A Study on the Water Consumption Characteristics of Fraxinus pennsylvanica Marshall During the Growing and Non-Growing Seasons and Their Response to Microclimate Variables
Abstract
1. Introduction
2. Material and Methods
2.1. Sites and Species
2.2. Sap Flow Measurement
2.3. Determination of Environmental Factors
2.4. Data Analysis and Processing
3. Results
3.1. Characteristics of Sap Flow
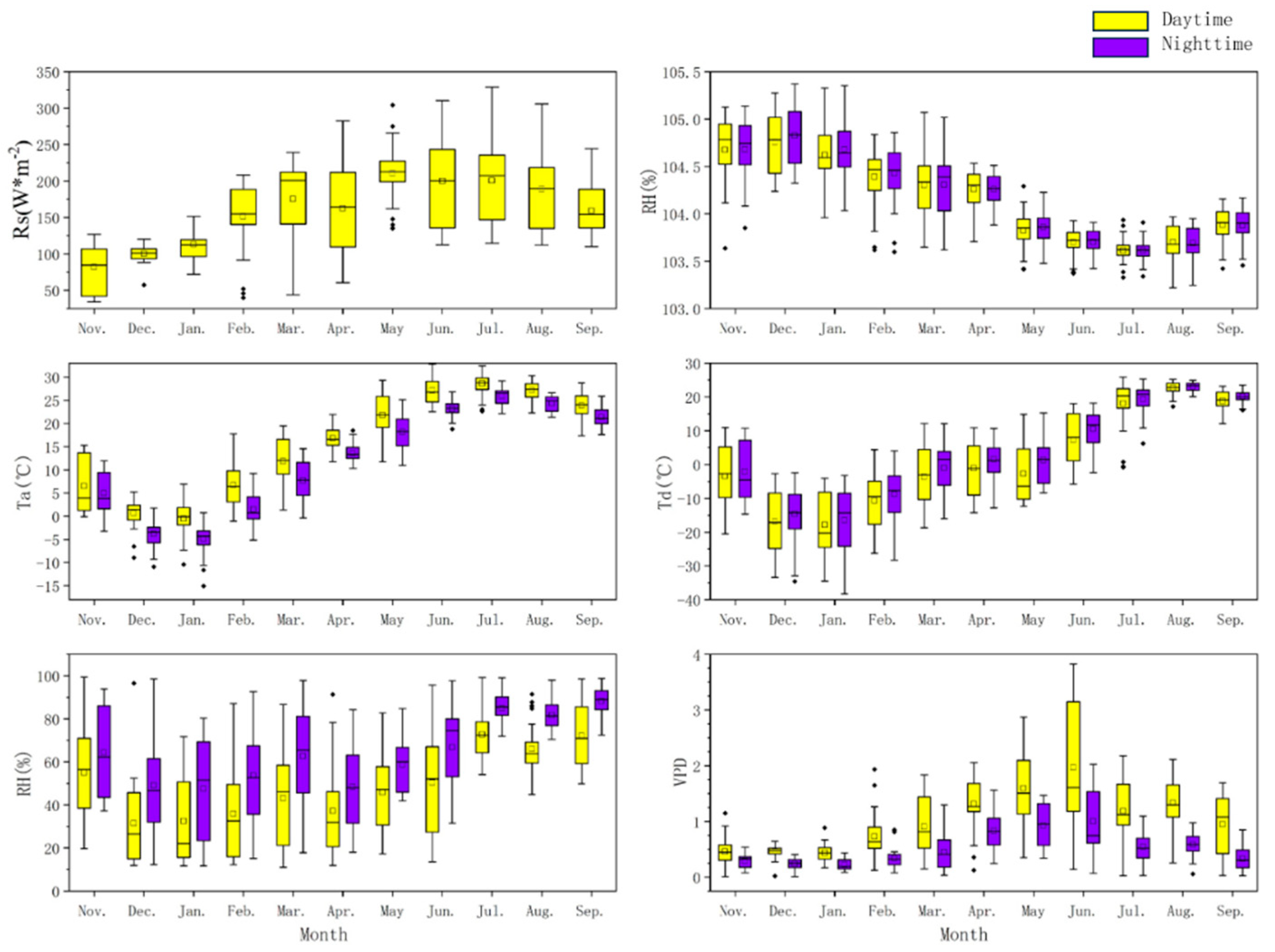
3.2. Meteorological Factor Characteristics
3.3. The Relationship Between Sap Flow and Meteorological Factors
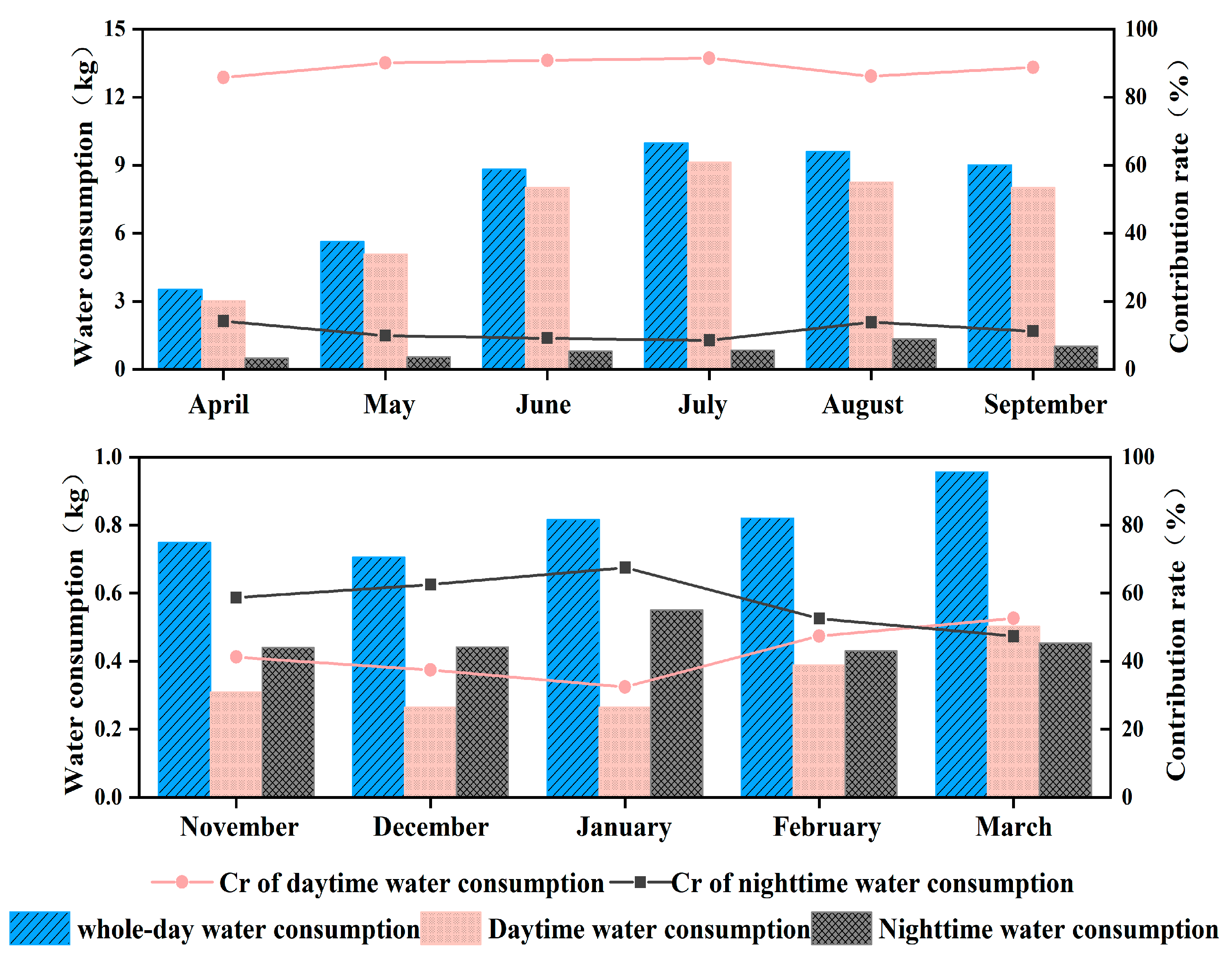
3.4. Daytime and Nighttime Water Consumption Characteristics
3.5. Daytime and Nighttime Water Consumption Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Period | Average Sap Flow Velocity at Different Scales (cm/h) | ||
|---|---|---|---|
| Whole Day | Daytime | Nighttime | |
| April | 2.63 | 4.59 | 0.68 |
| May | 3.47 | 6.12 | 0.77 |
| June | 5.03 | 7.62 | 1.41 |
| July | 5.39 | 8.30 | 1.32 |
| August | 5.35 | 7.96 | 1.68 |
| September | 5.17 | 8.08 | 1.73 |
| November | 0.41 | 0.37 | 0.44 |
| December | 0.38 | 0.31 | 0.44 |
| January | 0.44 | 0.31 | 0.55 |
| February | 0.46 | 0.42 | 0.49 |
| March | 0.49 | 0.45 | 0.52 |
| Period | Number of Individual Cases | Period | Number of Individual Cases |
|---|---|---|---|
| April | 20 | November | 19 |
| May | 18 | December | 25 |
| June | 17 | January | 14 |
| July | 23 | February | 11 |
| August | 21 | March | 20 |
| September | 15 | ||
| Growing season | 114 | Non-growing season | 89 |
References
- Ghimire, C.P.; van Meerveld, H.J.; Zwartendijk, B.W.; Bruijnzeel, L.A.; Ravelona, M.; Lahitiana, J.; Lubczynski, M.W. Vapour pressure deficit and solar radiation are the major drivers of transpiration in montane tropical secondary forests in eastern Madagascar. Agric. For. Meteorol. 2022, 326, 109159. [Google Scholar] [CrossRef]
- Jasechko, S.; Sharp, Z.D.; Gibson, J.J.; Birks, S.J.; Yi, Y.; Fawcett, P.J. Terrestrial water fluxes dominated by transpiration. Nature 2013, 496, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Jiao, L.; Lu, N.; Li, M.; Li, Z.; Keyimu, M.; Zhang, L.; Wang, H. Disentangling the independent and interacting impacts of biophysical factors on the transpiration of a black locust (Robinia pseudoacacia) plantation in the semiarid Loess Plateau, China. Land Degrad. Dev. 2022, 34, 1767–1777. [Google Scholar] [CrossRef]
