Abstract
Microbial residual carbon (MRC) is a key component of soil organic carbon (SOC) and crucial for SOC stabilization, contributing to the formation of a stable soil carbon pool. However, the accumulation patterns of MRC in different plantation forest types remain unclear. In this study, based on the principle of site condition similarity and supported by field investigations, soils from Populus alba, Salix matsudana Koidz, and Pinus tabuliformis in Beijing were selected as the research objects. The physical and chemical properties of the soils, as well as the microbial residual carbon content, were measured. Correlation analysis and redundancy analysis (RDA) were then conducted to explore the accumulation patterns of microbial residual carbon across different plantation forest types and to identify the factors influencing these patterns. Results showed that fungal residue carbon, bacterial residue carbon, and total MRC were highest in Populus alba, followed by Salix matsudana Koidz and Pinus tabuliformis. The contributions of fungal, bacterial, and total MRC to SOC were greatest in Populus alba, followed by Pinus tabuliformis and Salix matsudana Koidz. In this study, Populus alba were found to be more effective in sequestering microbial residue carbon. Fungal residue carbon content and its contribution to SOC were greater than bacterial residue carbon in all plantation types. Soil organic carbon, nitrate nitrogen, and available potassium were significantly correlated with both MRC content and its contribution to SOC. These findings deepen our understanding of microbial-driven soil carbon accumulation and provide a foundation for enhancing the carbon sequestration potential of plantation forests.
1. Introduction
Forest ecosystems serve as the planet’s most significant carbon reservoirs on land, holding a staggering 80% of the Earth’s vegetation-based carbon and roughly 39% of the carbon stored in global soils. These ecosystems are absolutely critical to the intricate dance of the global carbon cycle, acting as a linchpin in maintaining the balance [1]. Soil organic carbon (SOC) is the primary carrier of soil nutrient element cycling and drives soil microbial activity [2]. The main components of soil organic carbon include plant and animal residues, soil humus, and microbial carbon [3]. Soil humus, which forms as a result of microbial activity and the work of soil-dwelling organisms breaking down soil organic carbon (SOC), plays a crucial role in enhancing soil structure. It fosters the development of soil aggregates, boosts fertility, and refines the soil’s physical and chemical characteristics, making it more conducive to plant growth and overall ecosystem health [4]. Soil microorganisms are essential for the breakdown, alteration, and recycling of nutrients within Earth’s ecosystems, with soil organic matter (SOM) acting as a vital energy source for these tiny life forms [2].
Microorganisms are crucial in shaping soil organic carbon and serve as the main agents of carbon cycling in soils [5]. Microbial residual carbon, often referred to as the organic carbon left behind in soil following the demise of microorganisms, is a key player in the soil’s stable carbon reservoir. This carbon form is integral to the long-term storage of soil organic carbon, contributing significantly to carbon sequestration. The creation and breakdown of microbial residual carbon are shaped by a variety of elements, such as the soil’s carbon and nitrogen levels, the chemical makeup of microbial remnants, and prevailing environmental conditions. Grasping the patterns and behavior of microbial residual carbon is vital for precisely evaluating soil carbon reserves and for crafting effective approaches to combat climate change [6]. Microbial residual carbon accumulation is a key contributor to stable soil organic carbon pool formation [7,8,9]. Microorganisms are essential in decomposing plant remains (e.g., dead leaves and roots) into microbial-derived organic carbon, a process where microorganisms function as “carbon pumps”, significantly contributing to soil organic carbon accumulation [5]. Specifically, microorganisms drive this transformation in two key ways: first, by decomposing plant residues to extract nutrients, and second, by recycling the carbon from their own remains after death, thereby ensuring the long-term retention of this carbon in the soil. Furthermore, the carbon found in these microbial remnants plays an ongoing role in generating microbial residue carbon via the “reburial effect”, which bolsters the process of stabilizing and locking carbon within the soil [10]. Microbial residue carbon can be physically protected in mineral assemblages and is more stable than plant residue carbon in soil [5]. Studies have shown that microbial residue carbon contributes 30%–62% to global forest soil organic carbon [11]. However, due to the varying physicochemical properties of different plants, the content of microbial residue carbon produced by microbial decomposition differs, leading to variations in its accumulation characteristics and influencing factors. Shi et al. focused on plantation forests and found that forest rewilding resulted in the linear accumulation of soil microbial residue carbon [12]. Sun et al. identified altitude as a key factor influencing the accumulation of soil microbial residue carbon [13]. Xu et al. determined that both the composition of the soil microbial community and the fertility of the soil work together to influence the decline in microbial residual carbon levels when subtropical primary forests are transformed into plantation forests [14]. These findings suggest that microbial residue carbon content is regulated by various factors, such as vegetation type and soil physicochemical properties, exhibiting different accumulation characteristics [15,16]. Apoplastic litter is the main source of carbon in forest soils [17,18], and the lignin and nitrogen content of these materials differs in various plantation forests, resulting in varying inputs of organic matter to the soil. This diversity in organic matter inputs warrants further investigation into microbial residual carbon and its contribution to soil organic carbon.
Since 2012, Beijing has carried out two rounds of extensive afforestation and greening projects, resulting in a significant expansion of planted forests, which continue to contribute organic carbon to the soil. Although a significant body of research in this area has zeroed in on soil physicochemical properties, the storage of soil organic carbon, and its diverse fractions, the dynamics of microbial residual carbon accumulation in typical planted forests have yet to be fully understood. To address this gap, the current study focuses on various types of plantation forests in Beijing. It combines an examination of soil physicochemical properties with an analysis of microbial biomass. The primary objective is to explore the following questions: (1) How do the patterns of microbial residual carbon accumulation differ among the different plantation forest types found in Beijing? (2) What are the differences in the contributions of soil microbial residue carbon to soil organic carbon (SOC) among different plantation forest types in Beijing? (3) What are the primary factors influencing the accumulation of soil microbial residual carbon in the different plantation forest types of Beijing?
2. Materials and Methods
2.1. Overview of Study Area
The study area is located in Beijing, China (115°20′ E–117°30′ E, 39°28′ N–41°05′ N), the capital city of China and a major urban center in North China. The climate in this region is classified as a warm temperate semi-humid and semi-arid monsoon climate. It is primarily characterized by four distinct seasons: long, cold, and dry winters; hot and rainy summers; and relatively short springs and autumns. The average annual temperature in the plains region ranges from 11 °C to 13 °C, with the average annual humidity being approximately 57%. The annual precipitation averages around 570 mm. Beijing’s landscape gradually descends from the northwest to the southeast, with the northwestern areas boasting higher altitudes while the southeastern parts lie at lower elevations. The region’s terrain is shaped by two primary geomorphic features: the mountainous areas and the plains, with the mountainous areas covering about 62% of the total area and the plains making up the remaining 38%. The dominant tree species in these areas are Pinus tabuliformis, Robinia pseudoacacia L., and Salix matsudana Koidz.
2.2. Experimental Design and Sample Collection
In June 2024, based on the principle of site condition similarity and field surveys, three plantation forests with adjacent locations and comparable climatic conditions were selected: Populus alba (PA), Salix matsudana Koidz (SM), and Pinus tabuliformis (PT). In each plantation, three 20 m × 20 m sample plots were set up as replicates. In each plot, five sampling points were chosen via the “five-point sampling method”. Sampling was conducted following the principles of equal quantity and randomness. After removing any dead plant material and fallen debris covering the soil surface, soil samples were collected from three soil layers: 0–10 cm, 10–30 cm, and 30–60 cm. Samples collected from the same depth at each location were thoroughly blended, securely packed in resealable bags, and transported to the lab for processing. After removing any plant debris or foreign particles, the samples were left to air-dry and subsequently pulverized. They were then sifted using 1 mm and 0.149 mm mesh screens, respectively, before being stored for later examination. Table 1 outlines the soil type, texture, and bulk density measurements for the three plantation forests.

