Abstract
Southwest China’s karst region represents a global hotspot for ecological restoration, with natural succession on abandoned farmland emerging as a pivotal mechanism under recent land-use transitions. Despite its ecological significance, empirical data remain scarce regarding tree growth characteristics in this fragile ecosystem. This seven-year study (2018–2024) at Puding Karst Ecosystem Research Station quantified the spatiotemporal patterns of tree growth through monthly diameter at breast height (DBH) measurements for dominant species, coupled with microhabitat characterization (rock exposure, competition indices, and canopy architecture). Key findings revealed that the mean annual DBH increment was 5.74 mm/a, while biomass accumulation averaged 9.38 kg/a; growing-season drought duration significantly modulated interannual growth variation; and microhabitat heterogeneity and tree size significantly influenced the spatial variance of tree growth. These results substantiate natural succession as an effective carbon sequestration strategy, particularly in nutrient-depleted karst terrains. We advocate for the policy prioritization of passive restoration over active afforestation in marginal croplands.
1. Introduction
Southwest China is one of the three largest contiguous karst areas in the world [1], with about 426,000 km2 of exposed carbonate rocks [2]. Water and nutrient conditions are limited due to a high rock exposure ratio and a low amount of soil [3] in the area compared with the non-karst areas [4,5,6]. At the same time, soils in karst areas are generally characterized by high pH and high calcium and magnesium content [7,8,9,10], which are stressful conditions for vegetation growth. As a result, the net primary productivity and biomass of vegetation in the karst region of Southwest China are significantly lower than those of non-karst areas in the same climatic zone [11,12,13,14].
However, Southwest China has emerged as a global hotspot in terms of increasing woody plant cover in the first two decades of the 21st century [15]. Despite a slight decline in annual rainfall and soil moisture in recent years [16], studies have shown that the growing-season vegetation cover has increased from 69% to 81% during the 1999–2017 period [15]. The growing-season normalized difference vegetation index (GSNDVI) has increased at an average rate of 0.011 per year [17]. The region’s vegetation biomass and net primary productivity have also shown an increasing trend [17,18,19,20], with the total net primary productivity growing at an average annual rate of 20.58 gC·m−2 in the region [21]. As a result, Southwest China’s karst region was one of the world’s largest biomass accumulation regions, accounting for 5% of the world’s biomass increase between 2000 and 2018 [15].
This transformative greening is predominantly driven by China’s economic development [17,22] and the implementation of projects such as Grain for Green, the comprehensive management of rocky desertification, and ecological migration [22,23,24]. The Chinese government implemented the Returning Farmland to Forest project (also known as the Sloping Land Conversion Program and the Grain for Green Program) in 1999, which involved 25 provinces in central and western China [22]. By 2011, the area of forest had increased by about 28.94 million hectares through afforestation and reforestation [22]. At the same time, large numbers of people, including many of working age, moved out of the region, which promoted forest restoration by reducing the negative disturbances of forest areas and promoting the abandonment of sloping cultivated land [24]. For example, the rural population decreased by 4.8 million people between 2005 and 2015 in the karst region of Southwest China [24].
In addition, the mean carbon sequestration rate in the rocky desertification management area in Southwestern China’s karst region (1.32 tC/ha/a) was higher than that in the non-managed area (1.08 tC/ha/a) and the non-karst area (1.28 tC/ha/a) [19,25]. Over the past 20 years, the rate of vegetation recovery in Southwest China’s karst region has been 1.8 times higher than that of the non-karst areas [26]. Furthermore, the growth of net primary productivity in the karst areas is higher than that of the non-karst areas [23]. Both the increase in vegetation area and vegetation carbon density favor carbon sequestration [19,25]. It is estimated that forest ecosystems in Southwest China’s karst region will have an additional carbon sequestration potential of 5.32 PgC in the future [19].
As a large amount of sloping arable land was converted to forest in the region [27], trees are likely the main contributor of regional net primary productivity and carbon sequestration [28]. It is of great significance to study the growth process of trees and their main influencing factors in Southwest China’s karst region. However, the continuous observation of tree growth and biomass accumulation at the early stage of natural succession on abandoned farmland remains strikingly scarce, hindering the evidence-based management of natural regeneration processes.
In this study, an abandoned farmland that had been under natural succession since 2010, located inside the Puding Karst Ecosystem Research Station, was analyzed. Our aim was to examine the characteristics of tree growth among different species and diverse microhabitats, as well as the influence of climatic anomalies. The results can be valuable in verifying the effectiveness of natural restoration in carbon fixation at its early stage through rapid biomass accumulation in Southwest China’s karst region.
2. Materials and Methods
2.1. Study Area
The study area is situated within the Puding Karst Ecosystem Research Station, in Guizhou province, Southwest China (26°22′03″ N, 105°45′08″ E). The site is about 8 hectares, and the elevation is around 1170 m. The region features a subtropical monsoon climate characterized by distinct seasonal variations, with an annual mean temperature of 15.3 °C and an annual mean precipitation of 1390 mm. Notably, approximately 70% of the total precipitation is concentrated during the monsoon season from May to October, following the typical East Asian summer monsoon pattern. The bedrock of the study area consists of limestone from the Guanling Formation of the Triassic System, exhibiting an average rock exposure ratio of 42%. The predominant soil type is yellow limestone soil, with a mean thickness of 66 cm between rock outcrops. When considering exposed rock areas as having zero soil depth, the overall mean soil thickness across the study area is 32 cm.
