Carbon in Woody Debris and Charcoal Layer in Cold Temperate Coniferous Forest 13 Years After a Severe Wildfire
Abstract
1. Introduction
- The PyC formed a distinct charcoal layer on the ground, which has been the primary component of the above-ground PyC storage.
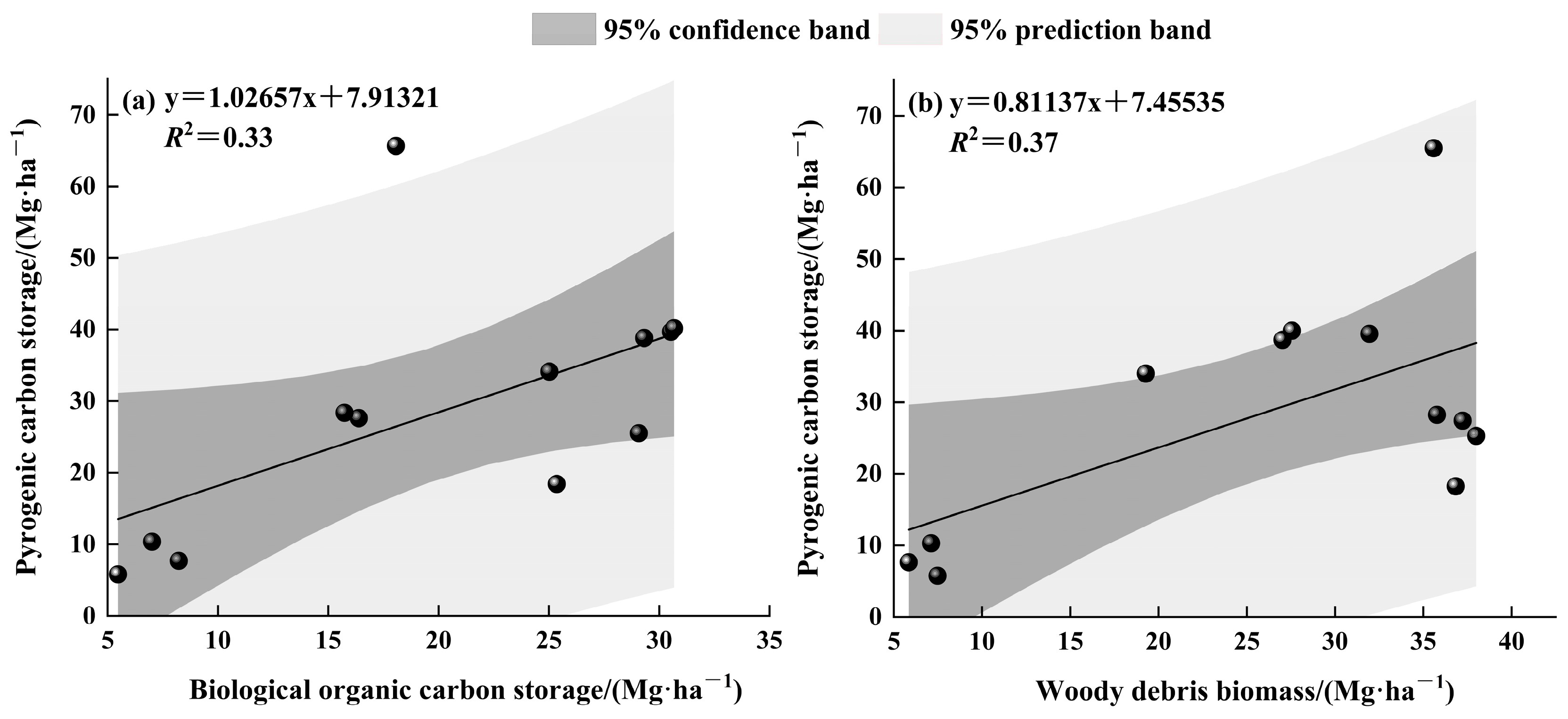
- Uncharred BOC has been effectively sequestered and demonstrated a strong correlation with PyC storage.
- The activated carbon in PyC has largely decomposed, and PyC that now exists is stable carbon.
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Sampling
2.2.1. Sampling of Woody Debris
- Standing trees. All snags (>1.37 m in height) and stumps (<1.37 m in height) were measured in each sample plot [27]. The following parameters were recorded for each tree: species, height, diameter at breast height (DBH), basal diameter, char height, char upper diameter, and char depth, the investigation methods followed the approach described by Donato et al. [12]. Specifically, the distance between charred and uncharred wood was measured to the nearest millimeter. These measurements were used to calculate the biomass of standing trees. In each sample plot, for different tree species, disks approximately 5 cm thick were collected at 1/4, 2/4, and 3/4 of the trunk height (representing the lower, middle, and upper sections, respectively) to determine the content of BOC. Bark samples were randomly collected from 20 cm wide strips at 1/2 char height to determine the content of PyC. Each sample was replicated three times.
