Whole-Genome Identification and Expression Profiles of WRKY Genes Related to the Leaf Expansion Period in the Camphor Tree
Abstract
:1. Introduction
2. Results
2.1. Identification of WRKY Protein Family
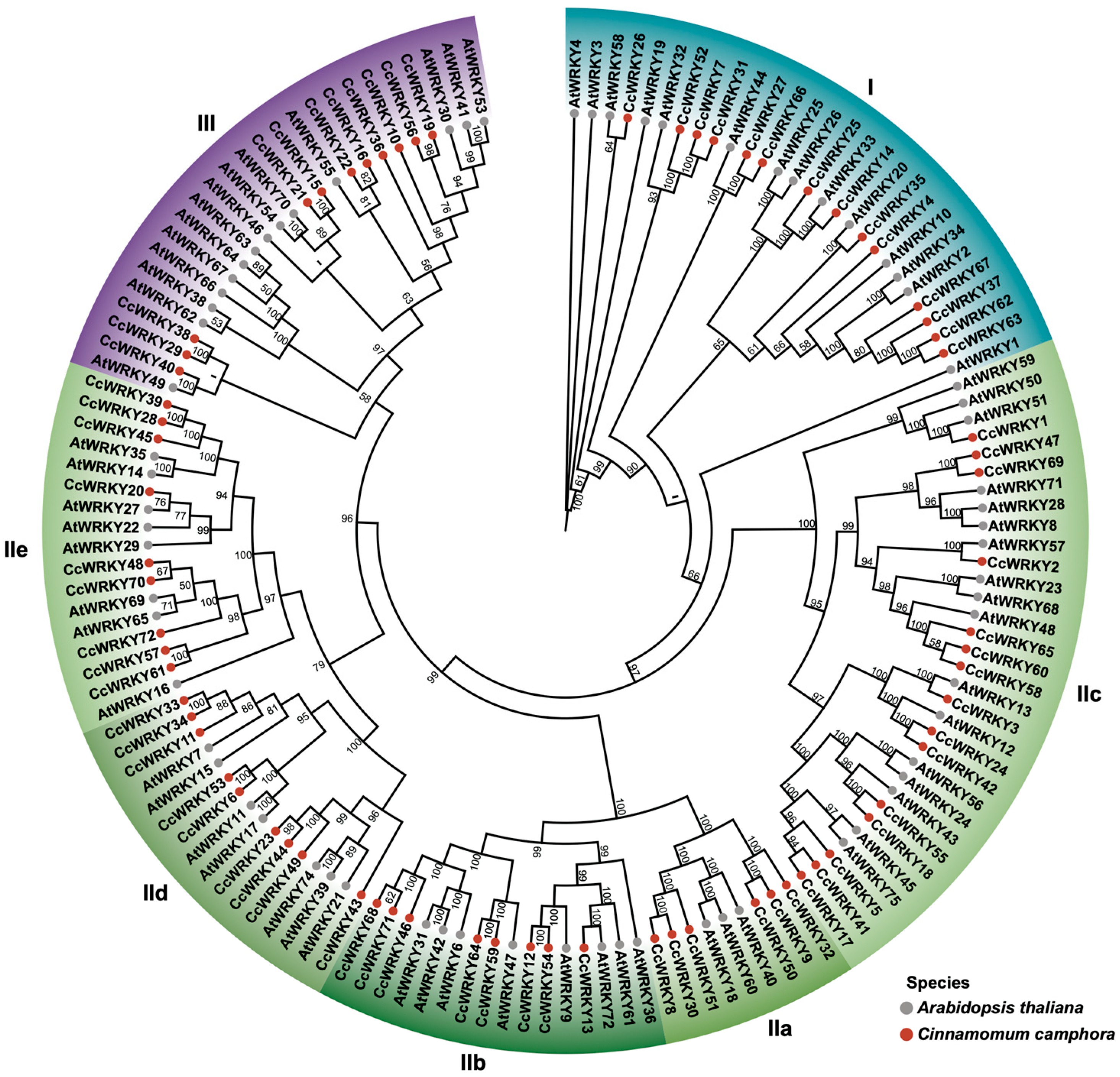
2.2. Classification and Phylogenetic Analysis
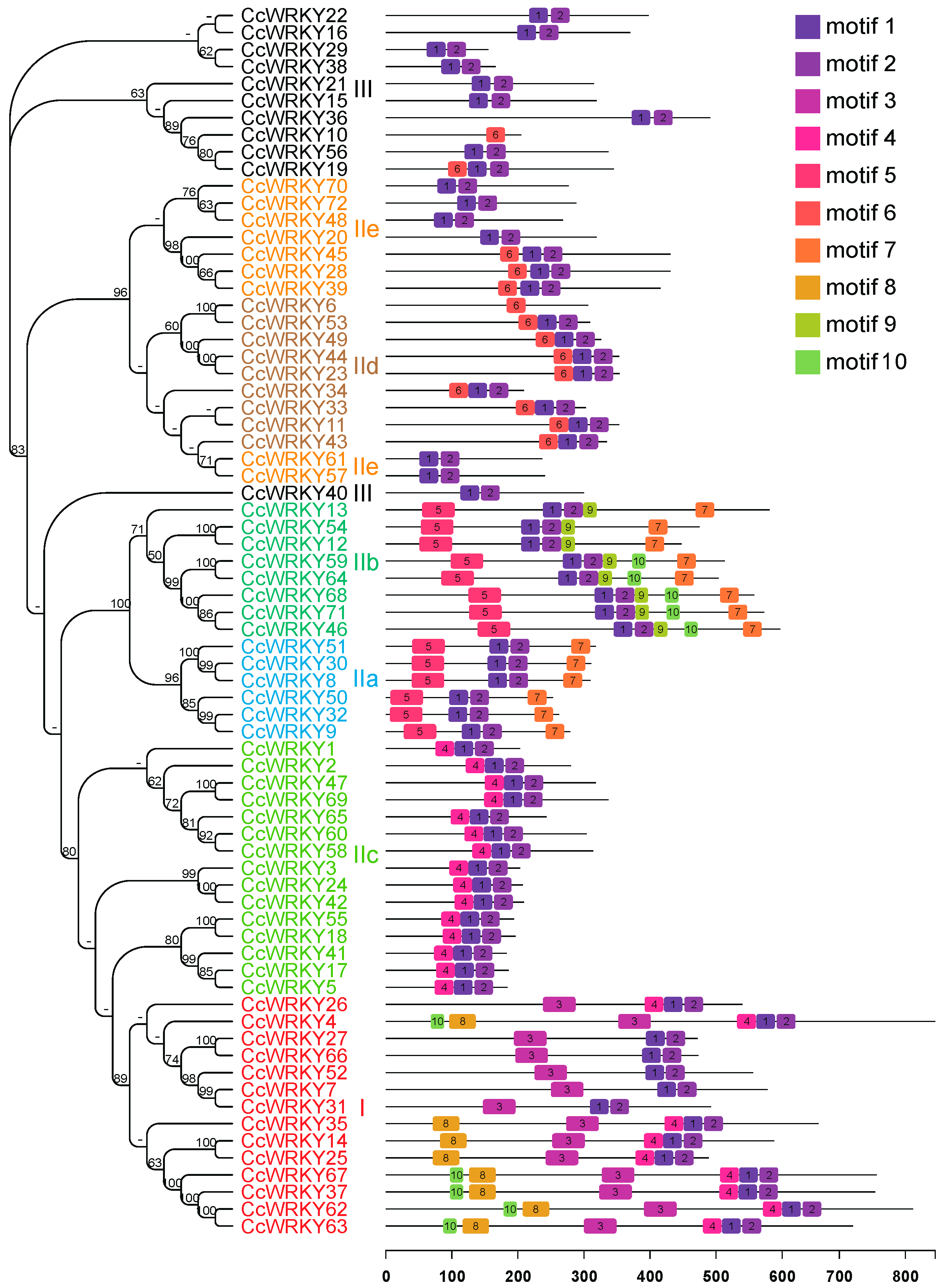
2.3. Motif Analysis of the WRKY in C. camphora
2.4. Identification of Differential Expression Patterns of WRKY Genes
3. Discussion
4. Materials and Methods
4.1. Identification and Physicochemical Properties Analysis
4.2. Phylogenetic Tree Analysis
4.3. Analysis of Conserved Motifs of CcWRKY TFs
4.4. Sample Collection and Transcriptome Sequencing
4.5. Differential Expression Patterns of CcWRKY Genes
4.6. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishiguro, S.; Nakamura, K. Characterization of a CDNA Encoding a Novel DNA-Binding Protein, SPF1, That Recognizes SP8 Sequences in the 5′ Upstream Regions of Genes Coding for Sporamin and β-Amylase from Sweet Potato. Mol. Gen. Genet. MGG 1994, 244, 563–571. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Xu, Z.; Chen, M.; Yu, D. Functions of WRKYs in Plant Growth and Development. Trends Plant Sci. 2023, 28, 630–645. [Google Scholar] [CrossRef]
- Song, H.; Cao, Y.; Zhao, L.; Zhang, J.; Li, S. Review: WRKY Transcription Factors: Understanding the Functional Divergence. Plant Sci. 2023, 334, 111770. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY Superfamily of Plant Transcription Factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY Transcription Factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L. The WRKY Transcription Factor Superfamily: Its Origin in Eukaryotes and Expansion in Plants. BMC Evol. Biol. 2005, 5, 1. [Google Scholar] [CrossRef]
- Rogers, H.J. From Models to Ornamentals: How Is Flower Senescence Regulated? Plant Mol. Biol. 2013, 82, 563–574. [Google Scholar] [CrossRef]
- Chen, F.; Hu, Y.; Vannozzi, A.; Wu, K.; Cai, H.; Qin, Y.; Mullis, A.; Lin, Z.; Zhang, L. The WRKY Transcription Factor Family in Model Plants and Crops. CRC Crit. Rev. Plant Sci. 2017, 36, 311–335. [Google Scholar] [CrossRef]
- Woo, H.R.; Kim, H.J.; Lim, P.O.; Nam, H.G. Leaf Senescence: Systems and Dynamics Aspects. Annu. Rev. Plant Biol. 2019, 70, 347–376. [Google Scholar] [CrossRef]
- Luo, M.; Dennis, E.S.; Berger, F.; Peacock, W.J.; Chaudhury, A. MINISEED3 (MINI3), a WRKY Family Gene, and HAIKU2 (IKU2), a Leucine-Rich Repeat (LRR) KINASE Gene, Are Regulators of Seed Size in Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 17531–17536. [Google Scholar] [CrossRef]