- Maurel, C.; Verdoucq, L.; Rodrigues, O. Aquaporins and plant transpiration. Plant Cell Environ. 2016, 39, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Guo, J.; Liu, Z.; Wang, Y.; Xu, L.; Yu, P.; He, L. Impacts of environmental and canopy conditions on the nighttime sap flow of larch plantations in the Liupan Mountains, China. J. For. Res. 2023, 34, 1927–1940. [Google Scholar] [CrossRef]
- Tie, Q.; Hu, H.; Tian, F.; Guan, H.; Lin, H. Environmental and physiological controls on sap flow in a subhumid mountainous catchment in North China. Agric. For. Meteorol. 2017, 240–241, 46–57. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Sun, Y.; Li, K.; Zhang, C. Sap flow characteristics of three afforestation species during the wet and dry seasons in a dry-hot valley in Southwest China. J. For. Res. 2017, 28, 51–62. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, Y.; Yin, L.; Wenninger, J.; Zhang, J.; Hou, G.; Zhang, E.; Uhlenbrook, S. Climatic controls on sap flow dynamics and used water sources of Salix psammophila in a semi-arid environment in northwest China. Environ. Earth Sci. 2014, 73, 289–301. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, J.; Shi, W.; Yamanaka, N.; Du, S. Interannual variation in stand transpiration estimated by sap flow measurement in a semi-arid black locust plantation, Loess Plateau, China. Ecohydrology 2014, 8, 137–147. [Google Scholar] [CrossRef]
- Fang, W.; Lv, N.; Fu, B. Research advances in nighttime sap flow density, its physiological implications, and influencing factors in plants. Acta Ecol. Sin. 2018, 38, 7521–7529. [Google Scholar]
- Fu, Z.Q.; Hu, X.; Tian, Q.R.; Ge, Y.L.; Zhou, H.J.; Wu, X.Y.; Chen, L.X. Nocturnal sap flow characteristics of two typical forest tree species and responses to environmental factors in the loess region of West Shanxi, China. Chin. J. Plant Ecol. 2024, 48, 1128–1142. [Google Scholar]
