Abstract
The alien leaf miner Cameraria ohridella (Lepidoptera, Gracillariidae) is damaging horse chestnuts in European countries. Since native natural enemies cannot control the moth, integrated control strategies must be implemented. In north-eastern Italy, from 1997 to 2020, sampling was performed on 55 sites to record the presence of and estimate the leaf damage caused by C. ohridella and the fungus Guignardia aesculi. The level of leaf removal from the ground in autumn was estimated, and information on altitude (199–1294 m a.s.l.), average annual temperature (4.4–11.9 °C) and rainfall (954–1394 mm), and the occurrence of trunk injection with abamectin was collected. Damage caused by the leaf miner and the fungus declined with decreasing temperatures, with negligible damage at sites with average annual temperatures below 7 °C (above 1000 m a.s.l. in the study area). Since, in the study sites, complete leaf removal provided comparable efficacy to trunk injections with abamectin, we suggest adopting this environmentally friendly practice to maintain C. ohridella below damaging levels. Interspecific competition occurred between C. ohridella and G. aesculi because leaf damage from one competitor decreased as damage from the other increased.
1. Introduction
The horse chestnut leaf miner, Cameraria ohridella Deschka and Dimić (Lepidoptera, Gracillariidae), which was first reported as causing damage to Aesculus hippocastanum L. (Sapindales: Sapindaceae) in Macedonia in the 1980s [1], spread to most European countries in only a few years, including Italy [2,3]. After a long debate on its origin [1,4,5], it has been definitively established on the basis of ancient herbarium collections and molecular analysis that the moth originates from natural horse chestnut stands located in the Balkan Mountains [6,7,8].
Cameraria ohridella lays eggs on all species of Aesculus, but complete larval development is observed only on A. hippocastanum and the phylogenetically closely related Aesculus spp. [9,10], as well as on Acer spp. [5,11]. The leaf miner overwinters in the pupal stage in dead fallen leaves and completes two to four generations a year depending on the temperature conditions, which, in turn, depend on latitude and altitude [3,12,13,14,15].
The larvae of C. ohridella dig blotch mines in leaf mesophyll, causing aesthetic damage at first, and when the entire leaf surface is covered with mines, early leaf fall can occur, sometimes followed by a second flowering [1,3,16]. Leaf damage can compromise the plants’ vitality, growth and reproduction [17,18,19]. However, in urban areas, total defoliation does not seem to affect the survival and growth of mature trees [17,20], but the possibility that C. ohridella infestations increase the susceptibility of horse chestnut trees to some diseases cannot be excluded [21,22]. Disposing of leaves that have already fallen in summer and replacing the horse chestnut with other plant species entails high costs for private entities and municipal administrations [23].
The infestation level and damage by the leaf miner decrease with altitude, both in urban areas [3] and in natural stands [24], especially due to the lower generation number.
In areas where C. ohridella has been introduced, native natural enemies have adapted to feed on the leaf miner but cannot keep moth populations below damage levels. Cameraria ohridella larvae are commonly preyed upon by insects, spiders and birds [14,25,26,27] and parasitized by hymenopteran parasitoids, particularly Eulophidae [3,28,29,30,31,32,33]. Pathogen fungi are important mortality factors, mostly for overwintering pupae [34,35,36]. An inter-specific competition between C. ohridella and the fungus Guignardia aesculi (Peck) V. B. Stewart (Ascomycota, Botryosphaeriaceae) has been reported [37,38]. Leaf infections by the fungus Erysiphe flexuosa (Peck) U. Braun & S. Takamatsu (Ascomycota, Erysiphales) reduce moth egg-laying [39].
To maintain C. ohridella below damage levels, chemical and cultural control methods have been adopted. Chemical control has been implemented by aerial spraying [40], soil injection [41] and trunk injections, with the latter preferred for the fewer negative side effects on the environment and human health [42,43,44,45]. Cultural control strategies include the removal from the ground of leaves carrying overwintering pupae [16,46,47] and the application of sticky bands on tree trunks [48].
Guignardia aesculi, as well as other foliar fungal pathogens, can cause foliage loss and disfigurement of ornamental trees [49,50]. To control G. aesculi, fungicides, also combined with induced resistance agents, and leaf removal were reported [50,51].
Since native natural enemies cannot keep the leaf miner below damage levels, knowing the relative importance of factors influencing C. ohridella infestations and damage is essential to implementing integrated control strategies. In this context, a multi-year study was conducted on C. ohridella infestations. The first aim was to record the spread of the leaf miner over the years in newly introduced areas to verify whether the moth would colonize A. hyppocastanum in all sites where the plant was recorded. The second aim was to verify how temperature (altitude) influences leaf miner damage, considering their influence on the number of moth generations per year. The third objective was to verify how much the removal of leaves contributes to reducing the damage caused by C. ohridella. The fourth aim was to determine the persistent effect over the years of abamectin injections in the trunk on leaf miner infestation and, for this reason, samplings were extended over several years, also evaluating the effects on egg-laying and mortality of newly-hatched larvae. The multi-year study also had the aim of verifying whether the infestation level by C. ohridella would decrease over the years, assuming an improvement in the biological control of the pest. Since A. hyppocastanum leaves were also damaged by G. aesculi, the influence of climatic conditions and leaf removal on the fungus were also evaluated.
2. Materials and Methods
2.1. Localities and Sites
This study was conducted in north-eastern Italy and covered a period of twenty-four years (from 1997 to 2020) and 25 localities (55 sampling sites) belonging to the regions of Friuli Venezia Giulia (Udine district, UD) and Veneto (Belluno district, BL) (Figure S1, Tables S1 and S2 of the Supplementary Materials). The names of the localities usually coincided with the municipalities, but sampling sites within the same municipality placed at very different altitudes were considered independent localities.
In all 25 localities, sampling was carried out in 1997–2001, 2005, 2012 and 2016. The three years between 2017–2019 were also considered in only eight localities, and in one of these sampling was extended until 2020 (Table S1 of the Supplementary Materials).
The sampled sites were distinguished for altitude, climatic conditions (i.e., average annual temperature and rainfall) and the level of removal of fallen leaves from the ground in autumn (Table S2 of the Supplementary Materials). Climatic data (i.e., average annual temperature and average annual rainfall) for the different localities were obtained from the “https://it.climate-data.org” website (accessed on 11 April 2024).
In some sites, C. ohridella was not present when the study began. In six sites, the level of leaf removal varied over the years because the site transitioned from uncultivated (no leaf removal) to managed (60%–90% leaf removal) or vice versa (Table S2 of the Supplementary Materials). Chemical control with trunk injections was adopted in six localities and different years (Table S2 of the Supplementary Materials: locality N. 2 in 2003; N. 6 in 2003; N. 9 in 2003; N. 13 in 2001; N. 15 in 2003, 2007, 2012; N. 16 in 2003). The active ingredient applied was abamectin (Vertimec ECs, Syngenta Italia S.p.A., Milano, Italy), which was forced through injection holes of 3.8 mm into the trunk under a controlled pressure of about 3–4 atm using a compressor with a pump. No specific practices were adopted to control G. aesculi in the study area.
