The Impact of Pollinating Fig Wasps’ Entry on Fig Development and the Hormonal Regulation of Sex Differentiation in Ficus hispida
Abstract
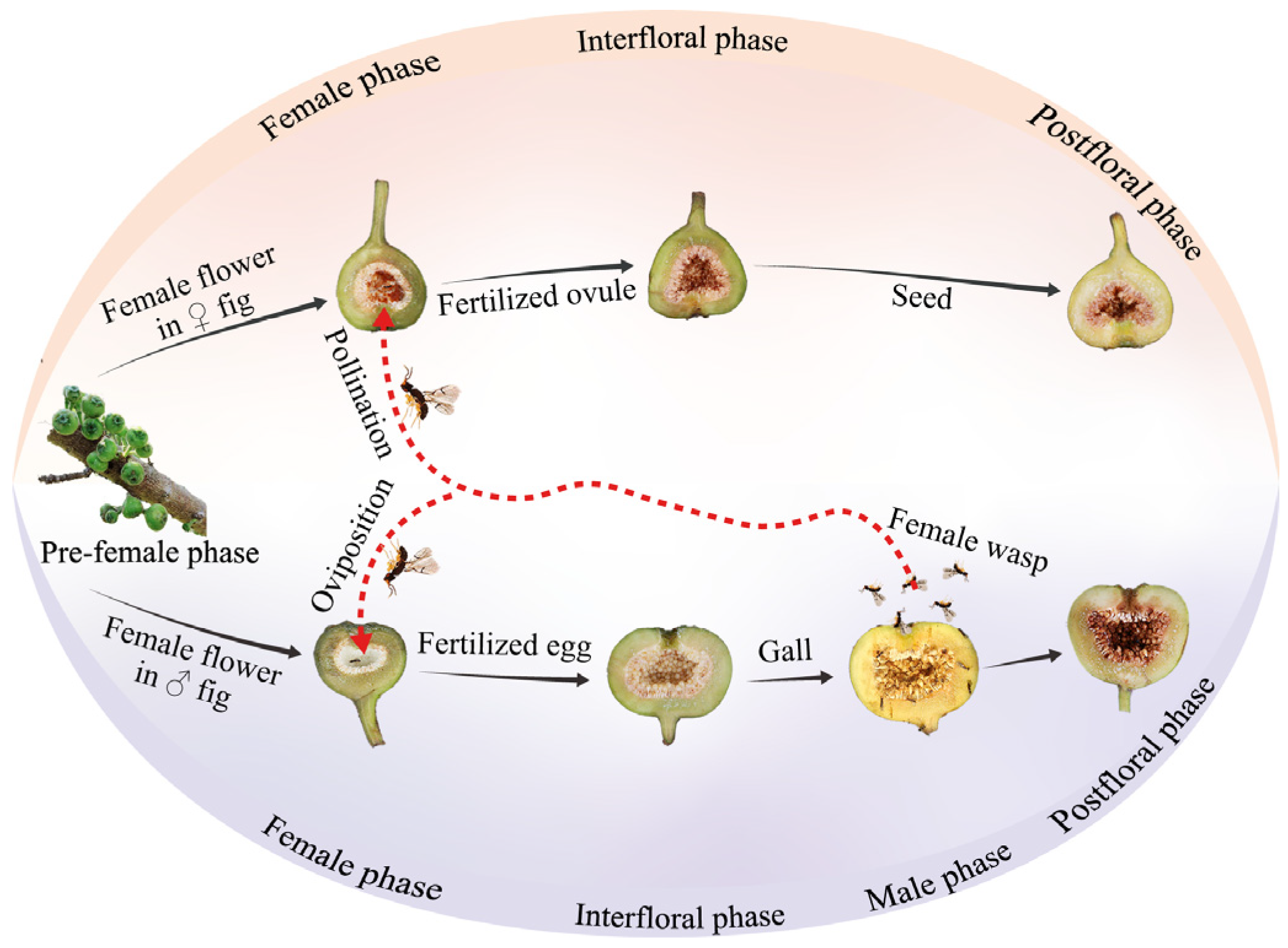
1. Introduction
2. Materials and Methods
2.1. Experimental Tree and Location
2.2. General Biology of Ficus hispida and Collection of Pollinating Fig Wasps
2.3. Identification of Female Phase
2.4. Introducing Pollinating Fig Wasps and Documenting Fig Abscission Rates
2.5. Collections of Plant Material
2.6. The Isolation and Purification of Endogenous Hormones
2.7. Determination of Endogenous Hormones
2.8. Data Analysis
3. Results
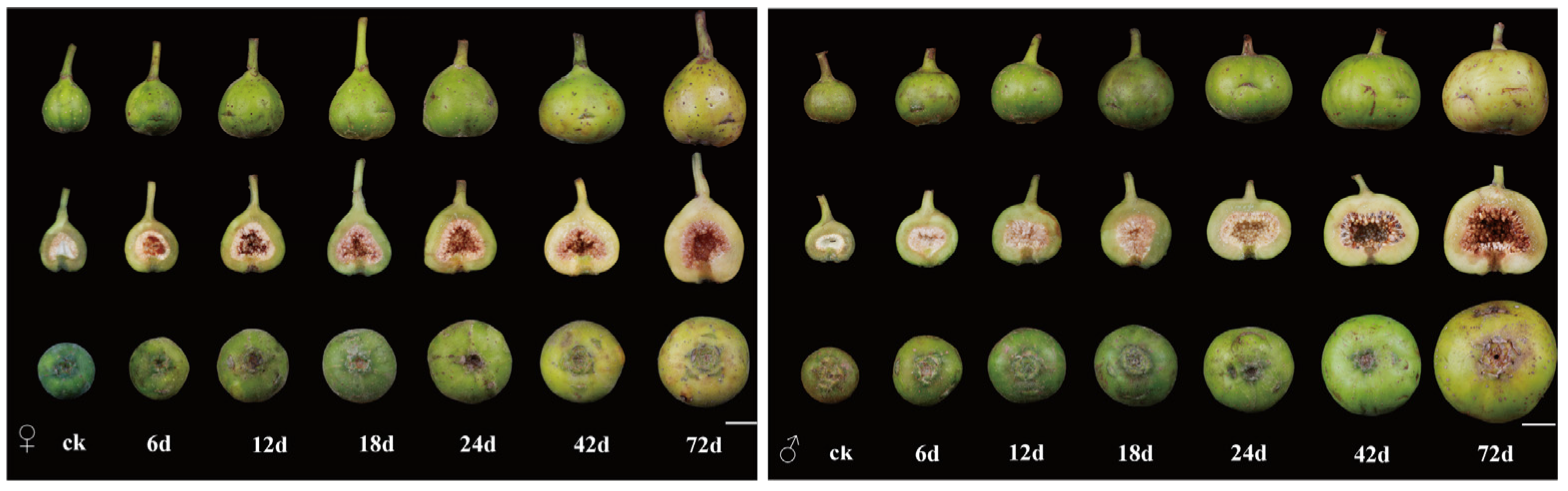
3.1. Developmental Processes and Morphological Characteristics of Male and Female Figs Following Wasp Entry
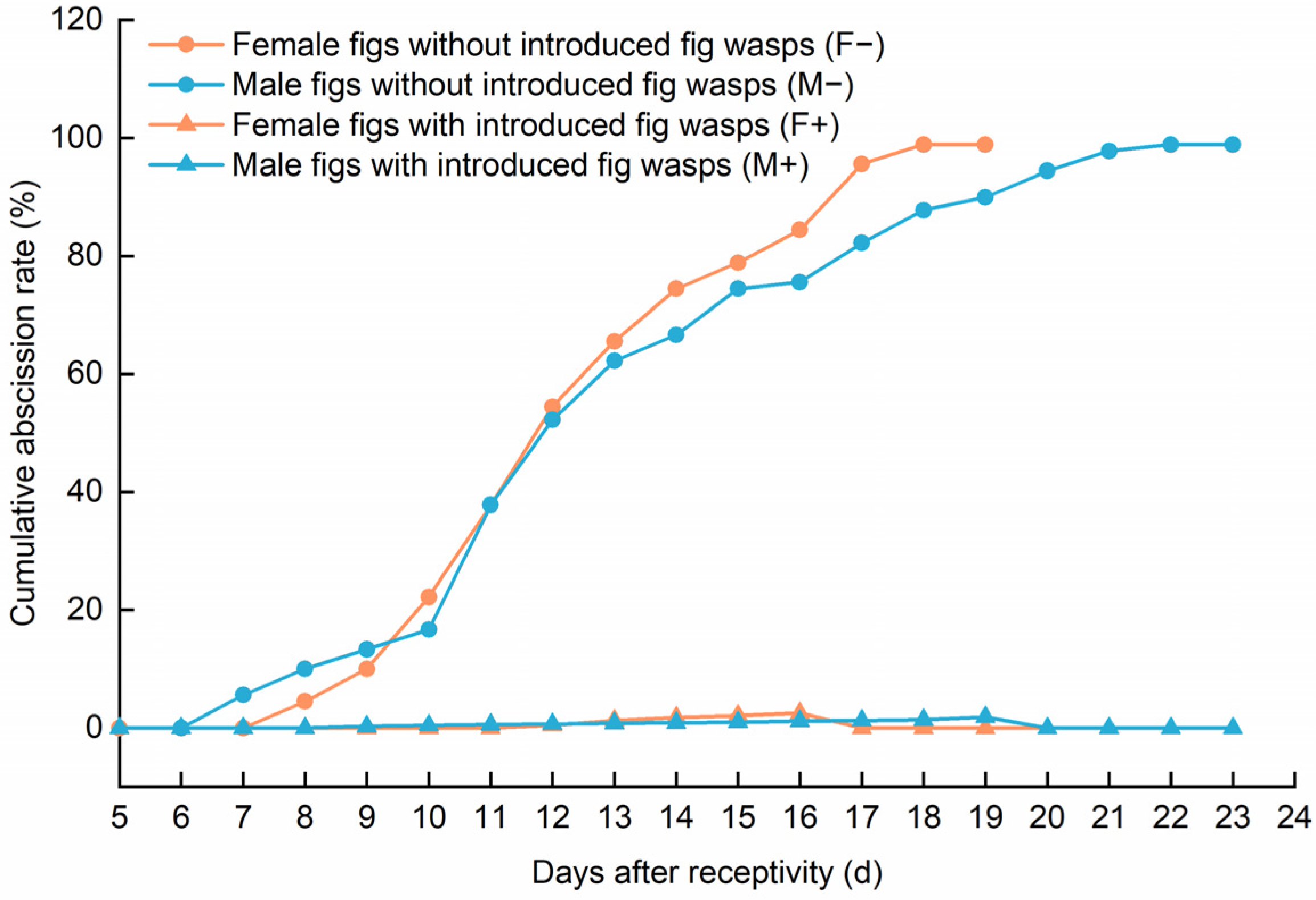
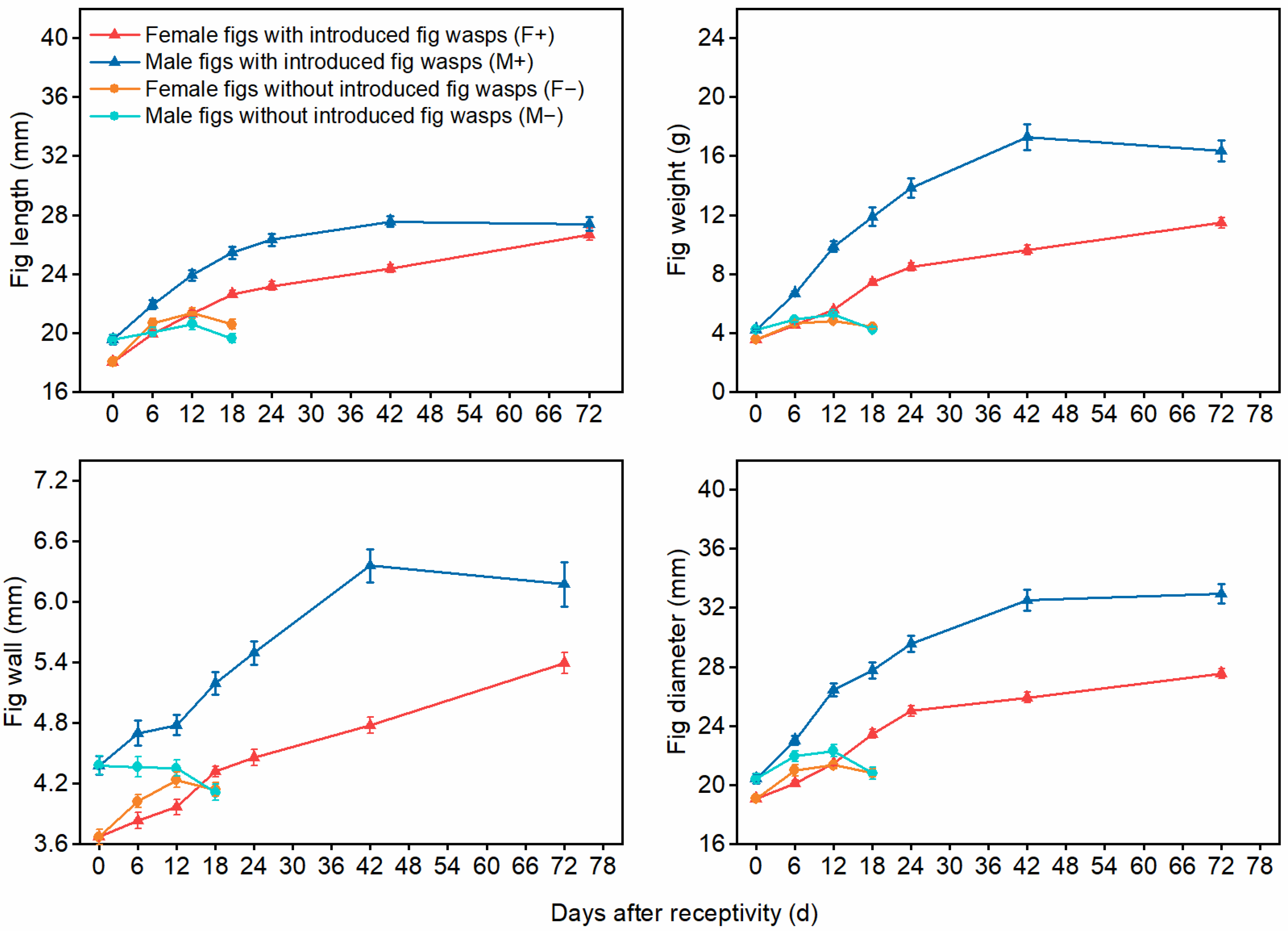
3.2. The Impact of Wasp Entry on Fig Abscission Rates and Developmental Indices for Male and Female Figs
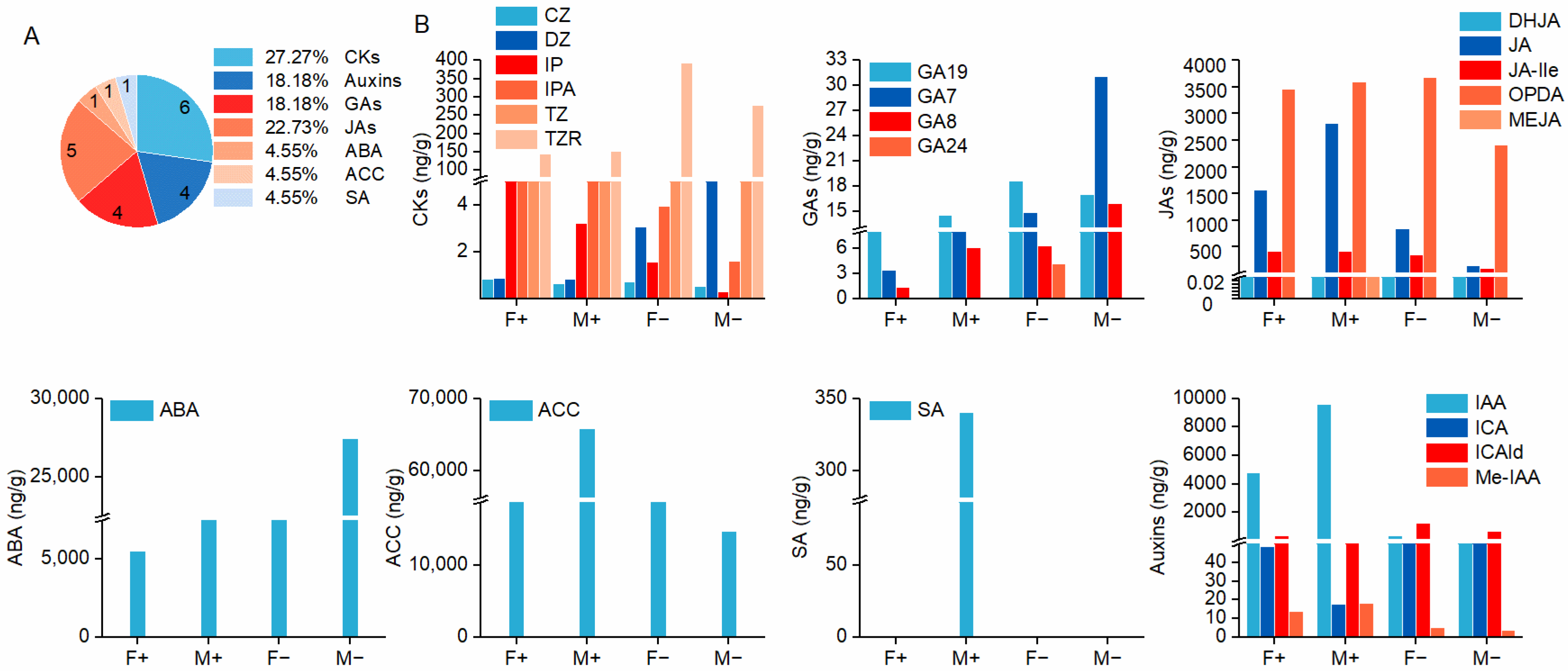
3.3. The Classification of Endogenous Phytohormones
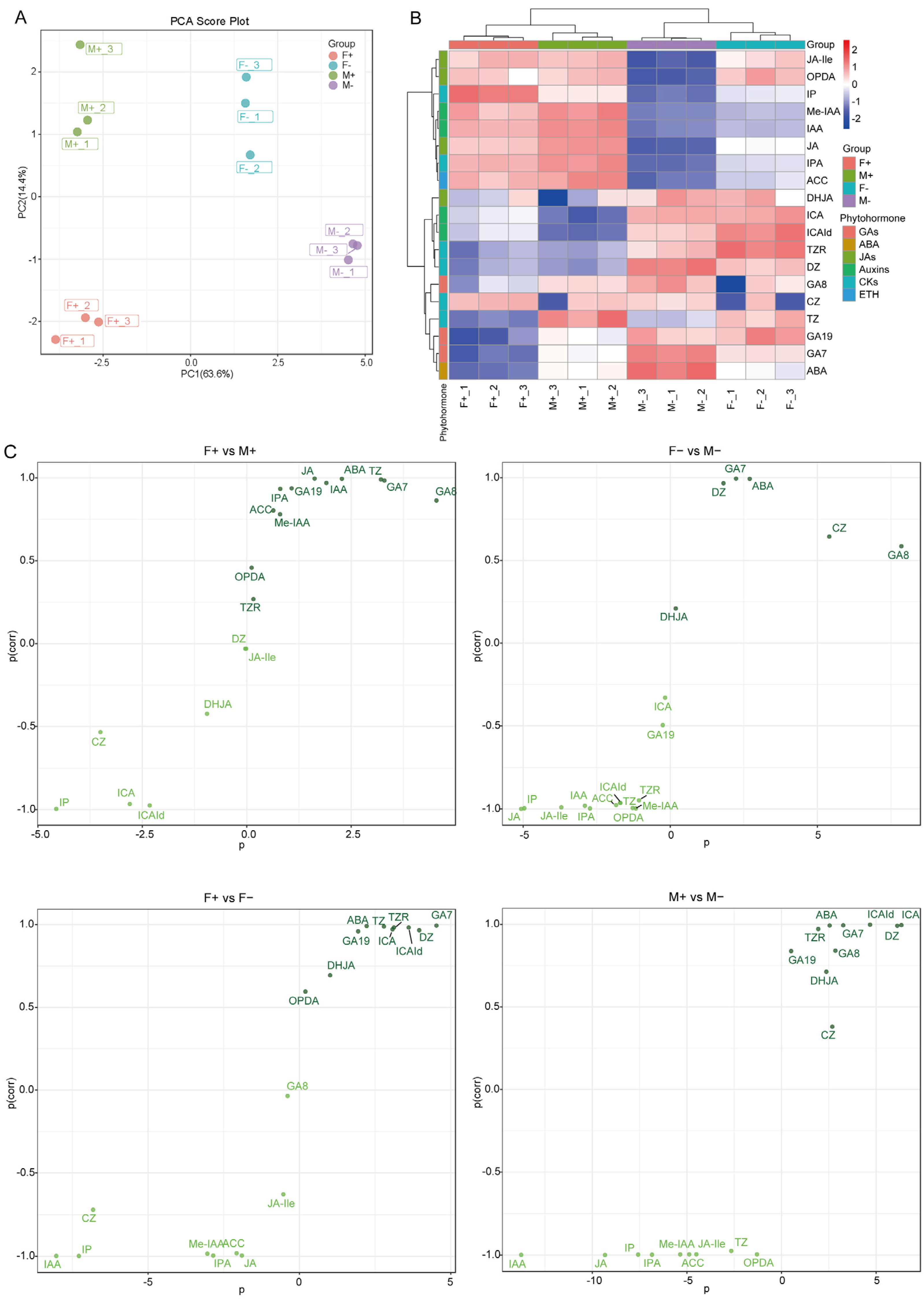
3.4. Multivariate Statistical Analysis of Hormone Metabolites in Male and Female Figs
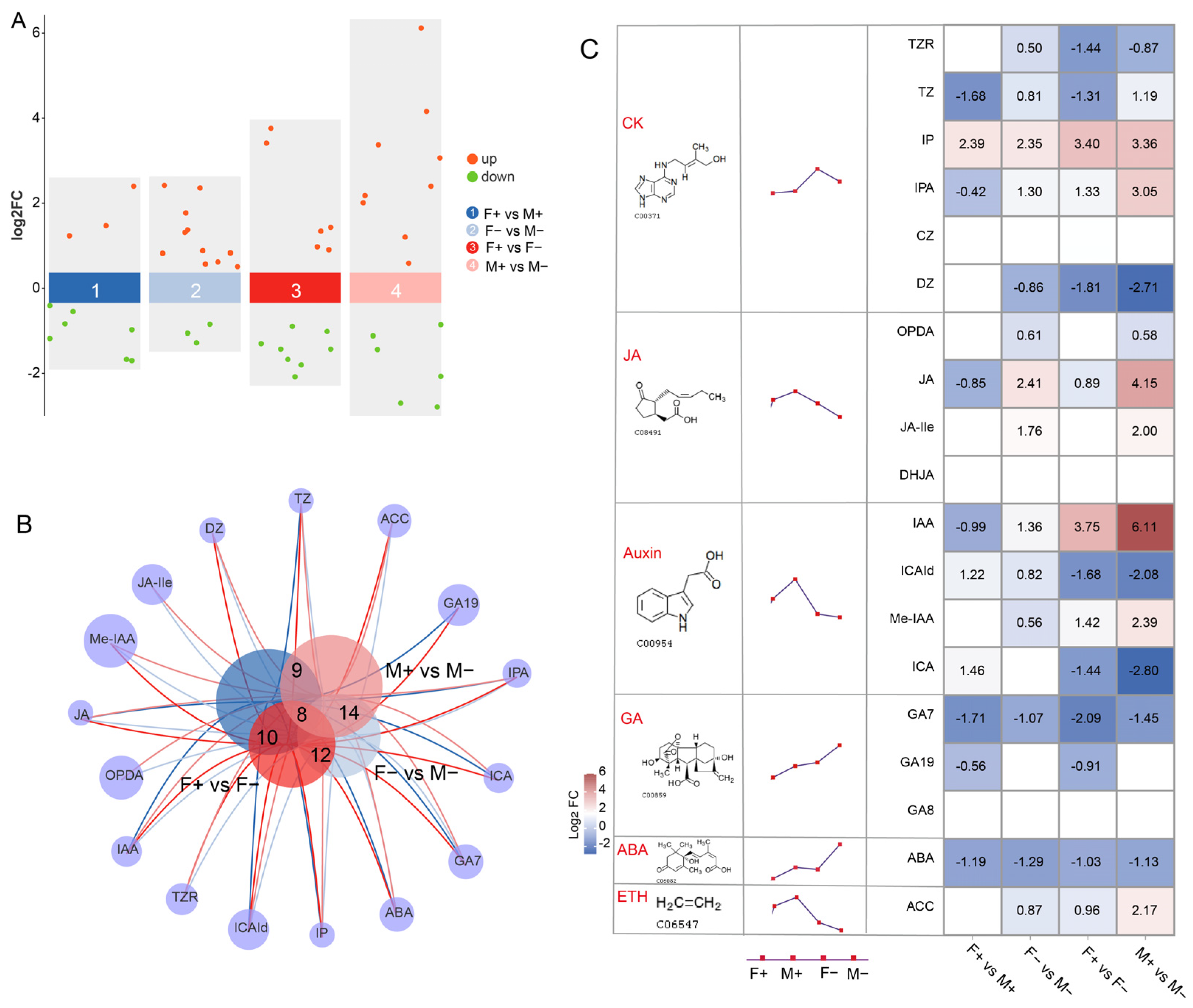
3.5. Comparative Analysis of Hormone Metabolites in Male and Female Figs
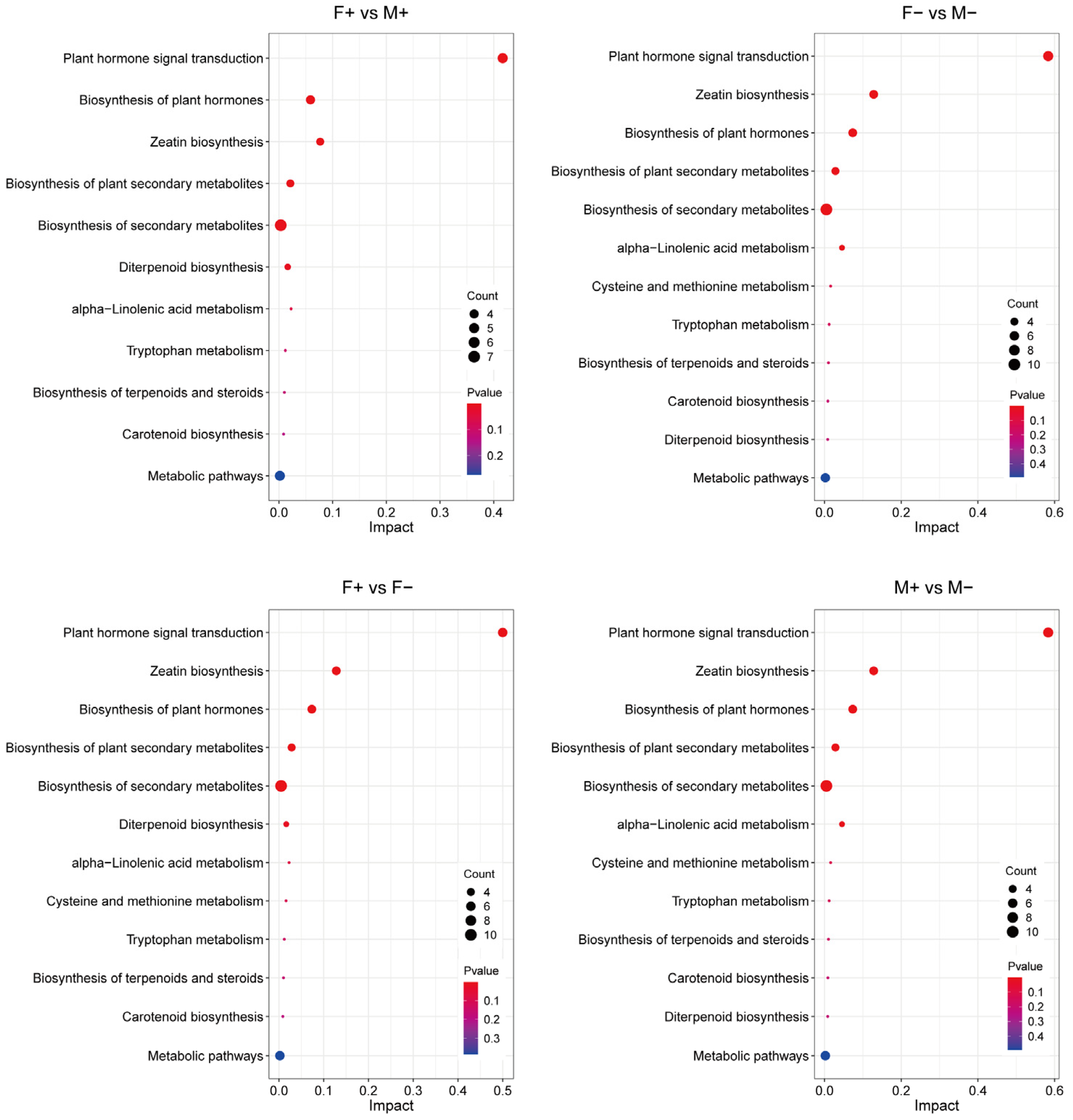
3.6. Analysis of KEGG Metabolic Pathways in Male and Female Figs
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruce, T.J. Interplay between insects and plants: Dynamic and complex interactions that have coevolved over millions of years but act in milliseconds. J. Exp. Bot. 2015, 66, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Whitney, H.M.; Glover, B.J. Coevolution: Plant-Insect. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2013. [Google Scholar]