- Yi, L.; Huang, J.; Wang, X.; Dong, L.; Ma, H.; Zhang, J. Characteristics of night time sap flow of Salix matsudana and Populus simonii in Yulin, Shaanxi. J. Northwest A F Univ. (Nat. Sci. Ed.) 2013, 41, 85–90. [Google Scholar]
- Zhu, H.; Li, H.; Wang, X.; Jiang, T.; Sun, L.; Luo, Y. Water use characteristics of the artificial forests black locust (Robinia pseudoacacia) and Chinese pine (Pinus tabulaeformis) on the Loess Plateau of China. Ecohydrology 2023, 16, e2526. [Google Scholar] [CrossRef]
- Chan, A.M.; Bowling, D.R. Assessing the thermal dissipation sap flux density method for monitoring cold season water transport in seasonally snow-covered forests. Tree Physiol. 2017, 37, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Taneda, H.; Tateno, M. Hydraulic conductivity, photosynthesis and leaf water balance in six evergreen woody species from fall to winter. Tree Physiol. 2005, 25, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Liu, Z.; Xiong, K.; Li, Y.; Li, K.; Yu, X. Influence of Environmental Factors on the Sap Flow Activity of the Golden Pear in the Growth Period of Karst Area in Southern China. Water 2022, 14, 1707. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Zhu, J.; Gong, L.; Xu, L.; Jin, G.; Li, J.; Hauer, R.; Xu, C. Nocturnal sap flow is mainly caused by stem refilling rather than nocturnal transpiration for Acer truncatum in urban environment. Urban For. Urban Green. 2020, 56, 126800. [Google Scholar] [CrossRef]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Buras, A.; Schunk, C.; Zeiträg, C.; Herrmann, C.; Kaiser, L.; Lemme, H.; Straub, C.; Taeger, S.; Gößwein, S.; Klemmt, H.J.; et al. Are Scots pine forest edges particularly prone to drought-induced mortality? Environ. Res. Lett. 2018, 13, 025001. [Google Scholar] [CrossRef]
- Dang, H.; Han, H.; Chen, S.; Li, M. A fragile soil moisture environment exacerbates the climate change-related impacts on the water use by Mongolian Scots pine (Pinus sylvestris var. mongolica) in northern China: Long-term observations. Agric. Water Manag. 2021, 251, 106857. [Google Scholar] [CrossRef]