The area was primarily utilized as sloping arable land prior to its enclosure in 2010. It has been under natural succession since 2010 (Figure 1), while some locations have initiated natural succession six to seven years earlier. Currently, the area is at the early stages of natural succession, dominated by tree species including Toona sinensis, Catalpa fargesii, Populus adenopoda, Broussonetia papyrifera, Platyosprion platycarpum, Albizia kalkora, Rhus chinensis, Melia azedarach, and Celtis sinensis.
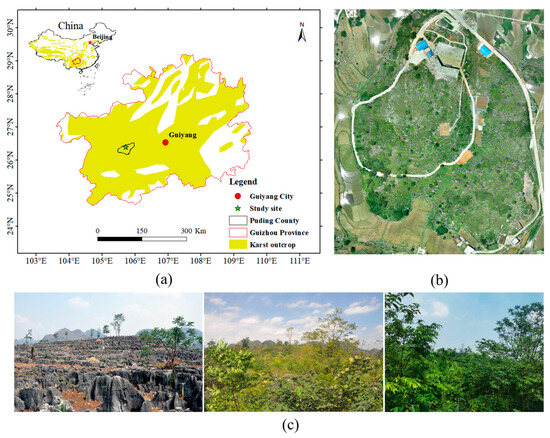
Figure 1.
Study site: (a) location of the study site [29], (b) aerial view of the study site, and (c) vegetation restoration at the study site (photos taken in 2010, 2013, and 2020).
2.2. Methods
2.2.1. Tree Selection and Dendrometer Installation
A comprehensive field survey was conducted to quantify species composition and the relative abundance of the dominant tree species in March 2018. Based on these metrics, 19 dominant tree species were identified, from which 100 individuals were selected for monitoring (Table 1). Within each species, at least 3 trees were selected to encompass a broad diameter range to capture intraspecific growth variability. High-resolution dendrometers (D1, METER, Pullman, WA, USA) were installed at breast height (1.3 m) on each monitored tree.

Table 1.
Basic information of monitored trees and allometric growth equations used [30].
The dendrometers were initially installed on 13 April 2018, with baseline DBH recorded at the same time. Subsequent measurements have been taken at regular monthly intervals since then. During the observation period up to 12 September 2024, 11 monitored trees died, including 4 Populus canadensis (PC), 3 Rhus chinensis (RC), 1 Melia azedarach (MA), 1 Catalpa ovata (CO), 1 Broussonetia papyrifera (BP), and 1 Albizia kalkora (AK). One dendrometer installed on Populus adenopoda sustained mechanical failure and was excluded from subsequent analyses. Continuous datasets were obtained from 88 surviving individuals throughout the study period. Climatic data such as temperature (HMP45A/D, Vaisala, Helsinki, Finland) and precipitation (SM1-1, Shanghai Meteorological Instrument Factory Co., Ltd., Shanghai, China) were collected from the weather station of Puding Karst Ecosystem Research Station inside the study site.
2.2.2. Microhabitat Survey
Microhabitats were classified into five types (SCa: stone cavern; SCr: stone crevices; SG: stone gully; SS: stone surface; ES: earthy surface) based on the morphology of the exposed rock [31]. Microhabitat parameters associated with each monitored tree were surveyed in early November 2024. The investigation encompassed multiple ecological parameters, including soil depth, rock exposure ratios, crown exposure to sunlight, microhabitat types, and the species composition, DBH, and height of all neighboring plants (DBH > 3 cm) within a 3 m radius of each monitored tree [32]. The survey protocol included the following: (1) soil depth was measured using a calibrated measuring rod at seven or more randomly selected points within 3 times the crown width radius [33]; (2) rock exposure ratio was quantified within the same sampling area by visual examination; (3) crown exposure to sunlight was assessed for each monitored tree by visual examination and classified into upper, middle, and lower crown types; and (4) all competing trees (DBH > 3 cm) within a 3 m radius were identified and measured for species, DBH, and height. Additionally, the presence of parasitic organisms on monitored trees was systematically documented to account for potential biotic influences on tree growth. During the field measurements, three instruments were employed: (1) a 3.3 m soil depth probe (steel chisel) for quantifying soil thickness; (2) a caliper with 0.1 mm measurement accuracy for determining DBH; and (3) a height-measuring rod with 10 cm precision for assessing vertical height.
2.3. Data Analysis
Biomass was estimated using allometric equations established from comparable regions adjacent to the study area [30]. When species-specific equations were unavailable, generalized allometric equations (Table 1) were applied. The change in the cross-sectional area of each individual tree was calculated as the difference in circular area derived from successive DBH measurements (assuming stem circularity). Statistical analyses were conducted to examine the temporal dynamics and environmental drivers of biomass and DBH increments. Both monthly and annual variations in biomass and DBH were quantified, and interspecific differences in biomass accumulation were assessed using one-way ANOVA tests. Multiple regression models were used to evaluate the synergistic effects of microhabitat parameters on tree growth. The impact of drought duration during the growing season on the annual DBH change was analyzed through Pearson linear correlation analyses. The relationship between microhabitat conditions and tree growth was further explored by classifying 63 selected trees (excluding dead trees, parasitized trees, and trees located at site boundaries to minimize edge effects). Mean annual DBH increments (ΔDBH) across different microhabitat categories were compared. “Stone surface” represents all kinds of microhabitat surfaces except earthy surfaces. Competition intensity was categorized as high or low based on the sum of the DBH of neighboring trees within a 3 m radius, using a threshold of 90 cm determined by prior sensitivity analyses. Data processing utilized Microsoft Excel 2007 (Microsoft, Redmond, USA), statistical analyses (correlation and ANOVA) were performed in SPSS PASW Statistics 18 (IBM, Chicago, IL, USA), and figures were generated using Origin 2021 (OriginLab, Northampton, MA, USA).