- Gu, Y.; Li, W.; Jiang, H.; Wang, Y.; Gao, H.; Liu, M.; Chen, Q.; Lai, Y.; He, C. Differential Expression of a WRKY Gene between Wild and Cultivated Soybeans Correlates to Seed Size. J. Exp. Bot. 2017, 68, 2717–2729. [Google Scholar] [CrossRef]
- Lei, R.; Li, X.; Ma, Z.; Lv, Y.; Hu, Y.; Yu, D. Arabidopsis WRKY2 and WRKY34 Transcription Factors Interact with VQ20 Protein to Modulate Pollen Development and Function. Plant J. 2017, 91, 962–976. [Google Scholar] [CrossRef]
- Yang, Y.; Chi, Y.; Wang, Z.; Zhou, Y.; Fan, B.; Chen, Z. Functional Analysis of Structurally Related Soybean GmWRKY58 and GmWRKY76 in Plant Growth and Development. J. Exp. Bot. 2016, 67, 4727–4742. [Google Scholar] [CrossRef]
- Cheng, Y.; JalalAhammed, G.; Yu, J.; Yao, Z.; Ruan, M.; Ye, Q.; Li, Z.; Wang, R.; Feng, K.; Zhou, G.; et al. Putative WRKYs Associated with Regulation of Fruit Ripening Revealed by Detailed Expression Analysis of the WRKY Gene Family in Pepper. Sci. Rep. 2016, 6, 39000. [Google Scholar] [CrossRef]
- Tiika, R.J.; Wei, J.; Ma, R.; Yang, H.; Cui, G.; Duan, H.; Ma, Y. Identification and Expression Analysis of the WRKY Gene Family during Different Developmental Stages in Lycium ruthenicum Murr. Fruit. PeerJ 2020, 8, e10207. [Google Scholar] [CrossRef]
- Ay, N.; Irmler, K.; Fischer, A.; Uhlemann, R.; Reuter, G.; Humbeck, K. Epigenetic Programming via Histone Methylation at WRKY53 Controls Leaf Senescence in Arabidopsis Thaliana. Plant J. 2009, 58, 333–346. [Google Scholar] [CrossRef]
- Brusslan, J.A.; Rus Alvarez-Canterbury, A.M.; Nair, N.U.; Rice, J.C.; Hitchler, M.J.; Pellegrini, M. Genome-Wide Evaluation of Histone Methylation Changes Associated with Leaf Senescence in Arabidopsis. PLoS ONE 2012, 7, e33151. [Google Scholar] [CrossRef]
- Fan, Z.; Tan, X.-L.; Shan, W.; Kuang, J.; Lu, W.; Chen, J. Characterization of a Transcriptional Regulator, BrWRKY6, Associated with Gibberellin-Suppressed Leaf Senescence of Chinese Flowering Cabbage. J. Agric. Food Chem. 2018, 66, 1791–1799. [Google Scholar] [CrossRef]
- Price, A.M.; Aros Orellana, D.F.; Salleh, F.M.; Stevens, R.; Acock, R.; Buchanan-Wollaston, V.; Stead, A.D.; Rogers, H.J. A Comparison of Leaf and Petal Senescence in Wallflower Reveals Common and Distinct Patterns of Gene Expression and Physiology. Plant Physiol. 2008, 147, 1898–1912. [Google Scholar] [CrossRef]