- He, C. Characteristics of Agrometeorological Conditions in Xiqing District of Tianjin in Recent 30 Years. J. Agric. Catastrophology 2019, 9, 23–24. [Google Scholar]
- Feng, R.; Zhao, J.; Zhang, F.; Wang, J.; Wei, J.; Liu, J. Transcriptomic changes in response of Fraxinus velutina Torr phloem to Agrilus planipennis Fairmaire hazards. For. Res. 2021, 34, 47–55. [Google Scholar]
- Moon, M.; Kim, T.; Park, J.; Cho, S.; Ryu, D.; Kim, H.S. Variation in sap flux density and its effect on stand transpiration estimates of Korean pine stands. J. For. Res. 2015, 20, 85–93. [Google Scholar] [CrossRef]
- Berry, Z.C.; Gotsch, S.G.; Holwerda, F.; Muñoz-Villers, L.E.; Asbjornsen, H. Slope position influences vegetation-atmosphere interactions in a tropical montane cloud forest. Agric. For. Meteorol. 2016, 221, 207–218. [Google Scholar] [CrossRef]
- Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, S.; Ke, J.; Zhu, A. Effects of meteorological factors and groundwater depths on plant sap flow velocities in karst critical zone. Sci. Total Environ. 2021, 781, 146764. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, P.; Zhu, L.; Wang, Q.; Hu, Y.; Cranston, B.M.; Kaplick, J.; Lei, O.; Chen, X.; Ni, G.; et al. Exploring the Influence of Biological Traits and Environmental Drivers on Water Use Variations across Contrasting Forests. Forests 2021, 12, 161. [Google Scholar] [CrossRef]
- Lyu, J.; He, Q.-Y.; Yang, J.; Chen, Q.-W.; Cheng, R.-R.; Yan, M.-J.; Yamanaka, N.; Du, S. Sap flow characteristics in growing and non-growing seasons in three tree species in the semiarid Loess Plateau region of China. Trees 2020, 34, 943–955. [Google Scholar] [CrossRef]
- Lu, Z.; Wei, Y.; Li, Z.; Guo, X.; Zhou, Y. Characteristics of sap flow and its influencing factors of Pinus sylvestris var. Characteristics of sap flow and its influencing factors of Pinus sylvestris var. mongolica in sandy land of Northwest Liaoning. Chin. J. Ecol. 2017, 36, 3182–3189. [Google Scholar]
- Di, N.; Yang, S.; Liu, Y.; Fan, Y.; Duan, J.; Nadezhdina, N.; Li, X.; Xi, B. Soil-Moisture-Dependent Nocturnal Water Use Strategy and its Responses to Meteorological Factors in a Seasonal-Arid Poplar Plantation. SSRN Electron. J. 2022, 274, 107984. [Google Scholar]