Table 1.
Soil types, textures, and bulk densities at various soil layers in different types of planted forests.
2.3. Assessment of Soil Physicochemical Characteristics
Soil pH was determined using a pH meter by mixing soil samples with distilled water in a 1:2.5 ratio, thoroughly mixing the suspension, and allowing it to stand for a specific period. The pH of the soil–water suspension was then measured using a pH meter to assess its acidity or alkalinity [19]. Soil organic carbon (SOC) content was measured using the potassium dichromate oxidation-external heating method, which facilitates the oxidation of organic carbon by mixing the soil samples with excess potassium dichromate solution and sulfuric acid, followed by heating. The unreacted potassium dichromate was quantified through titration, and the soil’s organic carbon content was subsequently calculated [19]. The concentration of available potassium (AK) in the soil was assessed through flame photometry. To extract soluble potassium, an ammonium acetate solution was applied to the soil samples. The extracted solution was then fed into a flame photometer. By referencing a standard calibration curve, the potassium levels in the soil were quantified, providing a precise measurement of its availability [19]. The assessment of available phosphorus (AP) in the soil was carried out through the molybdenum-antimony colorimetric technique, utilizing a hydrochloric acid–sulfuric acid extractant to effectively draw out the phosphorus from the soil matrix. The extract was then mixed with ammonium molybdate and ascorbic acid solutions, forming a phosphomolybdenum blue complex. The absorbance of this complex was measured at 710 nm using a spectrophotometer(UV-6100, METASH, Shanghai, China), and the effective phosphorus content was calculated from a standard calibration curve [19]. Soil alkaline nitrogen (AN) levels were measured using the alkaline hydrolysis-diffusion technique. In this process, the soil was exposed to a sodium hydroxide solution, which facilitated the release of nitrogen. The liberated nitrogen subsequently diffused into an absorbent solution for analysis. The nitrogen content in the absorbent solution was measured, and the alkaline nitrogen content in the soil was calculated [19]. Soil nitrate nitrogen (NO3−) was determined by UV spectrophotometry, utilizing the UV absorption properties of nitrate in the 210–225 nm wavelength range to quantify its concentration in the soil [19].
2.4. Determination of Soil Microbiomass Carbon and Nitrogen
In this research, the measurement of soil microbial biomass carbon (MBC) and nitrogen (MBN) was conducted through the chloroform fumigation technique. Freshly collected soil samples underwent chloroform exposure to eradicate the microorganisms, after which the organic matter was extracted using a 0.5 mol/L K2SO4 solution. The fumigation process resulted in a significant increase in the organic carbon and nitrogen content of the soil extract. By assessing the rises in organic carbon and nitrogen, the carbon and nitrogen levels in the microbial biomass within the soil can be estimated [20].
2.5. Determination of Soil Amino Sugars
In this study, soil samples from the 0–10 cm soil layer of the three plantation sites were utilized to measure soil amino sugars via gas chromatography [21]. Amino sugars were first extracted from the soil, and the extract was reacted with derivatization reagents to form volatile derivatives. These derivatives were then injected into a gas chromatograph, where they were separated and detected by a column. The amino sugar content was calculated from a standard calibration curve. Microbial residual carbon can be characterized by quantifying soil amino sugars. Glucosamine (GluN), which mainly comes from the cell walls of fungi, along with muramic acid (MurA), sourced solely from bacterial cell walls, are utilized to assess the leftover carbon content from both fungi and bacteria [11,22]. The residual carbon content of microbes was determined using the following formula:
FRC = [AGluN/179.17 − 2 × AMurA/251.23] × 179.17 × 9
BRC = AMurA × 45