- Down wood. Three 5 × 5 m sample squares were established within each sample plot to measure the length, large head diameter, small head diameter, middle diameter, and char depth of all down wood. A classification system using five classes was used to assess decay level [28], which was used to calculate the biomass of down wood. Samples were randomly collected from each tree species at different decay levels in charred and uncharred forms within each sample square, with each sample being repeated three times to determine down wood carbon content.
- Twig. Sample squares of 1 × 1 m were established within each 5 × 5 m sample square. All twigs within these sample squares were collected to determine their biomass and carbon content.
2.2.2. Sampling of the Charcoal Layer
2.3. Laboratory Analysis and Determination
- Charred WD. Pyrogenic carbon from the bark samples of standing trees was manually selected and weighed to determine the PyC mass per unit volume [29,30,31]. Pyrogenic carbon was visually characterized as black particles with a silvery gloss [32]. The PyC samples were carefully scraped from down wood and twigs using a blade [3], then ground with a mortar and passed through a 0.25 mm sieve. Carbonate content was assessed by adding 10% HCl to representative PyC subsamples. The absence of effervescence suggested a negligible carbonate content (<1%) [33]. Consequently, inorganic carbon was considered insignificant in the samples, and total carbon was equivalent to organic carbon.
- Uncharred WD. Samples were ground using an ultra-fine grinder and passed through a 0.25 mm sieve.
- Charcoal layer. All charcoal was gently sieved and separated into four particle size fractions: >2 mm, 2–1 mm, 1–0.5 mm and <0.5 mm. The dry weights of each fraction were recorded. The samples for each particle size fraction were ground using a mortar and passed through a 0.15 mm sieve. Pyrogenic carbon samples were extracted using the chemical oxidation method using HF/HCl treatment and a mixed solution of K2Cr2O7 and H2SO4 [34,35].
2.4. Carbon Storage Calculation
2.4.1. Woody Debris Carbon Storage Calculation
- Standing trees. The biomass of the uncharred snag was determined using the allometric growth equation [37]. The regression relationship between the volume and biomass of the snag was employed to calculate the biomass of the uncharred stump. The surface area of the charred WD was calculated based on field measurement data [12], and multiplied by the mass of PyC per unit volume to obtain the PyCmass of standing trees (Equation (1)).