- Novick, K.A.; Oren, R.; Stoy, P.C.; Siqueira, M.B.S.; Katul, G.G. Nocturnal evapotranspiration in eddy-covariance records from three co-located ecosystems in the Southeastern U.S.: Implications for annual fluxes. Agric. For. Meteorol. 2009, 149, 1491–1504. [Google Scholar] [CrossRef]

| Tree Number | Age | Height(m) | Stem Diameter (cm) | Crown Diameter (m) | Sapwood Area (cm2) |
|---|---|---|---|---|---|
| 1 | 15 | 9.30 | 15.60 | 6.17 | 160.86 |
| 2 | 8 | 6.90 | 10.20 | 4.68 | 76.40 |
| 3 | 30 | 10.40 | 27.20 | 8.73 | 517.19 |
| 4 | 17 | 8.10 | 16.70 | 7.35 | 177.42 |
| Period | Ta | RH | VPD | p | Td | Rs | |
|---|---|---|---|---|---|---|---|
| April | Daytime | 0.633 ** | −0.371 ** | 0.544 ** | −0.068 | −0.456 * | 0.277 ** |
| Nighttime | 0.273 ** | −0.237 ** | 0.354 ** | −0.201 ** | −0.169 ** | ||
| May | Daytime | 0.399 ** | −0.441 ** | 0.439 ** | −0.020 | −0.121 * | 0.459 ** |
| Nighttime | 0.468 ** | −0.491 ** | 0.637 ** | −0.142 | −0.152 * | ||
| June | Daytime | 0.597 ** | −0.558 ** | 0.517 ** | −0.200 * | −0.082 | 0.432 ** |
| Nighttime | 0.616 ** | −0.586 ** | 0.653 ** | −0.054 | −0.305 * | ||
| July | Daytime | 0.851 ** | −0.731 ** | 0.752 ** | −0.055 | −0.024 | 0.508 ** |
| Nighttime | 0.567 ** | −0.325 ** | 0.381 ** | −0.081 | −0.107 | ||
| August | Daytime | 0.781 ** | −0.751 ** | 0.747 ** | −0.113 | −0.390 * | 0.476 ** |
| Nighttime | 0.408 ** | −0.508 ** | 0.562 ** | −0.108 | −0.308 ** | ||
| September | Daytime | 0.751 ** | −0.745 ** | 0.701 ** | −0.024 | −0.382 * | 0.504 ** |
| Nighttime | 0.746 ** | −0.924 ** | 0.707 ** | −0.689 * | −0.593 ** | ||
| Growing season | Daytime | 0.78 ** | −0.25 ** | 0.55 ** | 0.09 | 0.20 ** | 0.38 ** |
| Nighttime | 0.58 ** | −0.03 | 0.31 ** | 0.02 | 0.21 ** | ||
| November | Daytime | −0.269 ** | −0.008 | −0.073 | 0.306 * | −0.245 | −0.366 ** |
| Nighttime | −0.242 ** | −0.329 ** | −0.307 * | 0.320 * | −0.340 * | ||
| December | Daytime | −0.220 | −0.243 * | −0.300 * | 0.299 * | −0.104 | −0.145 |
| Nighttime | −0.293 ** | −0.264 * | −0.224 * | 0.311 * | −0.182 * | ||
| January | Daytime | 0.034 | −0.237 * | 0.253 * | 0.201 | −0.177 | −0.068 |