- Meng, L.; Yang, H.; Yang, J.; Wang, Y.; Ye, T.; Xiang, L.; Chan, Z.; Wang, Y. Tulip Transcription Factor TgWRKY75 Activates Salicylic Acid and Abscisic Acid Biosynthesis to Synergistically Promote Petal Senescence. J. Exp. Bot. 2024, 75, 2435–2450. [Google Scholar] [CrossRef]
- Wu, X.; Shiroto, Y.; Kishitani, S.; Ito, Y.; Toriyama, K. Enhanced Heat and Drought Tolerance in Transgenic Rice Seedlings Overexpressing OsWRKY11 under the Control of HSP101 Promoter. Plant Cell Rep. 2009, 28, 21–30. [Google Scholar] [CrossRef]
- Ma, P.; Guo, G.; Xu, X.; Luo, T.; Sun, Y.; Tang, X.; Heng, W.; Jia, B.; Liu, L. Transcriptome Analysis Reveals Key Genes Involved in the Response of Pyrus betuleafolia to Drought and High-Temperature Stress. Plants 2024, 13, 309. [Google Scholar] [CrossRef]
- Ge, M.; Tang, Y.; Guan, Y.; Lv, M.; Zhou, C.; Ma, H.; Lv, J. TaWRKY31, a Novel WRKY Transcription Factor in Wheat, Participates in Regulation of Plant Drought Stress Tolerance. BMC Plant Biol. 2024, 24, 27. [Google Scholar] [CrossRef]
- Luo, D.; Xian, C.; Zhang, W.; Qin, Y.; Li, Q.; Usman, M.; Sun, S.; Xing, Y.; Dong, D. Physiological and Transcriptomic Analyses Reveal Commonalities and Specificities in Wheat in Response to Aluminum and Manganese. Curr. Issues Mol. Biol. 2024, 46, 367–397. [Google Scholar] [CrossRef]
- Gonzalez, A.; Brown, M.; Hatlestad, G.; Akhavan, N.; Smith, T.; Hembd, A.; Moore, J.; Montes, D.; Mosley, T.; Resendez, J.; et al. TTG2 Controls the Developmental Regulation of Seed Coat Tannins in Arabidopsis by Regulating Vacuolar Transport Steps in the Proanthocyanidin Pathway. Dev. Biol. 2016, 419, 54–63. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, G.; Yu, D. Activated Expression of WRKY57 Confers Drought Tolerance in Arabidopsis. Mol. Plant 2012, 5, 1375–1388. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Yu, D. Arabidopsis WRKY Transcription Factors WRKY12 and WRKY13 Oppositely Regulate Flowering under Short-Day Conditions. Mol. Plant 2016, 9, 1492–1503. [Google Scholar] [CrossRef]
- Ma, Z.; Li, W.; Wang, H.; Yu, D. WRKY Transcription Factors WRKY12 and WRKY13 Interact with SPL10 to Modulate Age-mediated Flowering. J. Integr. Plant Biol. 2020, 62, 1659–1673. [Google Scholar] [CrossRef]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A Fumigant during the Black Death and a Coveted Fragrant Wood in Ancient Egypt and Babylon—A Review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, W. Conservation and Applications of Camphor Tree (Cinnamomum camphora) in China: Ethnobotany and Genetic Resources. Genet. Resour. Crop Evol. 2016, 63, 1049–1061. [Google Scholar] [CrossRef]