- Botha, A.M. A coevolutionary conundrum: The arms race between Diuraphis noxia (Kurdjumov) a specialist pest and its host Triticum aestivum (L.). Arthropod-Plant Plant Interact. 2013, 7, 359–372. [Google Scholar] [CrossRef]
- Ehrlich, P.R.; Raven, P.H. Butterflies and plants: A study in coevolution. Evolution 1964, 18, 586–608. [Google Scholar] [CrossRef]
- Maron, J.L.; Agrawal, A.A.; Schemske, D.W. Plant-herbivore coevolution and plant speciation. Ecology 2019, 100, e02704. [Google Scholar] [CrossRef]
- McKenna, D.D.; Shin, S.; Ahrens, D.; Balke, M.; Beza-Beza, C.; Clarke, D.J.; Donath, A.; Escalona, H.E.; Friedrich, F.; Letsch, H.; et al. The evolution and genomic basis of beetle diversity. Proc. Natl. Acad. Sci. USA 2019, 116, 24729–24737. [Google Scholar] [CrossRef] [PubMed]
- Ashra, H.; Nair, S. Review: Trait plasticity during plant-insect interactions: From molecular mechanisms to impact on community dynamics. Plant. Sci. 2022, 317, 111188. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, Y.; Jing, Y.; Segar, S.T.; Zhang, Y.; Wang, G.; Chen, J.; Liu, Q.F.; Chen, S.; Chen, Y.; et al. Molecular mechanisms of mutualistic and antagonistic interactions in a plant-pollinator association. Nat. Ecol. Evol. 2021, 5, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.R.; Peng, Y.Q.; Song, Q.S.; Zhang, G.M.; Wang, R.W.; Zhao, T.Z.; Wang, Q.Y. Pollination biology of Ficus hispida in the tropical rainforests of Xishuangbanna, China. Acta Bot. Sin. 2002, 44, 519–526. [Google Scholar]
- Wiebes, J.T. Co-evolution of figs and their insect pollinators. Annu. Rev. Ecol. Syst. 1979, 10, 1–12. [Google Scholar] [CrossRef]
- Corlett, R.T. Figs (Ficus, Moraceae) in urban Hong Kong, South China. Biotropica 2006, 38, 116–121. [Google Scholar] [CrossRef]
- Yuan, S.; Yin, T.; He, H.; Liu, X.; Long, X.; Dong, P.; Zhu, Z. Phenotypic, metabolic and genetic adaptations of the Ficus species to abiotic stress response: A comprehensive review. Int. J. Mol. Sci. 2024, 25, 9520. [Google Scholar] [CrossRef]