- Down wood. The calculation of the down wood volume was conducted for the entire particle cylinder, which included the charred part (Equation (2)). For an inner uncharred cylinder whose diameter depends on char depth (Equation (3)). The volume difference between these two measurements represented the PyC volume (Equation (4)) [12,38].
- Twig. By calculating the mass ratio of PyC to unburned twig [3], we determined the biomass of PyC and unburned twig in each sample plot.
2.4.2. Charcoal Layer Carbon Storage Calculation
2.5. Data Analysis
3. Results
3.1. Carbon in Woody Debris
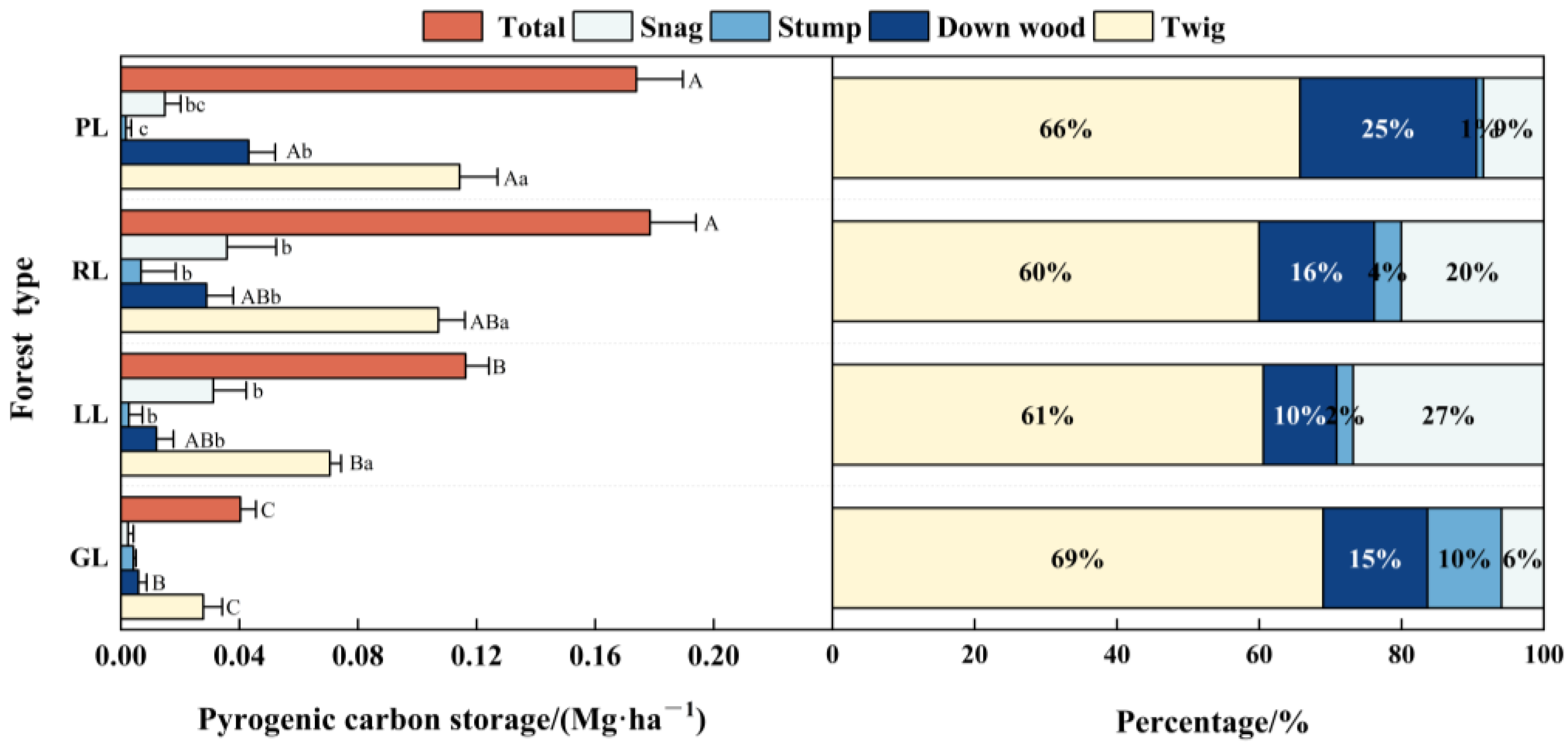
3.1.1. Pyrogenic Carbon on Woody Debris
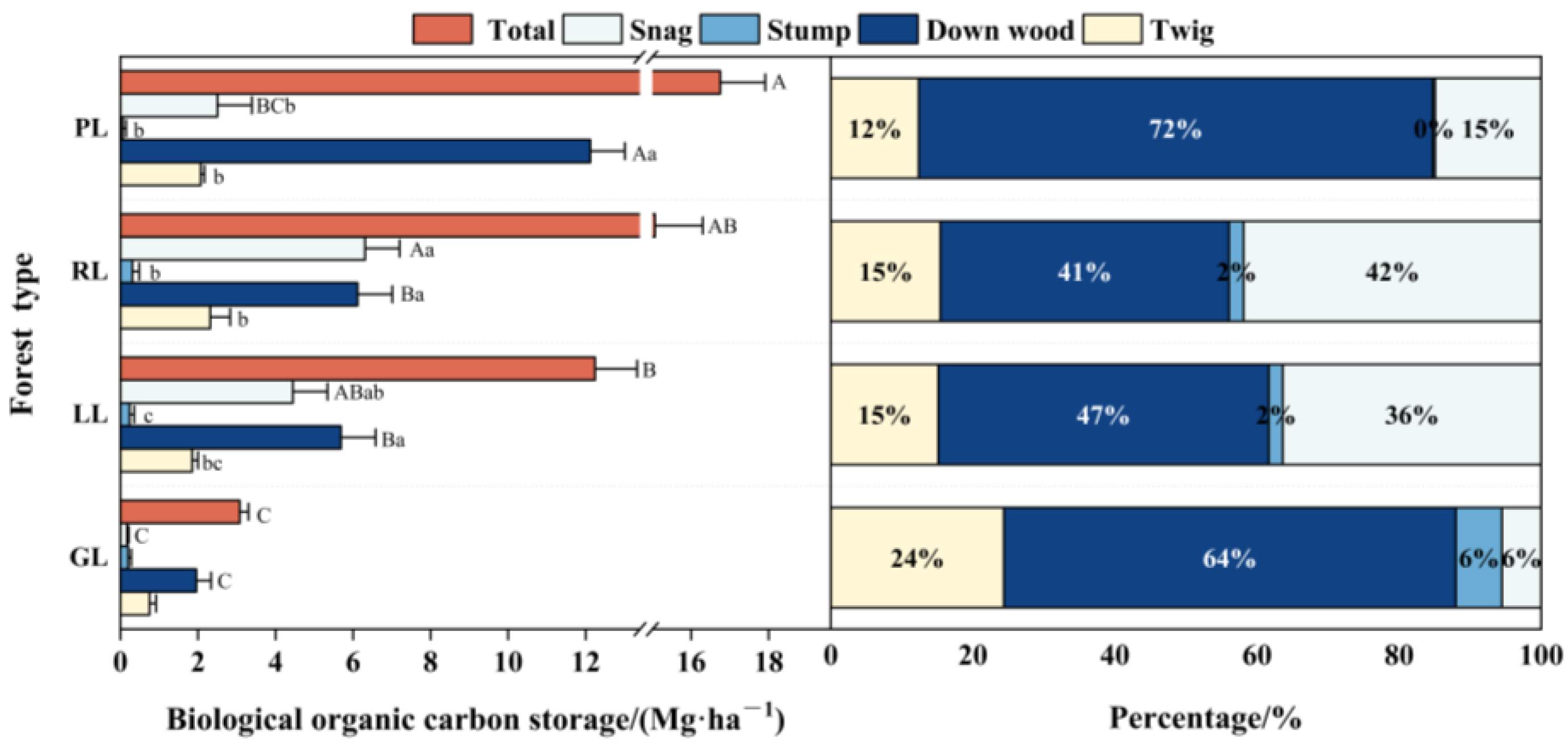
3.1.2. Biological Organic Carbon Protected by Pyrogenic Carbon
3.2. Carbon in the Charcoal Layer
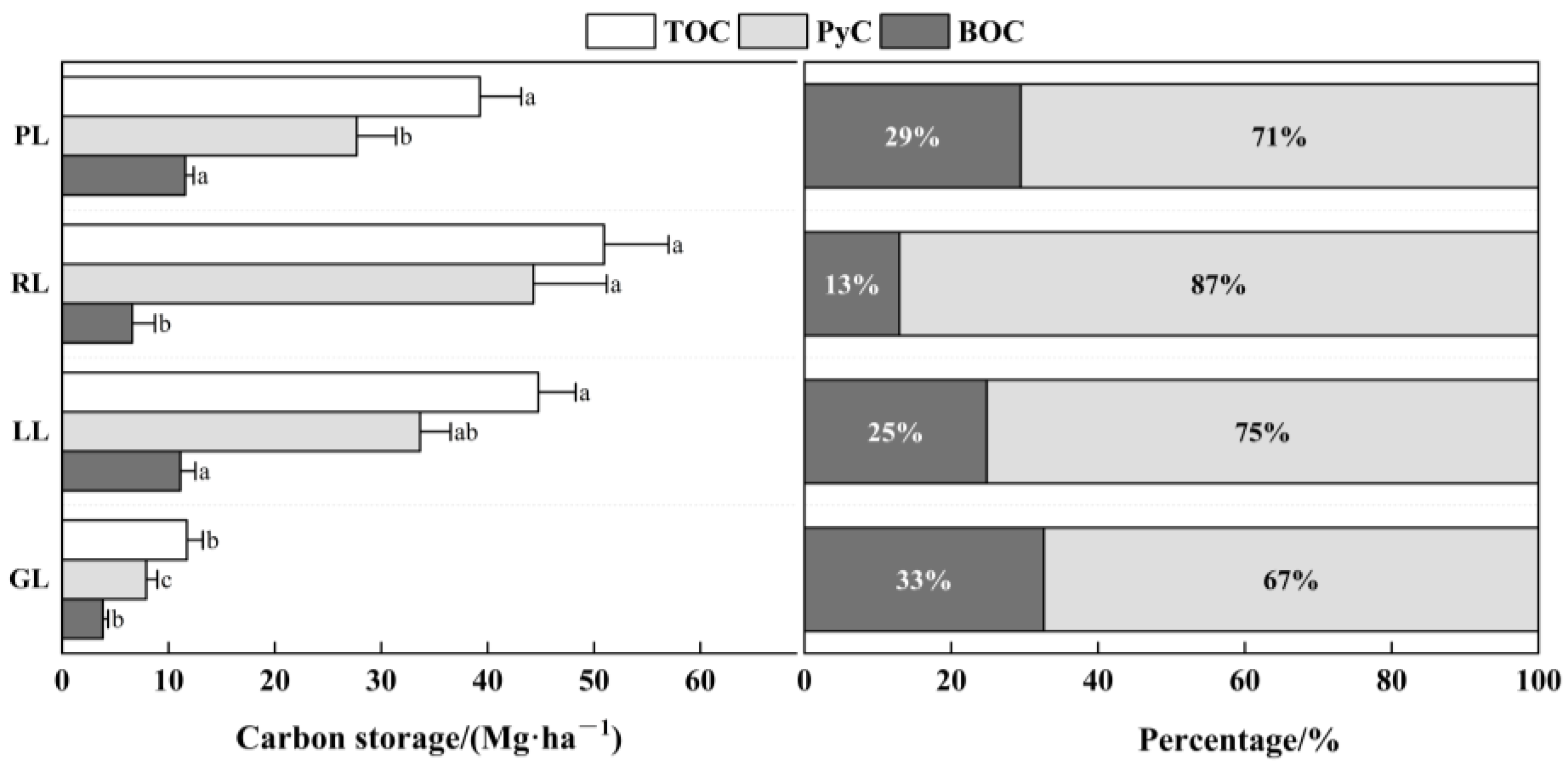
3.3. Carbon Storage and Distribution Patterns in the Woody Debris and Charcoal Layer
3.4. Pyrogenic Carbon Element Analysis
4. Discussion
4.1. Carbon in Woody Debris
4.2. Carbon in the Charcoal Layer
4.3. Properties of Pyrogenic Carbon
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santín, C.; Doerr, S.H.; Preston, C.M.; González-Rodríguez, G. Pyrogenic organic matter production from wildfires: A missing sink in the global carbon cycle. Glob. Change Biol. 2015, 21, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.T.; Hu, Y.M.; Chang, Y.; Liu, M.; Zhang, H.X.; Zhang, W. Advances on research of pyrogenic carbon in forests. Chin. J. Ecol. 2017, 36, 3257. [Google Scholar] [CrossRef]
- Alexis, M.A.; Rasse, D.P.; Rumpel, C.; Bardoux, G.; Péchot, N.; Schmalzer, P.; Drake, B.; Mariotti, A. Fire impact on C and N losses and charcoal production in a scrub oak ecosystem. Biogeochemistry 2006, 82, 201–216. [Google Scholar] [CrossRef]
- Hanke, U.M.; Eglinton, T.I.; Braun, A.L.L.; Reddy, C.M.; Wiedemeier, D.B.; Schmidt, M.W.I. Decoupled sedimentary records of combustion: Causes and implications. Geophys. Res. Lett. 2016, 43, 5098–5108. [Google Scholar] [CrossRef]
- Czimczik, C.I.; Schmidt, M.W.I.; Schulze, E. Effects of increasing fire frequency on black carbon and organic matter in Podzols of Siberian Scots pine forests. Eur. J. Soil Sci. 2004, 56, 417–428. [Google Scholar] [CrossRef]
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.D.; Seneviratne, S.I.; Zscheischler, J.; Beer, C.; Buchmann, N.; Frank, D.C.; et al. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef]
- Ottmar, R.D. Wildland fire emissions, carbon, and climate: Modeling fuel consumption. For. Ecol. Manag. 2014, 317, 41–50. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. Change Biol. Bioener. 2016, 8, 512–523. [Google Scholar] [CrossRef]
- Bird, M.I.; Wynn, J.G.; Saiz, G.; Wurster, C.M.; McBeath, A. The Pyrogenic Carbon Cycle. Annu. Rev. Earth Planet. Sci. 2015, 43, 273–298. [Google Scholar] [CrossRef]