MRC = FRC + BRC
Note: AGluN indicates glucosamine levels (mg/kg), AMurA represents the muramic acid content (mg/kg), FRC refers to fungal residue carbon, BRC refers to bacterial residue carbon, and MRC refers to total microbial residue carbon. The values 179.17 and 251.23 are the molecular weights of glucosamine and muramic acid, respectively, and 9 is the conversion factor used to convert glucosamine from the fungal source into fungal residue carbon. The value 45 is the conversion factor for converting muramic acid into bacterial residue carbon. Equation (1) was used to calculate fungal residue carbon (FRC) from glucosamine and muramic acid, while Equation (2) was used to calculate bacterial residue carbon (BRC) based on muramic acid content. Finally, Equation (3) calculated total microbial residue carbon (MRC) by combining fungal residue carbon (FRC) and bacterial residue carbon (BRC).
2.6. Data Processing and Analysis
This study utilized Excel 2016 for data processing and SPSS 20.0 for performing ANOVA. Correlation analysis was conducted using the Pearson method. Redundancy analysis (RDA) was performed with Canoco 5 software, while graphical representations were created using Origin 2021 software.
3. Results
3.1. Differences in Soil Physical and Chemical Properties and Microbial Carbon and Nitrogen Content of Different Plantation Forest Types
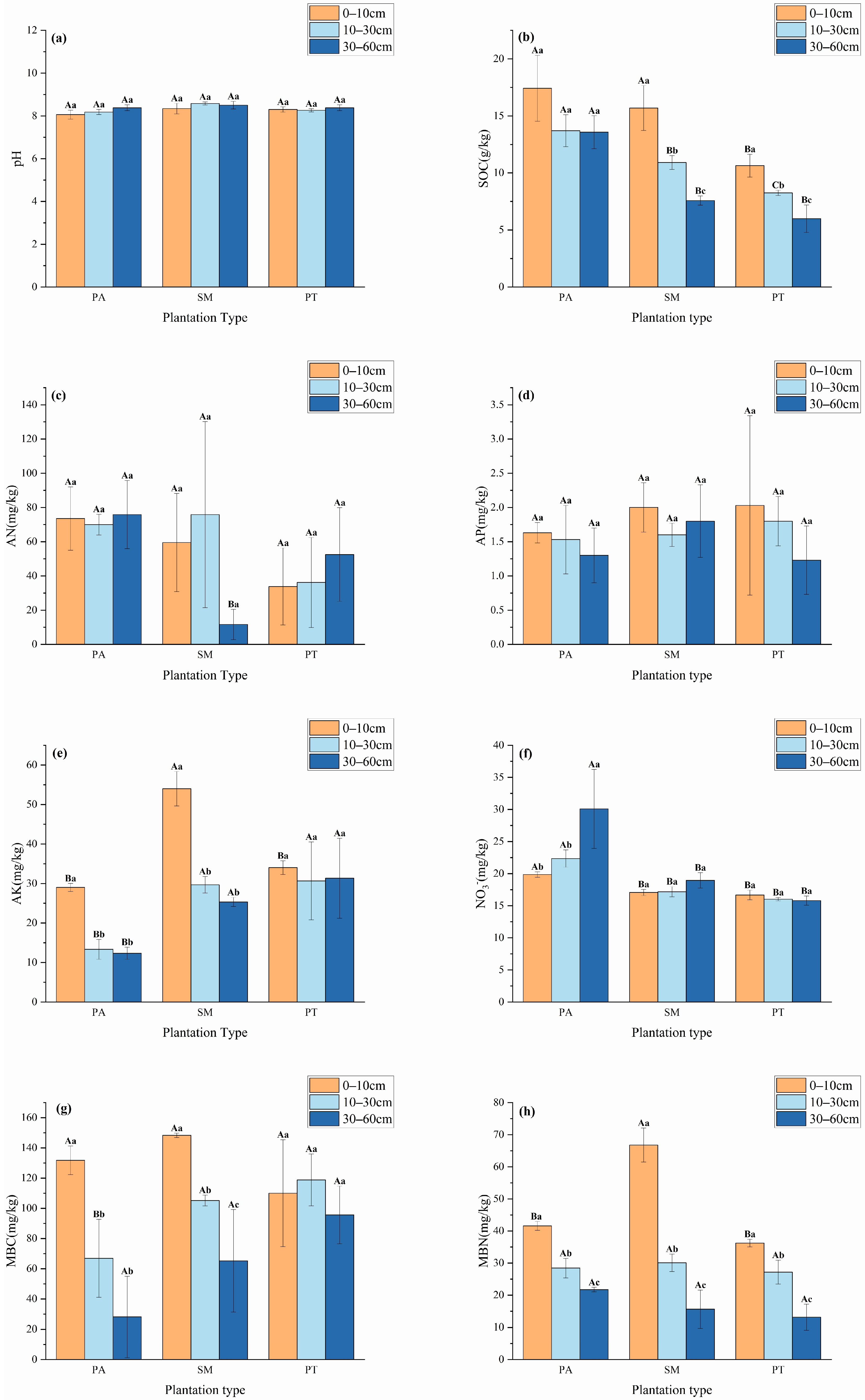
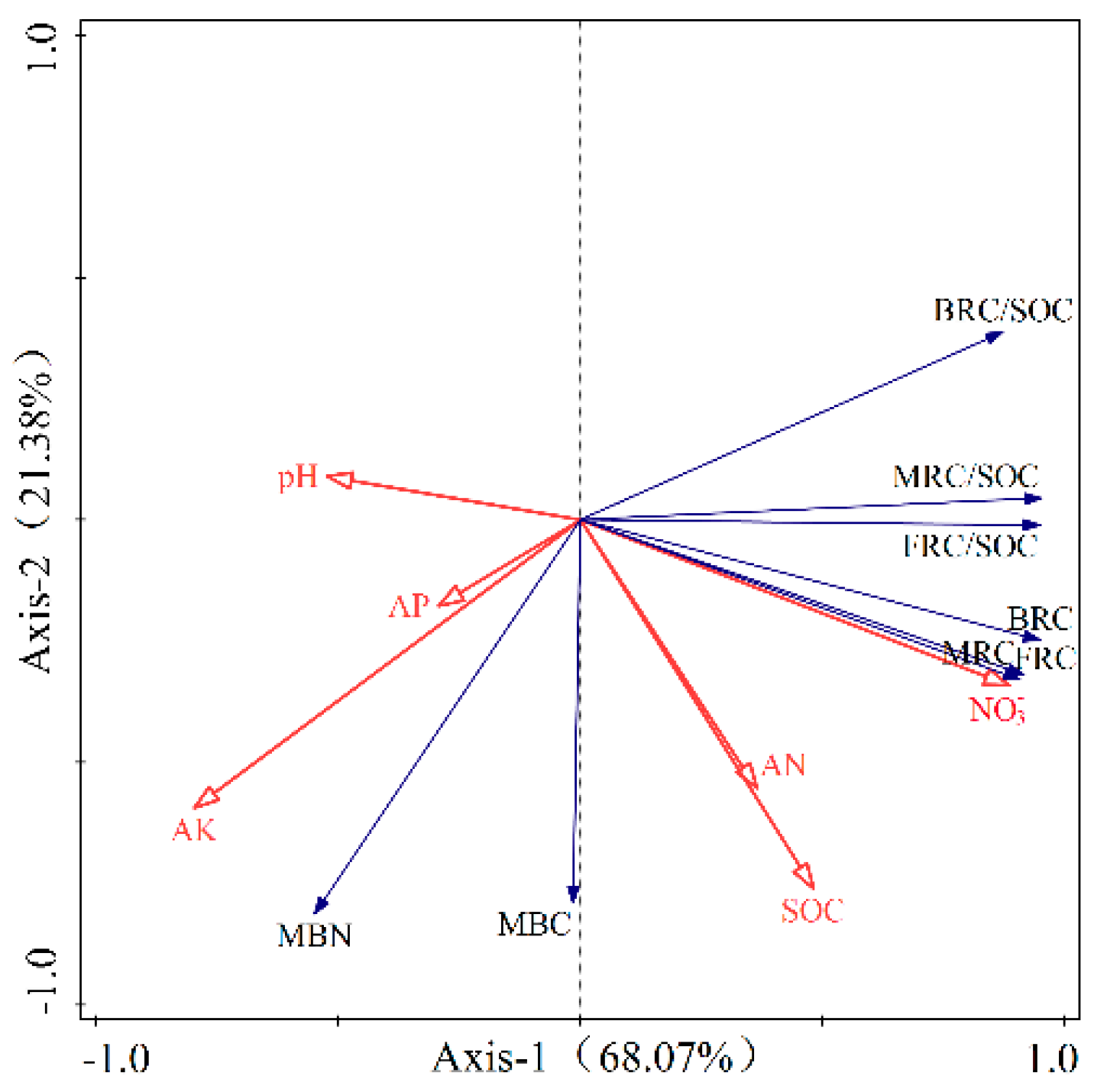
As depicted in Figure 1, notable differences (p < 0.05) emerged in the physicochemical characteristics, the content of microbial biomass carbon (MBC), and microbial biomass nitrogen (MBN) in the soils of various plantation forest types. Soils from all three plantation forest types turned out to be alkaline. In the 0–10 cm soil layer, the soil organic carbon (SOC) contents of the Populus alba (PA) and Salix matsudana Koidz (SM) plantations were significantly higher than those of the Pinus tabuliformis (PT) plantation (p < 0.05). In the 10–30 cm soil layer, the SOC contents of the three plantation forest types differed significantly (p < 0.05), with the order being PA > SM > PT. Within the soil layer of 30–60 cm, the soil organic carbon (SOC) levels were markedly elevated in PA compared to both SM and PT, with statistical significance (p < 0.05). At various depths, the SOC concentrations in SM and PT revealed notable distinctions, arranged in descending order as follows: 0–10 cm > 10–30 cm > 30–60 cm (p < 0.05). The content of alkaline nitrogen (AN) displayed variability among the different plantation forest types, where in the 30–60 cm layer, both PA and PT exhibited significantly higher AN levels than SM (p < 0.05). The peak concentration of available phosphorus (AP) for each type was found in the 0–10 cm soil layer. Meanwhile, the available potassium (AK) levels were significantly higher in SM compared to PA and PT in both the 0–10 cm and 30–60 cm depths (p < 0.05). Notably, the AK content in PA and SM revealed a significant higher concentration in the 0–10 cm layer relative to the 10–30 cm and 30–60 cm layers. The nitrate–nitrogen (NO3−) levels in PA surpassed those of both SM and PT across all three soil layers, demonstrating statistical significance (p < 0.05). Furthermore, PA showed an especially high concentration of NO3− in the 0–10 cm layer compared to the two deeper layers (p < 0.05). Even at the 30–60 cm depth, the NO3− levels in PA remained significantly higher than those found in the 0–10 cm and 10–30 cm layers (p < 0.05). In contrast, the microbial biomass carbon (MBC) in PA was significantly lower than that of SM and PT in the 10–30 cm layer (p < 0.05), whereas the MBC in SM exhibited a significant decline as soil depth increased (p < 0.05). The 0–10 cm layer for PA recorded significantly higher MBC levels than the other two layers. Additionally, in the 0–10 cm layer, the microbial biomass nitrogen (MBN) concentration in SM was notably greater than that in PA and PT (p < 0.05). All three forest types displayed a significant decrease in MBN levels with increasing soil depth, in the sequence of 0–10 cm > 10–30 cm > 30–60 cm (p < 0.05).