3. Results
3.1. Characteristics of Tree Growth Among Dominant Species
Significant interspecific variation in DBH and biomass accumulation was observed (Figure 2; Table 2). The three fastest-growing species were Choerospondias axillaris (CA), Broussonetia papyrifera (BP), and Albizia kalkora (AK), with mean annual DBH increments of 11.69 ± 3.33, 11.56 ± 5.61, and 10.40 ± 0.02 mm/a, respectively. Conversely, the three slowest-growing species were Lindera communis (LC), Catalpa fargesii (CF), and Pyrus pyrifolia (PPy), with mean annual DBH increments of 2.09 ± 0.10, 1.27 ± 0.81, and 0.99 ± 0.90 mm/a, respectively. Regarding biomass accumulation, the three species with the highest annual biomass accumulation were Cunninghamia lanceolata (CL), Broussonetia papyrifera (BP), and Celtis sinensis (CS), with mean annual biomass accumulation values of 24.45, 19.04, and 17.44 kg/a, respectively. Conversely, the species with the lowest annual biomass accumulation were Pyrus pyrifolia (PPy), Lindera communis (LC), and Rhus chinensis (RC), with annual biomass accumulation values of 0.43, 0.87, and 2.11 kg/a, respectively. The species with the highest biomass growth rate was 56.86 times higher than that of the slowest-growing species.
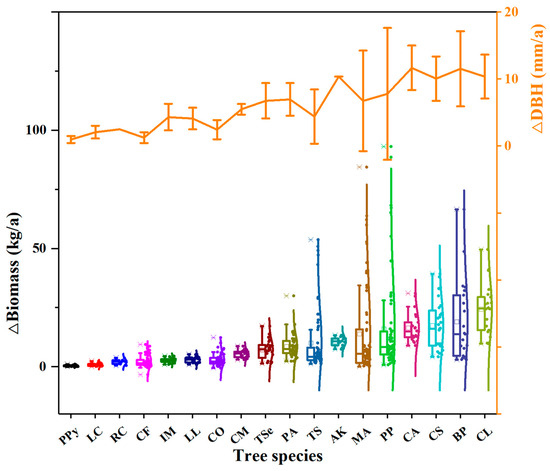
Figure 2.
Annual increments of biomass and annual average increments of DBH for each tree species.

Table 2.
Statistical analysis of DBH and biomass variation for each species.
Intraspecific variability was particularly pronounced in DBH and biomass accumulation too (Figure 2; Table 2). The largest differences were found in Platyosprion platycarpum (PP), Melia azedarach (MA), and Broussonetia papyrifera (BP) (Figure 2); the difference between the maximum and minimum biomass accumulation of individuals reached 92.33, 84.43, and 63.63 kg/a, respectively. The species with the smallest difference in biomass growth among plants was Pyrus pyrifolia (PPy), with a difference of only 0.98 kg/a.
3.2. Temporal Characteristics of Tree Growth
DBH increments exhibit pronounced seasonal variation (Figure 3). The increase in DBH peaked in June (1.8 mm/month) from 2018 to 2020. However, this rate decreased from 1.5 mm/month to about 1.2 mm/month from 2021 to 2024. Biomass accumulation demonstrated a distinct phenological pattern too (Figure 3). The accumulation of biomass is extremely slow from October to March, with a mean rate of less than 0.15 kg/month. The fastest growth period is from May to July, with a mean biomass accumulation rate of 1.8 kg/month. Mean annual biomass accumulation reached 9.5 kg/a, where 65% of the annual biomass accumulation occurred during May–July, while October–March merely contributed about 3%. Notably, maximum productivity occurred in June–July 2020 (2.64 ± 0.38 kg/month). The seasonal pattern of DBH increments and biomass accumulation variation followed well with the change in temperature and precipitation at the study site (Figure 3).
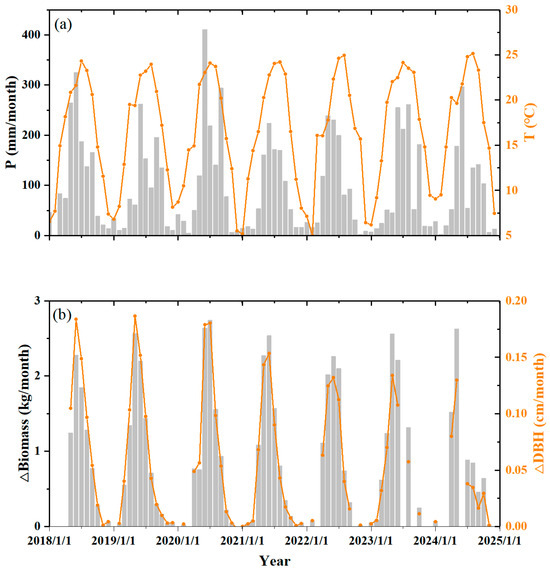
Figure 3.