- Janzen, D.H. How many parents do the wasps from a fig have? Biotropica 1979, 11, 127–129. [Google Scholar] [CrossRef]
- Weiblen, G.D. How to be a fig wasp. Annu. Rev. Entomol. 2002, 47, 299–330. [Google Scholar] [CrossRef]
- Cruaud, A.; Rønsted, N.; Chantarasuwan, B.; Chou, L.S.; Clement, W.L.; Couloux, A.; Cousins, B.; Genson, G.; Harrison, R.D.; Hanson, P.E.; et al. An extreme case of plant-insect codiversification: Figs and fig-pollinating wasps. Syst. Biol. 2012, 61, 1029–1047. [Google Scholar] [CrossRef]
- Anstett, M.C.; Bronstein, G.L.; Hossacrt-McKcy, M. Resource allocation: A conflict in the fig/fig wasp mutualism. J. Evol. Biol. 1996, 9, 417–428. [Google Scholar] [CrossRef]
- Anstett, M.C. Unbeatable strategy, constraint and coevolution, or how to resolve evolutionary conflicts: The case of the fig/wasp mutualism. Oikos 2001, 95, 476–484. [Google Scholar] [CrossRef]
- Derek, W.D. Stability in fig tree-fig wasp mutualisms: How to be a cooperative fig wasp. Biol. J. Linn. Soc. Lond. 2020, 130, 1–17. [Google Scholar]
- Chetty, A.; Glennon, K.L.; Venter, S.M.; Cron, G.V.; Witkowsk, E.T.F. Reproductive ecology of the African baobab: Floral features differ among individuals with different fruit production. Forest Ecol. Manag. 2021, 489, 119077. [Google Scholar] [CrossRef]
- Xia, Z.; Chen, B.J.W.; Korpelainen, H.; Niinemets, Ü.; Li, C. Belowground ecological interactions in dioecious plants: Why do opposites attract but similar ones repel? Trends Plant Sci. 2024, 29, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Korpelainen, H.; Li, C.Y. Sexual differences and sex ratios of dioecious plants under stressful environments. J. Plant Ecol. 2021, 14, 920–933. [Google Scholar] [CrossRef]