- Fazmiya, M.J.A.; Sultana, A.; Rahman, K.; Heyat, M.B.B.; Sumbul; Akhtar, F.; Khan, S.; Appiah, S.C.Y. Current Insights on Bioactive Molecules, Antioxidant, Anti-Inflammatory, and Other Pharmacological Activities of Cinnamomum camphora Linn. Oxid. Med. Cell. Longev. 2022, 2022, 9354555. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Chen, X.; Liao, X.; Peng, D.; Han, X.; Zhu, C.; Wang, P.; Hufnagel, D.E.; Wang, L.; Li, K.; et al. A Chromosome-Level Genome of the Camphor Tree and the Underlying Genetic and Climatic Factors for Its Top-Geoherbalism. Front. Plant Sci. 2022, 13, 827890. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kim, D.-S.; Park, S.-H.; Park, H. Phytochemistry and Applications of Cinnamomum camphora Essential Oils. Molecules 2022, 27, 2695. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lin, H.-Y.; Wang, X.; Bi, B.; Gao, Y.; Shao, L.; Zhang, R.; Liang, Y.; Xia, Y.; Zhao, Y.-P.; et al. Genome and Whole-Genome Resequencing of Cinnamomum camphora Elucidate Its Dominance in Subtropical Urban Landscapes. BMC Biol. 2023, 21, 192. [Google Scholar] [CrossRef]
- Wu, Z.; Raven, P.H. (Eds.) Cinnamomum camphora. In Flora of China 7, 102; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2008. [Google Scholar]
- Lee, H.J.; Hyun, E.-A.; Yoon, W.J.; Kim, B.H.; Rhee, M.H.; Kang, H.K.; Cho, J.Y.; Yoo, E.S. In Vitro Anti-Inflammatory and Anti-Oxidative Effects of Cinnamomum camphora Extracts. J. Ethnopharmacol. 2006, 103, 208–216. [Google Scholar] [CrossRef]
- Marouf, A.; Harras, F.; Shehata, E.; Abd-Allah, G. Efficacy of Camphor Oil and Its Nano Emulsion on The Cotton Leafworm, Spodoptera Littoralis. Egypt. Acad. J. Biol. Sci. F Toxicol. Pest Control 2021, 13, 103–108. [Google Scholar] [CrossRef]
- Zhou, L.; Dong, L.; Huang, Y.; Shi, S.; Zhang, L.; Zhang, X.; Yang, W.; Li, L. Spatial Distribution and Source Apportionment of Polycyclic Aromatic Hydrocarbons (PAHs) in Camphor (Cinnamomum camphora) Tree Bark from Southern Jiangsu, China. Chemosphere 2014, 107, 297–303. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, Z.; Li, Z.; Liu, Y.; Fang, S. Predictive Modeling of Suitable Habitats for Cinnamomum camphora (L.) Presl Using Maxent Model under Climate Change in China. Int. J. Environ. Res. Public Health 2019, 16, 3185. [Google Scholar] [CrossRef]
