A Deciduous Forest’s CO2 Exchange Within the Mixed-Humid Climate of Kentucky, USA
Abstract
1. Introduction
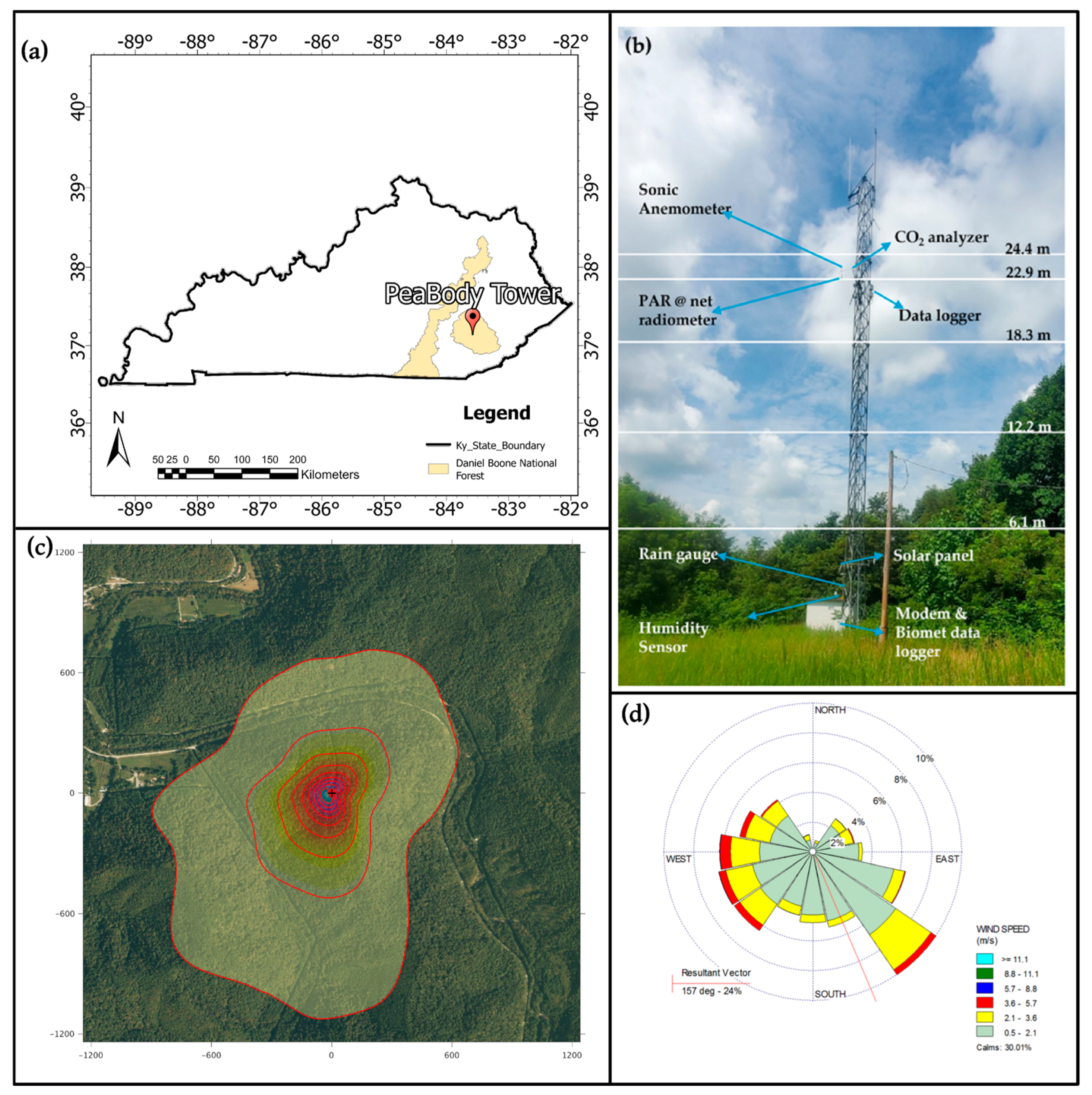
2. Materials and Methods
3. Results
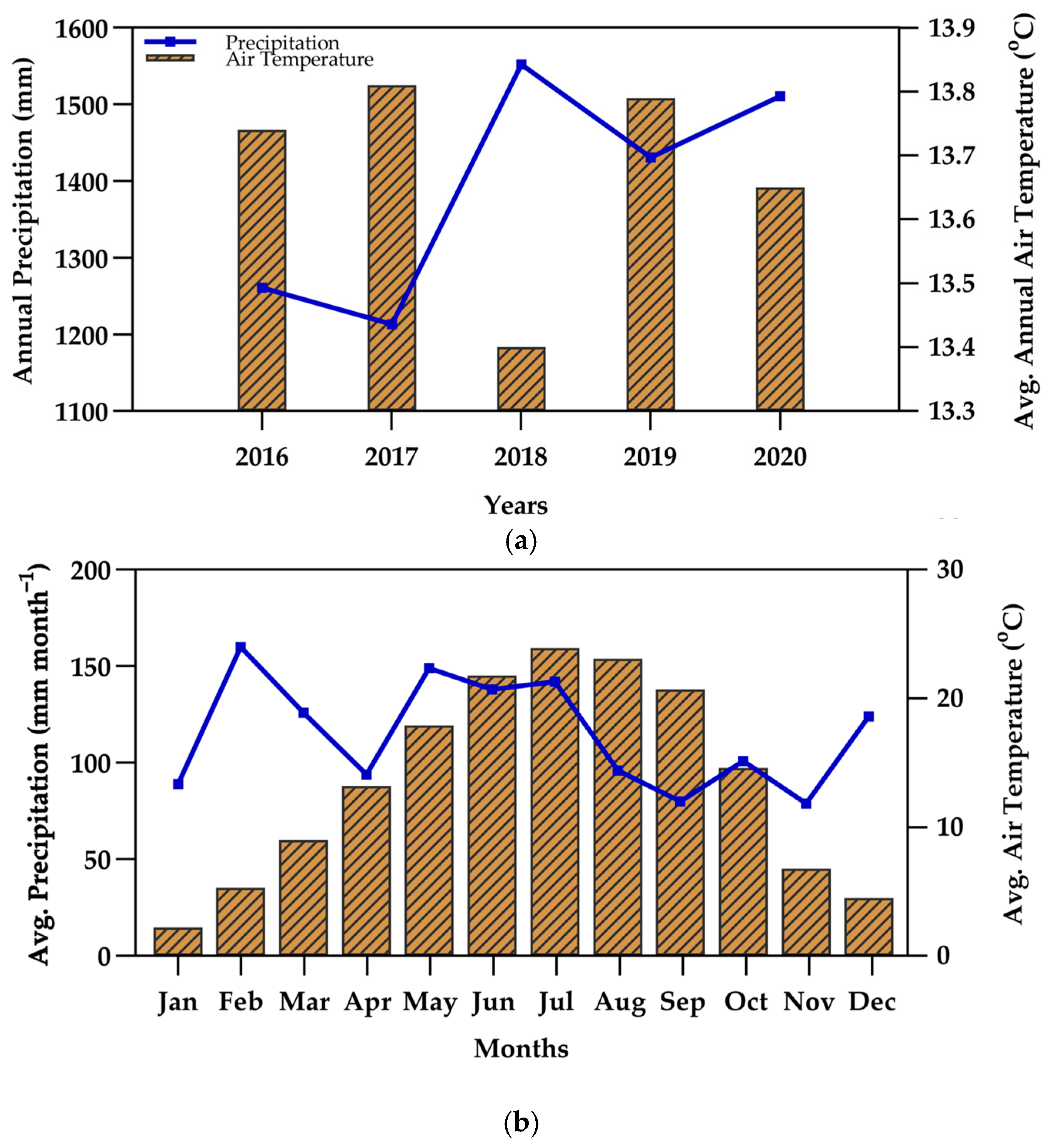
3.1. Weather and Climate
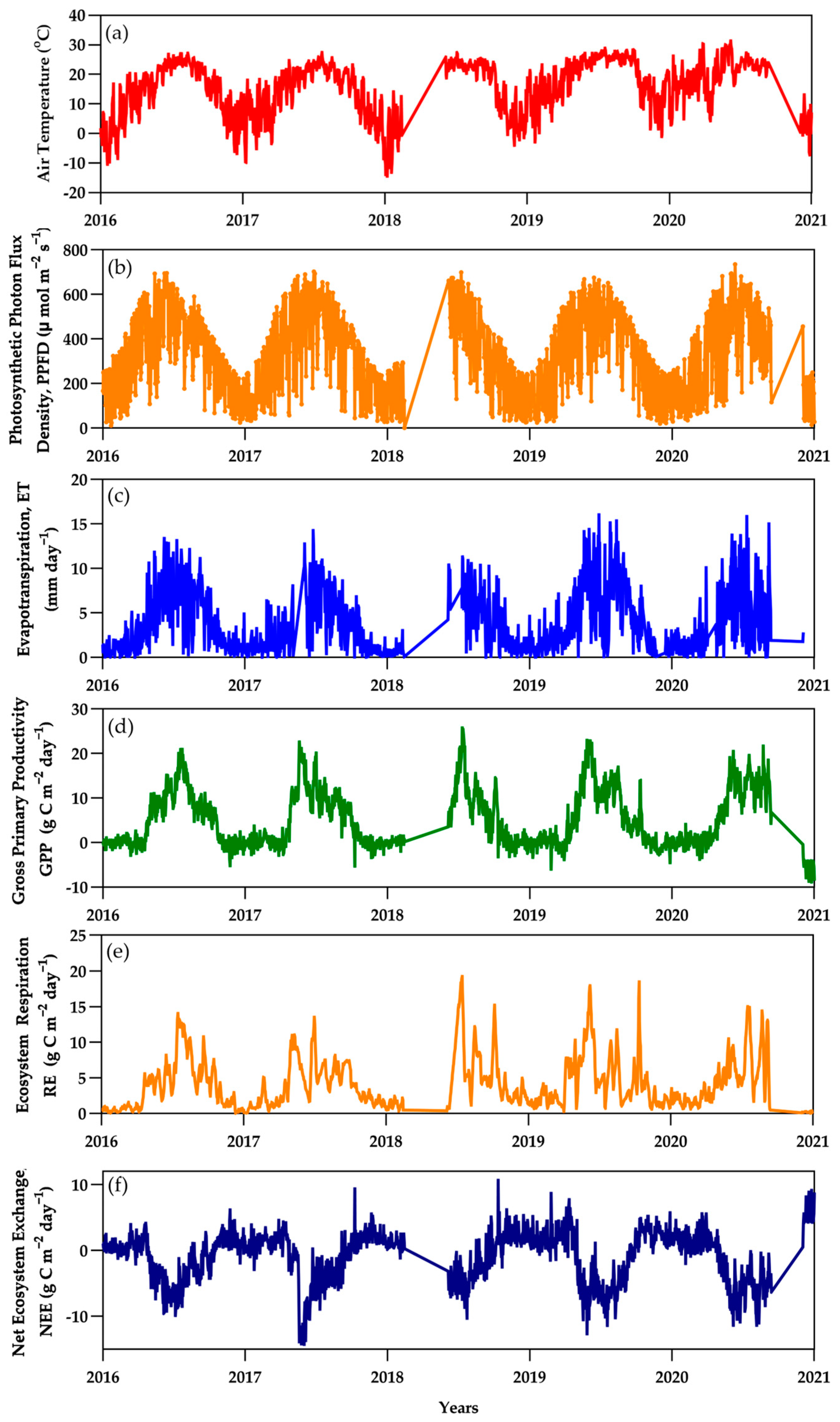
3.2. Ecosystem Carbon Dioxide Fluxes
3.3. Cumulative Ecosystem Exchange
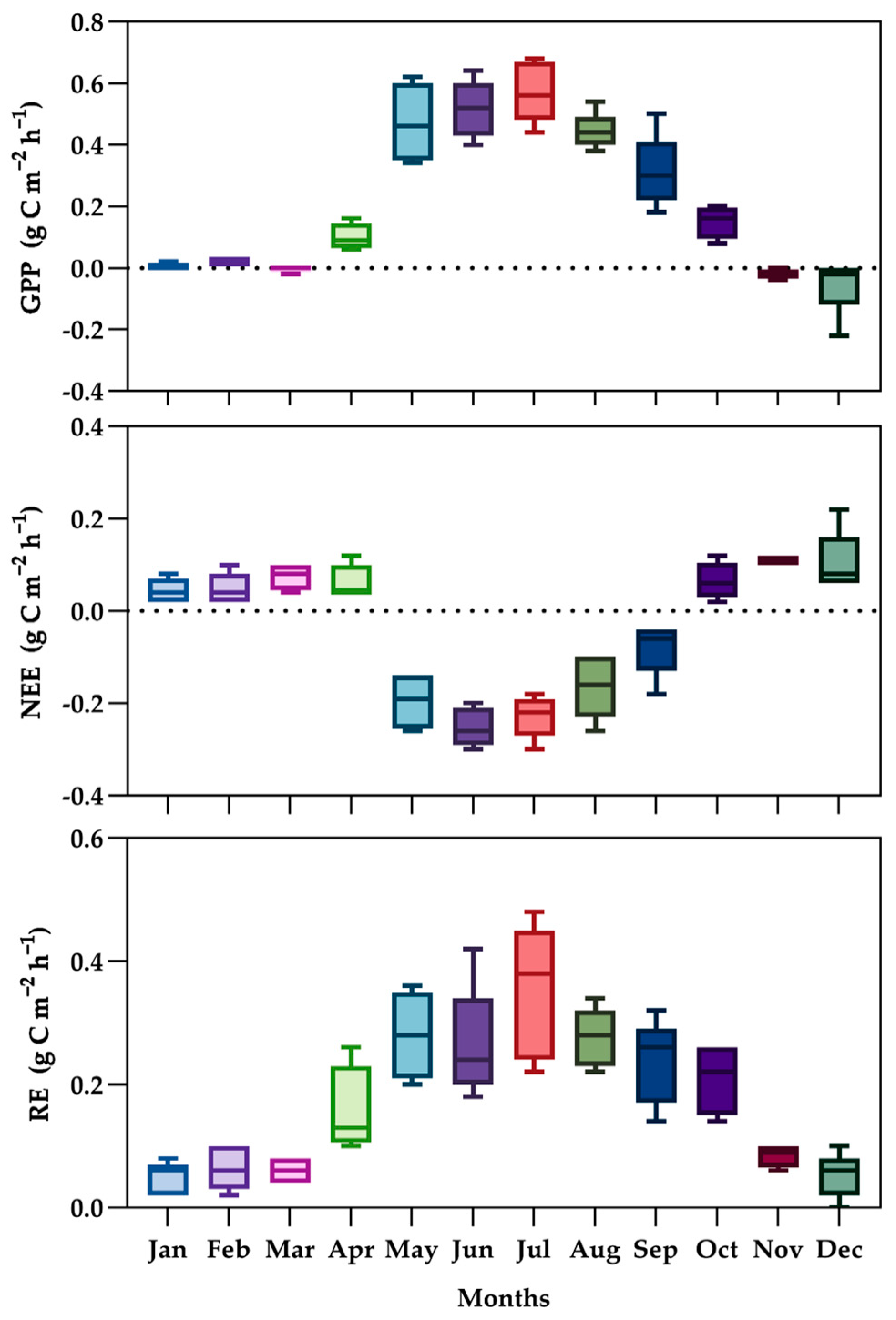
3.4. Seasonal Distribution of Gross Primary Productivity (GPP), Ecosystem Respiration (RE), and Net Ecosystem Exchange (NEE)
3.5. Diurnal Pattern of Gross Primary Productivity (GPP), Ecosystem Respiration (RE), and Net Ecosystem Exchange (NEE)
3.6. Environmental Control of Carbon Dioxide Exchange
3.6.1. Monthly Light Response Curves (Michelis–Menten Curves)
3.6.2. Monthly Temperature Response Curves for the Year 2016
3.6.3. Correlational Analysis
3.6.4. Seasonal Regression (Four Seasons)
4. Discussion
4.1. Ecosystem Scale Exchanges
4.2. Controlling Variables
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NEE | Net Ecosystem Exchange |
| GPP | Gross Primary Productivity |
| RE | Ecosystem Respiration |
References
- Haight, R.G.; Bluffstone, R.; Kline, J.D.; Coulston, J.W.; Wear, D.N.; Zook, K. Estimating the Present Value of Carbon Sequestration in U.S. Forests, 2015–2050, for Evaluating Federal Climate Change Mitigation Policies. Agric. Resour. Econ. Rev. 2019, 49, 150–177. [Google Scholar] [CrossRef]
- USDA. The USDA Forest Service Forest Inventory & Analysis Program Supplies Annual Updates of Forest Resources in Each State Based on an Inventory Conducted by the FIA Program in Cooperation with State Forestry Agencies. Available online: https://research.fs.usda.gov/programs/fia#data-and-tools (accessed on 24 April 2024).
- Hoover, K.; Riddle, A.A. U.S. Forest Carbon Data: In Brief. 2023. Congressional Research Service. Available online: https://sgp.fas.org/crs/misc/R46313.pdf (accessed on 10 March 2025).
- Birdsey, R.; Pregitzer, K.; Lucier, A. Forest Carbon Management in the United States. J. Environ. Qual. 2006, 35, 1461–1469. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and Climate Change: Forcings, Feedbacks, and the Climate Benefits of Forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Gebremedhin, M.T.; Loescher, H.W.; Tsegaye, T.D. Carbon Balance of No-till Soybean with Winter Wheat Cover Crop in the Southeastern United States. Agron. J. 2012, 104, 1321–1335. [Google Scholar] [CrossRef]
- Gilmanov, T.G.; Baker, J.M.; Bernacchi, C.J.; Billesbach, D.P.; Burba, G.G.; Castro, S.; Chen, J.; Eugster, W.; Fischer, M.L.; Gamon, J.A.; et al. Productivity and Carbon Dioxide Exchange of Leguminous Crops: Estimates from Flux Tower Measurements. Agron. J. 2014, 106, 545–559. [Google Scholar] [CrossRef]
- Yu, G.R.; Zhang, L.M.; Sun, X.M.; Fu, Y.L.; Wen, X.F.; Wang, Q.F.; Li, S.G.; Ren, C.Y.; Song, X.; Liu, Y.F.; et al. Environmental Controls over Carbon Exchange of Three Forest Ecosystems in Eastern China. Glob. change Biol. 2008, 14, 2555–2571. [Google Scholar] [CrossRef]
- Gilmanov, T.G.; Soussana, J.F.; Aires, L.; Allard, V.; Ammann, C.; Balzarolo, M.; Barcza, Z.; Bernhofer, C.; Campbell, C.L.; Cernusca, A.; et al. Partitioning European Grassland Net Ecosystem CO2 Exchange into Gross Primary Productivity and Ecosystem Respiration Using Light Response Function Analysis. Agric. Ecosyst. Environ. 2007, 121, 93–120. [Google Scholar] [CrossRef]