- Miao, B.G.; Liu, M.X.; Wang, B.; Peng, Y.Q.; Lesne, A.; Kjellberg, F.; Jandér, K.C. Active pollination in a functionally dioecious Ficus species: An interplay between pollinator behaviour and floral morphology. Flora 2023, 302, 152274. [Google Scholar] [CrossRef]
- Martinson, E.O.; Hackett, J.D.; Machado, C.A.; Arnold, A.E. Metatranscriptome analysis of fig flowers provides insights into potential mechanisms for mutualism stability and gall induction. PLoS ONE 2015, 10, e0130745. [Google Scholar] [CrossRef]
- Zidi, K.; Kati, D.E.; Bachir-bey, M.; Genva, M.; Fauconnier, M.L. Comparative study of fig volatile compounds using headspace solid-phase microextraction-gas chromatography / mass spectrometry: Effects of cultivars and ripening stages. Front. Plant Sci. 2021, 12, 667809. [Google Scholar] [CrossRef]
- Malik, N.U.A.; Fajer, O.; Amin, L.; Khalid, A.R.; Khan, N.; Bhatti, M.F.; Munir, F.; Haider, G.; Amir, R.; Gul, A. Phytohormones, plant growth and development. In Phytohormones and Stress Responsive Secondary Metabolites; Academic Press: Cambridge, MA, USA, 2023; pp. 175–186. [Google Scholar]
- Nambara, E.; Van Wees, S.C.M. Plant hormone functions and interactions in biological systems. Plant J. 2021, 105, 287–289. [Google Scholar] [CrossRef]
- Izawa, T. What is going on with the hormonal control of flowering in plants? Plant J. 2021, 105, 431–445. [Google Scholar] [CrossRef]
- Chandler, J.W. The hormonal regulation of flower development. J. Plant Growth Regul. 2011, 30, 242–254. [Google Scholar] [CrossRef]
- Chai, L.; Chai, P.; Chen, S.; Flaishman, M.A.; Ma, H. Transcriptome analysis unravels spatiotemporal modulation of phytohormone-pathway expression underlying gibberellin-induced parthenocarpic fruit set in San Pedro-type fig (Ficus carica L.). BMC Plant Biol. 2018, 18, 100. [Google Scholar] [CrossRef]