Therefore, based on the data collected, each locality gave information on one or more of the following aspects regarding C. ohridella: (i) the progression of leaf miner damage over the years in newly colonized areas; (ii) the influence of leaf removal, altitude, climatic conditions and competitors (C. ohridella or G. aesculi) on leaf miner and fungus damage; and (iii) the influence of trunk injections with abamectin on leaf miner infestation over the years (Table S1 of the Supplementary Materials).
2.2. Sampling over the Years in Different Localities and Sites Within Localities
In the different sites and years, samplings were carried out to estimate the leaf surface damage by C. ohridella and G. aesculi and the level of leaf removal.
Samplings were always conducted on the same plants and by the same two members of the research team at the beginning of July and October to estimate the leaf surface covered by C. ohridella mines and G. aesculi necrotic spots. Based on the sampling method of Gilbert and Grégoire [52], which was appropriately modified, the following classes of leaf surface covered by C. ohridella leaf mines were considered: 1 = 0% (no leaf mines found), 2 = 0.0%–0.1% (very few leaf mines), 3 = 0.1%–1% (a few leaf mines), 4 = 1%–5%, 5 = 6%–10%, 6 = 11%–25%, 7 = 26%–50%, 8 = 51%–75%, 9 = 76%–100% and 10 = 100%. Attribution to the first two classes was made by carefully examining the crowns of the trees from the ground, also with the aid of binoculars. Attribution to the 1%–5% to 51%–75% classes was made by the two samplers collecting and observing the leaves of 5 branches (collected from 5 trees, when the number of plants allowed); if the assigned class was different between the two samplers, another 5 branches were sampled. The 76%–100% infestation class was assigned when most of the leaves had fallen, and class 100% was assigned in the case of total anticipated leaf fall and re-flowering. The same classes used for C. ohridella infestation were utilized to estimate the leaf surface covered by G. aesculi necrotic spots.
To establish the leaf removal level, a survey was carried out at the end of winter before the horse chestnut trees reached the budding stage. Five levels of leaf removal from the ground were considered: 0%, 30%, 60%, 90% and 100%. The latter level was assigned to horse chestnuts growing in fully concreted or asphalted sites without flowerbeds or hedges and from which fallen leaves could not be collected.
Multiple regression analyses were performed to establish the influence of different variables on leaf damage by C. ohridella and G. aesculi. The variables of altitude, average annual temperature, average annual rainfall and leaf removal level were considered for both the leaf miner and the fungus. Moreover, for each of the competitors, the influence of the other leaf damage agent was evaluated (i.e., G. aesculi leaf damage for C. ohridella and vice versa). For the leaf damage values, the average leaf surface with symptoms of the four sampling years (2001, 2005, 2012 and 2016) was calculated for 46 out of 55 sites, i.e., excluding sites whose variability in leaf damage among the years was influenced by the effects of trunk injection with abamectin or variations in leaf removal levels. To calculate the average leaf damage by C. ohridella and G. aesculi over the years, an intermediate value was assigned to each class (i.e., 0%, 0.05%, 0.5%, 3%, 8%, 18%, 38%, 63%, 87.5% and 100%). The independent variables that determined a close correlation in the Pearson correlation analysis were not considered in the same multiple regression analysis. Before performing multiple regression analyses, normality and homoscedasticity were verified. For significant independent variables, the percentage contribution to the total variability was calculated by dividing each variable’s SRC (standardized coefficient of regression) by the sum of the SRCs of all variables and multiplying by 100. The SRC of each independent variable was calculated by multiplying the slope of each independent variable by its standard deviation and dividing by the standard deviation of the dependent variable.
Multiple regression analyses and Pearson’s correlation were performed using Statistics Kingdom’s 2017 online tool [53].
For sites at four localities (N. 9, 14, 15 and 17), the dynamics over the years of C. ohridella damage were plotted to represent the effects of trunk injections with abamectin and the variation in leaf removal levels.
2.3. Specific Study on Long-Lasting Effects of Trunk Injections
In locality N. 13, on 13 August 2019, five branches were collected from five horse chestnut trees (one branch per plant) subjected to chemical control with trunk injections in 2001 and five branches from plants that were never treated. The leaves of the former group of branches had no C. ohridella mines, whereas the latter had some mines. The two groups of branches were separately enclosed in plastic bags, labelled and kept cool until they were transferred to the laboratory, where each branch was individually placed in a clear glass jar of water. For the comparison, selected leaves were removed from each branch to make the leaf numbers and sizes uniform. Then, each of the ten potted branches was placed inside individual cubic plastic cages (sides 30 cm), the interiors of which were accessible from one side via a tulle net that opened and closed with a clothespin. Approximately 30 adults of C. ohridella were released into each cage. These adults were collected on the same day from the bark of an infested horse chestnut tree using a mouth aspirator. The collected adults were divided into 10 groups of around 30 adults, with each group enclosed separately in plastic bags containing a leaf and stored at a cool temperature until transferred to the plastic cages. After three days, the eggs laid on the leaves of each branch were counted. A t-test was used to compare the number of eggs in the two branch groups.
In locality N. 13, on 8 October 2019, five branches were collected from five plants (one branch per plant) subjected to chemical control with trunk injections in 2001. Each branch was enclosed separately in a plastic bag, labelled and stored at a cool temperature until transferral to the laboratory. The number of hatched eggs was counted on 10 leaves per branch (50 leaves in total) under a dissecting microscope.
In locality N. 13 in 2019, some C. ohridella eggs were observed on the leaves of plants subjected to chemical control with trunk injections in 2001. To confirm this observation, on 27 August of the following year, four branches were collected from each of three horse chestnut trees, two of which were subjected to chemical control with trunk injections in 2001 and one was untreated. Each branch was enclosed separately in a plastic bag, labeled and stored at a cool temperature until transferral to the laboratory. For treated and untreated plants, a variable number of central leaflets of some leaves per branch were observed under a dissecting microscope up to 50 mines. This value was achieved by observing three to five leaflets, with the highest number required for treated plants. The mines were split into developed mines (i.e., with living larvae or cocooned/hatched chrysalises) and non-developed mines (i.e., with dead larvae). The latter were further divided into two classes based on the diameter of the circular part of the mine: class 1 = <2 mm, class 2 = 3–5 mm. The proportion of developed mines and, among the non-developed ones, the proportion of tiny mines (class 1) across the three trees (i.e., the two treated and the one untreated) were compared with Ryan’s test [54].
In the N. 13 and N. 14 localities, on 8 July 2021, one horse chestnut branch was collected from each of five trees subjected to trunk injections up to 2012, and these were taken separately to the laboratory where they were kept in a refrigerator for one day. Subsequently, a selection of leaves from each locality, in equal numbers from each plant, were enclosed in two plastic bags that were placed in a polystyrene container with ice packs and shipped by courier to an accredited laboratory (Chelab S.r.l., Resana, TV, Italy) for residue determination. The two leaf samples were tested via multi-residue analysis according to the food standard UNI EN 15662:2018.