- Niu, Y.Y.; Li, Y.Q.; Wang, X.Y.; Gong, X.W.; Luo, Y.Q.; Tian, D.Y. Characteristics of Annual Variation in Net Carbon Dioxide Flux in a Sandy Grassland Ecosystem during Dry Years. Acta Prataculturae Sin. 2018, 27, 215. [Google Scholar] [CrossRef]
- Mendes, K.R.; Campos, S.; da Silva, L.L.; Mutti, P.R.; Ferreira, R.R.; Medeiros, S.S.; Perez-Marin, A.M.; Marques, T.V.; Ramos, T.M.; de Lima Vieira, M.M.; et al. Seasonal Variation in Net Ecosystem CO2 Exchange of a Brazilian Seasonally Dry Tropical Forest. Sci. Rep. 2020, 10, 9454. [Google Scholar] [CrossRef]
- Nandy, P.; Ghose, M. Photosynthesis and Water-Use Characteristics in Indian Mangroves. J. Plant Biol. 2005, 48, 245–252. [Google Scholar] [CrossRef]
- Barr, A.; Black, T.A.; McCaughey, H. Climatic and Phenological Controls of the Carbon and Energy Balances of Three Contrasting Boreal Forest Ecosystems in Western Canada. In Phenology of Ecosystem Processes: Applications in Global Change Research; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Luxmoore, R.J.; Wullschleger, S.D.; Hanson, P.J. Forest Responses to CO2 Enrichment and Climate Warming. Water Air Soil. Pollut. 1993, 70, 309–323. [Google Scholar] [CrossRef]
- Liu, J.; Lai; Derrick, Y.F. Subtropical Mangrove Wetland Is a Stronger Carbon Dioxide Sink in the Dry than Wet Seasons. Agric. For. Meteorol. 2019, 278, 107644. [Google Scholar] [CrossRef]
- Rodda, S.R.; Thumaty, K.C.; Fararoda, R.; Jha, C.S.; Dadhwal, V.K. Unique Characteristics of Ecosystem CO2 Exchange in Sundarban Mangrove Forest and Their Relationship with Environmental Factors. Estuar. Coast. Shelf Sci. 2022, 267, 107764. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.M.; Ju, W.; Zhang, Y. Seasonal Variations in Leaf Maximum Photosynthetic Capacity and Its Dependence on Climate Factors Across Global FLUXNET Sites. J. Geophys. Res. Biogeosci. 2022, 127, e2021JG006709. [Google Scholar] [CrossRef]
- Raihan, A. A Review of Forest’s Contribution to Mitigating Climate Change. In Proceedings of the International Conference on Forests and Climate Change, Brussels, Belgium, 4–5 February 2020; 2024. [Google Scholar]
- Li, X.; Du, H.; Zhou, G.; Mao, F.; Zhu, D.; Zhang, M.; Xu, Y.; Zhou, L.; Huang, Z. Spatiotemporal Patterns of Remotely Sensed Phenology and Their Response to Climate Change and Topography in Subtropical Bamboo Forests during 2001-2017: A Case Study in Zhejiang Province, China. GIsci. Remote Sens. 2023, 60, 2163575. [Google Scholar] [CrossRef]
- Psistaki, K.; Tsantopoulos, G.; Paschalidou, A.K. An Overview of the Role of Forests in Climate Change Mitigation. Sustainability 2024, 16, 6089. [Google Scholar] [CrossRef]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate Change, Phenology, and Phenological Control of Vegetation Feedbacks to the Climate System. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Keenan, T.F.; Gray, J.; Friedl, M.A.; Toomey, M.; Bohrer, G.; Hollinger, D.Y.; Munger, J.W.; O’Keefe, J.; Schmid, H.P.; Wing, I.S.; et al. Net Carbon Uptake Has Increased through Warming-Induced Changes in Temperate Forest Phenology. Nat. Clim. change 2014, 4, 598–604. [Google Scholar] [CrossRef]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant Phenology and Global Climate Change: Current Progresses and Challenges. Glob. change Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef]