- Xiang, Z.; Zhao, M.; Ogbodo, U.S. Accumulation of Urban Insect Pests in China: 50 Years’ Observations on Camphor Tree (Cinnamomum Camphora). Sustainability 2020, 12, 1582. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, C.; Huang, Y.; An, W.; Liu, S.; Huang, S.; Zheng, X. Metabolism and Transcriptome Profiling Provides Insight into the Genes and Transcription Factors Involved in Monoterpene Biosynthesis of Borneol Chemotype of Cinnamomum camphora Induced by Mechanical Damage. PeerJ 2021, 9, e11465. [Google Scholar] [CrossRef]
- Luan, X.; Xu, W.; Zhang, J.; Shen, T.; Chen, C.; Xi, M.; Zhong, Y.; Xu, M. Genome-Scale Identification, Classification, and Expression Profiling of MYB Transcription Factor Genes in Cinnamomum camphora. Int. J. Mol. Sci. 2022, 23, 14279. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Luan, X.; Chen, C.; Gong, X.; Li, X.; Li, H.; Wu, Z.; Liu, Q.; Xu, M.; Zhong, Y. A Systematic Genome-Wide Analysis and Screening of the NAC Family Genes Related to Wood Formation in Cinnamomum camphora. Genomics 2023, 115, 110631. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Chen, Z. Identification of Genes Encoding Receptor-like Protein Kinases as Possible Targets of Pathogen- and Salicylic Acid-Induced WRKY DNA-Binding Proteins in Arabidopsis. Plant J. 2000, 24, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Chen, C.; Chen, Z. Expression Profiles of the Arabidopsis WRKY Gene Superfamily during Plant Defense Response. Plant Mol. Biol. 2003, 51, 21–37. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Zhou, X.; Liu, S.; Zhuang, Y. Identification of WRKY Gene Family and Characterization of Cold Stress-Responsive WRKY Genes in Eggplant. PeerJ 2020, 8, e8777. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, D.; Yang, C.; Kong, N.; Shi, Z.; Zhao, P.; Nan, Y.; Nie, T.; Wang, R.; Ma, H.; et al. Genome-Wide Identification of the Potato WRKY Transcription Factor Family. PLoS ONE 2017, 12, e0181573. [Google Scholar] [CrossRef]
- Dai, X.; Wang, Y.; Zhang, W.-H. OsWRKY74, a WRKY Transcription Factor, Modulates Tolerance to Phosphate Starvation in Rice. J. Exp. Bot. 2016, 67, 947–960. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, R.; Su, W.; Sun, T.; Qi, M.; Zhang, X.; Wei, F.; Yu, Z.; Xiao, F.; Yan, L.; et al. A Comprehensive Analysis of the WRKY Family in Soybean and Functional Analysis of GmWRKY164-GmGSL7c in Resistance to Soybean Mosaic Virus. BMC Genom. 2024, 25, 620. [Google Scholar] [CrossRef]
