Carbon and Nitrogen Content and CO2 Efflux from Coarse Woody Debris of Norway Spruce, Black Alder, and Silver Birch
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sampling of Experimental Material
2.3. CO2 Measurements and Sample Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Physical and Chemical Properties
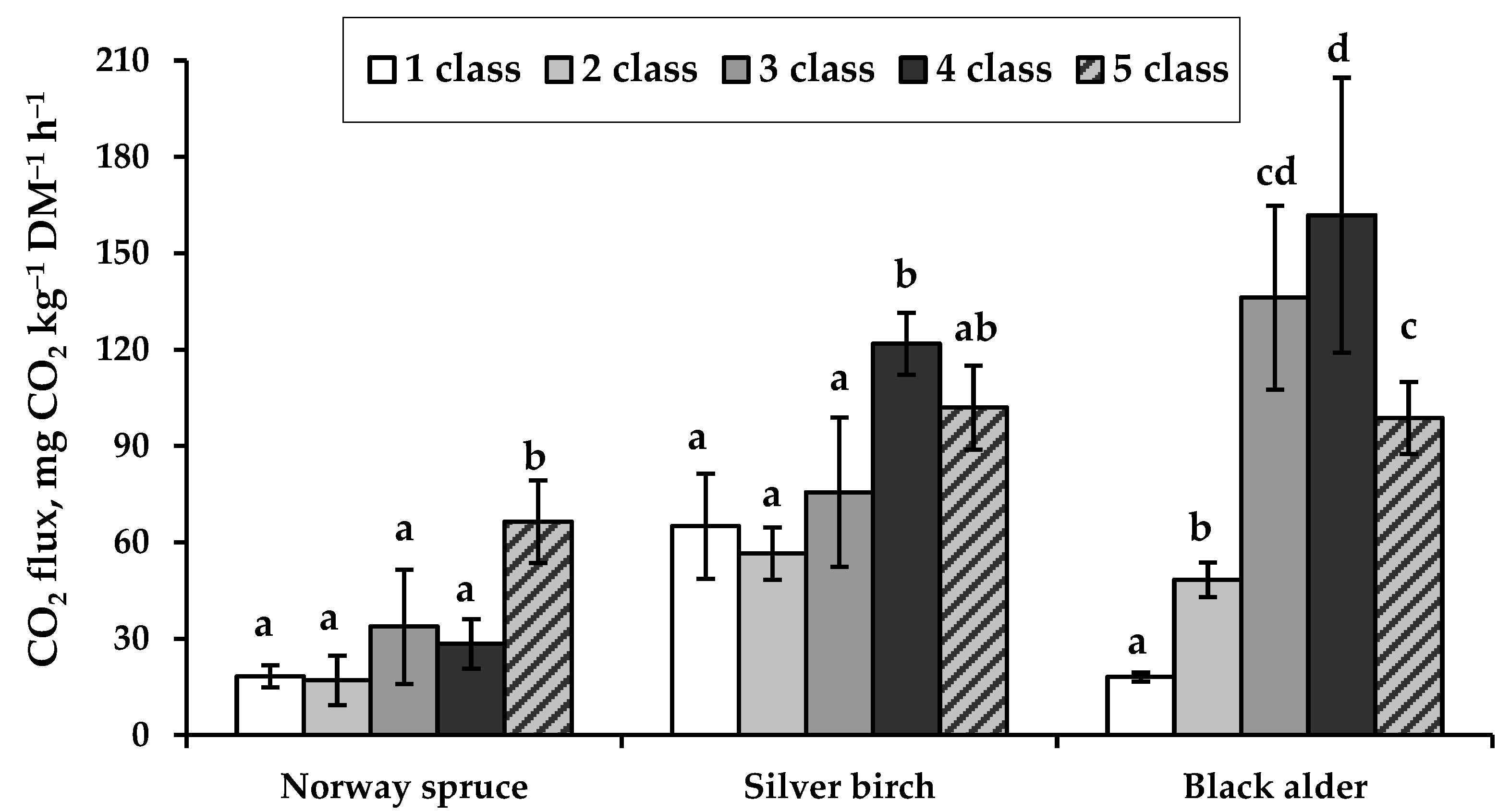
3.2. CO2 Efflux from Coarse Woody Debris
3.3. Relations Among CWD Properties and CO2 Efflux
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bastrup-Birk, A.; Neville, P.; Chirici, G.; Houston, T. The Biosoil Forest Biodiversity Field Manual; ICP Forests: Hamburg, Germany, 2007; Available online: https://www.icp-forests.org/pdf/manual/BioSoil/MANUALForestFocus_Biosoil_FieldManual_v1_0-1_1-1_1A_2006.pdf (accessed on 27 December 2024).