- Geosciences LibreTexts. 7.5.4: Humid Subtropical Climate—Geosciences LibreTexts. Available online: https://geo.libretexts.org/Courses/Kansas_State_University/Physical_Geography%3A_our_Beautiful_World/07%3A_Climate_Systems/7.05%3A_Midlatitude_and_Subtropical_Climates/7.5.04%3A_Humid_Subtropical_Climate (accessed on 23 December 2024).
- Antonopoulos, C.; Gilbride, T.; Margiotta, E.; Cragin, M.; Kaltreider, C. Guide to Determining Climate Zone by County: Building America and IECC 2021 Updates (No. PNNL-33270). Available online: https://www.osti.gov/servlets/purl/1893981/ (accessed on 8 January 2025).
- Hicks, D.J.; Chabot, B.F. Deciduous Forest. In Physiological Ecology of North American Plant Communities; Springer: Dordrecht, The Netherlands, 1985; pp. 257–277. [Google Scholar] [CrossRef]
- Busgen, M.; Munch, F.; Thomson, T. The Structure and Life of Forest Trees. Soil Sci. 1930, 29, 159. [Google Scholar] [CrossRef]
- Hari, P.; Kulmala, L.; Havimo, M. Introduction to Physical, Physiological and Causal Forest Ecology. In Physical and Physiological Forest Ecology; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Meyers, T.P. A Comparison of Summertime Water and CO2 Fluxes over Rangeland for Well Watered and Drought Conditions. Agric. For. Meteorol. 2001, 106, 205–214. [Google Scholar] [CrossRef]
- Flanagan, L.B.; Wever, L.A.; Carlson, P.J. Seasonal and Interannual Variation in Carbon Dioxide Exchange and Carbon Balance in a Northern Temperate Grassland. Glob. change Biol. 2002, 8, 599–615. [Google Scholar] [CrossRef]
- Law, B.E.; Falge, E.; Gu, L.; Baldocchi, D.D.; Bakwin, P.; Berbigier, P.; Davis, K.; Dolman, A.J.; Falk, M.; Fuentes, J.D.; et al. Environmental Controls over Carbon Dioxide and Water Vapor Exchange of Terrestrial Vegetation. Agric. For. Meteorol. 2002, 113, 97–120. [Google Scholar] [CrossRef]
- Arain, M.A.; Black, T.A.; Barr, A.G.; Griffis, T.J.; Morgenstern, K.; Nesic, Z. Year-Round Observations of the Energy and Water Vapour Fluxes above a Boreal Black Spruce Forest. Hydrol. Process 2003, 17, 3581–3600. [Google Scholar] [CrossRef]
- Tanja, S.; Berninger, F.; Vesala, T.; Markkanen, T.; Hari, P.; Mäkelä, A.; Ilvesniemi, H.; Hänninen, H.; Nikinmaa, E.; Huttula, T.; et al. Air Temperature Triggers the Recovery of Evergreen Boreal Forest Photosynthesis in Spring. Glob. change Biol. 2003, 9, 1410–1426. [Google Scholar] [CrossRef]
- Angert, A.; Biraud, S.; Bonfils, C.; Henning, C.C.; Buermann, W.; Pinzon, J.; Tucker, C.J.; Fung, I. Drier Summers Cancel out the CO2 Uptake Enhancement Induced by Warmer Springs. Proc. Natl. Acad. Sci. USA 2005, 102, 10823–10827. [Google Scholar] [CrossRef]
- Barford, C.C.; Wofsy, S.C.; Munger, J.W.; Goulden, M.L.; Pyle, H.E.; Urbanski, S.P.; Hutyra, L.; Saleska, S.R.; Fitzjarrald, D.; Moore, K. Factors Controlling Long- and Short-Term Sequestration of Atmospheric CO2 in a Mid-Latitude Forest. Science 2001, 294, 1688–1691. [Google Scholar] [CrossRef]
- Curtis, P.S.; Hanson, P.J.; Bolstad, P.; Barford, C.; Randolph, J.C.; Schmid, H.P.; Wilson, K.B. Biometric and Eddy-Covariance Based Estimates of Annual Carbon Storage in Five Eastern North American Deciduous Forests. Agric. For. Meteorol. 2002, 113, 3–19. [Google Scholar] [CrossRef]
- Dunn, A.L.; Barford, C.C.; Wofsy, S.C.; Goulden, M.L.; Daube, B.C. A Long-Term Record of Carbon Exchange in a Boreal Black Spruce Forest: Means, Responses to Interannual Variability, and Decadal Trends. Glob. change Biol. 2007, 13, 577–590. [Google Scholar] [CrossRef]