3. Results
3.1. Spatial Distribution of Cameraria ohridella and Its Spread over the Years
In 1997, C. ohridella was recorded in around 50% of the sampled localities, but as early as 1998, horse chestnut mines were observed in 76% of the localities (Figure 1). The localities that were not yet infested by 1997 were almost always contiguous to each other along certain road directions, such as (i) along the SS52 road from locality N. 25 (infested in 1997) to localities N. 9 and 1 (infested from 1998), (ii) along SS52 road from locality N. 2 (infested in 1997) to localities N. 14 (infested since 1998), (iii) along the SS52 and SP23 roads from locality N. 24 (infested in 1997) to locality N. 15 (infested since 1998), (iv) along the SR355 road from locality N. 25 (infested in 1998) to localities N. 8 (infested since 1999) and locality N. 10 (infested in 2005), (v) along the SR355 road from locality N. 20 (infested in 1997) to locality N. 19 (infested since 2000) and (vi) along the SS51bis road from locality N. 4 (infested in 1997) to locality N. 3 (infested since 1998) (Figure S1 in Supplementary Materials). In 1999, the leaf miner was absent in only two localities (N. 10 and 19), and from 2000 to 2004, only one locality was free of the species (N. 10). In 2005, C. ohridella was also recorded in this last site.
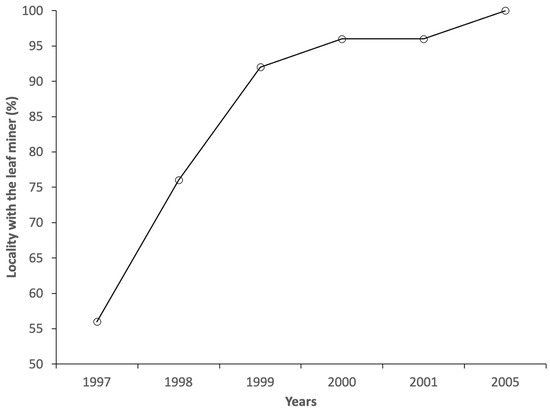
Figure 1.
Percentage of localities out of 22 studied with horse chestnut infested by Cameraria ohridella over the sampling years.
The progression of infestation from the first year of recording C. ohridella was very rapid, as the maximum infestation class reached at each site was usually observed by the third year, with only three cases in the fourth year. These latter three sites were located at higher altitudes (over 900 m a.s.l.).
3.2. Factors Influencing the Damage from Cameraria ohridella and Guignardia aesculi
The independent variables of altitude and average annual temperature were found to be negatively and strongly correlated (R = 0.9819, p < 0.0001); therefore, they were not considered together in the multiple regression analyses.
In the study area, the altitude, average annual temperature and average annual rainfall ranged from 199 to 1294 m a.s.l., from 4.4 to 11.9 °C and from 954 to 1394 mm, respectively.
Multiple regression analyses showed that altitude (or average annual temperature), leaf removal level and leaf surface covered by necrotic spots due to G. aesculi significantly influenced the leaf surface covered by mines, with average annual rainfall not being a significant predictor of the dependent variable (Table 1). The leaf surface covered by C. ohridella mines decreased as temperature decreased (or altitude increased) and leaf removal increased. The increase in the leaf surface covered by necrotic spots due to G. aesculi reduced the level of leaf damage by C. ohridella. The three significant independent variables represent over 75% of the total variability. The percentage contribution to the regression was maximum for the average annual temperature or altitude (around 47%); however, the leaf removal level was also important (around 32%), as was G. aesculi leaf damage (21%). At altitudes over 1000 m or average annual temperatures below 7 °C, the leaf damage never exceeded 5%, regardless of the leaf removal level. At 90% leaf removal, the leaf damage never exceeded 25%, irrespective of altitude.

Table 1.
Multiple regression analyses conducted to evaluate the influence of different factors (variables) on damage to horse chestnut leaves by Cameraria ohridella. C = contribution of each variable in the regression analysis.
Multiple regression analyses showed that altitude (or average annual temperature), leaf removal level and leaf surface area covered by C. ohridella mines significantly influenced the leaf surface covered by G. aesculi necrotic spots, with average annual rainfall not being a significant predictor of the dependent variable (Table 2). The leaf surface covered by necrotic spots decreased with the decrease in temperature (or increase in altitude) and the increase in the leaf removal level. The increase in leaf damage by C. ohridella decreased the leaf surface covered by G. aesculi necrotic spots. However, the three significant independent variables only accounted for about 35% of the total variability, suggesting the involvement of other important factors. The percentage contribution to the regression was maximum for the average annual temperature or altitude (51%) and lower for leaf removal (around 26%) and C. ohridella (22%). It is noteworthy that above a 1000 m altitude and below a 6 °C average annual temperature, the percentage of leaf surfaces with necrotic spots was always below 5%.

Table 2.
Multiple regression analyses conducted to evaluate the influence of different factors (variables) on the damage to horse chestnut leaves caused by Guignardia aesculi. C = contribution of each variable in the regression analysis.
3.3. Effect of Abamectin Applications via Trunk Injections
A clear reduction in leaf damage was observed in the six sites where trunk injection of the insecticide abamectin was applied. After the insecticide applications, the positive effect persisted for many years (Table 3). Persistent effects of the trunk injections also occurred in sites with a low level of leaf removal (localities N. 2, 6 and 16) or located in proximity to heavily infested plants, which are potential sources of egg-laying females (localities N. 6, 9 and 13). This last occurrence was particularly evident in locality N. 13, with three possible sources of egg-laying females less than 150 m away (Figure 2).

Table 3.
Leaf damage classes recorded before and after applications of abamectin with trunk injections against Cameraria ohridella.
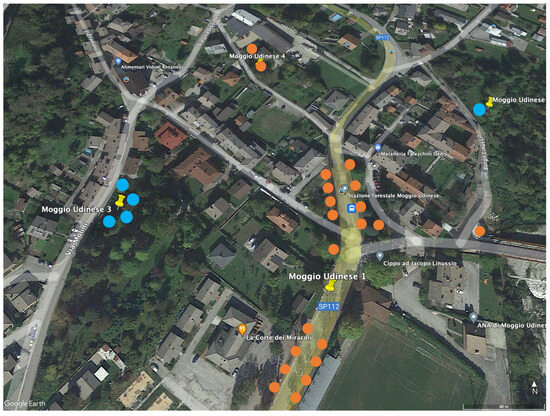
Figure 2.
Position of the four sites at locality N. 13. The plants subjected to trunk injections of abamectin are indicated in orange and the untreated ones in blue. The distance between the untreated plants and the closest treated plants was always less than 150 m.
3.4. Effect of Variation in Leaf Removal Levels
Increases in leaf removal levels from 0%–30% to over 90% (localities N. 9 and 15) or 60%–90% (locality N. 9) caused decreases in leaf damage below 5% and 10%, respectively (Figure 3A,B). In locality N. 14, where the level of leaf removal decreased from 90% to 30%, there was an increase in leaf damage to over 80% (Figure 3C). In locality N. 17, an increase in the leaf removal level from 30%–60% to 60%–90% resulted in a reduction in leaf damage at an average level of less than 10% (Figure 3D).