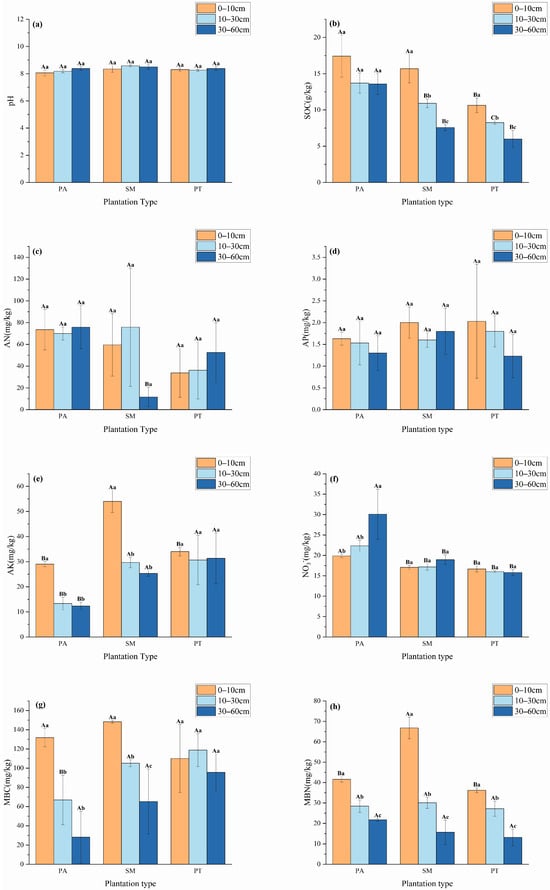
Figure 1.
Differences in physicochemical properties (pH (a), SOC (b), AN (c), AP (d), AK (e), NO3− (f)) and microbial carbon and nitrogen (MBC (g), MBN (h)) content between different plantation forest types. Different capital letters indicate significant (p < 0.05) differences between stand types at same soil depth, and different lowercase letters indicate significant (p < 0.05) differences between stand types at same soil depth. Bars indicate mean ± SD. p < 0.05.
3.2. Differences in Soil Microbial Residual Carbon Content Among Plantation Forest Types
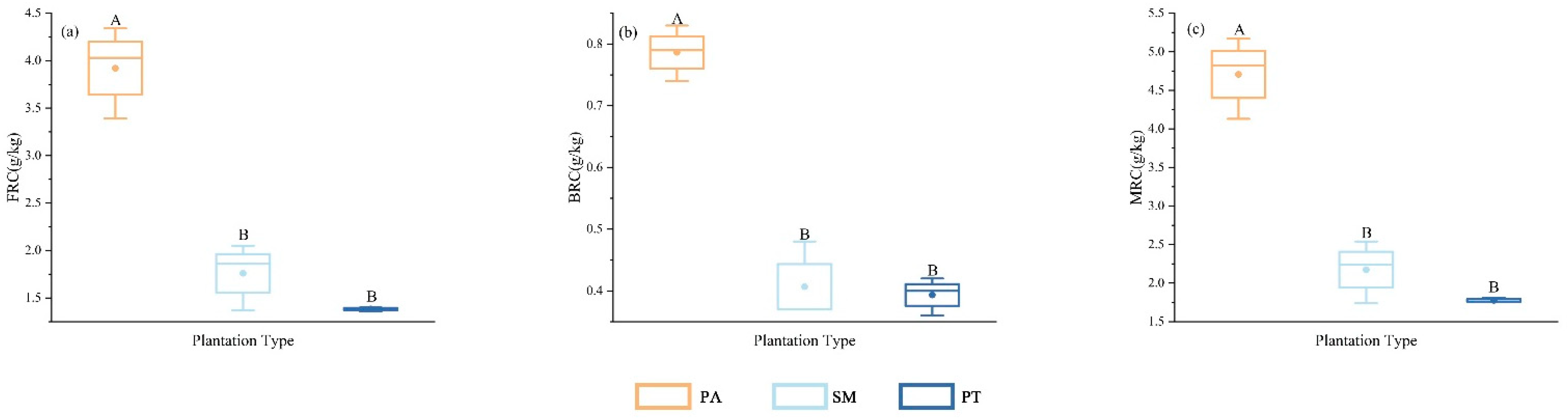
As illustrated in Figure 2, the levels of fungal residual carbon (FRC), bacterial residual carbon (BRC), and total microbial residual carbon (MRC) varied considerably across the various plantation forest soil types, with statistically significant differences observed (p < 0.05). The FRC content in the Populus alba (PA) plantation was significantly higher than that of the Salix matsudana Koidz (SM) and Pinus tabuliformis (PT) plantations (p < 0.05). Furthermore, the FRC content in SM was slightly higher than that in PT, although the difference between SM and PT did not reach the significance level. The BRC content in PA was significantly higher than in both SM and PT (p < 0.05). Specifically, the BRC content in PA was 92.68% higher than that in SM and 102.56% higher than that in PT among the three plantation forest types. Regarding MRC, the content in PA was significantly higher than in both SM and PT (p < 0.05), with the MRC content in SM being slightly higher than that in PT. The MRC values in SM ranged from 1.74 to 2.54 g/kg, while those in PT ranged from 1.76 to 1.81 g/kg, respectively.

Figure 2.
Differences in soil microbial residue carbon content among different plantation forest types (FRC, (a); BRC, (b); MRC, (c)). At same soil depth, different capital letters denote significant (p < 0.05) differences between various stand types. Bars indicate mean ±SD. (p < 0.05).
3.3. Differences in Contribution of Soil Microbial Residue Carbon to Soil Organic Carbon in Different Plantation Forest Types
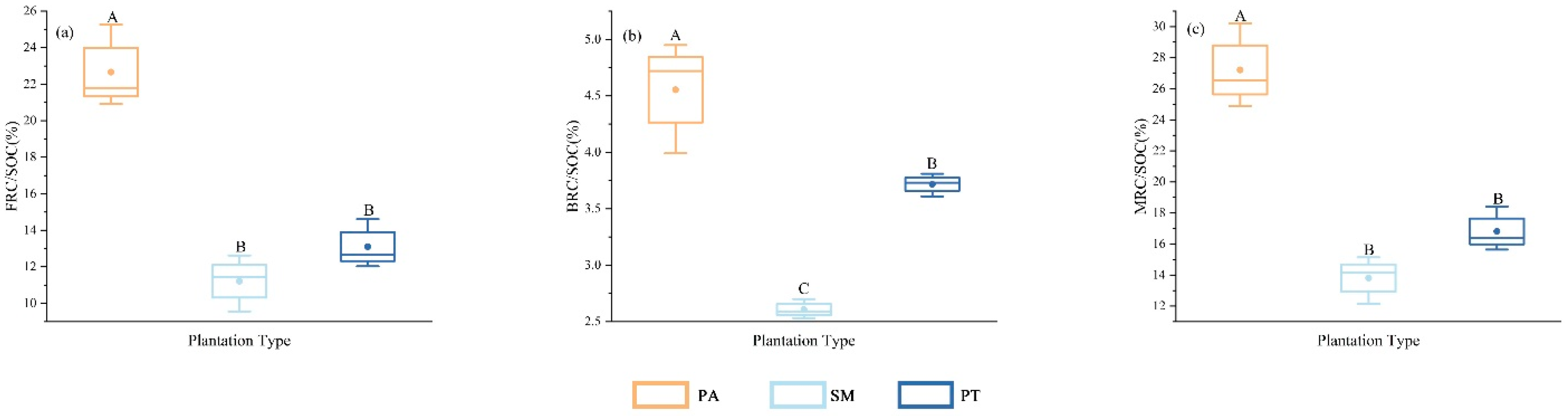
Figure 3 illustrates that the contributions of fungal residual carbon (FRC), bacterial residual carbon (BRC), and the overall microbial residual carbon (MRC) to soil organic carbon (SOC) displayed notable discrepancies (p < 0.05) across the various types of plantation forests. The FRC/SOC ratio in the Populus alba (PA) plantation was significantly higher than that in the Salix matsudana Koidz (SM) and Pinus tabuliformis (PT) plantations. The FRC/SOC ratio in SM was slightly lower than that in PT, although the difference between SM and PT did not reach statistical significance. The BRC/SOC ratio among the three plantation forest types differed significantly (p < 0.05), with the order of BRC/SOC being PA > PT > SM. The MRC/SOC ratio in PA was significantly higher (p < 0.05) than in both SM and PT, being 97.1% higher than that in SM and 61.93% higher than that in PT. The MRC/SOC ratio in PT was slightly higher than that in SM, although the difference between PT and SM was not statistically significant.