Seasonal variations in tree growth along with climate: (a) monthly precipitation (P) and monthly mean air temperature (T); (b) monthly mean biomass accumulation (ΔBiomass) and monthly mean DBH increment (ΔDBH).
Interannual climate variability and tree growth showed nonlinear trends during the study period (Figure 4). Meteorological conditions remained relatively stable, with a mean annual temperature of 16.6 ± 0.6 °C and a mean annual precipitation of 1156 ± 160 mm. However, drought duration within the growing season varied a lot among years. DBH increments averaged 5.74 mm/a across all individual trees (Figure 4), peaking in 2019 (6.75 mm/a) before declining progressively. Biomass accumulation peaked in 2021 (10.22 kg/a) before declining progressively. The interannual variation in the sectional area was similar to the change in biomass accumulation and exhibited a significantly negative correlation with drought duration (Figure 4).
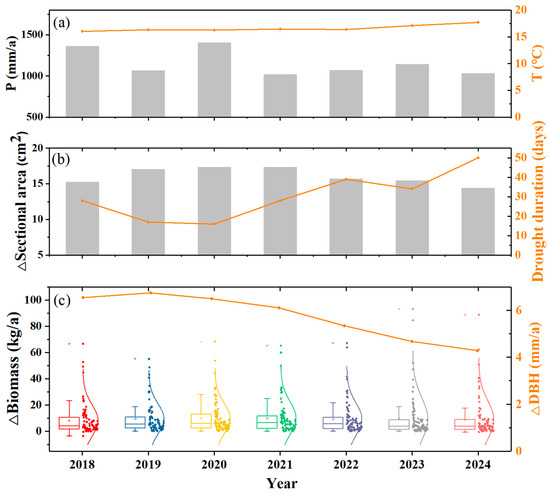
Figure 4.
Interannual variations in tree growth along with climate: (a) annual precipitation and annual mean air temperature; (b) annual mean sectional area change and drought duration; (c) annual biomass accumulation for each tree and annual mean DBH increment.
3.3. Distribution of Microhabitats in the Study Area
Trees monitored in this study were located in five types of microhabitats, namely, stone caves, stone crevices, stone gullies, stone surfaces, and soil surfaces [34] (Figure 5). A larger proportion of trees were located in stone gullies and soil surfaces, while the proportion of trees located in stone crevices, stone surfaces, and stone caves was less than 5%. The rock exposure ratio of all surveyed plots was 45%, with the highest ratio of rock exposure of 80% for the stone surface microhabitat and 32% for the soil surface. The mean soil depth was 37 cm, with the soil surface microhabitat showing the largest mean soil depth of 46 cm, while the mean soil depths in stone caves, stone crevices, and stone surface microhabitats were all less than 23 cm (Figure 5). Overall, there is a negative correlation between the rock exposure ratio and soil depth. There was a total of 706 trees (DBH > 3 cm) within a 3 m radius of each monitored tree, representing 32 species, with a mean DBH of 7.9 cm and a mean height of 7 m (Table 3). The average crown exposure to sunlight was 86%, 53%, and 30% for trees with upper crowns (80% of monitored trees), middle crowns (17% of monitored trees), and lower crowns, respectively (Table 3). A total of 10 monitored trees were parasitized, and 4 of these trees died during the study period.
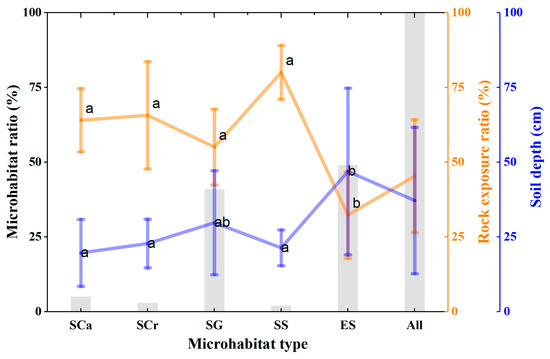
Figure 5.
Distribution of microhabitats (SCa: stone cavern; SCr: stone crevices; SG: stone gully; SS: stone surface; ES: earthy surface). Different letters indicate significant differences (p < 0.05) between microhabitat types.

Table 3.
Summary of microhabitat survey results.
4. Discussion
4.1. Comparison with Other Research in Similar Climatic Zones
The mean annual DBH increment in this study was 5.74 mm/a, exceeding the DBH growth values for Garuga floribunda var. gamblei in tropical karst forests (2.87 mm/a) [35], but slightly lower than subtropical monsoon hilly evergreen species (8.7 mm/a) [36]. This value intermediates between the growth rates of Ailao Mountain’s evergreen (3 mm/a) and deciduous (10.6 mm/a) broad-leaved forests [37]. Biomass accumulation averaged 9.38 kg/a, surpassing rates of Guizhou karst broad-leaf (2.4 kg/a) and conifer (4.6 kg/a) [38], while intermediating between Guizhou non-karst broad-leaves (5.6 kg/a) and conifers (12.4 kg/a). Notably, this value significantly exceeds mature deciduous (2.41 kg/a) and evergreen (1.46 kg/a) trees in the karst region of Southwestern China [12].