- Herrmann, S.; Bauhus, J. Nutrient retention and release in coarse woody debris of three important central European tree species and the use of NIRS to determine deadwood chemical properties. For. Ecosyst. 2018, 5, 22. [Google Scholar] [CrossRef]
- Sena, K.L.; Flynn, J.K.; Leuenberger, W.; Kolka, R.; Barton, C.D. Long-term changes in coarse woody debris abundance in three Appalachian headwater streams with differing best management practices. Front. For. Glob. Chang. 2023, 6, 1242878. [Google Scholar] [CrossRef]
- Khanina, L.; Bobrovsky, M.; Smirnov, V.; Romanov, M. Wood decomposition, carbon, nitrogen, and pH values in logs of 8 tree species 14 and 15 years after a catastrophic windthrow in a mesic broad-leaved forest in the East European plain. For. Ecol. Manag. 2023, 545, 121275. [Google Scholar] [CrossRef]
- Forzieri, G.; Girardello, M.; Ceccherini, G.; Spinoni, J.; Feyen, L.; Hartmann, H.; Beck, P.S.A.; Camps-Valls, G.; Chirici, G.; Mauri, A.; et al. Emergent vulnerability to climate-driven disturbances in European forests. Nat. Commun. 2021, 12, 1081. [Google Scholar] [CrossRef] [PubMed]
- Patacca, M.; Lindner, M.; Lucas-Borja, M.E.; Cordonnier, T.; Fidej, G.; Gardiner, B.; Hauf, Y.; Jasinevičius, G.; Labonne, S.; Linkevičius, E.; et al. Significant Increase in Natural Disturbance Impacts on European Forests since 1950. Glob. Chang. Biol. 2023, 29, 1359–1376. [Google Scholar] [CrossRef]
- Puletti, N.; Canullo, R.; Mattioli, W.; Gawryś, R.; Corona, P.; Czerepko, J. A dataset of forest volume deadwood estimates for Europe. Ann. For. Sci. 2019, 76, 68. [Google Scholar] [CrossRef]
- State Forest Service. Lietuvos Miškų Valstybinė Apskaita, 2024-01-01 State Inventory of Lithuanian Forests as of January 1, 2024. Available online: https://amvmt.lrv.lt/lt/atviri-duomenys-1/misku-statistikos-leidiniai/valstybine-misku-apskaita/20240101/ (accessed on 7 January 2025).
- Eggleston, H.S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. (Eds.) IPCC Guidelines for National Greenhouse Gas Inventories. In The National Greenhouse Gas Inventories Programme, The Intergovernmental Panel on Climate Change; IPCC: Hayama, Japan, 2006. [Google Scholar]
- Barbosa, R.I.; de Castilho, C.V.; de Oliveira Perdiz, R.; Damasco, G.; Rodrigues, R.; Fearnside, P.M. Decomposition rates of coarse woody debris in undisturbed Amazonian seasonally flooded and unflooded forests in the Rio Negro-Rio Branco basin in Roraima, Brazil. For. Ecol. Manag. 2017, 397, 1–9. [Google Scholar] [CrossRef]
- Dai, Z.; Trettin, C.C.; Burton, A.J.; Jurgensen, M.F.; Page-Dumroese, D.S.; Forschler, B.T.; Schilling, J.S.; Lindner, D.L. Coarse woody debris decomposition assessment tool: Model development and sensitivity analysis. PLoS ONE 2021, 16, e0251893. [Google Scholar] [CrossRef] [PubMed]
- Krankina, O.N.; Harmon, M.E. Dynamics of the dead wood carbon pool in northwestern Russian boreal forests. Water Air Soil Pollut. 1995, 82, 227–238. [Google Scholar] [CrossRef]