- Fang, Y.; Singh, B.P.; Krull, E. Biochar carbon stability in four contrasting soils. Eur. J. Soil Sci. 2014, 65, 60–71. [Google Scholar] [CrossRef]
- Mašek, O.; Brownsort, P.; Cross, A.; Sohi, S. Influence of production conditions on the yield and environmental stability of biochar. Fuel 2013, 103, 151–155. [Google Scholar] [CrossRef]
- Donato, D.C.; Campbell, J.L.; Fontaine, J.B.; Law, B.E. Quantifying Char in Postfire Woody Detritus Inventories. Fire Ecol. 2009, 5, 104–115. [Google Scholar] [CrossRef]
- Chao, L.; Zhang, W.; Wang, S. Review on Biochar Decomposition and Priming Effect. Earth Environ. 2017, 45, 686–697. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Maestrini, B.; Nannipieri, P.; Abiven, S. A meta-analysis on pyrogenic organic matter induced priming effect. GCB Bioenergy 2015, 7, 577–590. [Google Scholar] [CrossRef]
- Esteves, B.M.; Pereira, H.M. Wood modification by heat treatment: A review. Bioresources 2009, 4, 370–404. [Google Scholar] [CrossRef]
- Preston, C.M.; Schmidt, M.W.I. Black (pyrogenic) carbon: A synthesis of current knowledge and uncertainties with special consideration of boreal regions. Biogeosciences 2006, 3, 397–420. [Google Scholar] [CrossRef]
- Haukenes, V.L.; Asplund, J.; Åsgård, L.; Rolstad, J.; Storaunet, K.O.; Ohlson, M. Proportion of charcoal carbon in the forest floor carbon pool in relation to fire history in a boreal forest landscape. Ecosphere 2023, 14, e4713. [Google Scholar] [CrossRef]
- Eckdahl, J.A.; Rodriguez, P.C.; Kristensen, J.A.; Metcalfe, D.B.; Ljung, K. Mineral Soils Are an Important Intermediate Storage Pool of Black Carbon in Fennoscandian Boreal Forests. Glob. Biogeochem. Cycles 2022, 36, e2022GB007489. [Google Scholar] [CrossRef]
- Sayer, E.J. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol. Rev. 2006, 81, 1–31. [Google Scholar] [CrossRef]
- Dymov, A.A. Soils of Post-Pyrogenic Forests. Eurasian Soil Sci. 2023, 56, S84–S113. [Google Scholar] [CrossRef]
- Jenkins, M.E.; Bell, T.L.; Poon, L.F.; Aponte, C.; Adams, M.A. Production of pyrogenic carbon during planned fires in forests of East Gippsland, Victoria. For. Ecol. Manag. 2016, 373, 9–16. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, P.; Yang, H.; Li, H.; Luo, C.; Jia, H.; Huang, Y. Fire effects on soil carbon cycling pools in forest ecosystems: A global meta-analysis. Sci. Total. Environ. 2023, 895, 165001. [Google Scholar] [CrossRef] [PubMed]
- Mantel, S.; Dondeyne, S.; Deckers, S. World reference base for soil resources (WRB). Encycl. Soils Environ. 2023, 4, 206–217. [Google Scholar] [CrossRef]
- Chen, H.; Hu, Y.; Chang, Y.; Bu, R.; Li, Y.; Liu, M.; Xiong, Z. Simulation of the effect of forest harvest mode on forest landscape: A case study in Huzhong forest region of Daxing’ anling Mountains, China. Chin. J. Ecol. 2013, 32, 1888–1895. [Google Scholar] [CrossRef]