- Rodda, S.R.; Thumaty, K.C.; Praveen, M.S.S.; Jha, C.S.; Dadhwal, V.K. Multi-Year Eddy Covariance Measurements of Net Ecosystem Exchange in Tropical Dry Deciduous Forest of India. Agric. For. Meteorol. 2021, 301–302, 108351. [Google Scholar] [CrossRef]
- Teklemariam, T.; Staebler, R.M.; Barr, A.G. Eight Years of Carbon Dioxide Exchange above a Mixed Forest at Borden, Ontario. Agric. For. Meteorol. 2009, 149, 2040–2053. [Google Scholar] [CrossRef]
- Urbanski, S.; Barford, C.; Wofsy, S.; Kucharik, C.; Pyle, E.; Budney, J.; McKain, K.; Fitzjarrald, D.; Czikowsky, M.; Munger, J.W. Factors Controlling CO2 Exchange on Timescales from Hourly to Decadal at Harvard Forest. J. Geophys. Res. Biogeosci. 2007, 112. [Google Scholar] [CrossRef]
- RedRiverGorge.com. Hiking Trails Red River | Flora & Fauna. (n.d.). Red River Gorge. Retrieved February 6, 2023.
- Kljun, N.; Calanca, P.; Rotach, M.W.; Schmid, H.P. A Simple Two-Dimensional Parameterisation for Flux Footprint Prediction (FFP). Geosci. Model. Dev. 2015, 8, 3695–3713. [Google Scholar] [CrossRef]
- USGS. LANDFIRE Map Viewer: LANDFIRE 2020 Biophysical Settings (BPS) CONUS. Available online: https://www.landfire.gov/viewer/ (accessed on 9 March 2025).
- Schimel, D.; Melillo, J.; Tian, H.; McGuire, A.D.; Kicklighter, D.; Kittel, T.; Rosenbloom, N.; Running, S.; Thornton, P.; Ojima, D.; et al. Contribution of Increasing CO2 and Climate to Carbon Storage by Ecosystems in the United States. Science 2000, 287, 2004–2006. [Google Scholar] [CrossRef]
- Wofsy, S.C.; Goulden, M.L.; Munger, J.W.; Fan, S.-M.; Bakwin, P.S.; Daube, B.C.; Bassow, S.L.; Bazzaz, F.A. Net Exchange of CO2 in a Mid-Latitude Forest. Science 1993, 260, 1314–1317. [Google Scholar] [PubMed]
- Thornley, J.H.; Johnson, I.R. Plant and Crop Modelling; Clarendon: Oxford, UK, 1990. [Google Scholar]
- Ruimy, A.; Jarvis, P.G.; Baldocchi, D.D.; Saugier, B. CO2 Fluxes over Plant Canopies and Solar Radiation: A Review. Adv. Ecol. Res. 1995, 26, 1–68. [Google Scholar] [CrossRef]
- Falge, E.; Baldocchi, D.; Olson, R.; Anthoni, P.; Aubinet, M.; Bernhofer, C.; Burba, G.; Ceulemans, R.; Clement, R.; Dolman, H.; et al. Gap Filling Strategies for Defensible Annual Sums of Net Ecosystem Exchange. Agric. For. Meteorol. 2001, 107, 43–69. [Google Scholar] [CrossRef]
- Lloyd, J.; Taylor, J.A. On the Temperature Dependence of Soil Respiration. Funct. Ecol. 1994, 8, 315. [Google Scholar] [CrossRef]
- Falge, E.; Baldocchi, D.; Tenhunen, J.; Aubinet, M.; Bakwin, P.; Berbigier, P.; Bernhofer, C.; Burba, G.; Clement, R.; Davis, K.J.; et al. Seasonality of Ecosystem Respiration and Gross Primary Production as Derived from FLUXNET Measurements. Agric. For. Meteorol. 2002, 113, 53–74. [Google Scholar] [CrossRef]
- Moffat, A.M.; Papale, D.; Reichstein, M.; Hollinger, D.Y.; Richardson, A.D.; Barr, A.G.; Beckstein, C.; Braswell, B.H.; Churkina, G.; Desai, A.R.; et al. Comprehensive Comparison of Gap-Filling Techniques for Eddy Covariance Net Carbon Fluxes. Agric. For. Meteorol. 2007, 147, 209–232. [Google Scholar] [CrossRef]
- Baldocchi, D. Assessing the Eddy Covariance Technique for Evaluating Carbon Dioxide Exchange Rates of Ecosystems: Past, Present and Future. Glob. change Biol. 2003, 9, 479–492. [Google Scholar]
- Baldocchi, D. “Breathing” of the Terrestrial Biosphere: Lessons Learned from a Global Network of Carbon Dioxide Flux Measurement Systems. Aust. J. Bot. 2008, 56, 1–26. [Google Scholar] [CrossRef]