DBH and biomass accumulation dynamics reflect combined controls by environmental factors (climate/soil) and biological determinants (tree size/species) [14,39,40,41]. Caution is warranted as monitored conifers (Cunninghamia lanceolata only) may bias results toward broad-leaf dynamics in this study. The growth rate was higher than that of broad-leaf trees in other studies, probably because the sample plots in this study were at the early stage of natural succession, where the competition between trees is still small [38]. In addition, some of the monitored trees on the boundary of the study site may be affected by fertilizer nutrients applied on adjacent cropland.
4.2. Main Factors Affecting Tree Growth
4.2.1. Microhabitat Factors
Water and nutrient availability in karst ecosystems are fundamentally regulated by soil–rock configurations, where heterogeneous lithology creates microhabitats with divergent edaphic conditions [42]. Soil water content directly influences tree growth through transpiration regulating [43], making soil moisture the key limiting factor for tree growth [44]. Compared to non-karst areas, karst landscapes possess less soil volume and lower water retention capacity on average. Even at saturation, karst soils can sustain plant water demand for only 7–10 days [5], explaining the characteristically low net primary productivity (8.41 t/ha) and high plant mortality rates observed in these ecosystems [11,45]. Despite enough total annual precipitation in the study area, uneven temporal distribution amplifies drought risks. Our data confirm significant growth suppression under growing-season droughts (sig < 0.001) (Figure 4), which is consistent with enhanced drought vulnerability documented in karst forests [46]. During the 2022 to 2024 study period, drought events exceeding 15 days occurred in growing seasons. These prolonged drought events may directly account for the observed decreasing trend in biomass accumulation from 2021 to 2024 (Figure 4). Overall, multiple regression analyses showed that the explanation of initial DBH, soil depth, crown exposure to sunlight, and competition on DBH variation reached 0.56.
Notably, certain monitored trees exhibited growth enhancement during the extreme droughts from 2023 to 2024, highlighting microhabitat-mediated climate resilience (Figure 4). The complex soil–rock architecture in the karst terrain generates distinct microhabitats [42,47]. These microhabitats may lead to differences in root-accessible water reserves, translating to marked growth divergences. Our study revealed that tree growth on the earthy surface was generally 1.6 times higher than that on the stone surface (Figure 6). Paradoxically, existing research suggests that the high rock exposure rate may provide hydrological compensation through enhanced rainwater funneling to soil pockets, partially mitigating drought effects [48]. This creates a dual role for rock exposure: while it reduces the available soil distribution area, potentially hindering plant growth, it may simultaneously provide a mechanism for drought alleviation to some degree.
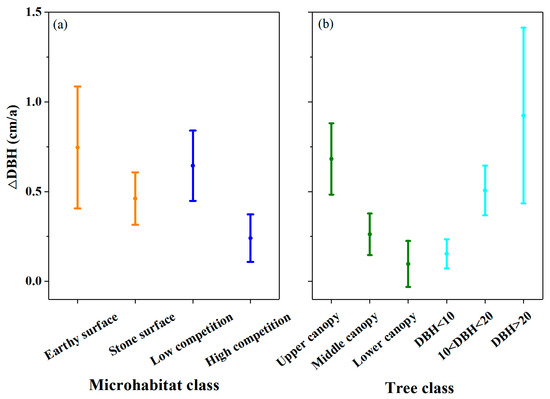
Figure 6.
Factors impact tree growth: (a) microhabitat; (b) crown location and tree size.
4.2.2. Biological Elements
Tree growth is influenced by multiple biological factors. Research on the drought responses of karst species demonstrates differential susceptibility to extreme drought conditions [44,49]. Furthermore, drought coping mechanisms and post-drought recovery capacities vary significantly across functional plant types [48,50,51]. Our findings reveal divergent growth rates among species under comparable environmental conditions (Table 2; Figure 2). Biomass accumulation shows strong correlations with plant size [39,41]. A study on plantation forests, secondary birch forests, and mixed conifer forests revealed that DBH growth increases with diameter class [39]. Similarly, our study indicates 6 times higher growth rates in larger diameter classes compared to smaller diameter classes (Figure 6). Specifically, the DBH growth rate of tree classes with upper crowns was 7 times higher than tree classes with lower crowns, while tree classes with low competition exhibited 2.7 times higher DBH growth rates than tree classes with high competition (Figure 6). The post-2021 biomass accumulation decline likely reflects drought impacts (Figure 4). Concurrently, intensified competition during succession could also contribute to the slowdown in tree biomass growth [38] (Figure 6).
Notably, all four monitored Populus canadensis Moench experienced mortality, primarily due to heavy parasitization by Scurrula parasitica, which affected nearly all the branches (Table 1). Parasitization was observed in 10% of the 100 continuously monitored trees. While parasitic plants substantially reduce host growth through resource competition, impacts on non-Populus species remained limited due to the smaller parasite size.