- Lama, K.; Harlev, G.; Shafran, H.; Peer, R.; Flaishman, M.A. Anthocyanin accumulation is initiated by abscisic acid to enhance fruit color during fig (Ficus carica L.) ripening. J. Plant Physiol. 2020, 251, 153192. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kobayashi, Y.; Yamamoto, S.; Daimon, Y.; Yamaguchi, A.; Ikeda, Y.; Ichinoki, H.; Notaguchi, M.; Goto, K.; Araki, T. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 2005, 309, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Obeso, J.R. The costs of reproduction in plants. New Phytol. 2002, 155, 321–348. [Google Scholar] [CrossRef] [PubMed]
- Nefdt, R.J.; Compton, S.G. Regulation of seed and pollinator production in the fig-fig wasp mutualism. J. Anim. Ecol. 1996, 65, 170–182. [Google Scholar] [CrossRef]
- Shi, Z.H.; Yang, D.R.; Peng, Y.Q. The style-length of the female florets and their fate in two dioecious species of Xishuangbanna, China. Trees 2006, 20, 410–415. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, T.; Lei, W.; Wang, Y.; Yu, J.; Wang, Y.; Chai, K.; Wang, G.; Zhang, H.; Zhang, X. A telomere-to-telomere reference genome of ficus (Ficus hispida) provides new insights into sex determination. Hortic. Res. 2023, 11, uhad257. [Google Scholar] [CrossRef]
- Yang, W.; Xiang, P. Changes of fruit abscission and carbohydrates, hormones, related gene expression in the fruit and pedicel of macadamia under starvation stress. Horticulturae 2022, 8, 398. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, C.; Yan, X.; Zhang, J.; Xu, J. Simultaneous analysis of ten phytohormones in Sargassum horneri by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. J. Sep. Sci. 2016, 39, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.; Wang, Y.; Yan, S.; Shi, J.; Huang, W.; Zayed, M.Z.; Peng, C.; Chen, X.; Wu, G. Simultaneous analysis of endogenous plant growth substances during floral sex differentiation in Jatropha curcas L. using HPLC-ESI-MS/MS. Sci. Hortic. 2018, 241, 209–217. [Google Scholar] [CrossRef]