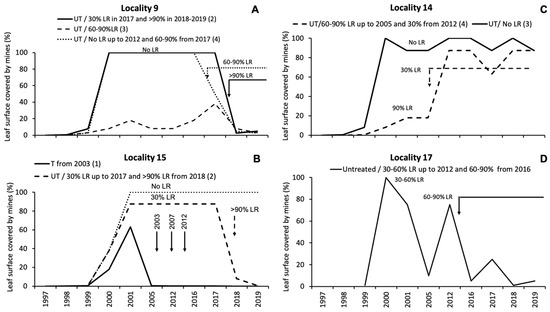
Figure 3.
Dynamics over the years of leaf damage from Cameraria ohridella in sites at four localities (A–D) subjected to trunk injections with abamectin (T) or variations in leaf removal levels (LR) compared to control sites. UT = untreated. The number of the site inside each locality, according to Table S2 of the Supplementary Materials, is reported between breaks in the legends.
3.5. Insights into the Persistent Effect of Trunk Injections of Abamectin
In the no-choice experiment carried out in 2019 in the laboratory, there was no significant difference in the number of eggs laid by C. ohridella females on leaves of branches collected from trees treated with abamectin in 2001 (33.8 ± 19.8 eggs per leaf) or left untreated (36.4 ± 13.9 eggs per leaf) (t = 0.11, d.f. = 8, p = 0.92). Therefore, the leaves of plants treated many years before did not deter oviposition.
Late in the 2019 season, an average number of 11.8 ± 10.6 eggs per leaf was observed on leaves of plants treated in 2001, confirming that the treated leaves did not deter oviposition, and at the same time, suggesting that egg-laying females had migrated from nearby untreated plants.
In 2020, in the branches of the two plants treated in 2012, the percentage of well-developed mines was significantly lower than in the untreated plant (Ryan’s test, p < 0.01) (Figure 4A). Furthermore, in the two treated plants, the percentage of non-developed mines with a diameter less than 2 mm was significantly higher than in the untreated plant (Ryan’s test, p < 0.01) (Figure 4B).
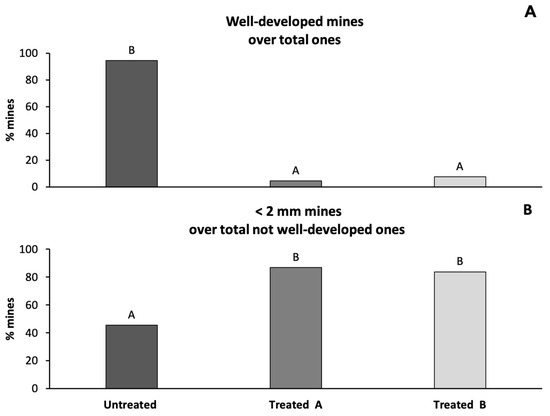
Figure 4.
Percentage of well-developed mines over total mines ((A), above) and percentage of mines < 2 mm over total not-well-developed mines ((B), under) in one untreated plant and two plants treated with abamectin. Different capital letters above columns indicate significant differences at the 0.01 level with Ryan’s test.
Despite the high mortality of larvae in the first phases of their development, abamectin residues (avermectin B1a and B1b with reliability limits of 0.01 mg/kg) were not detected in the leaves of treated plants.
4. Discussion
This multi-year study, conducted at locations across a broad altitudinal range, provided robust data on the relative importance of climatic and cultural factors influencing C. ohridella infestations and G. aesculi infections.
4.1. Spread of Cameraria ohiridella in the Introduced Area
In the first sampling year, C. ohridella was not found in most localities but was practically present in all sites after four years. Because the gradually colonized localities were often located along the same road, it is evident that the spread of the leaf miner is favored by human activity, as already suggested by other studies [2,55,56,57]. According to Valade et al. [6], natural long-distance dispersal is unlikely considering that the moth took a long time to migrate from natural horse chestnut stands in the Balkans to planted trees in urban areas. When the host plant of the pest is an ornamental tree that is not distributed continuously in the landscape, it is inconceivable that the leaf miner could spread naturally over many kilometers.
C. ohridella can be established on horse chestnut at altitudes approaching 1300 m a.s.l. with an average annual temperature of 4–4.5 °C. This geographical distribution agrees with that recorded by Hellrigl and Ambrosi [3] in another mountain area of north-eastern Italy. Based on our data, the leaf miner can colonize A. hippocastanum plants in all localities where the tree is found, including the coldest ones. After all, the overwintering pupae of C. ohridella may survive even at temperatures between −19.5 and −23 °C [58].
The biotic potential of the leaf miner was high, as the maximum class damage characteristic of each locality was usually reached within three years, even at altitudes above 1200 m and with high levels of leaf removal (60%–90%). This agrees with the rapid rise in C. ohridella damage during the first three years after initial colonization [34].
4.2. Natural Control of Cameraria ohridella in the Introduced Area
In some sites that already displayed high damage levels by 1997, the infestation of C. ohridella was maintained at the same level over 20 years, indicating that natural enemies cannot keep leaf miner populations below damage levels, even in natural environments. Usually, leaf miner moths are efficiently controlled by density-dependent parasitoids, but this is not the case with C. ohridella (parasitization often less than 10%), which is mostly due to poor synchronization between parasitoid emergence in spring and C. ohridella phenology and the inability of the native parasitoids to locate a leaf miner living on a host plant that does not have any native congener in Europe [29,30,33,59]. The higher parasitism rates by Pediobius saulius (Walker) (Hymenoptera, Eulophidae) in the Balkans, the area of origin of the leaf miner, suggest its possible use in the context of classical biological control [60].
4.3. Factors Affecting Cameraria ohridella and Guignardia aesculi
In the investigated mountain area, higher altitudes were associated with the lowest level of leaf damage by C. ohridella, as reported in other studies [3,24]. This occurs because altitude is closely correlated with average annual temperature and, in turn, with the number of generations per year [3]. Above 1000 m altitude or below an average annual temperature of 7 °C, the leaf miner never caused damage greater than 10% of the leaf surface, even if the leaf removal level was low. Therefore, since the arrival of C. ohridella, horse chestnut should be planted only in the highest mountain localities (i.e., above 1000 m altitude), even though infestation rates there are still higher than in the Balkans where effective biological control is present [60]. Planting in areas with altitudes between 700 and 1000 m or even lower can be associated with acceptable leaf damage only if thorough leaf removal can be achieved. In particular, leaf damage did not exceed 25% in sites up to 200 m altitude when the leaf removal level was at least 90%. Because temperature may be the only reference parameter at different latitudes, we suggest new plantings of A. hippocastanum in localities with an average annual temperature below 9 °C where leaf removal is possible or below 7 °C where it is not.
The classical biological control approach of guaranteeing low population levels similar to the place of origin of the horse chestnut and C. ohridella (i.e., the Balkan Mountains) is achievable only if the allochthonous natural enemies adapt to all the environmental conditions in which horse chestnut has been planted. At altitudes lower than 700 m, A. hippocastanum could be substituted with hybrid Aesculus × carnea Zeyher, which is resistant to the leaf miner [61,62,63].