Figure 3.
Differences in soil organic carbon contribution by soil microbial residue carbon in different plantation forest types (FRC/SOC, (a); BRC/SOC, (b); MRC/SOC, (c)). At same soil depth, different capital letters denote significant (p < 0.05) differences between various stand types. Bars indicate mean ±SD. (p < 0.05).
3.4. Main Factors Affecting Carbon Accumulation in Microbial Residues
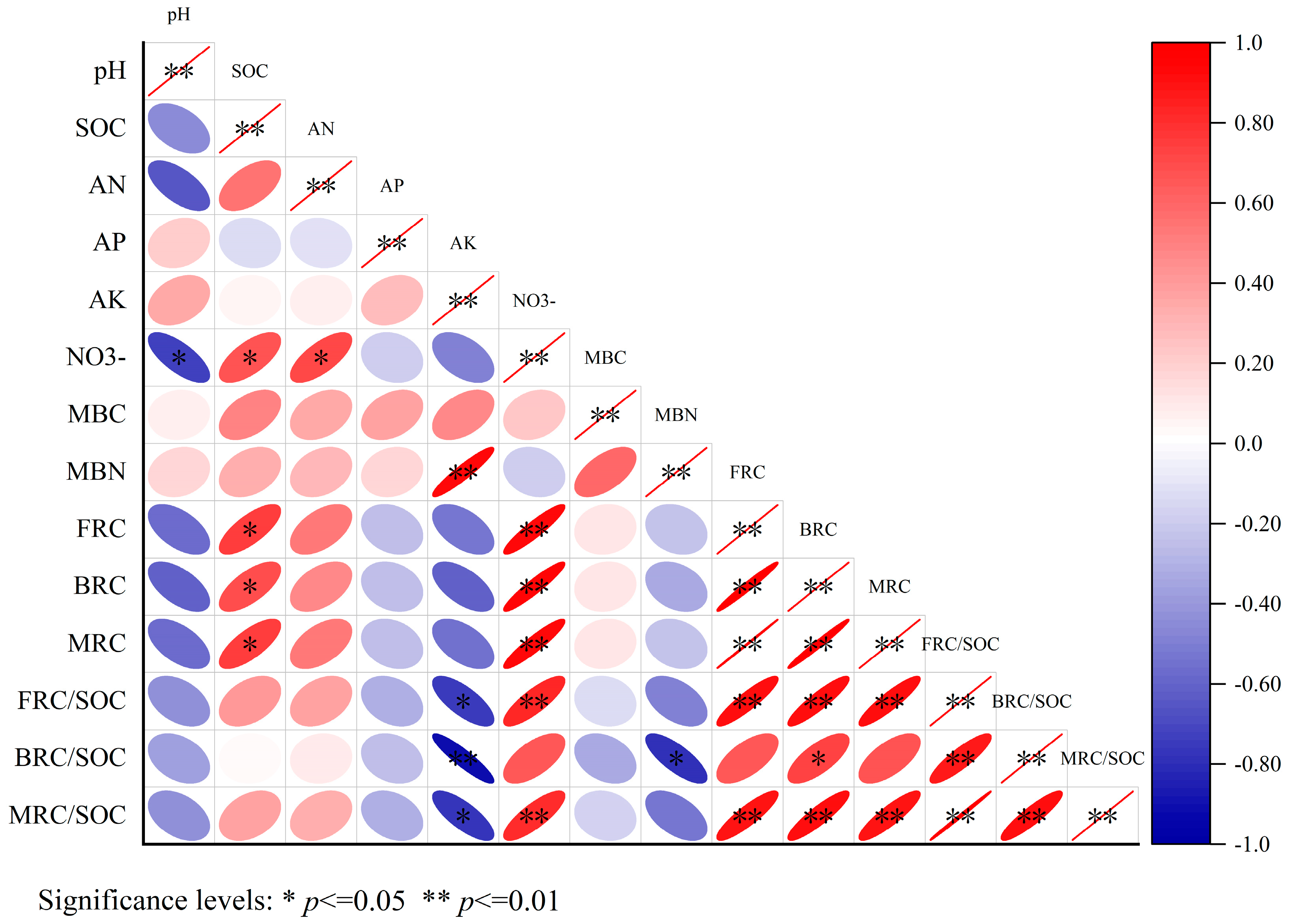
In Figure 4, the findings of the correlation analysis indicated that microbial residue carbon (MRC), fungal residual carbon (FRC), and bacterial residual carbon (BRC) were strongly positively correlated with soil organic carbon (SOC) and nitrate–nitrogen (NO3−) (p < 0.05). In other words, there was a significant positive association between these variables. The MRC/SOC and FRC/SOC ratios were significantly positively correlated with NO3− (p < 0.05) and significantly negatively correlated with available potassium (AK) (p < 0.05). Additionally, FRC/SOC was significantly negatively correlated with both AK and microbial biomass nitrogen (MBN) (p < 0.05).
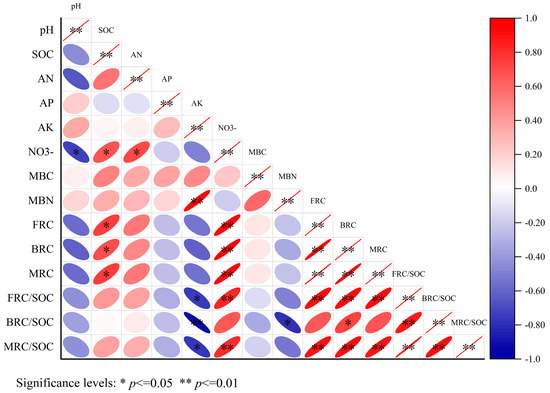
Figure 4.
Correlation analysis of factors influencing soil microbial residual carbon.
In Figure 5, the redundancy analysis (RDA) findings revealed that microbial residue carbon content and its role in soil organic carbon (SOC) were primarily accounted for by 68.97% along axis 1 and 21.38% along axis 2. Together, these two axes captured a combined explanatory power of 90.35%, painting a comprehensive picture of the relationship between microbial residues and SOC. This high cumulative variance suggests that the RDA could better reflect the relationship between microbial residue carbon content, its contribution to SOC, and various influencing factors. In the RDA plots, solid arrows represented the microbial residue carbon content and its contribution to SOC, while the influencing factors such as SOC, available nitrogen (AN), available phosphorus (AP), available potassium (AK), pH, and NO3− were represented by hollow arrows. The angle between the arrows of microbial residue carbon content and its contribution to SOC and those of the influencing factors indicates the nature of the correlation: if the angle is acute, it suggests a positive correlation; if it is obtuse, it suggests a negative correlation; and if it is a right angle, it suggests no correlation. The cosine value of the angle reflects the strength of the correlation. The RDA findings showed a positive correlation between microbial residue carbon levels, their SOC contribution, and NO3−, AN, and SOC, and were negatively correlated with pH, available phosphorus, and available potassium.