- Papale, D.; Reichstein, M.; Aubinet, M.; Canfora, E.; Bernhofer, C.; Kutsch, W.; Longdoz, B.; Rambal, S.; Valentini, R.; Vesala, T.; et al. Towards a Standardized Processing of Net Ecosystem Exchange Measured with Eddy Covariance Technique: Algorithms and Uncertainty Estimation. Biogeosciences 2006, 3, 571–583. [Google Scholar] [CrossRef]
- Wilson, K.; Goldstein, A.; Falge, E.; Aubinet, M.; Baldocchi, D.; Berbigier, P.; Bernhofer, C.; Ceulemans, R.; Dolman, H.; Field, C.; et al. Energy Balance Closure at FLUXNET Sites. Agric. For. Meteorol. 2002, 113, 223–243. [Google Scholar] [CrossRef]
- Jung, M.; Reichstein, M.; Margolis, H.A.; Cescatti, A.; Richardson, A.D.; Arain, M.A.; Arneth, A.; Bernhofer, C.; Bonal, D.; Chen, J.; et al. Global Patterns of Land-Atmosphere Fluxes of Carbon Dioxide, Latent Heat, and Sensible Heat Derived from Eddy Covariance, Satellite, and Meteorological Observations. J. Geophys. Res. Biogeosci. 2011, 116, G3. [Google Scholar] [CrossRef]
- Foken, T.; Leuning, R.; Oncley, S.R.; Mauder, M.; Aubinet, M. Corrections and Data Quality Control. In Eddy Covariance; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A.; et al. On the Separation of Net Ecosystem Exchange into Assimilation and Ecosystem Respiration: Review and Improved Algorithm. Glob. change Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Lasslop, G.; Reichstein, M.; Papale, D.; Richardson, A.; Arneth, A.; Barr, A.; Stoy, P.; Wohlfahrt, G. Separation of Net Ecosystem Exchange into Assimilation and Respiration Using a Light Response Curve Approach: Critical Issues and Global Evaluation. Glob. change Biol. 2010, 16, 187–208. [Google Scholar] [CrossRef]
- Knohl, A.; Baldocchi, D.D. Effects of Diffuse Radiation on Canopy Gas Exchange Processes in a Forest Ecosystem. J. Geophys. Res. Biogeosci. 2008, 113, G2. [Google Scholar] [CrossRef]
- Clement, R.J.; Jarvis, P.G.; Moncrieff, J.B. Carbon Dioxide Exchange of a Sitka Spruce Plantation in Scotland over Five Years. Agric. For. Meteorol. 2012, 153, 106–123. [Google Scholar] [CrossRef]
- Harmon, M.E.; Bond-Lamberty, B.; Tang, J.; Vargas, R. Heterotrophic Respiration in Disturbed Forests: A Review with Examples from North America. J. Geophys. Res. Biogeosci. 2011, 116, G4. [Google Scholar] [CrossRef]
- Valentini, R.; Matteucci, G.; Dolman, A.J.; Schulze, E.D.; Rebmann, C.J.; Moors, E.J.; Granier, A.; Gross, P.; Jensen, N.O.; Pilegaard, K.; et al. Respiration as the Main determinant of Carbon Balance in European Forests. Nature 2000, 404, 861–865. [Google Scholar] [PubMed]
- Nilsson, O. Winter Dormancy in Trees. Curr. Biol. 2022, 32, R630–R634. [Google Scholar] [CrossRef]
- Fadón, E.; Fernandez, E.; Behn, H.; Luedeling, E. A Conceptual Framework for Winter Dormancy in Deciduous Trees. Agronomy 2020, 10, 241. [Google Scholar] [CrossRef]
- Allona, I.; Ramos, A.; Ibáñez, C.; Contreras, A.; Casado, R.; Aragoncillo, C. Review. Molecular Control of Winter Dormancy Establishment in Trees. Span. J. Agric. Res. 2008, 6, 201–210. [Google Scholar] [CrossRef]
- Hirata, R.; Hirano, T.; Saigusa, N.; Fujinuma, Y.; Inukai, K.; Kitamori, Y.; Takahashi, Y.; Yamamoto, S. Seasonal and Interannual Variations in Carbon Dioxide Exchange of a Temperate Larch Forest. Agric. For. Meteorol. 2007, 147, 110–124. [Google Scholar] [CrossRef]
- Law, R.D.; Crafts-Brandner, S.J. Inhibition and Acclimation of Photosynthesis to Heat Stress Is Closely Correlated with Activation of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase. Plant Physiol. 1999, 120, 173–182. [Google Scholar] [CrossRef]