Furthermore, the leaf damage by G. aesculi was significantly influenced by the altitude (average annual temperature) or by the level of leaf removal, even though the influence only appeared important for temperature, considering the low coefficient of determination of multiple regressions. Average annual rainfall was not significantly correlated with fungus damage. Still, this fact should not lead one to believe that rainfall is not important, because it is known that rainfall and hours of wetness are favorable to fungal infections [38]. Furthermore, since the variability in the average annual rainfall among the sampled sites is likely smaller than among different years inside the same locality, the average annual rainfall is probably not a good variable to explain differences in the incidence of the disease among the localities. Moreover, the wetness hours are greatly influenced by microclimatic conditions in which individual plants are located. Since the variables considered in the multiple regression explained only a third of the total variability, the availability of data on wetting hours would likely have meant that the regression explained a much higher percentage of the total variability.
Leaf damage by C. ohridella decreased as leaf damage by G. aesculi increased, and vice versa, indicating competition between the leaf miner and the fungus. This agrees with the interspecific competition between C. ohridella and G. aesculi reported in the literature [37,38]. Although the percentage contribution of the competitor to the variability was similar in the multiple regressions related to C. ohridella (21.3%) and G. aesculi (22.4%), we can assume that the latter had a more negative influence on the former than vice versa because the angular coefficient relative to the competitor was much higher in the C. ohridella than in the G. aesculi regressions (0.812 vs. 0.387). This agrees with Kopačka et al. [38] but not with Jagiełło et al. [37]. However, our study confirms that an interspecific competition occurs.
4.4. Effectiveness of Trunk Injections with Abamectin
The leaf damage by C. ohridella was significantly reduced by insecticide applications via trunk injections with abamectin. Leaf damage was negligible for at least 19 years after insecticide injection. This high persistence is confirmed by the literature [64,65,66]. In particular, the study of Schenke and Jäckel [65] reported abamectin residues on horse chestnut leaves up to six years from the last trunk injection and the absence of leaf miner infestations during the same period. Persistent effects on leaf miner infestations and the detection of residues many years after trunk injection were also reported for imidacloprid [45,67].
The persistent effect of abamectin trunk injections occurred despite the untreated and very infested trees being less than 150 m away from treated and non-infested trees in three out of the six treated sites. The presence of C. ohridella eggs on treated plants proximal to untreated plants confirmed that egg-laying females can colonize these plants. Moreover, the laboratory two-choice test showed that the leaves of treated plants were not oviposition deterrents. Our field sampling showed that the persistent effect was associated with larval mortalities in the first phases of their development, as most mines did not develop after egg hatching.
The detection of abamectin residues in the leaves for 6 years after trunk injection [65] and the mortality of larvae after 19 years from the last trunk injection (the present study) suggest that abamectin is still present after many years inside leaves at lethal doses for newly hatched larvae of C. ohridella. It can be assumed that for many consecutive years the insecticide is translocated from leaves to the woody parts in autumn and translocated again to the leaves in spring at a concentration sufficient to kill the newly hatched larvae.
Abamectin is toxic to honeybees, and an impact on the expression of genes associated with their vitality has been demonstrated [68]. The persistence of abamectin inside the plant for many years and the fact that emamectin benzoate, the synthetic analogue of abamectin, has been detected in the nectar and pollen of apple plants when applied in the previous spring [69] suggest that trunk injections may be harmful to bees in the years following treatment. Risks for honeybees associated with trunk injections with imidacloprid were recently also suggested by the study of Walczak et al. [67]. Therefore, trunk injections may not be as free of negative side effects as previously assumed [43] because, in addition to the high cost of application and the problem of wounds that do not heal [42,44,70], there are also possible adverse effects on pollinators. We believe that the data reported in this study on the persistence of abamectin, when applied by trunk injection, should be taken into account in discussions regarding the approval or withdrawal of this active substance in the European Union.
In the context of integrated pest management to reduce the negative side effects of trunk insecticide injections, the present study demonstrated that a similar efficacy for C. ohridella control can be achieved with thorough leaf removal. The coupling of leaf removal with strategies of biological control based on the selective emergence of parasitoids and C. ohridella from stored dead leaves [47,71,72] and the installation of bird nesting boxes [73] could further reduce leaf damage, particularly at the lowest altitudes where infestation levels may be too high when adopting leaf removal alone. Moreover, attract-and-kill strategies based on sexual pheromones, effective when C. ohridella has a low population density, could be integrated with leaf removal [74,75]. In the future, in the context of integrated pest management, the use of systemic resistance inductors [76] or natural substances produced by Aesculus spp. resistant to C. ohridella [77] could represent further possibilities for controlling the leaf miner.
5. Conclusions
Until it is possible to bring the leaf miner, C. ohridella, under effective biological control, it is necessary to adopt an integrated approach.
Insecticide applications by trunk injections can provide excellent and persistent effectiveness. However, the surprisingly very long persistence (up to at least 19 years) recorded in the present study could represent a problem for pollinators if insecticide residues remain inside leaves, nectar and pollen over several years. For this reason, we believe that trunk injections with abamectin should no longer be adopted. Removal of fallen leaves from the ground in autumn can guarantee effectiveness that is not much lower than trunk injections. Therefore, we suggest adopting this environmentally friendly practice as rigorously as possible to maintain C. ohridella below damaging levels. Leaf removal should also be encouraged for the control of G. aesculi, although its efficacy against the fungus is less than that against C. ohridella. Finally, the search for further eco-friendly control strategies should be continued.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f16020284/s1, Figure S1: Map of sample sites in the area of north-eastern Italy where the study was conducted (from Google Earth Pro); Table S1: Localities in which the multi-year study was conducted. The number of sampling sites, the sampling years and the studied effects are reported for each locality; Table S2: The characteristics and cultural practices adopted at the sampled sites in each locality.