4.3. Several Suggestions on Vegetation Restoration in the Karst Region of Southwest China
In the karst region, trees undergoing natural succession on abandoned limestone slope farmland exhibit accelerated growth rates and enhanced carbon accumulation. Therefore, it is imperative to implement rigorous land management strategies in these areas to systematically retire agriculturally unsuitable land. Observational data suggest that rapidly growing species, including Cunninghamia lanceolata, Broussonetia papyrifera, and Celtis sinensis, are promising candidates for plantation efforts in the region. Furthermore, the pronounced spatial variability of karst soils, coupled with the profound impact of soil depth on biomass productivity, underscores the importance of selecting microhabitats with deeper soils, such as stone gullies and soil-rich surfaces, for afforestation. The karst region of Southwest China is experiencing decreasing rainfall trends [15], with projections indicating increased frequency and severity of extreme droughts due to global climate change. This necessitates a priority towards drought-resistant species in ecological restoration projects, such as Broussonetia papyrifera and Celtis sinensis [49]. Additionally, high-frequency monitoring of the tree growth process across diverse karst microhabitats is required to better study their response and adaptations to climate change.
5. Conclusions
The karst region of Southwest China has experienced significant greening induced by vegetation restoration in recent years. However, the role of natural succession on abandoned farmland remains to be clarified. Here, we found that tree growth was generally rapid and stable at the early stage of natural succession on abandoned farmland in the area. The average growth rate of dominant tree species reached approximately 5.74 mm/a, with a biomass accumulation rate of 9.38 kg/a. These results demonstrate that natural restoration serves as an effective land management strategy for marginal cropland under the Returning Farmland to Forest project in the region. However, prolonged drought duration may significantly decrease tree growth in the future under uncontrolled climate change. Furthermore, as succession progresses, intensified competition for limited nutrient resources in karst habitats may further inhibit tree growth. To promote natural restoration in the area, long-term monitoring studies across different ecological succession stages are required.
Author Contributions
Conceptualization, W.L. and Y.W. (Yanwei Wang); methodology, Y.W. (Yanwei Wang); software, X.C. and Y.W. (Yanwei Wang); validation, X.C. and Y.W. (Yanwei Wang); formal analysis, X.C., Y.W. (Yanwei Wang) and Y.W. (Yangyang Wu); investigation, X.C. and L.Z.; resources, W.L. and S.W.; data curation, X.C.; writing—original draft preparation, X.C. and Y.W. (Yanwei Wang); writing—review and editing, W.L. and J.C.; visualization, X.C. and Y.W. (Yanwei Wang); supervision, W.L.; project administration, W.L.; funding acquisition, W.L., J.C. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was jointly supported by the National Natural Science Foundation of China (W2412149, 42273021, 42407352, and U2244216), the China Postdoctoral Science Foundation (2021M703187), the Guizhou Provincial Science and Technology Projects (Qiankehejichu-ZK [2022] Yiban564 and Qiankehejichu-ZK [2023] Yiban475), and the Guizhou Provincial Major Scientific and Technological Program ([2024]013).
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
We sincerely thank the editors and anonymous reviewers for their valuable comments and suggestions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GSNDVI | Growing-season normalized difference vegetation index |
| DBH | Diameter at breast height |
| MA | Melia azedarach |
| PP | Platyosprion platycarpum |
| CF | Catalpa fargesii |
| PA | Populus adenopoda |
| TS | Toona sinensis |
| CO | Catalpa ovata |
| BP | Broussonetia papyrifera |
| PC | Populus canadensis Moench |
| CS | Celtis sinensis |
| TSe | Triadica sebifera |
| RC | Rhus chinensis |
| CM | Castanea mollissima |
| IM | Ilex macrocarpa |
| PPy | Pyrus pyrifolia |
| CA | Choerospondias axillaris |
| LL | Ligustrum lucidum |
| AK | Albizia kalkora |
| CL | Cunninghamia lanceolata |
| LC | Lindera communis |
References
- Wang, Y.; Li, Y. Occurrence Law of Karst Water in Down-Faulted Basins; Yunnan Science & Technology Press: Kunming, China, 2003; p. 140. [Google Scholar]
- Wang, S. The most serious eco-geologically environmental problem in Southwestern China—Karst rocky desertification. Bull. Mineral. Petrol. Geochem. 2003, 22, 120–126. [Google Scholar]
- Shi, Y.; Wang, L.; Zhu, W.; Su, W.; Li, P. The model of water resource utilization in karst area in Southwest China. Sci. Technol. Rev. 2005, 23, 52–55. [Google Scholar]
- Zhang, X.; Wang, K. Ponderation on the shortage of mineral nutrients in the soil-vegetation ecosystem in carbonate rock distributed mountain regions in Southwest China. Earth Environ. 2009, 37, 337–341. [Google Scholar]