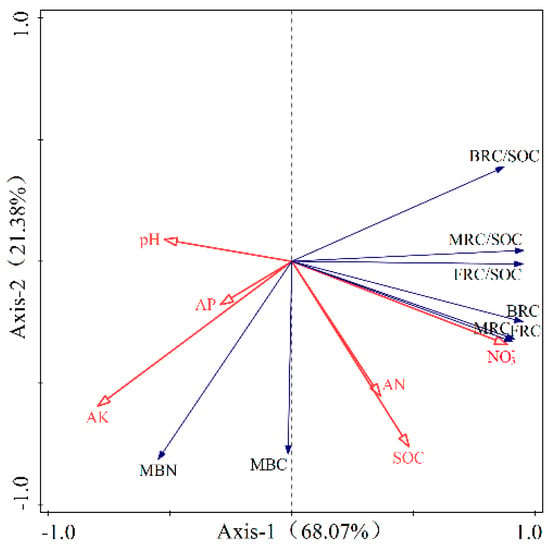
Figure 5.
Redundancy analysis of soil microbial residual carbon content and its contribution to soil organic carbon with influence factors.
Table 2 demonstrates the explanation rate and contribution of each influencing factor to the microbial residue carbon (MRC) content and its contribution to soil organic carbon (SOC). The findings revealed that nitrate–nitrogen (NO3−) played the most significant role in influencing both microbial residue carbon levels and their contribution to soil organic carbon. It accounted for an impressive 55.8% of the explanatory power and contributed a substantial 59.9% to the overall soil organic carbon pool. This was followed by available potassium (AK) and pH, which also contributed significantly. The influencing factor with the lowest explanation rate and contribution was available nitrogen (AN), which accounted for only 0.9%. The analysis of the p-value indicated that NO3− exhibited a notable positive correlation with both the carbon content of microbial residues and their role in organic carbon (p < 0.05). Conversely, AK displayed a significant negative correlation with these same factors (p < 0.05). On the other hand, pH, soil organic carbon (SOC), available phosphorus (AP), and ammonium nitrogen (AN) did not demonstrate any significant correlation with the carbon content of microbial residues or their contribution to organic carbon.

Table 2.
Correlation of impact factors with soil microbial residual carbon and its contribution to soil organic carbon.
4. Discussion
4.1. Soil Microbial Residue Carbon Accumulation in Different Plantation Forest Types
Soil microorganisms are fundamental drivers of organic carbon sequestration, and the accumulation of microbial residual carbon (MRC) plays a critical role in the formation of stable soil organic carbon (SOC) pools [7,8,9]. Investigating the accumulation patterns of microbial residue carbon in different plantation forest types is therefore of great significance for formulating effective carbon storage strategies within plantation forest ecosystems. The results of this study demonstrated that the MRC content in Pinus tabuliformis (PT) was significantly higher than that in Salix matsudana Koidz (SM) and Populus alba (PA), and that the MRC content of SM was marginally higher than that of ST. This difference can be attributed to the varying amounts and types of apoplastic inputs across the different plantation forest types. Apoplastic input is known to be a key factor influencing microbial residue carbon accumulation [23], and several studies have indicated that broadleaf forests tend to accumulate more SOC compared to coniferous forests [24,25,26]. These findings align with the results of the present study, which observed that PA exhibited higher apoplastic inputs compared to SM and PT, thus providing a more available substrate for microorganisms. Additionally, in PT, the apoplastic matter was found to be rich in lignin and cellulose, compounds that are more resistant to decomposition in soil [6]. This difference in decomposition rates may explain why PA and SM could release more carbon sources and nutrients into the soil during the decomposition process than PT [27], creating conditions that are more favorable for microbial growth and reproduction.
The fungal residue carbon (FRC) content was consistently higher than the bacterial residue carbon (BRC) content in all three plantation forest types, which corroborates the findings of Wang et al. [28]. This difference is likely because fungal biomass generally exceeds bacterial biomass in forest ecosystems [29]. Previous studies have shown that fungal biomass can be approximately four times greater than bacterial biomass in forest soils [30]. Fungi are also more effective at decomposing complex organic materials [31]. They possess the ability to breakdown lignin, cellulose, and other components of apoplastic materials by secreting extracellular enzymes and organic acids [32], which facilitates the higher accumulation of fungal biomass in surface soils compared to bacteria. The “entombing effect” of microbial residue carbon is predominantly due to fungal residue accumulation, as the main components of fungal cell walls—such as chitin—are more resistant to decomposition and easily combine with clay mineral particles in the soil, resulting in greater stability for fungal residue carbon. Additionally, fungal residues can be stabilized through the formation of fungal residue–tannin complexes [31]. In contrast, bacterial cell walls are primarily composed of peptidoglycan, which is easily degraded [33]. Consequently, bacterial residues are often utilized as a carbon source by fungi, which then incorporate them into fungal residues [34]. Thus, in the various plantation forest types examined, fungal residue carbon dominates the process of microbial residue carbon accumulation.
4.2. Characterization of Contribution of Microbial Residual Carbon to Soil Organic Carbon in Different Plantation Forest Types
Characterizing the contribution of microbial residual carbon (MRC) to soil organic carbon (SOC) is crucial for understanding the stability and sustainability of soil ecosystems. In this study, we found that the apoplastic input in the Salix matsudana Koidz plantation (SM) was larger compared to the Pinus tabuliformis plantation (PT), which resulted in a larger amount of plant-derived carbon being introduced into the soil. This, in turn, contributed to a significantly higher SOC content in SM than in PT (p < 0.05). However, although the apoplastic input was higher in SM, the bacterial residue carbon (BRC) content in SM was not significantly different from that in PT. Moreover, the contribution of bacterial residue carbon to SOC in SM was significantly lower compared to PT (p < 0.05). This suggests that the bacterial residue carbon in SM did not contribute as significantly to SOC as it did in PT. This difference might also be due to the fact that microbial residual carbon is not always fully stabilized in the soil and can be re-mineralized or recycled by other microorganisms [35], which could lead to a lower BRC/SOC ratio in SM.
In all three plantation forest types (Populus alba plantation (PA), Salix matsudana Koidz plantation (SM), and Pinus tabuliformis plantation (PT)), the ratio of fungal residue carbon to soil organic carbon (FRC/SOC) was found to be higher than that of bacterial residue carbon to soil organic carbon (BRC/SOC). This observation is consistent with the findings of Wang et al. [28] and Li et al. [36]. This result can be explained by the fact that this study focused on the surface soil layer, where fungi, being aerobic organisms, predominantly utilize apoplastic material as their carbon source [37]. Fungi have a competitive advantage over bacteria in the surface soil due to their preference for decomposing complex and refractory organic matter. Moreover, fungi are more capable of utilizing recalcitrant compounds such as lignin and cellulose as energy sources, leading to the production of more residual carbon after biomass turnover. As a result, fungi contribute more to the formation of soil organic carbon (SOC) than bacteria do [38]. This is why the fungal residue carbon in the soil was more prevalent and contributed more to SOC than bacterial residue carbon in all plantation forest types examined in this study.