- Guo, K.; Fang, J.-Y.; Wang, G.-H.; Tang, Z.-Y.; Xie, Z.-Q.; Shen, Z.-H.; Wang, R.-Q.; Qiang, S.; Liang, C.-Z.; DA, L.-J.; et al. A revised scheme of vegetation classification system of China. Chin. J. Plant Ecol. 2020, 44, 111–127. [Google Scholar] [CrossRef]
- Yang, D.; He, H.-S.; Wu, Z.-W.; Liang, Y.; Huang, C.; Luo, X.; Xiao, J.-T.; Zhang, Q.-L. Influence of fire disturbance on aboveground deadwood debris carbon storage in Huzhong forest region of Great Xing’an Mountains, Northeast China. Yingyong Shengtai Xuebao 2015, 26, 331–339. [Google Scholar]
- Yan, E.; Wang, X.; Huang, J. Concept and Classification of Coarse Woody Debris in Forest Ecosystems. Front. Biol. China 2005, 1, 76–84. [Google Scholar] [CrossRef]
- Preston, C.M.; Simard, M.; Bergeron, Y.; Bernard, G.M.; Wasylishen, R.E. Charcoal in Organic Horizon and Surface Mineral Soil in a Boreal Forest Fire Chronosequence of Western Quebec: Stocks, Depth Distribution, Chemical Properties and a Synthesis of Related Studies. Front. Earth Sci. 2017, 5, 98. [Google Scholar] [CrossRef]
- Nocentini, C.; Certini, G.; Knicker, H.; Francioso, O.; Rumpel, C. Nature and reactivity of charcoal produced and added to soil during wildfire are particle-size dependent. Org. Geochem. 2010, 41, 682–689. [Google Scholar] [CrossRef]
- Rumpel, C.; Alexis, M.; Chabbi, A.; Chaplot, V.; Rasse, D.; Valentin, C.; Mariotti, A. Black carbon contribution to soil organic matter composition in tropical sloping land under slash and burn agriculture. Geoderma 2006, 130, 35–46. [Google Scholar] [CrossRef]
- Ohlson, M.; Tryterud, E. Interpretation of the charcoal record in forest soils: Forest fires and their production and deposition of macroscopic charcoal. Holocene 2000, 10, 519–525. [Google Scholar] [CrossRef]
- Rayment, G.E.; Lyons, D.J. Soil Chemical Methods: Australasia; CSIRO Publishing: Clayton, Australia, 2011; Volume 3. [Google Scholar]
- Sun, J.; Sang, Y.; Song, J.; Cui, X. Content and distribution of black carbon in typical forest soils in Changbaishan Mountains. J. For. Res. 2016, 29, 34–40. [Google Scholar] [CrossRef]
- Lim, B.; Cachier, H. Determination of black carbon by chemical oxidation and thermal treatment in recent marine and lake sediments and Cretaceous-Tertiary clays. Chem. Geol. 1996, 131, 143–154. [Google Scholar] [CrossRef]
- Cai, H.; Di, X.; Jin, G. Carbon density of coarse woody debris in a spruce-fir valley forest in Xiaoxing’an Mountains, China. Acta Ecol. Sin. 2015, 35, 8194–8201. [Google Scholar] [CrossRef]