| Nighttime | −0.069 | −0.249 * | 0.301 * | −0.027 | −0.193 * | ||
| February | Daytime | 0.266 ** | −0.318 ** | 0.412 ** | −0.137 | −0.260 | 0.127 |
| Nighttime | 0.306 ** | −0.351 ** | 0.401 ** | −0.040 | −0.099 | ||
| March | Daytime | 0.594 ** | −0.520 ** | 0.501 ** | −0.306 * | −0.351 * | 0.359 * |
| Nighttime | 0.314 ** | −0.407 ** | 0.420 ** | −0.011 * | −0.322 ** | ||
| Non-growing season | Daytime | −0.31 ** | −0.35 ** | 0.13 | 0.10 | −0.30 ** | 0.01 |
| Nighttime | −0.49 ** | −0.46 ** | 0.43 ** | 0.16 | −0.14 |
| Period | Correlation | Regression Equation |
|---|---|---|
| April | 0.584 ** | |
| May | 0.536 ** | |
| June | 0.722 ** | |
| July | 0.707 ** | |
| August | 0.699 ** | |
| September | 0.568 ** | |
| Growing season | 0.663 ** | |
| November | 0.407 ** | |
| December | 0.577 * | |
| January | 0.471 | |
| February | 0.741 * | |
| March | 0.463 ** | |
| Non-growing season | 0.364 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Su, Y.; Liu, F.; Zhang, Y.; Wu, H. A Study on the Water Consumption Characteristics of Fraxinus pennsylvanica Marshall During the Growing and Non-Growing Seasons and Their Response to Microclimate Variables. Forests 2025, 16, 401. https://doi.org/10.3390/f16030401
Han Y, Su Y, Liu F, Zhang Y, Wu H. A Study on the Water Consumption Characteristics of Fraxinus pennsylvanica Marshall During the Growing and Non-Growing Seasons and Their Response to Microclimate Variables. Forests. 2025; 16(3):401. https://doi.org/10.3390/f16030401
Chicago/Turabian StyleHan, Yuehao, Yu Su, Fude Liu, Yan Zhang, and Hailong Wu. 2025. "A Study on the Water Consumption Characteristics of Fraxinus pennsylvanica Marshall During the Growing and Non-Growing Seasons and Their Response to Microclimate Variables" Forests 16, no. 3: 401. https://doi.org/10.3390/f16030401
APA StyleHan, Y., Su, Y., Liu, F., Zhang, Y., & Wu, H. (2025). A Study on the Water Consumption Characteristics of Fraxinus pennsylvanica Marshall During the Growing and Non-Growing Seasons and Their Response to Microclimate Variables. Forests, 16(3), 401. https://doi.org/10.3390/f16030401