- Haldimann, P.; Feller, U. Inhibition of Photosynthesis by High Temperature in Oak (Quercus pubescens L.) Leaves Grown under Natural Conditions Closely Correlates with a Reversible Heat-Dependent Reduction of the Activation State of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase. Plant Cell Environ. 2004, 27, 1169–1183. [Google Scholar] [CrossRef]
- Familusi, I.; Gebremedhin, M.; Ries, I.; Brown, J.; Gyawali, B. The Productivity and Carbon Exchange of an Intensively Managed Pasture in Central Kentucky. Atmosphere 2024, 15, 348. [Google Scholar] [CrossRef]
- Thomas, M.V.; Malhi, Y.; Fenn, K.M.; Fisher, J.B.; Morecroft, M.D.; Lloyd, C.R.; Taylor, M.E.; McNeil, D.D. Carbon Dioxide Fluxes over an Ancient Broadleaved Deciduous Woodland in Southern England. Biogeosciences 2011, 8, 1595–1613. [Google Scholar] [CrossRef]
- Wilkinson, M.; Eaton, E.L.; Broadmeadow, M.S.J.; Morison, J.I.L. Inter-Annual Variation of Carbon Uptake by a Plantation Oak Woodland in South-Eastern England. Biogeosciences 2012, 9, 5373–5389. [Google Scholar] [CrossRef]
- Pilegaard, K.; Mikkelsen, T.N.; Beier, C.; Jensen, N.O.; Ambus, P.; Ro-Poulsen, H. Field Measurements of Atmosphere-Biosphere Interactions in a Danish Beech Forest. Boreal Environ. Res. 2003, 8, 315. [Google Scholar]
- Nakai, Y.; Kitamura, K.; Suzuki, S.; Abe, S. Year-Long Carbon Dioxide Exchange above a Broadleaf Deciduous Forest in Sapporo, Northern Japan. Tellus B Chem. Phys. Meteorol. 2003, 55, 305–312. [Google Scholar] [CrossRef]
- Shibata, H.; Hiura, T.; Tanaka, Y.; Takagi, K.; Koike, T. Carbon Cycling and Budget in a Forested Basin of Southwestern Hokkaido, Northern Japan. Ecol. Res. 2005, 20, 325–331. [Google Scholar] [CrossRef]
- Saigusa, N.; Yamamoto, S.; Murayama, S.; Kondo, H.; Nishimura, N. Gross Primary Production and Net Ecosystem Exchange of a Cool-Temperate Deciduous Forest Estimated by the Eddy Covariance Method. Agric. For. Meteorol. 2002, 112, 203–215. [Google Scholar] [CrossRef]
- Yasuda, Y.; Watanabe, T.; Ohtani, Y.; Okano, M.; Nakayama, K. Seasonal Variation of CO2 Flux over a Broadleaf Deciduous Forest. J. Jpn. Soc. Hydrol. Water Resour. 1998, 11, 575–585. [Google Scholar] [CrossRef][Green Version]
- Saigusa, N.; Yamamoto, S.; Murayama, S.; Kondo, H. Inter-Annual Variability of Carbon Budget Components in an AsiaFlux Forest Site Estimated by Long-Term Flux Measurements. Agric. For. Meteorol. 2005, 134, 4–16. [Google Scholar] [CrossRef]
- Yang, Y.; Li, P.; Ding, J.; Zhao, X.; Ma, W.; Ji, C.; Fang, J. Increased Topsoil Carbon Stock across China’s Forests. Glob. change Biol. 2014, 20, 2687–2696. [Google Scholar] [CrossRef]
- Durand, M.; Murchie, E.H.; Lindfors, A.V.; Urban, O.; Aphalo, P.J.; Robson, T.M. Diffuse Solar Radiation and Canopy Photosynthesis in a Changing Environment. Agric. For. Meteorol. 2021, 311, 108684. [Google Scholar] [CrossRef]
- Liu, L.; Gudmundsson, L.; Hauser, M.; Qin, D.; Li, S.; Seneviratne, S.I. Soil Moisture Dominates Dryness Stress on Ecosystem Production Globally. Nat. Commun. 2020, 11, 4892. [Google Scholar] [CrossRef]




| χ2 | df | p | ε2 | |
|---|---|---|---|---|
| NEE | 6766 | 3 | <0.001 | 0.0863 |
| RE | 33,970 | 3 | <0.001 | 0.4332 |
| GPP | 22,488 | 3 | <0.001 | 0.2868 |
| Year | NEE | RE | GPP | RE/GPP | Status |
|---|---|---|---|---|---|
| 2016 | −403 | 1353 | 1756 | 0.771 | Sink |