- Harmon, M.E.; Krankina, O.N.; Sexton, J. Decomposition vectors: A new approach to estimating woody detritus decomposition dynamics. Can. J. For. Res. 2000, 30, 76–84. [Google Scholar] [CrossRef]
- Yatskov, M.; Harmon, M.E.; Krankina, O.N. A chronosequence of wood decomposition in the boreal forests of Russia. Can. J. For. Res. 2003, 33, 1211–1226. [Google Scholar] [CrossRef]
- Mäkinen, H.; Hynynen, J.; Siitonen, J.; Sievänen, R. Predicting the decomposition of Scots pine, Norway spruce, and birch stems in Finland. Ecol. Appl. 2006, 16, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Palviainen, M.; Finér, L. Decomposition and nutrient release from Norway spruce coarse roots and stumps–A 40-year chronosequence study. For. Ecol. Manag. 2015, 358, 1–11. [Google Scholar] [CrossRef]
- Shorohova, E.; Kapitsa, E. The decomposition rate of non-stem components of coarse woody debris (CWD) in European boreal forests mainly depends on site moisture and tree species. Eur. J. For. Res. 2016, 135, 593–606. [Google Scholar] [CrossRef]
- Mukhortova, L.; Pashenova, N.; Meteleva, M.; Krivobokov, L.; Guggenberger, G. Temperature sensitivity of CO2 and CH4 fluxes from coarse woody debris in Northern Boreal forests. Forests 2021, 12, 624. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Sass-Klaassen, U.; Poorter, L.; Van Geffen, K.; Van Logtestijn, R.S.P.; Van Hal, J.; Goudzwaard, L.; Sterck, F.J.; Klaassen, R.K.W.M.; Freschet, G.T.; et al. Controls on coarse wood decay in temperate tree species: Birth of the LOGLIFE Experiment. Ambio 2012, 41, 231–245. [Google Scholar] [CrossRef]
- Taminskas, J.; Pileckas, M.; Šimanauskiene, R.; Linkeviciene, R. Lietuvos šlapynės: Klasifikacija ir sklaida Wetlands of Lithuania: Classification and distribution. Baltica 2011, 24, 151–162. [Google Scholar]
- Valatka, S.; Stoškus, A.; Pileckis, M. Lietuvos durpynai: Kiek jų turime, ar racionaliai naudojame? In Lithuanian Peatlands: How Many Do We Have, and Are We Using Them Rationally? Gamtos paveldo fondas: Vilnius, Lithuania, 2018; 91p. [Google Scholar]
- Vigricas, E.; Čiuldienė, D.; Armolaitis, K.; Valujeva, K.; Laiho, R.; Jauhiainen, J.; Schindler, T.; Bārdule, A.; Lazdiņš, A.; Butlers, A.; et al. Total soil CO2 efflux from drained Terric Histosols. Plants 2024, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- LHMT Lithuanian Hydrometeorological Service. Standard Climate Normals. Available online: https://www.meteo.lt/en/climate/lithuanian-climate/standard-climate-normals/ (accessed on 28 December 2024).
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Hunter, M.L., Jr. Wildlife, Forests, and Forestry: Principles of Managing Forests For Biological Diversity; Prentice Hall: Englewood Cliffs, NJ, USA, 1990; 370p. [Google Scholar]
- Weggler, K.; Dobbertin, M.; Jüngling, E.; Kaufmann, E.; Thürig, E. Dead wood volume to dead wood carbon: The issue of conversion factors. Eur. J. For. Res. 2012, 131, 1423–1438. [Google Scholar] [CrossRef]
- PP Systems. Operator’s Manual, Version 1.03: PP Systems Inc. 2018. Available online: https://ppsystems.com/download/technical_manuals/80109-1-EGM-5_Operation_V103.pdf (accessed on 7 January 2025).