- Wang, X.-L.; Chang, Y.; Chen, H.-W.; Hu, Y.-M.; Jiao, L.-L.; Feng, Y.-T.; Wu, W.; Wu, H.-F. Spatial pattern of forest biomass and its influencing factors in the Great Xing’an Mountains, Heilongjiang Province, China. J. Appl. Ecol. 2014, 25, 974–982. [Google Scholar] [CrossRef]
- Waddell, K.L. Sampling coarse woody debris for multiple attributes in extensive resource inventories. Ecol. Indic. 2002, 1, 139–153. [Google Scholar] [CrossRef]
- Guochun, L. Brief introduction to statistical software―SPSS. J. Evid.-Based Med. 2003, 3, 249–251,253. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; González-Vila, F.J.; Almendros, G.; Knicker, H. The effect of fire on soil organic matter—A review. Environ. Int. 2004, 30, 855–870. [Google Scholar] [CrossRef]
- Fuente, E.; Menéndez, J.A.; Díez, M.A.; Suárez, D.; Montes-Morán, M.A. Infrared Spectroscopy of Carbon Materials: A Quantum Chemical Study of Model Compounds. J. Phys. Chem. B 2003, 107, 6350–6359. [Google Scholar] [CrossRef]
- El-Hendawy, A.-N.A. Variation in the FTIR spectra of a biomass under impregnation, carbonization and oxidation conditions. J. Anal. Appl. Pyrolysis 2006, 75, 159–166. [Google Scholar] [CrossRef]
- Knicker, H.; Hilscher, A.; González-Vila, F.J.; Almendros, G. A new conceptual model for the structural properties of char produced during vegetation fires. Org. Geochem. 2008, 39, 935–939. [Google Scholar] [CrossRef]
- Tinker, D.B.; Knight, D.H. Coarse Woody Debris following Fire and Logging in Wyoming Lodgepole Pine Forests. Ecosystems 2000, 3, 472–483. [Google Scholar] [CrossRef]
- Righi, C.A.; de Alencastro Graça, P.M.L.; Cerri, C.C.; Feigl, B.J.; Fearnside, P.M. Biomass burning in Brazil’s Amazonian “arc of deforestation”: Burning efficiency and charcoal formation in a fire after mechanized clearing at Feliz Natal, Mato Grosso. For. Ecol. Manag. 2009, 258, 2535–2546. [Google Scholar] [CrossRef]
- Lehmann, J.; Solomon, D.; Kinyangi, J.; Dathe, L.; Wirick, S.; Jacobsen, C. Spatial complexity of soil organic matter forms at nanometre scales. Nat. Geosci. 2008, 1, 238–242. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Subbotina, I.; Chen, H.; Bogomolova, I.; Xu, X. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol. Biochem. 2009, 41, 210–219. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Major, J.; Lehmann, J.; Rondon, M.; Goodale, C. Fate of soil-applied black carbon: Downward migration, leaching and soil respiration. Glob. Chang. Biol. 2010, 16, 1366–1379. [Google Scholar] [CrossRef]
- Brodowski, S.; Amelung, W.; Haumaier, L.; Abetz, C.; Zech, W. Morphological and chemical properties of black carbon in physical soil fractions as revealed by scanning electron microscopy and energy-dispersive X-ray spectroscopy. Geoderma 2005, 128, 116–129. [Google Scholar] [CrossRef]