Author Contributions
Conceptualization, F.P. and P.Z.; methodology, F.P. and P.Z.; formal analysis, F.P. and P.Z.; field investigation, F.P. and P.Z.; data curation, F.P. and P.Z.; writing—original draft preparation, F.P. and P.Z.; writing—review and editing, F.P. and P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Deschka, G.; Dimić, N. Cameraria ohridella sp. n. (Lep., Lithocolletidae) aus Mazedonien, Jugoslawien. Acta Entomol. Jugosl. 1986, 22, 11–23. [Google Scholar]
- Pavan, F.; Zandigiacomo, P. Distribuzione di Cameraria ohridella in Italia ed entità delle infestazioni su ippocastano. Inform. Fitopatol. 1998, 48, 57–60. [Google Scholar]
- Hellrigl, K.; Ambrosi, P. Die Verbreitung der Roßkastanien-Miniermotte Cameraria ohridella Desch. & Dimic (Lepid., Gracillariidae) in der Region Südtirol-Trentino. Anz. Schädlingskunde 2000, 73, 25–32. [Google Scholar]
- Grabenweger, G.; Grill, R. On the place of origin of Cameraria ohridella Deschka & Dimic (Lepidoptera: Gracillariidae). Beitr. Entomofaun. 2000, 1, 9–17. [Google Scholar]
- Hellrigl, K. Neue Erkenntnisse und Untersuchungen über die Roßkastanien-Miniermotte Cameraria ohridella Deschka & Dimic, 1986 (Lepidoptera, Gracillariidae). Gredleriana 2001, 1, 9–81. [Google Scholar]
- Valade, R.; Kenis, M.; Hernandez-Lopez, A.; Augustin, S.; Mena, N.M.; Magnoux, E.; Rougerie, R.; Lakatos, F.; Roques, A.; Lopez-Vaamonde, C. Mitochondrial and microsatellite DNA markers reveal a Balkan origin for the highly invasive horse-chestnut leaf miner Cameraria ohridella (Lepidoptera, Gracillariidae). Mol. Ecol. 2009, 18, 3458–3470. [Google Scholar] [CrossRef] [PubMed]
- Lees, D.C.; Lack, H.W.; Rougerie, R.; Hernandez-Lopez, A.; Raus, T.; Avtzis, N.D.; Augustin, S.; Lopez-Vaamonde, C. Tracking origins of invasive herbivores through herbaria and archival DNA: The case of the horse-chestnut leaf miner. Front. Ecol. Environ. 2011, 9, 322–328. [Google Scholar] [CrossRef]
- Kirichenko, N.I.; Karpun, N.N.; Zhuravleva, E.N.; Shoshina, E.I.; Anikin, V.V.; Musolin, D.L. Invasion genetics of the horse-chestnut leaf miner, Cameraria ohridella (Lepidoptera: Gracillaridae), in European Russia: A case of successful involvement of citizen science in studying an alien insect pest. Insects 2023, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, L.; Simmonds, M.S.J.; Straw, N.; Castagneyrol, B.; Koricheva, J. Leaf traits influencing oviposition preference and larval performance of Cameraria ohridella on native and novel host plants. Entomol. Exp. Appl. 2014, 152, 157–164. [Google Scholar] [CrossRef]
- Walczak, U.; Baraniak, E.; Zduniak, P. Survival, body mass and potential fecundity of the invasive moth Cameraria ohridella (Lepidoptera: Gracillariidae) on its original host plant Aesculus hippocastanum and Aesculus glabra. Eur. J. Entomol. 2017, 114, 295–300. [Google Scholar] [CrossRef][Green Version]
- Péré, C.; Augustin, S.; Turlings, T.C.J.; Kenis, M. The invasive alien leaf miner Cameraria ohridella and the native tree Acer pseudoplatanus: A fatal attraction? Agr. Forest Entomol. 2010, 12, 151–159. [Google Scholar] [CrossRef]
- Santi, F.; Accinelli, G.; Maini, S. Cameraria ohridella, minatore fogliare dell’ippocastano: Catture con trappole sessuali e note di biologia. Inform. Fitopatol. 2000, 50, 7–11. [Google Scholar]
- Del Bene, G.; Gargani, E. Cameraria ohridella Deschka & Dimic (Lep. Gracillariidae) and its natural enemies in Tuscany. Redia 2003, 86, 115–127. [Google Scholar]
- Girardoz, S.; Quicke, D.L.J.; Kenis, M. Factors favouring the development and maintenance of outbreaks in an invasive leaf miner Cameraria ohridella (Lepidoptera: Gracillariidae): A life table study. Agric. For. Entomol. 2007, 9, 141–158. [Google Scholar] [CrossRef]
- Fora, C.G.; Lauer, K.F.; Fora, A.; Damianov, S.; Moatăr, M. The flight of the Cameraria ohridella population in the city of Timisoara, Romania. J. Hortic. For. Biotechnol. 2010, 14, 197–201. [Google Scholar]
- Pavan, F.; Barro, P.; Bernardinelli, I.; Gambon, N.; Zandigiacomo, P. Cultural control of Cameraria ohridella on horsechestnut in urban areas by removing fallen leaves in autumn. J. Arboricul. 2003, 29, 253–258. [Google Scholar]
- Salleo, S.; Nardini, A.; Raimondo, F.; Lo Gullo, M.A.; Pace, F.; Giacomich, P. Effects of defoliation caused by the leaf miner Cameraria ohridella on wood production and efficiency in Aesculus hippocastanum growing in north-eastern Italy. Trees—Struct. Funct. 2003, 17, 367–375. [Google Scholar] [CrossRef]
- Percival, G.C.; Barrow, I.; Noviss, K.; Keary, I.; Pennington, P. The impact of horse chestnut leaf miner (Cameraria ohridella Deschka and Dimic; HCLM) on vitality, growth and reproduction of Aesculus hippocastanum L. Urban For. Urban Green. 2011, 10, 11–17. [Google Scholar] [CrossRef]
- Tyburska-Woś, J.; Nowak, K.; Kieliszewska-Rokicka, B. Influence of leaf damage by the horse chestnut leafminer (Cameraria ohridella Deschka & Dimić) on mycorrhiza of Aesculus hippocastanum L. Mycorrhiza 2018, 29, 61–67. [Google Scholar]
- Raimondo, F.; Ghirardelli, L.A.; Nardini, A.; Salleo, S. Impact of the leaf miner Cameraria ohridella on photosynthesis, water relations and hydraulics of Aesculus hippocastanum leaves. Trees—Struct. Funct. 2003, 17, 376–382. [Google Scholar] [CrossRef]
- Mirchev, S.; Hristov, B.; Zafirov, N. Impact of horse chestnut leaf miner (Cameraria ohridella Deschka et Dimic) on horse chestnut (Aesculus hippocastanum L.). Dendrochronological analysis. J. Balkan Ecol. 2013, 16, 63–71. [Google Scholar]
- Percival, G.C.; Banks, J.M. Studies of the interaction between horse chestnut leaf miner (Cameraria ohridella) and bacterial bleeding canker (Pseudomonas syringae pv. aesculi). Urban For. Urban Green. 2014, 13, 403–409. [Google Scholar] [CrossRef]
- Reinhardt, F.; Herle, M.; Bastiansen, F.; Streit, B. Economic Impact of the Spread of Alien Species in Germany. Research Report 201 86 211; Federal Environmental Agency: Berlin, Germany, 2003.