- Šimura, J.; Antoniadi, I.; Široká, J.; Tarkowská, D.; Strnad, M.; Ljung, K.; Novák, O. Plant hormonomics: Multiple phytohormone profiling by targeted metabolomics. Plant Physiol. 2018, 177, 476–489. [Google Scholar] [CrossRef]
- Wei, L.; Lu, Z.; Wang, Y.; Gao, G. The effects of globose scale (Sphaerolecanium prunastri) infestation on the growth of wild apricot (Prunus armeniaca) trees. Forests 2023, 14, 2032. [Google Scholar] [CrossRef]
- Srivastava, A.; Handa, A.K. Hormonal regulation of tomato fruit development: A molecular perspective. J. Plant Growth Regul. 2005, 24, 67–82. [Google Scholar] [CrossRef]
- Osianski, Y.; Doron-Faigenboim, A.; Freiman, Z.E.; Lama, K.; Milo-Cochavi, S.; Dahan, Y.; Kerem, Z.; Flaishman, M.A. Tissue-specific transcriptome and hormonal regulation of pollinated and parthenocarpic fig (Ficus carica L.) fruit suggest that fruit ripening is coordinated by the reproductive part of the syconium. Front. Plant Sci. 2016, 7, 1696. [Google Scholar]
- Zhang, Y.; Yang, D.R.; Peng, Y.Q.; Compton, S.G. Costs of inflorescence longevity for an Asian fig tree and its pollinator. Evol. Ecol. 2012, 26, 513–527. [Google Scholar] [CrossRef]
- Ahmad, S.; Lu, C.; Gao, J.; Wei, Y.; Xie, Q.; Jin, J.; Zhu, G.; Yang, F. Integrated proteomic, transcriptomic, and metabolomic profiling reveals that the gibberellin-abscisic acid hub runs flower development in the Chinese orchid Cymbidium sinense. Hortic. Res. 2024, 11, uhae073. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ma, N.; Jia, Y.; Zhang, Y.; Feng, M.; Jiang, C.Z.; Ma, C.; Gao, J. An ethylene-induced regulatory module delays flower senescence by regulating cytokinin content. Plant Physiol. 2016, 173, 853–862. [Google Scholar] [CrossRef]