- Piaszczyk, W.; Błońska, E.; Lasota, J.; Lukac, M. A comparison of C:N:P stoichiometry in soil and deadwood at an advanced decomposition stage. Catena 2019, 179, 1–5. [Google Scholar] [CrossRef]
- Stakėnas, V.; Varnagirytė-Kabašinskienė, I.; Sirgedaitė-Šėžienė, V.; Armolaitis, K.; Araminienė, V.; Muraškienė, M.; Žemaitis, P. Dead wood density and carbon estimates for the main tree species in the Lithuanian hemiboreal forest. Eur. J. For. Res. 2020, 139, 1045–1055. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Ascher-Jenull, J.; Bardelli, T.; Fornasier, F.; Fravolini, G.; Arfaioli, P.; Ceccherini, M.T.; Pietramellara, G.; Lamorski, K.; Sławiński, C.; et al. Physico-chemical and microbiological evidence of exposure effects on Picea abies—Coarse woody debris at different stages of decay. For. Ecol. Manag. 2017, 391, 376–389. [Google Scholar] [CrossRef]
- Bani, A.; Pioli, S.; Ventura, M.; Panzacchi, P.; Borruso, L.; Tognetti, R.; Tonon, G.; Brusetti, L. The role of microbial community in the decomposition of leaf litter and deadwood. Appl. Soil Ecol. 2018, 126, 75–84. [Google Scholar] [CrossRef]
- Strukelj, M.; Brais, S.; Quideau, S.A.; Angers, V.A.; Kebli, H.; Drapeau, P.; Oh, S.-W. Chemical transformations in downed logs and snags of mixed boreal species during decomposition. Can. J. For. Res. 2013, 43, 785–798. [Google Scholar] [CrossRef]
- Petrillo, M.; Cherubini, P.; Sartori, G.; Abiven, S.; Ascher, J.; Bertoldi, D.; Egli, M. Decomposition of Norway spruce and European larch coarse woody debris (CWD) in relation to different elevation and exposure in an Alpine setting. iForest 2016, 9, 154–164. [Google Scholar] [CrossRef]
- Pastorelli, R.; Agnelli, A.E.; De Meo, I.; Graziani, A.; Paletto, A.; Lagomarsino, A. Analysis of microbial diversity and greenhouse gas production of decaying pine logs. Forests 2017, 8, 224. [Google Scholar] [CrossRef]
- Pastorelli, R.; Paletto, A.; Agnelli, A.E.; Lagomarsino, A.; De Meo, I. Microbial communities associated with decomposing deadwood of downy birch in a natural forest in Khibiny Mountains (Kola Peninsula, Russian Federation). For. Ecol. Manag. 2020, 455, 117643. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Wang, C.; Gower, S.T. Annual carbon flux from woody debris for a boreal black spruce fire chronosequence. J. Geophys. Res. 2002, 108, 8220. [Google Scholar] [CrossRef]
- Herrmann, S.; Bauhus, J. Effects of moisture, temperature and decomposition stage on respirational carbon loss from coarse woody debris (CWD) of important European tree species. Scand. J. For. Res. 2013, 28, 346–357. [Google Scholar] [CrossRef]
- Laihonen, A.; Aalto, S.L.; Pihlatie, M.; Tiirola, M. Production of greenhouse gases by logging residue in boreal clear-cut forests. Eur. J. For. Res. 2024, 4, 1267–1281. [Google Scholar] [CrossRef]
- Lagomarsino, A.; De Meo, I.; Agnelli, A.E.; Paletto, A.; Mazza, G.; Bianchetto, E.; Pastorelli, R. Decomposition of black pine (Pinus nigra J. F. Arnold) deadwood and its impact on forest soil components. Sci. Total Environ. 2021, 754, 142039. [Google Scholar] [CrossRef]
- Christiansen, C.T.; Mack, M.C.; DeMarco, J.; Grogan, P. Decomposition of senesced leaf litter is faster in tall compared to low birch shrub tundra. Ecosystems 2018, 21, 1564–1579. [Google Scholar] [CrossRef]