- Walas, Ł.; Dering, M.; Ganatsas, P.; Pietras, M.; Pers-Kamczyc, E.; Iszkuło, G. The present status and potential distribution of relict populations of Aesculus hippocastanum L. in Greece and the diverse infestation by Cameraria ohridella Deschka & Dimić. Plant Biosyst. 2018, 152, 1048–1058. [Google Scholar]
- Radeghieri, P. Cameraria ohridella (Lepidoptera Gracillariidae) predation by Crematogaster scutellaris (Hymenoptera Formicidae) in Northern Italy (Preliminary note). Bull. Insectology 2004, 57, 63–64. [Google Scholar]
- Grabenweger, G.; Kehrli, P.; Schlick-Steiner, B.; Steiner, F.; Stolz, M.; Bacher, S. Predator complex of the horse chestnut leafminer Cameraria ohridella: Identification and impact assessment. J. Appl. Entomol. 2005, 129, 353–362. [Google Scholar] [CrossRef]
- Petrova, V.; Voitkane, S.; Jankevica, L.; Cera, I. Spider community on the horse-chestnut Aesculus hippocastanum L.—Preliminary results. Acta Biol. Univ. Daugavp. 2013, 13, 77–84. [Google Scholar]
- Freise, J.F.; Heitland, W.; Tosevski, I. Parasitism of the horse-chestnut leaf miner, Cameraria ohridella Deschka and Dimic (Lep., Gracillariidae), in Serbia and Macedonia. J. Pest Sci. 2002, 75, 152–157. [Google Scholar] [CrossRef]
- Grabenweger, G.; Avtzis, N.; Girardoz, S.; Hrasovec, B.; Tomov, R.; Kenis, M. Parasitism of Cameraria ohridella (Lepidoptera, Gracillariidae) in natural and artificial horse-chestnut stands in the Balkans. Agric. For. Entomol. 2005, 7, 291–296. [Google Scholar] [CrossRef]
- Grabenweger, G.; Hopp, H.; Jäckel, B.; Balder, H.; Koch, T.; Schmolling, S. Impact of poor host-parasitoid synchronisation on the parasitism of Cameraria ohridella (Lepidoptera: Gracillariidae). Eur. J. Entomol. 2007, 104, 153–158. [Google Scholar] [CrossRef]
- Girardoz, S.; Kenis, M.; Quicke, D.L.J. Recruitment of native parasitoids by an exotic leaf miner, Cameraria ohridella: Host-parasitoid synchronization and influence of the environment. Agric. For. Entomol. 2006, 8, 49–56. [Google Scholar] [CrossRef]
- Ferracini, C.; Alma, A. Evaluation of the community of native eulophid parasitoids on Cameraria ohridella Deschka and Dimic in urban areas. Environ. Entomol. 2007, 36, 1147–1153. [Google Scholar] [CrossRef]
- Pocock, M.J.O.; Evans, D.M. The success of the horse-chestnut leaf-miner, Cameraria ohridella, in the UK revealed with hypothesis-led citizen science. PLoS ONE 2014, 9, e86226. [Google Scholar] [CrossRef] [PubMed]
- Samek, T.; Novotný, D.; Jankovský, L. Infection of wintering pupae of horse-chestnut leafminer Cameraria ohridella Deschka et Dimić by Verticillium lecanii (Zimmerman) Viégas. J. Forest Sci. 2006, 52, 136–140. [Google Scholar] [CrossRef]
- Metla, Z.; Voitkāne, S.; Sešķēna, R.; Petrova, V.; Jankevica, L. Presence of entomopathogenic fungi and bacteria in Latvian population of horse-chestnut leaf miner Cameraria ohridella. Acta Biol. Univ. Daugavp. 2013, 13, 69–76. [Google Scholar]
- Schemmer, R.; Chládeková, P.; Medo, J.; Barta, M. Natural prevalence of entomopathogenic fungi in hibernating pupae of Cameraria ohridella (Lepidoptera: Gracillariidae) and virulence of selected isolates. Plant Prot. Sci. 2016, 52, 199–208. [Google Scholar] [CrossRef]
- Jagiełło, R.; Baraniak, E.; Karolewski, P.; Łakomy, P.; Behnke-Borowczyk, J.; Walczak, U.; Giertych, M.J. Ecophysiological aspects of the interaction between Cameraria ohridella and Guignardia aesculi on Aesculus hippocastanum. Dendrobiology 2017, 78, 146–156. [Google Scholar] [CrossRef]
- Kopačka, M.; Nachman, G.; Zemek, R. Seasonal changes and the interaction between the horse chestnut leaf miner Cameraria ohridella and horse chestnut leaf blotch disease caused by Guignardia aesculi. Forests 2021, 12, 952. [Google Scholar] [CrossRef]
- Johne, A.B.; Weissbecker, B.; Schütz, S. Approaching risk assessment of complex disease development in horse chestnut trees: A chemical ecologist’s perspective. J. Appl. Entomol. 2008, 132, 349–359. [Google Scholar] [CrossRef]
- Percival, G.C.; Banks, J.; Keary, I. Evaluation of organic, synthetic and physical insecticides for the control of horse chestnut leaf miner (Cameraria ohridella). Urban For. Urban Green. 2012, 11, 426–431. [Google Scholar] [CrossRef]
- Lohrer, T.; Gerlach, W.W.P.; Fischer, P.; Fuchsbichler, G.; Eichinger, H.M. Untersuchungen zur Laub- und Kompostbelastung nach einer Bodenapplikation mit Imidacloprid zur Bekämpfung der Kastanienminiermotte Cameraria ohridella (Lepidoptera, Gracillariidae). Nachrichtenbl. Deut. Pflanzenschutzd. 2003, 55, 240–241. [Google Scholar]
- Gargani, E.; Russo, R.; Del Bene, G. Control tests of the horse-chestnut leafminer using methods with low environmental impact. Redia 2002, 85, 131–141. [Google Scholar]
- Mešić, A.; Barčić, J.; Barčić, J.I.; Miličević, T.; Duralija, B.; Ćuljak, T.G. A low environmental impact method to control horse chestnut leaf miner Cameraria ohridella (Deschka & Dimić). J. Food Agric. Environ. 2008, 6, 421–427. [Google Scholar]
- Ferracini, C.; Alma, A. How to preserve horse chestnut trees from Cameraria ohridella in the urban environment. Crop Prot. 2008, 27, 1251–1255. [Google Scholar] [CrossRef]
- Jagiełło, R.; Walczak, U.; Iszkuło, G.; Karolewski, P.; Baraniak, E.; Giertych, M.J. Impact of Cameraria ohridella on Aesculus hippocastanum growth and long-term effects of trunk injection with pesticides. Int. J. Pest Manag. 2019, 65, 33–43. [Google Scholar] [CrossRef]
- Gilbert, M.; Svatoš, A.; Lehmann, M.; Bacher, S. Spatial patterns and infestation processes in the horse chestnut leafminer Cameraria ohridella: A tale of two cities. Entomol. Exp. Appl. 2003, 107, 25–37. [Google Scholar] [CrossRef]
- Kehrli, P.; Bacher, S. How to safely compost Cameraria ohridella-infested horse chestnut leaf litter on private compost heaps. J. Appl. Entomol. 2004, 128, 707–709. [Google Scholar] [CrossRef]
- Percival, G.C. Evaluation of insect barrier glue bands and liquid glue for the management of horse chestnut leaf miner (Cameraria ohridella). Arboric. J. 2016, 38, 134–142. [Google Scholar] [CrossRef]
- Hersh, M.H.; Vilgalys, R.; Clark, J.S. Evaluating the impacts of multiple generalist fungal pathogens on temperate tree sedling survival. Ecology 2012, 93, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Percival, G.C.; Graham, S. Evaluation of inducing agents and synthetic fungicide combinations for management of foliar pathogens of urban trees. Arboric. Urban For. 2021, 47, 85–95. [Google Scholar] [CrossRef]
- Del Bene, G.; Gargani, E.; Landi, S.; Bonifacio, A. Cameraria ohridella e malattie fogliari dell’ippocastano in Toscana. Italus Hortus 2001, 8, 41–49. [Google Scholar]
- Gilbert, M.; Grégoire, J.-C. Visual, semi-quantitative assessments allow accurate estimates of leafminer population densities: An example comparing image processing and visual evaluation of damage by the horse chestnut leafminer Cameraria ohridella (Lep., Gracillariidae). J. Appl. Entomol. 2003, 127, 354–359. [Google Scholar] [CrossRef]
- Statistics Kingdom. 2017. Available online: https://www.statskingdom.com (accessed on 11 April 2024).