4.3. Factors Affecting Microbial Residue Carbon Accumulation and Its Contribution to Soil Organic Carbon
Soil physical and chemical properties, along with microbial populations, are environmental factors that can influence microbial community structure, diversity, and carbon utilization efficiency. These factors, in turn, affect the accumulation of microbial residual carbon (MRC) and the contribution of MRC to soil organic carbon (SOC). Consequently, the accumulation of microbial residual carbon and its contribution to SOC vary across different plantation forest types [39]. From the correlation and redundancy analyses, it is clear that SOC, NO3−, and AK are critical factors that influence both the content of microbial residual carbon and its contribution to soil organic carbon.
Among these factors, microbial residual carbon was positively correlated with SOC, which is consistent with the findings of Jing et al. [40]. This positive relationship can be attributed to the fact that a soil environment rich in carbon sources promotes microbial growth and metabolic processes. This leads to an increase in microbial respiration, improved microbial carbon utilization efficiency, and indirectly supports the accumulation of microbial residual carbon [40,41]. Additionally, microbial residual carbon and its contribution to soil organic carbon were significantly and positively correlated with NO3−, which may be due to the role of the soil nitrogen cycle in enhancing the accumulation of microbial residual carbon. Increased nitrogen availability likely boosts the content of agglomerated carbon and suppresses the microbial decomposition of soil organic matter [42]. On the other hand, in soils where nitrogen is deficient, microbial residual carbon undergoes decomposition via soil extracellular enzymes and is used by microorganisms as a nitrogen source, leading to a decrease in the microbial residual carbon content [43].
The negative correlation between available potassium (AK) and microbial residual carbon content, as well as its contribution to soil organic carbon, might be due to the fact that potassium is an essential nutrient for plant growth and microbial metabolism. An increase in AK content can stimulate microbial activity in the soil, leading to an increase in the amount of plant-derived carbon entering the soil. However, microbial residual carbon is not entirely stable and may be utilized by other microorganisms. Therefore, elevated levels of fast-acting potassium can inhibit the accumulation of microbial residual carbon and its contribution to soil organic carbon [35].
In summary, microbial residual carbon accumulation and its contribution to soil organic carbon are influenced by a variety of factors. The interactions between soil physical and chemical properties and microbial populations play a significant role in shaping microbial residual carbon dynamics. To gain a better understanding of these processes and their effects on soil organic carbon storage, further studies are needed to explore these interactions in greater detail and to examine the dynamic nature of microbial residual carbon accumulation.
5. Conclusions
Microbial residue carbon plays a vital role as a significant component of the soil stabilization carbon pool. It is crucial in the fixation of soil organic carbon (SOC) and substantially contributes to the formation of the soil stabilization carbon pool. This study demonstrated that the contents of fungal residue carbon, bacterial residue carbon, and total microbial residue carbon in the soils of three different plantation forest types were ranked as follows: Populus alba plantation > Salix matsudana Koidz plantation > Pinus tabuliformis plantation. The contributions of fungal residue carbon, bacterial residue carbon, and total microbial residue carbon to SOC also varied, following this order: Populus alba plantation > Pinus tabuliformis plantation > Salix matsudana Koidz plantation. In this study, Populus alba were found to be more effective in sequestering microbial residue carbon. Across all three plantation forest types, the content of fungal residue carbon and its contribution to SOC were consistently higher than that of bacterial residue carbon. Furthermore, this study identified soil organic carbon, nitrate nitrogen, and available potassium as the key factors influencing microbial residual carbon accumulation. Based on these findings, future research integrating the stabilization mechanisms of soil microbial residue carbon with dynamic changes in soil organic carbon may offer valuable insights into the microbial-driven processes that underpin soil organic carbon accumulation in forest ecosystems.
Author Contributions
Conceptualization, X.K. and S.L.; Methodology, C.W.; Validation, X.K., C.W., J.L. and J.X.; Investigation, X.K.; Writing—Original Draft Preparation, X.K.; Writing—Review and Editing, S.L.; Visualization, X.K.; Funding Acquisition, X.S. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Plains Ecological Forest Management Monitoring and Evaluation Project (662404201).
Data Availability Statement
The data presented in this study are available upon request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The Structure, Distribution, and Biomass of the World’s Forests. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 593–622. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.R.; Dou, Y.X.; Xue, Z.J.; Sun, H.; Wang, Y.Q.; Liang, C.; An, S.S. Advances in the research of transformation and stabilization of soil organic carbon from plant and microbe. Chin. J. Appl. Ecol. 2024, 35, 111–123. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Kolbe, H.; Zhang, R.L. Research Progress of SOC Functions andTransformation Mechanisms. Sci. Agric. Sin. 2020, 53, 317–331. [Google Scholar]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The Importance of Anabolism in Microbial Control over Soil Carbon Storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Yu, Y.C.; Zhang, X.U.; Dai, X.Q.; Lü, S.D.; Yang, Y. Distributions and influencing factors of microbial residue carbon contents in forest soil profiles in subtropical red soil region. Acta Ecol. Sin. 2022, 42, 1108–1117. [Google Scholar] [CrossRef]
- Cai, M.; Zhao, G.; Zhao, B.; Cong, N.; Zheng, Z.; Zhu, J.; Duan, X.; Zhang, Y. Climate Warming Alters the Relative Importance of Plant Root and Microbial Community in Regulating the Accumulation of Soil Microbial Necromass Carbon in a Tibetan Alpine Meadow. Glob. Change Biol. 2023, 29, 3193–3204. [Google Scholar] [CrossRef]
- Shao, P.S.; Xie, H.T.; Bao, X.L.; Liang, C. Variation of Microbial Residues during Forest Secondary Succession in Topsoil and Subsoil. Acta Pedol. Sin. 2021, 58, 1050–1059. [Google Scholar]
- Shao, P.S.; Han, H.Y.; Zhang, Y.H.; Fang, Y. Variation of Soil Microbial Residues Under Different Salinity Concentrations in the Yellow River Delta. Sci. Geogr. Sin. 2022, 42, 1307–1315. [Google Scholar] [CrossRef]
- Ludwig, M.; Achtenhagen, J.; Miltner, A.; Eckhardt, K.-U.; Leinweber, P.; Emmerling, C.; Thiele-Bruhn, S. Microbial Contribution to SOM Quantity and Quality in Density Fractions of Temperate Arable Soils. Soil Biol. Biochem. 2015, 81, 311–322. [Google Scholar] [CrossRef]
- Ni, X.; Liao, S.; Tan, S.; Peng, Y.; Wang, D.; Yue, K.; Wu, F.; Yang, Y. The Vertical Distribution and Control of Microbial Necromass Carbon in Forest Soils. Glob. Ecol. Biogeogr. 2020, 29, 1829–1839. [Google Scholar] [CrossRef]