- Zhou, Y.; Pan, G. Adaptation and adjustment of Maolan forest ecosystem to karst environment. Carsologica Sin. 2001, 20, 47–52. [Google Scholar]
- Jiang, Z.; Liu, H.; Wang, H.; Peng, J.; Meersmans, J.; Green, S.; Quine, T.; Wu, X.; Song, Z. Bedrock geochemistry influences vegetation growth by regulating the regolith water holding capacity. Nat. Commun. 2020, 11, 2392. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ren, M.; Lu, Z.; Wang, N. Research on the property of soil geochemistry in typical karst area in Guizhou Province. Carsologica Sin. 2010, 29, 246–252. [Google Scholar]
- Wang, S.; Ji, H.; Ouyang, Z.; Zhou, D.; Zheng, L.; Li, T. A preliminary study on the weathering of carbonate rocks. Sci. China (Ser. D) 1999, 29, 441–449. [Google Scholar]
- Yuan, D. On the karst ecosystem. Acta Ecol. Sin. 2001, 75, 336–338. [Google Scholar]
- Zhu, S. Ecological Research on Karst Forest (II); Guizhou Science and Technology Press: Guiyang, China, 1997. [Google Scholar]
- Yu, W.; Dong, D.; Ni, J. Comparisons of biomass and net primary productivity of karst and non-karst forest in moutainuous areas, Southwestern China. J. Subtrop. Resour. Environ. 2010, 5, 25–30. [Google Scholar]
- Ni, J.; Xu, H.; Liu, L. Low net primary productivity of dominant tree species in a karst forest, southwestern China: First evidences from tree ring width and girth increment. Acta Geochim. 2017, 36, 482–485. [Google Scholar] [CrossRef]
- Yang, H.; Cheng, S. Study on biomass of the karst forest community in Maolan, Guizhou Province. Acta Ecol. Sin. 1991, 11, 307–313. [Google Scholar]
- Zhu, S.; Wei, L.; Chen, Z.; Zhang, C. A preliminary study on biomass components of karst forest in Maolan of Guizhou Province, China. Acta Phytoecol. Sin. 1995, 19, 358–367. [Google Scholar]
- Chen, C.; Park, T.; Wang, X.; Piao, S.; Xu, B. China and India lead in greening of the world through land-use management. Nat. Sustain. 2019, 2, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.; Yue, Y.; Wigneron, J.; Tong, X.; Tian, F.; Jepsen, M.; Xiao, X.; Verger, A.; Mialon, A.; Al-Yaari, A. Satellite-observed major greening and biomass increase in South China Karst during recent decade. Earth’s Future 2018, 6, 1017–1028. [Google Scholar] [CrossRef]
- Zhang, X. The Vegetation Carbon Sequestration Effect and Its Potential in The Southwestern Karst Region of China in the Past 20 Years. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2022. [Google Scholar]
- Yuan, E.; Zhou, Q.; Luo, Y. Contribution of socioeconomic factors to vegetation restoration in karst areas of southwest China. Acta Ecol. Sin. 2024, 44, 6265–6275. [Google Scholar]
- Han, Z.; Song, W. Spatiotemporal variations in cropland abandonment in the Guizhou–Guangxi karst mountain area, China. J. Clean. Prod. 2019, 238, 117888. [Google Scholar] [CrossRef]
- Dong, D.; Ni, J. Modeling changes of net primary productivity of karst vegetation in Southwestern China using the CASA model. Acta Ecol. Sin. 2011, 31, 1855–1866. [Google Scholar]
- Lv, Y.; Zhang, L.; Yan, H.; Ren, X.; Wang, J.; Niu, Z.; Gu, F.; He, H. Spatial and temporal pattern of changing vegetation and the influence of the environmental factors in the karst region of Southwest China. Acta Ecol. Sin. 2018, 38, 8774–8786. [Google Scholar]
- Cai, H.; Yang, X.; Wang, K.; Xiao, L. Is forest restoration in the Southwest China karst promoted mainly by climate change or human-induced factors? Remote Sens. 2014, 6, 9895–9910. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, K.; Liu, H.; Zhang, C.; Wang, J.; Yue, Y.; Qi, X. How ecological restoration alters ecosystem services: An analysis of vegetation carbon sequestration in the karst area of northwest Guangxi, China. Environ. Earth Sci. 2015, 74, 5307–5317. [Google Scholar] [CrossRef]
- Chang, J.; Yue, Y.; Tong, X.; Brandt, M.; Zhang, C.; Zhang, X. Rural outmigration generates a carbon sink in South China karst. Prog. Phys. Geogr. Earth Environ. 2023, 47, 655–667. [Google Scholar] [CrossRef]
- Li, P.; Zhu, J.; Hu, H.; Guo, Z.; Pan, Y.; Richard, B.; Fang, J. The relative contributions of forest growth and areal expansion to forest biomass carbon sinks in China. Biogeosci. Discuss. 2015, 12, 9587–9612. [Google Scholar]
- Qiao, Y.; Jiang, Y.; Zhang, C. Contribution of karst ecological restoration engineering to vegetation greening in southwest China during recent decade. Ecol. Indic. 2020, 121, 107181. [Google Scholar] [CrossRef]
- Tong, X.; Brandt, M.; Yue, Y.; Horion, S.; Wang, K.; Keersmaecker, W.; Tian, F.; Schurgers, G.; Xiao, X.; Luo, Y.; et al. Increased vegetation growth and carbon stock in China karst via ecological engineering. Nat. Sustain. 2018, 1, 44–50. [Google Scholar] [CrossRef]