- Ryan, T.H. Significance tests for multiple comparison of proportions, variances, and other statistics. Psychol. Bull. 1960, 57, 318–328. [Google Scholar] [CrossRef]
- Gilbert, M.; Grégoire, J.-C.; Freise, J.F.; Heitland, W. Long-distance dispersal and human population density allow the prediction of invasive patterns in the horse chestnut leafminer Cameraria ohridella. J. Anim. Ecol. 2004, 73, 459–468. [Google Scholar] [CrossRef]
- Gilbert, M.; Guichard, S.; Freise, J.; Grégoire, J.-C.; Heitland, W.; Straw, N.; Tilbury, C.; Augustin, S. Forecasting Cameraria ohridella invasion dynamics in recently invaded countries: From validation to prediction. J. Appl. Ecol. 2005, 42, 805–813. [Google Scholar] [CrossRef]
- Augustin, S.; Guichard, S.; Heitland, W.; Freise, J.; Svatoš, A.; Gilbert, M. Monitoring and dispersal of the invading Gracillariidae Cameraria ohridella. J. Appl. Entomol. 2009, 133, 58–66. [Google Scholar] [CrossRef]
- Kovács, Z.; Lakatos, F. Observations on the overwintering and ontogenesis of Cameraria ohridella (Deschka et Dimič 1986, Lep. Lithocolletidae). Növényvédelem 1999, 35, 57–59. [Google Scholar]
- Volter, L.; Prenerová, E.; Weyda, F.; Zemek, R. Changes in the parasitism rate and parasitoid community structure of the horse chestnut leafminer, Cameraria ohridella (Lepidoptera: Gracillariidae), in the Czech Republic. Forests 2022, 13, 885. [Google Scholar] [CrossRef]
- Hernández-López, A.; Rougerie, R.; Augustin, S.; Lees, D.C.; Tomov, R.; Kenis, M.; Çota, E.; Kullaj, E.; Hansson, C.; Grabenweger, G.; et al. Host tracking or cryptic adaptation? Phylogeography of Pediobius saulius (Hymenoptera, Eulophidae), a parasitoid of the highly invasive horse-chestnut leafminer. Evol. Appl. 2012, 5, 256–269. [Google Scholar] [CrossRef]
- Nardini, A.; Raimondo, F.; Scimone, M.; Salleo, S. Impact of the leaf miner Cameraria ohridella on whole-plant photosynthetic productivity of Aesculus hippocastanum: Insights from a model. Trees—Struct. Funct. 2004, 18, 714–721. [Google Scholar] [CrossRef]
- Irzykowska, L.; Werner, M.; Bocianowski, J.; Karolewski, Z.; Frużyńska-Jóźwiak, D. Genetic variation of horse chestnut and red horse chestnut and trees susceptibility to Erysiphe flexuosa and Cameraria ohridella. Biologia 2013, 68, 851–860. [Google Scholar] [CrossRef]
- Bačovský, V.; Vyhnánek, T.; Hanáček, P.; Mertelík, J.; Šafránková, I. Genetic diversity of chestnut tree in relation to susceptibility to the leaf miner (Cameraria ohridella Deschka & Dimič). Trees 2017, 31, 753–763. [Google Scholar]
- Doccola, J.J.; Wild, P.M. Tree injection as an alternative method of insecticide application. In Insecticides—Basic and Other Applications; Soloneski, S., Larramendy, M., Eds.; InTech: Rijeka, Croatia, 2012; pp. 61–78. [Google Scholar]
- Schenke, D.; Jäckel, B. Langzeit-Effekt von Abamectin auf die Kastanienminiermotte (Cameraria ohridella) in Blättern der Rosskastanie (Aesculus hippocastanum) nach Stamminjektion. Julius-Kühn-Arch. 2012, 438, 470. [Google Scholar]
- Pál, M.; Bálint, J.; Balog, A. Using the technique of vegetal endoterapy against the horse chestnut’s leaf miner (Lepidoptera: Cameraria ohridella Deschka & Dimie). Sci. Pap. Ser. B Hortic. 2014, 58, 353–358. [Google Scholar]
- Walczak, U.; Giertych, M.J.; Baraniak, E. Persistence of imidacloprid in trunk injected horse chestnut and its impact on Cameraria ohridella (Lepidoptera: Gracillariidae). Appl. Entomol. Zool. 2024, 59, 203–210. [Google Scholar] [CrossRef]
- Li, G.; Zhao, H.; Guo, D.; Liu, Z.; Wang, H.; Sun, Q.; Liu, Q.; Xu, B.; Guo, X. Distinct molecular impact patterns of abamectin on Apis mellifera ligustica and Apis cerana cerana. Ecotoxicol. Environ. Saf. 2022, 232, 113242. [Google Scholar] [CrossRef] [PubMed]
- Coslor, C.C.; Vandervoort, C.; Wise, J.C. Insecticide dose and seasonal timing of trunk injection in apples influence efficacy and residues in nectar and plant parts. Pest Manag. Sci. 2019, 75, 1453–1463. [Google Scholar] [CrossRef]
- Nicolotti, G.; Gonthier, P.; Giordano, L. Effetti collaterali di trattamenti endoterapici su legno di ippocastano. Inform. Fitopatol. 2006, 56, 34–39. [Google Scholar]
- Kehrli, P.; Lehmann, M.; Bacher, S. Mass-emergence devices: A biocontrol technique for conservation and augmentation of parasitoids. Biol. Contr. 2005, 32, 191–199. [Google Scholar] [CrossRef]
- Klug, T.; Meyhöfer, R.; Kreye, M.; Hommes, M. Native parasitoids and their potential to control the invasive leafminer, Cameraria ohridella Desch. & Dim. (Lep.: Gracillariidae). Bull. Entomol. Res. 2008, 98, 379–387. [Google Scholar] [PubMed]
- Mösch, S.; Eilers, E.J.; Hommes, M. Biocontrol of Cameraria ohridella by insectivorous birds in different landscape contexts. BioControl 2018, 63, 215–225. [Google Scholar] [CrossRef]
- Grabenweger, G.; Koch, T.; Balder, H.; Hopp, H.; Jäckel, B.; Schmolling, S. Possibilities to control the horse chestnut leaf miner (Cameraria ohridella) in urban environments. Commun. Agric. Appl. Biol. Sci. 2005, 70, 633–640. [Google Scholar] [PubMed]
- Sukovata, L.; Czokajlo, D.; Kolk, A.; Ślusarski, S.; Jabłoński, T. An attempt to control Cameraria ohridella using an attract-and-kill technique. J. Pest Sci. 2011, 84, 207–212. [Google Scholar] [CrossRef][Green Version]
- Percival, G.C.; Holmes, S.P. The influence of systemic inducing agents on horse chestnut leaf miner (Cameraria ohridella) severity in white flowering horse chestnut (Aesculus hoppicastanum L.). Urban For. Urban Green. 2016, 20, 97–102. [Google Scholar] [CrossRef]
- Ferracini, C.; Curir, P.; Dolci, M.; Lanzotti, V.; Alma, A. Aesculus pavia foliar saponins: Defensive role against the leafminer Cameraria ohridella. Pest Manag. Sci. 2010, 66, 767–772. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).