- Tsuji, K.; Kobayashi, K.; Hasegawa, E.; Yoshimura, J. Dimorphic flowers modify the visitation order of pollinators from male to female flowers. Sci. Rep. 2020, 10, 9965. [Google Scholar] [CrossRef] [PubMed]
- Boualem, A.; Troadec, C.; Camps, C.; Lemhemdi, A.; Morin, H.; Sari, M.A.; Fraenkel-Zagouri, R.; Kovalski, I.; Dogimont, C.; Perl-Treves, R. A cucurbit androecy gene reveals how unisexual flowers develop and dioecy emerges. Science 2015, 350, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, D. Roles of jasmonate signalling in plant inflorescence and flower development. Curr. Opin. Plant Biol. 2015, 27, 44–51. [Google Scholar]
- Luo, Y.; Pan, B.Z.; Li, L.; Yang, C.X.; Xu, Z.F. Developmental basis for flower sex determination and effects of cytokinin on sex determination in Plukenetia volubilis (Euphorbiaceae). Plant Reprod. 2020, 33, 21–34. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.Y.; Zhou, P.; Liao, Z.; Lin, H.; Yu, Q.; Ming, R. Sex biased expression of hormone related genes at early stage of sex differentiation in papaya flowers. Hortic. Res. 2021, 8, 147. [Google Scholar] [CrossRef]
- Segura, M.; García, A.; Benítez, Á.; Martínez, C.; Jamilena, M. Comparative RNA-Seq analysis between monoecious and androecious plants reveals regulatory mechanisms controlling female flowering in Cucurbita pepo. Int. J. Mol. Sci. 2023, 24, 17195. [Google Scholar] [CrossRef]
- Li, W.; Fu, W.; Hou, J.; Yang, Y.; Yin, T. Evolution of plant sex and molecular mechanisms underlying plants sex separation. For. Res. 2023, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Godoy, F.; Kühn, N.; Muñoz, M.; Marchandon, G.; Gouthu, S.; Deluc, L.; Delrot, S.; Lauvergeat, V.; Arce-Johnson, P. The role of auxin during early berry development in grapevine as revealed by transcript profiling from pollination to fruit set. Hortic. Res. 2021, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Hilker, M.; Fatouros, N.E. Plant responses to insect egg deposition. Annu. Rev. Entomol. 2015, 60, 493–515. [Google Scholar] [CrossRef] [PubMed]