- Shi, K.; Liao, J.; Zou, X.; Chen, H.Y.H.; Delgado-Baquerizo, M.; Yan, Z.; Ren, T.; Ruan, H. Accumulation of Soil Microbial Extracellular and Cellular Residues during Forest Rewilding: Implications for Soil Carbon Stabilization in Older Plantations. Soil Biol. Biochem. 2024, 188, 109250. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, X.; Zhong, A.; Guo, S.; Zhang, H. Variations in Microbial Residue and Its Contribution to SOC between Organic and Mineral Soil Layers along an Altitude Gradient in the Wuyi Mountains. Forests 2023, 14, 1678. [Google Scholar] [CrossRef]
- Xu, F.; Li, C.; Chen, Y.; Wu, J.; Bai, H.; Fan, S.; Yang, Y.; Zhang, Y.; Li, S.; Su, J. Soil Microbial Community Structure and Soil Fertility Jointly Regulate Soil Microbial Residue Carbon during the Conversion from Subtropical Primary Forest to Plantations. Geoderma 2024, 441, 116767. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, B.; Lü, X.; Wang, J.; Horwath, W.R. Parent Material and Conifer Biome Influence Microbial Residue Accumulation in Forest Soils. Soil Biol. Biochem. 2017, 107, 1–9. [Google Scholar] [CrossRef]
- Chen, G.; Ma, S.; Tian, D.; Xiao, W.; Jiang, L.; Xing, A.; Zou, A.; Zhou, L.; Shen, H.; Zheng, C.; et al. Patterns and Determinants of Soil Microbial Residues from Tropical to Boreal Forests. Soil Biol. Biochem. 2020, 151, 108059. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of Soil Organic Matter via Biochemical and Physical Pathways of Litter Mass Loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Wang, B.; Liang, C.; Yao, H.; Yang, E.; An, S. The Accumulation of Microbial Necromass Carbon from Litter to Mineral Soil and Its Contribution to Soil Organic Carbon Sequestration. Catena 2021, 207, 105622. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis; China Agricultural Press: Beijing, China, 2001. [Google Scholar]
- Zhang, H.Y.; Zhang, X.D.; Li, J.; Wang, D.M. Outline of Soil Microbial Biomass Measurement Methods. J. Microbiol. 2005, 25, 95–99. [Google Scholar]
- Zhang, X.; Amelung, W. Gas Chromatographic Determination of Muramic Acid, Glucosamine, Mannosamine, and Galactosamine in Soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- Jing, Y.; Ding, X.; Zhao, X.; Tian, P.; Xiao, F.; Wang, Q. Non-Additive Effects of Nitrogen and Phosphorus Fertilization on Microbial Biomass and Residue Distribution in a Subtropical Plantation. Eur. J. Soil Biol. 2022, 108, 103376. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, C.; Shao, S.; Chen, J.; Qin, H.; Xu, Q. Linkages of Litter and Soil C:N:P Stoichiometry with Soil Microbial Resource Limitation and Community Structure in a Subtropical Broadleaf Forest Invaded by Moso Bamboo. Plant Soil 2021, 465, 473–490. [Google Scholar] [CrossRef]
- Laganière, J.; Angers, D.A.; Paré, D. Carbon Accumulation in Agricultural Soils after Afforestation: A Meta-Analysis. Glob. Change Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Xu, W.; Wang, Y.; Wan, H.; Chen, D.; Tang, Z.; Tang, X.; Zhou, G.; Xie, Z.; et al. Plant Diversity Enhances Productivity and Soil Carbon Storage. Proc. Natl. Acad. Sci. USA 2018, 115, 4027–4032. [Google Scholar] [CrossRef]
- Liu, X.; Trogisch, S.; He, J.-S.; Niklaus, P.A.; Bruelheide, H.; Tang, Z.; Erfmeier, A.; Scherer-Lorenzen, M.; Pietsch, K.A.; Yang, B.; et al. Tree Species Richness Increases Ecosystem Carbon Storage in Subtropical Forests. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181240. [Google Scholar] [CrossRef]
- Pei, B.; Gao, G.R. Impact of Forest Litter Decomposition on Soil Carbon Pool: A Review. Chin. Agric. Sci. Bull. 2018, 34, 58–64. [Google Scholar]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial Necromass as the Source of Soil Organic Carbon in Global Ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Bailey, V.L.; Smith, J.L.; Bolton, H. Fungal-to-Bacterial Ratios in Soils Investigated for Enhanced C Sequestration. Soil Biol. Biochem. 2002, 34, 997–1007. [Google Scholar] [CrossRef]
- He, L.; Mazza Rodrigues, J.L.; Soudzilovskaia, N.A.; Barceló, M.; Olsson, P.A.; Song, C.; Tedersoo, L.; Yuan, F.; Yuan, F.; Lipson, D.A.; et al. Global Biogeography of Fungal and Bacterial Biomass Carbon in Topsoil. Soil Biol. Biochem. 2020, 151, 108024. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, Q.; Ding, X.L.; He, H.B.; Zhang, X.D. Research Progress on Accumulation, Turnover and Stabilization of Microbial Residues in Soil. Acta Pedol. Sin. 2022, 59, 1479–1491. [Google Scholar]
- Song, H.W. Effects of Soil Warming on Litter Decomposition in Subtropical Forests and Its Microbial Mechanism. Master’s Thesis, Fujian Normal University, Fuzhou, China, 2022. [Google Scholar]
- Li, T.; Zhang, J.; Wang, X.; Hartley, I.P.; Zhang, J.; Zhang, Y. Fungal Necromass Contributes More to Soil Organic Carbon and More Sensitive to Land Use Intensity than Bacterial Necromass. Appl. Soil Ecol. 2022, 176, 104492. [Google Scholar] [CrossRef]
- Zheng, T.; Miltner, A.; Liang, C.; Nowak, K.M.; Kästner, M. Turnover of Bacterial Biomass to Soil Organic Matter via Fungal Biomass and Its Metabolic Implications. Soil Biol. Biochem. 2023, 180, 108995. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, Q.; Noll, L.; Zhang, S.; Wanek, W. Direct Measurement of the in Situ Decomposition of Microbial-Derived Soil Organic Matter. Soil Biol. Biochem. 2020, 141, 107660. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Wei, L.; Wang, X.; Zhang, Q.; Guo, T.; Xu, X.; Zhao, N.; Xu, S. Variations in Microbial Residue Carbon and Its Contribution to Soil Organic Carbon after Vegetation Restoration on Farmland: The Case of Guinan County. Org. Geochem. 2024, 189, 104753. [Google Scholar] [CrossRef]
- Moritz, L.K.; Liang, C.; Wagai, R.; Kitayama, K.; Balser, T.C. Vertical Distribution and Pools of Microbial Residues in Tropical Forest Soils Formed from Distinct Parent Materials. Biogeochemistry 2009, 92, 83–94. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and Function of the Global Topsoil Microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Sokol, N.W.; Sanderman, J.; Bradford, M.A. Pathways of Mineral-Associated Soil Organic Matter Formation: Integrating the Role of Plant Carbon Source, Chemistry, and Point of Entry. Glob. Change Biol. 2019, 25, 12–24. [Google Scholar] [CrossRef]
- Jing, Y.L.; Li, X.H.; Zhang, Y.; Zhang, X.Y.; Liu, M.; Feng, Q.H. Effects of thinning on accumulation of soil microbial residue carbon of Picea asperata plantations in sub-alpine region of western Sichuan, China. Chin. J. Appl. Ecol. 2024, 35, 169–176. [Google Scholar] [CrossRef]
- Hu, J.W.; Liu, C.F.; Gou, M.M.; Chen, H.L.; Lei, L.; Xiao, W.F.; Zhu, S.F.; Hu, R.Y. Influencing mechanism of stand age to the accumulation of microbial residue carbon in the Pinus masso-niana plantations. Chin. J. Appl. Ecol. 2024, 35, 153–160. [Google Scholar] [CrossRef]
- Lu, X.; Hou, E.; Guo, J.; Gilliam, F.S.; Li, J.; Tang, S.; Kuang, Y. Nitrogen Addition Stimulates Soil Aggregation and Enhances Carbon Storage in Terrestrial Ecosystems of China: A Meta-Analysis. Glob. Chang. Biol. 2021, 27, 2780–2792. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, X.; Zhang, X.; Ju, W.; Duan, C.; Guo, X.; Wang, Y.; Fang, L. Soil Moisture Mediates Microbial Carbon and Phosphorus Metabolism during Vegetation Succession in a Semiarid Region. Soil Biol. Biochem. 2020, 147, 107814. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).