and the necessity of proactive measures. Key steps include prioritizing the protection of highly suitable habitats, establishing ecological corridors to facilitate migration, implementing habitat restoration programs, and preserving genetic diversity through seed banks and ex situ conservation. Community engagement and adaptive management are also essential to ensure the long-term survival of S. cochinchinensis.
This study fills a critical gap in previous research by providing a comprehensive analysis of S. cochinchinensis’s potential distribution under climate change and proposing actionable conservation strategies. The methodologies and findings are not limited to S. cochinchinensis but can be applied to other widespread tropical species facing similar climatic pressures. By integrating species distribution modeling (SDM) with climate scenarios, this research offers a robust framework for predicting habitat suitability and guiding conservation efforts for tropical biodiversity. This approach contributes to the broader goal of safeguarding ecosystem resilience in the face of global environmental change. Given its ecological significance and extensive distribution, the conservation of S. cochinchinensis is not only vital for its own survival but also for maintaining the stability and biodiversity of the ecosystems it inhabits.
For conservation practice, S. cochinchinensis serves as a critical indicator: its projected refugia (e.g., Eastern Himalayas) likely harbor other climate-resilient generalists, whereas its abandonment zones (e.g., lowland Sundaland) signal impending ecosystem reorganization. This study establishes a new framework—moving beyond rarity-focused models to assess biodiversity threats through the lens of keystone hyper-abundant niche space occupiers.
4.6. Ecological Significance of Widespread Species
Widespread species like S. cochinchinensis often play pivotal roles as pioneer species in ecosystems, facilitating ecological succession and creating conditions that support the establishment of other species. As a pioneer species, S. cochinchinensis contributes to soil stabilization, nutrient cycling, and the creation of microhabitats that benefit rare and specialized species. The results of this study reveal that S. cochinchinensis’s distribution is highly sensitive to climatic variables such as precipitation and temperature, with “Precipitation of Wettest Month” (Bio 13) and “Mean Temperature of Wettest Quarter” (Bio 8) being the most influential factors. This sensitivity underscores the potential impact of climate change on its ecological functions. If such key species are adversely affected by climate change, the cascading effects could extend to other components of the ecosystem, particularly rare and endemic species that depend on the ecological functions provided by these pioneers.
The projected shifts in S. cochinchinensis’s distribution—characterized by a reduction in barely suitable habitats and an expansion in highly suitable areas—highlight the species’ adaptive resilience but also its vulnerability to changing environmental conditions. These trends suggest that while S. cochinchinensis may thrive in new regions with suitable microclimates, its decline in traditional ranges could disrupt ecological processes and reduce habitat quality. Therefore, conserving S. cochinchinensis is not only essential for its own survival but also for maintaining the stability and biodiversity of the ecosystems it supports. This study provides a foundation for understanding the ecological significance of widespread species and underscores the importance of integrating climate change projections into conservation strategies to safeguard ecosystem resilience.
4.7. Methodological Contributions and Applications
By integrating species distribution models (SDMs) with climate scenarios, this study provides a framework to assess tropical species’ resilience: (1) Generalizable tools: the approach can be extended to other ecologically or economically significant tropical flora facing climatic pressures. Global climate change, under high-emission scenarios, is likely to render some currently suitable habitats unsuitable, leading to niche contraction and distributional shifts for widespread tropical tree species. (2) Conservation insights: findings emphasize the need to incorporate climate projections into biodiversity strategies (e.g., protecting potential expansion zones, monitoring at-risk populations). By addressing the vulnerabilities of widespread and ecologically significant species like S. cochinchinensis, we can better safeguard the resilience of entire ecosystems in the face of global climate change.