| 2017 | −364 | 1429 | 1793 | 0.797 | Sink |
| 2018 | −144 | 1259 | 1403 | 0.897 | Sink |
| 2019 | −254 | 1617 | 1871 | 0.864 | Sink |
| 2020 | −350 | 1212 | 1562 | 0.776 | Sink |
| Total (Mean) | −1515 (−303) | 6870 (1374) | 8385 (1677) | Sink |
| Month | Amax | α | Re (gCm−2) | Total NEE (gCm−2) | Mean NEE (gCm−2) | Mean PPFD (µMol/m2) | n | R2 (%) |
|---|---|---|---|---|---|---|---|---|
| January | 0.000 | 0.627 | 0.017 | 8.812 | 0.017 | 494.87 | 509 | 0.00 |
| February | 0.000 | 0.588 | 0.002 | 1.341 | 0.002 | 505.95 | 556 | 0.00 |
| March | 0.000 | 0.744 | 0.025 | 16.672 | 0.025 | 680.87 | 677 | 43.01 |
| April | 1.511 | 0.617 | 1.507 | 4.093 | 0.006 | 839.59 | 726 | 38.18 |
| May | 0.583 | 0.001 | 0.044 | −169.581 | −0.213 | 792.08 | 796 | 87.29 |
| June | 2.656 | 1.386 | 3.431 | −175.644 | −0.216 | 986.26 | 814 | 45.07 |
| July | 3.205 | 1.304 | 2.947 | −191.990 | −0.234 | 865.02 | 822 | 39.21 |
| August | 1.736 | 0.676 | 1.548 | −134.826 | −0.174 | 816.94 | 776 | 39.50 |
| September | 1.246 | 0.699 | 1.106 | −91.443 | −0.134 | 859.99 | 682 | 44.73 |
| October | 0.222 | 1.769 | 0.175 | −30.087 | −0.047 | 693.53 | 639 | 46.59 |
| November | 0.672 | 1.286 | 0.691 | 10.995 | 0.020 | 557.66 | 542 | 45.81 |
| December | 0.000 | 0.572 | 0.018 | 8.943 | 0.018 | 380.27 | 498 | 49.87 |
| β0 | β1 | Q10 raw data | Q10 modeled | Total RE (gCm−2) | Mean RE (gCm−2) | Mean Tair (°C) | R2 (%) | n | |
|---|---|---|---|---|---|---|---|---|---|
| Jan | 0.010 | 0.060 | 2.296 | 1.825 | 8.837 | 0.009 | −2.06 | 92.67 | 977 |
| Feb | 0.011 | 0.010 | 1.914 | 1.108 | 8.110 | 0.010 | 2.71 | 99.82 | 836 |
| Mar | 0.010 | 0.039 | 1.513 | 1.475 | 12.313 | 0.015 | 8.49 | 98.05 | 811 |
| Apr | 0.018 | 0.073 | 3.083 | 2.083 | 30.468 | 0.043 | 10.81 | 91.90 | 714 |
| May | 0.056 | 0.034 | 1.474 | 1.410 | 62.458 | 0.090 | 13.54 | 99.31 | 693 |
| Jun | 0.026 | 0.061 | 1.766 | 1.834 | 53.504 | 0.085 | 19.14 | 97.12 | 626 |
| Jul | 0.048 | 0.064 | 2.223 | 1.895 | 126.243 | 0.190 | 21.43 | 94.10 | 666 |
| Aug | 0.037 | 0.066 | 2.121 | 1.944 | 111.418 | 0.156 | 21.75 | 98.80 | 712 |
| Sep | 0.052 | 0.044 | 1.598 | 1.556 | 86.482 | 0.114 | 17.53 | 98.74 | 758 |
| Oct | 0.051 | 0.037 | 1.665 | 1.447 | 69.696 | 0.082 | 12.47 | 98.57 | 849 |
| Nov | 0.019 | 0.060 | 1.799 | 1.817 | 26.964 | 0.030 | 6.22 | 95.23 | 898 |
| Dec | 0.014 | 0.037 | 2.190 | 1.445 | 16.092 | 0.016 | 2.77 | 97.35 | 990 |
| Q10 modeled | Total RE (gCm−2) | Mean RE (gCm−2) | Mean Tair (°C) | Total GPP | Mean GPP | n | |
|---|---|---|---|---|---|---|---|
| 2016 | 2.697 | 612.57 | 0.064 | 10.30 | 1483.40 | 0.185 | 9530 |
| 2017 | 1.881 | 674.82 | 0.071 | 10.67 | 1416.17 | 0.176 | 9473 |
| 2018 | 2.002 | 620.09 | 0.092 | 10.19 | 1212.59 | 0.226 | 6770 |
| 2019 | 1.992 | 774.86 | 0.081 | 16.94 | 1592.45 | 0.199 | 9519 |
| 2020 | 2.046 | 533.33 | 0.073 | 16.12 | 921.51 | 0.185 | 7267 |
| NEE | RE | GPP | |
|---|---|---|---|
| LE | −0.58 ** | 0.32 ** | 0.61 ** |
| VPD | −0.16 ** | 0.19 ** | 0.21 ** |
| Tair | −0.30 ** | 0.60 ** | 0.48 ** |
| ET | −0.48 ** | 0.24 ** | 0.50 ** |
| PPFD | −0.39 ** | 0.25 ** | 0.42 ** |
| H | −0.22 ** | 0.00 | 0.19 ** |
| Season | NEE | Reco | GPP | |
|---|---|---|---|---|
| (a) winter | LE | 0.19 ** | 0.02 ** | −0.19 ** |
| VPD | 0.06 ** | 0.24 ** | −0.01 | |
| Tair | 0.04 ** | 0.49 ** | 0.06 ** | |
| ET | 0.27 ** | 0.03 ** | −0.26 ** | |
| PPFD | −0.08 ** | 0.09 ** | 0.10 ** | |
| H | −0.01 | −0.01 | 0.01 | |