- Wang, M.; Cui, X.; Li, S.; Zhang, W.; Zhao, H. Effects of topographic factors on soil black carbon storage in coniferous forests at the north end of Greater Khingan Mountains. J. Nanjing For. Univ. Nat. Sci. Ed. 2021, 45, 151–158. [Google Scholar] [CrossRef]
- Brodowski, S.; Rodionov, A.; Haumaier, L.; Glaser, B.; Amelung, W. Revised black carbon assessment using benzene polycarboxylic acids. Org. Geochem. 2005, 36, 1299–1310. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Camps-Arbestain, M.; Amonette, J.E.; Singh, B.; Wang, T.; Schmidt, H.P. A Biochar Classification System and Associated Test Methods, Biochar for Environmental Management; Routledge: London, UK, 2015. [Google Scholar] [CrossRef]
- Hammes, K.; Smernik, R.J.; Skjemstad, J.O.; Schmidt, M.W. Characterisation and evaluation of reference materials for black carbon analysis using elemental composition, colour, BET surface area and 13C NMR spectroscopy. Appl. Geochem. 2008, 23, 2113–2122. [Google Scholar] [CrossRef]
- A Spokas, K. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]




| Forest Type | Latitude and Longitude | Slope Position | Altitude (m) | Slope (°) | Mortality (%) | Pre-Fire Community Structure | Post-Fire Community Structure | Update Biomass (Mg·ha−1) | Thickness of PyC Layer |
|---|---|---|---|---|---|---|---|---|---|
| (cm) | |||||||||
| PL | 123°15′43″–123°26′17″ E 51°27′17″–51°45′64″ N | Upper slope | 1030.6–1061.3 | 10–22 | 100 | Tree Larix gmelinii, Betula platyphylla Shrub Pinus pumila, Vaccinium uliginosum Herb Cyperaceae, Roseceae | Tree Populus davidiana Dode, Betula platyphylla Shrub Vaccinium uliginosum Herb Cyperaceae, Roseceae | 35.6 | 4.3 |
| RL | 123°25′20″–123°25′27″ E 51°27′20″–51°27′25″ N | Middle slope | 948.6–960.4 | 13–17 | 100 | Tree Larix gmelinii, Betula platyphylla Shrub Pinus pumila, Rhododendron dauricum Herb Cyperaceae, Roseceae | Tree Populus davidiana Dode, Betula platyphylla Shrub Rhododendron dauricum Herb Cyperaceae, Roseceae | 33.5 | 6.1 |
| LL | 123°15′30″–123°15′38″ E 51°27′31″–51°27′34″ N | Lower slope | 853.2–871.3 | 18–19 | 100 | Tree Larix gmelinii, Betula platyphylla Shrub Pinus pumila, Ledum palustre Herb Cyperaceae, Roseceae | Tree Populus davidiana Dode, Betula platyphylla Shrub Ledum palustre, Vaccinium uliginosum Herb Cyperaceae, Roseceae | 27.4 | 7.1 |
| GL | 123°15′35″–123°15′43″ E 51°27′40″–51°27′45″ N | Valley | 727.2–731.5 | 14–16 | 100 | Tree Larix gmelinii, Betula platyphylla Shrub Vaccinium uliginosum, Ledum palustre Herb Cyperaceae, Deyeuxia angustifolia | Tree Populus davidiana Dode, Betula platyphylla Shrub Vaccinium uliginosum, Ledum palustre Herb Roseceae, Deyeuxia angustifolia | 6.8 | 1.8 |