- Wang, C.; Bond-Lamberty, B.; Gower, S.T. Environmental controls on carbon dioxide flux from black spruce coarse woody debris. Oecologia 2002, 132, 374–381. [Google Scholar] [CrossRef] [PubMed]


| Predictor | Betau | SE | Beta * | t | p | Betau | SE | Beta * | t | p | Betau | SE | Beta * | t | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Norway Spruce | Silver Birch | Black Alder | |||||||||||||
| Constant | −47,922.2 | 8438.7 | — | −5.7 | <0.001 | −156,215.8 | 38,737.2 | 0.0 | −4.0 | 0.00 | −162,359.6 | 37,177.1 | 0.0 | −4.4 | 0.00 |
| Decay class | 4225.9 | 889.3 | 2.6 | 4.8 | 0.001 | 9318.2 | 3389.9 | 0.9 | 2.7 | 0.00 | 14,037.1 | 3392.0 | 1.3 | 4.1 | 0.00 |
| Moisture | 187.5 | 96.4 | 0.5 | 1.9 | 0.078 | 712.1 | 296.5 | 0.3 | 2.4 | 0.00 | −516.3 | 152.8 | −0.5 | −3.4 | 0.00 |
| Density | 52.2 | 10.1 | 2.7 | 5.2 | <0.001 | 131.3 | 35.2 | 1.2 | 3.7 | 0.00 | 129.1 | 33.5 | 1.1 | 3.9 | 0.00 |
| TN | −21,555.4 | 16,292.0 | −0.6 | −1.3 | 0.20 | 73,975.7 | 63,542.2 | 0.4 | 1.2 | 0.30 | 103,077.9 | 61,836.9 | 0.5 | 1.7 | 0.10 |
| C | 564.7 | 128.6 | 0.9 | 4.4 | 0.001 | 1813.0 | 770.8 | 0.3 | 2.4 | 0.00 | 1830.4 | 730.3 | 0.3 | 2.5 | 0.00 |
| C/N | 1.4 | 4.5 | 0.098 | 0.30 | 0.80 | −8.7 | 25.9 | −0.1 | −0.3 | 0.70 | 10.7 | 25.7 | 0.1 | 0.4 | 0.70 |
| Model summary | R | R2 | R2adj | RMSE | R | R2 | R2adj | RMSE | R | R2 | R2adj | RMSE | |||
| 0.9 | 0.8 | 0.8 | 6503.9 | 0.8 | 0.6 | 0.5 | 9392.1 | 0.8 | 0.6 | 0.5 | 8977.6 | ||||
| MLR Equation: | CO2 efflux = −47,922.2 + 4225.9 × Decay class + 187.5 × Moisture + 52.2 × Density − 21,555.4 × TN + 564.7 × OC + 1.4 × C/N | CO2 efflux = −156,215.758 + 9318.202 × Decay class + 712.1 × Moisture + 131.3 × Density + 73,975.746 × TN + 1813.0 × OC − 8.7 × C/N | CO2 efflux = −162,359.563 + 14,037.067 × Decay class − 516.347 × Moisture + 129.112 × Density + 103,077.870 × TN + 1830.364 × OC + 10.736 × C/N | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čiuldienė, D.; Vigricas, E.; Galdikaitė, G.; Stakėnas, V.; Armolaitis, K.; Varnagirytė-Kabašinskienė, I. Carbon and Nitrogen Content and CO2 Efflux from Coarse Woody Debris of Norway Spruce, Black Alder, and Silver Birch. Forests 2025, 16, 293. https://doi.org/10.3390/f16020293
Čiuldienė D, Vigricas E, Galdikaitė G, Stakėnas V, Armolaitis K, Varnagirytė-Kabašinskienė I. Carbon and Nitrogen Content and CO2 Efflux from Coarse Woody Debris of Norway Spruce, Black Alder, and Silver Birch. Forests. 2025; 16(2):293. https://doi.org/10.3390/f16020293
Chicago/Turabian StyleČiuldienė, Dovilė, Egidijus Vigricas, Greta Galdikaitė, Vidas Stakėnas, Kęstutis Armolaitis, and Iveta Varnagirytė-Kabašinskienė. 2025. "Carbon and Nitrogen Content and CO2 Efflux from Coarse Woody Debris of Norway Spruce, Black Alder, and Silver Birch" Forests 16, no. 2: 293. https://doi.org/10.3390/f16020293
APA StyleČiuldienė, D., Vigricas, E., Galdikaitė, G., Stakėnas, V., Armolaitis, K., & Varnagirytė-Kabašinskienė, I. (2025). Carbon and Nitrogen Content and CO2 Efflux from Coarse Woody Debris of Norway Spruce, Black Alder, and Silver Birch. Forests, 16(2), 293. https://doi.org/10.3390/f16020293