| (b) spring | LE | −0.51 ** | 0.35 ** | 0.55 ** |
| VPD | −0.06 ** | 0.22 ** | 0.13 ** | |
| Tair | −0.21 ** | 0.57 ** | 0.36 ** | |
| ET | −0.38 ** | 0.27 ** | 0.42 ** | |
| PPFD | −0.29 ** | 0.19 ** | 0.31 ** | |
| H | −0.20 ** | −0.01 | 0.17 ** | |
| (c) summer | LE | −0.67 ** | 0.08 ** | 0.65 ** |
| VPD | −0.25 ** | 0.16 ** | 0.29 ** | |
| Tair | −0.28 ** | 0.23 ** | 0.34 ** | |
| ET | −0.59 ** | 0.07 ** | 0.58 ** | |
| PPFD | −0.43 ** | 0.12 ** | 0.45 ** | |
| H | −0.58 ** | 0.06 ** | 0.57 ** | |
| (d) fall | LE | −0.47 ** | 0.21 ** | 0.49 ** |
| VPD | −0.30 ** | 0.12 ** | 0.30 ** | |
| Tair | −0.28 ** | 0.45 ** | 0.44 ** | |
| ET | −0.41 ** | 0.16 ** | 0.42 ** | |
| PPFD | −0.37 ** | 0.17 ** | 0.39 ** | |
| H | −0.24 ** | −0.01 | 0.20 ** |
| Country | Forest Type | NEE (g C yr−1) | GPP (g C yr−1) | RE (g C yr−1) | Study Years | Location | Reference |
|---|---|---|---|---|---|---|---|
| USA | Mostly Deciduous | −303 | 1535 | 1374 | 2016–2020 | 37°8′17.16″ N, 83°34′46.82″ W | This study |
| “ | Deciduous broadleaf | −250 | 1400 | 1150 | - | 42°32′24″ N, 72°10′12″ W | [40] |
| “ | Mixed deciduous | −198 | 1297 | 1099 | 1993–2000 | 42.5 N, 72.2 W | [35] |
| “ | “ | −247 | 1400 | 1153 | 1992–2004 | 42.538 N, 72.171 W | [40] |
| Canada | Mixed deciduous & coniferous | −142 | 1118 | 976 | 1996–2003 | 44°19′ N, 79°56′ W | [39] |
| Brazil | Dry tropical | −168.7 | 414.7 | 246 | 2014 | 6°34′42″ S, 37°15′05″ W | [11] |
| “ | “ | −145 | 334 | 189 | 2015 | “ | “ |
| Iceland | Managed deciduous broadleaf | −100 | 710 | 610 | - | 63°49′48″ N, 20°13′12″ W | [63] |
| Denmark | “ | −150 | 1190 | 1040 | - | 55°28′48″ N, 11°38′24″ E | “ |
| UK | Deciduous | −130 | 2110 | 1980 | 2007–2009 | 51°46′12″ N, 1°19′48″ W | [71] |
| “ | Deciduous broadleaf | −486 | 2034 | 1548 | 1999–2010 | 51°07′12″ N, 0°51′00″ W | [72] |
| Germany | Deciduous broadleaf | −490 | 1560 | 1070 | - | 51°04′12″ N, 10°27′00″ E | [60] |
| Italy | Deciduous broadleaf | −660 | 1300 | 640 | - | 41°52′12″ N, 13°35′24″ E | [63] |
| Denmark | European beech | −157 | 1727 | 1570 | 1996–2009 | 55°29′13′′ N, 11°38′45′′ E | [73] |
| Japan | Deciduous broadleaf | −261 | 1118 | 857 | 2000 | 42°58′48″ N, 141°22′48″ E | [74] |
| “ | “ | −258 | - | - | 1999–2001 | 42°40′12″ N, 141°36′00″ E | [75] |
| “ | “ | −207 | 948 | 741 | - | 36°07′48″ N, 137°25′12″ E | [76] |
| “ | “ | −357 | - | - | 1997 | 35°52′12″ N, 139°28′48″ E | [77] |
| “ | Deciduous | −236 | 978 | 742 | 1994–2002 | 36°08′ N, 137°25′ E | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Familusi, I.; Gebremedhin, M.; Gyawali, B.; Chiluwal, A.; Brotzge, J. A Deciduous Forest’s CO2 Exchange Within the Mixed-Humid Climate of Kentucky, USA. Forests 2025, 16, 562. https://doi.org/10.3390/f16040562
Familusi I, Gebremedhin M, Gyawali B, Chiluwal A, Brotzge J. A Deciduous Forest’s CO2 Exchange Within the Mixed-Humid Climate of Kentucky, USA. Forests. 2025; 16(4):562. https://doi.org/10.3390/f16040562
Chicago/Turabian StyleFamilusi, Ife, Maheteme Gebremedhin, Buddhi Gyawali, Anuj Chiluwal, and Jerald Brotzge. 2025. "A Deciduous Forest’s CO2 Exchange Within the Mixed-Humid Climate of Kentucky, USA" Forests 16, no. 4: 562. https://doi.org/10.3390/f16040562
APA StyleFamilusi, I., Gebremedhin, M., Gyawali, B., Chiluwal, A., & Brotzge, J. (2025). A Deciduous Forest’s CO2 Exchange Within the Mixed-Humid Climate of Kentucky, USA. Forests, 16(4), 562. https://doi.org/10.3390/f16040562