| Forest Type | Standing Trees Density (N·hm−2) | Charred WD | Uncharred WD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Standing Trees | Down Wood | Standing Trees | Down Wood | ||||||||
| Tree Species Composition | Char Height (cm) | Char Depth (mm) | PyC Density (mg/cm3) | Tree Species Composition | Char Depth (mm) | Height (m) | DBH (cm) | Length (m) | Middle Diameter (cm) | ||
| PL | 461.1 | 98 Larix gmelinii 2 Betula platyphylla | 65.1 ± 10.3 | 1.1 ± 0.06 | 139.6 ± 4.9 | 82 Pinus pumila 10 Larix gmelinii 8 Betula platyphylla | 1.9 ± 0.7 | 7.1 ± 1.5 | 10.3 ± 0.3 | 2.1 ± 0.3 | 6.1 ± 1.3 |
| RL | 344.4 | 91 Larix gmelinii 9 Betula platyphylla | 80.4 ± 19.3 | 1.2 ± 0.06 | 147.4 ± 4.3 | 65 Pinus pumila 26 Larix gmelinii 9 Betula platyphylla | 1.4 ± 0.3 | 9.8 ± 3.3 | 18.5 ± 6.4 | 2.0 ± 0.3 | 5.6 ± 0.9 |
| LL | 661.1 | 95 Larix gmelinii 5 Betula platyphylla | 69.6 ± 21.3 | 1.0 ± 0.01 | 135.9 ± 10.0 | 65 Pinus pumila 29 Larix gmelinii 7 Betula platyphylla | 1.0 ± 0.2 | 10.2 ± 0.9 | 11.2 ± 1.6 | 2.3 ± 1.0 | 5.2 ± 1.1 |
| GL | 527.8 | 85 Larix gmelinii 15 Betula platyphylla | 56.4 ± 1.9 | 0.8 ± 0.07 | 129.4 ± 5.3 | 95 Larix gmelinii 5 Betula platyphylla | 1.3 ± 0.5 | 1.5 ± 0.3 | 5.4 ± 0.4 | 2.0 ± 0.3 | 3.0 ± 0.3 |
| Fraction | C Distribution % of Total | C (g·kg−1) | N (g·kg−1) | C/N | H/C | O/C |
|---|---|---|---|---|---|---|
| >2 mm | 8.55 ± 10.26 | 449.30 ± 90.58 a | 6.11 ± 1.70 a | 76.84 ± 16.54 a | 0.06 ± 0.01 c | 0.23 ± 0.04 c |
| 1–2 mm | 26.40 ± 9.56 | 249.04 ± 104.00 b | 4.27 ± 1.40 b | 58.97 ± 15.86 b | 0.12 ± 0.03 b | 0.43 ± 0.12 b |
| 0.5–1 mm | 25.28 ± 8.39 | 229.71 ± 53.83 b | 4.43 ± 1.17 b | 54.64 ± 15.06 bc | 0.13 ± 0.05 b | 0.46 ± 0.12 b |
| <0.5 mm | 39.77 ± 10.76 | 219.68 ± 90.60 b | 4.81 ± 1.61 b | 48.08 ± 15.93 c | 0.16 ± 0.04 a | 0.54 ± 0.17 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Shi, L.; Hou, X.; Zhang, Y. Carbon in Woody Debris and Charcoal Layer in Cold Temperate Coniferous Forest 13 Years After a Severe Wildfire. Forests 2025, 16, 685. https://doi.org/10.3390/f16040685
Peng Y, Shi L, Hou X, Zhang Y. Carbon in Woody Debris and Charcoal Layer in Cold Temperate Coniferous Forest 13 Years After a Severe Wildfire. Forests. 2025; 16(4):685. https://doi.org/10.3390/f16040685
Chicago/Turabian StylePeng, Yuanchun, Lina Shi, Xingyu Hou, and Yun Zhang. 2025. "Carbon in Woody Debris and Charcoal Layer in Cold Temperate Coniferous Forest 13 Years After a Severe Wildfire" Forests 16, no. 4: 685. https://doi.org/10.3390/f16040685
APA StylePeng, Y., Shi, L., Hou, X., & Zhang, Y. (2025). Carbon in Woody Debris and Charcoal Layer in Cold Temperate Coniferous Forest 13 Years After a Severe Wildfire. Forests, 16(4), 685. https://doi.org/10.3390/f16040685