- Liu, L.; Zhong, Q.; Zhang, Z. High mortality and low net change in live woody biomass of karst evergreen and deciduous broad-leaved mixed forest in Southwestern China. Forests 2018, 9, 263. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, W.; Zeng, G.; Peng, H.; Cheng, A.; Zhang, L.; Cai, X.; Chen, J.; Lyu, Y.; Yang, H.; et al. Characteristics of carbon, water, and energy fluxes on abandoned farmland revealed by critical zone observation in the karst region of southwest China. Agric. Ecosyst. Environ. 2020, 292, 106821. [Google Scholar] [CrossRef]
- Liu, C.; Wei, Y.; Liu, Y. Biomass of canopy and shrub layers of karst forest in Puding, Guizhou, China. Chin. J. Plant Ecol. 2009, 33, 698–705. [Google Scholar]
- Zhou, Z. Scientific Investigation Report of Maolan Karst Forest; Guizhou Science & Technology Press: Guiyang, China, 1987; pp. 1–23. (In Chinese) [Google Scholar]
- Ni, J.; Luo, D.; Xia, J.; Zhang, Z.; Hu, G. Vegetation in karst terrain of southwestern China allocates more biomass to roots. Solid Earth 2015, 6, 799–810. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, C.; Du, T.; Ma, Z.; Zhu, H.; Wang, Y.; Du, F. Three-dimensional visual simulation and root morphological parameter analysis of three slope protection plants in loess region. Res. Soil Water Conserv. 2025, 32, 218–226. [Google Scholar]
- Wang, S.; Lu, H.; Zhou, Y.; Xie, L.; Xiao, D. Spatial variability of soil organic carbon and representative soil sampling method in Maolan karst virgin forest. Acta Pedol. Sin. 2007, 44, 475–483. [Google Scholar]
- Liu, Y.; Wei, X.; Fan, Z.; Chen, L.; Lin, Y.; Fu, P. Annual growth dynamics of Garuga floribunda var.gamblei in tropical karst forests and their response to environmental factors. Acta Ecol. Sin. 2023, 43, 10171–10181. [Google Scholar]
- Liu, Q.; Chen, D.; Hong, W.; Zhuang, X. Growth analysis of 33 ecological broad-leaved tree species in Huizhou hilly area. Guangdong For. Sci. Technol. 2015, 31, 38–46. [Google Scholar]
- Zhou, B.; Fan, Z.; Qi, J. Intra-annual radial growth of evergreen and deciduous tree species and their response to climatic factors in a montane moist evergreen broad-leaved forest in the Ailao Mountains, Southwest China. Acta Ecol. Sin. 2020, 40, 1699–1708. [Google Scholar]
- Liu, L.; Xu, H.; Guo, Y.; Liang, H.; Lu, X.; Zhang, H.; Liang, E.; Ni, J. reconstruction of above-ground biomass and net primary productivity of dominant tree species in Guizhou forests over past five decades based on tree-ring data. Acta Ecol. Sin. 2022, 40, 3441–3451. [Google Scholar]
- Lee, W.; Gadow, K.; Chung, D.; Lee, J.; Shin, M. DBH growth model for Pinus densiflora and Quercus variabilis mixed forests in central Korea. Ecol. Model. 2004, 176, 187–200. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, S.; Zhang, X. Dynamics of stand biomass and volume of the tree layer in forests with different restoration approaches based on tree-ring analysis. Chin. J. Plant Ecol. 2012, 36, 117–125. [Google Scholar] [CrossRef]
- Michael, K.; Neupane, P.; Lotfiomran, N. The impact of tree age on biomass growth and carbon accumulation capacity: A retrospective analysis using tree ring data of three tropical tree species grown in natural forests of Suriname. PLoS ONE 2017, 12, e0181187. [Google Scholar]
- Liu, F.; Wang, S.; Luo, H.; Liu, Y.; Liu, H. Micro-habitats in karst forest ecosystem and variability of soil. Acta Pedol. Sin. 2008, 45, 1055–1062. [Google Scholar]
- Bréda, N.; Granier, A. Intra- and interannual variations of transpiration, leaf area index and radial growth of a sessile oak stand (Quercus petraea). Ann. Sci. Forest. 1996, 53, 521–536. [Google Scholar] [CrossRef]
- Xu, H.; Liu, L.; Guo, Y.; Liang, H.; Ni, J. Tree-rings of dominant species in karst forests in Southwest China and their responses to climate change. Earth Environ. 2018, 46, 23–31. [Google Scholar]
- Wang, B.; Yang, S.; Wang, Y. Estimation on net primary produtivity of vegetation in karst area of Guizhou province. Carsologica Sin. 2007, 26, 98–104. [Google Scholar]
- Shen, Y.; Chen, F. Large heterogeneity of water and nutrient supply derived from runoff of nearby rock outcrops in karst ecosystems in SW China. Catena 2019, 172, 125–131. [Google Scholar] [CrossRef]
- Zhu, S.; He, J.; Wei, L. Study on Feature of Micro-Habitas in Maolan Karst Forest (III); Guizhou Science and Technology Press: Guiyang, China, 2003; pp. 24–29. [Google Scholar]
- Su, J.; Gou, X.; Deng, Y.; Zhang, R.; Liu, W.; Zhang, F.; Lu, M.; Chen, Y.; Zheng, W. Tree growth response of Fokienia hodginsii to recent climate warming and drought in southwest China. Int. J. Biometeorol. 2017, 61, 2085–2096. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, S.; Rong, L.; Cheng, A.; Li, Y. Effects of extreme drought on plant species in Karst area of Guizhou Province, Southwest China. Chin. J. Appl. Ecol. 2011, 22, 1127–1134. [Google Scholar]
- Orwig, D.; Abrams, M. Variation in radial growth responses to drought among species, site, and canopy strata. Trees 1997, 11, 474–484. [Google Scholar] [CrossRef]
- Romà, O.; Josep, P. Tree growth, mortality, and above-ground biomass accumulation in a holm oak forest under a five-year experimental field drought. Plant Ecol. 2007, 189, 291–299. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).