- Lortzing, V.; Valsamakis, G.; Jantzen, F.; Hundacker, J.; Paniagua Voirol, L.R.; Schumacher, F.; Kleuser, B.; Hilker, M. Plant defensive responses to insect eggs are inducible by general egg-associated elicitors. Sci. Rep. 2024, 14, 1076. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.; Pickett, J.A. Plant defence signalling induced by biotic attacks. Curr. Opin. Plant Biol. 2007, 10, 387–392. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.; Zhang, Y.; Li, Z.; Wang, Y.; Chen, C.; Yang, X.; Gao, J.; Miao, B.; Peng, Y.; Zhang, Y. The Impact of Pollinating Fig Wasps’ Entry on Fig Development and the Hormonal Regulation of Sex Differentiation in Ficus hispida. Forests 2025, 16, 286. https://doi.org/10.3390/f16020286
Guan Y, Zhang Y, Li Z, Wang Y, Chen C, Yang X, Gao J, Miao B, Peng Y, Zhang Y. The Impact of Pollinating Fig Wasps’ Entry on Fig Development and the Hormonal Regulation of Sex Differentiation in Ficus hispida. Forests. 2025; 16(2):286. https://doi.org/10.3390/f16020286
Chicago/Turabian StyleGuan, Yunfang, Ying Zhang, Zongbo Li, Yan Wang, Changqi Chen, Xiaoyan Yang, Jinxia Gao, Baige Miao, Yanqiong Peng, and Yuan Zhang. 2025. "The Impact of Pollinating Fig Wasps’ Entry on Fig Development and the Hormonal Regulation of Sex Differentiation in Ficus hispida" Forests 16, no. 2: 286. https://doi.org/10.3390/f16020286
APA StyleGuan, Y., Zhang, Y., Li, Z., Wang, Y., Chen, C., Yang, X., Gao, J., Miao, B., Peng, Y., & Zhang, Y. (2025). The Impact of Pollinating Fig Wasps’ Entry on Fig Development and the Hormonal Regulation of Sex Differentiation in Ficus hispida. Forests, 16(2), 286. https://doi.org/10.3390/f16020286