- Guo, X.; Yan, X.; Li, Y. Genome-Wide Identification and Expression Analysis of the WRKY Gene Family in Rhododendron henanense subsp. lingbaoense. PeerJ 2024, 12, e17435. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, B.; Gu, G.; Yuan, J.; Shen, S.; Jin, L.; Lin, Z.; Lin, J.; Xie, X. Genome-Wide Identification and Expression Analysis of the R2R3-MYB Gene Family in Tobacco (Nicotiana Tabacum L.). BMC Genom. 2022, 23, 432. [Google Scholar] [CrossRef]
- Hu, W.; Ren, Q.; Chen, Y.; Xu, G.; Qian, Y. Genome-Wide Identification and Analysis of WRKY Gene Family in Maize Provide Insights into Regulatory Network in Response to Abiotic Stresses. BMC Plant Biol. 2021, 21, 427. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Fan, D.; Wang, G. Heteromeric Geranyl (Geranyl) Diphosphate Synthase Is Involved in Monoterpene Biosynthesis in Arabidopsis Flowers. Mol. Plant 2015, 8, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Wang, N.; Yin, Q.; Li, H.; Wu, A.-M.; Qin, G. Activation Tagging Identifies WRKY14 as a Repressor of Plant Thermomorphogenesis in Arabidopsis. Mol. Plant 2022, 15, 1725–1743. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhang, J.; Zhang, B.; Jin, X.; Zhang, H.; Jin, Z. Transcriptional Analysis of Metabolic Pathways and Regulatory Mechanisms of Essential Oil Biosynthesis in the Leaves of Cinnamomum Camphora (L.). Presl. Front. Genet. 2020, 11, 598714. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Y.; Fu, C.; Yang, H.; Liu, X.; Qiu, F.; Wang, X.; Wang, Z. Chemical Variation and Environmental Influence on Essential Oil of Cinnamomum camphora. Molecules 2023, 28, 973. [Google Scholar] [CrossRef]
- Chen, G.; Mostafa, S.; Lu, Z.; Du, R.; Cui, J.; Wang, Y.; Liao, Q.; Lu, J.; Mao, X.; Chang, B.; et al. The Jasmine (Jasminum sambac) Genome Provides Insight into the Biosynthesis of Flower Fragrances and Jasmonates. Genom. Proteom. Bioinform. 2023, 21, 127–149. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhong, X.-L.; Zhu, S.-H.; Wang, K.; Tan, G.-F.; Meng, P.-H.; Zhang, J. Research Advances in Toona Sinensis, a Traditional Chinese Medicinal Plant and Popular Vegetable in China. Diversity 2022, 14, 572. [Google Scholar] [CrossRef]
- Wu, L.; Liu, J.; Huang, W.; Wang, Y.; Chen, Q.; Lu, B. Exploration of Osmanthus Fragrans Lour.’s Composition, Nutraceutical Functions and Applications. Food Chem. 2022, 377, 131853. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E. Biochemical and Molecular Genetic Aspects of Floral Scents. Plant Physiol. 2000, 122, 627–634. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, Function and Metabolic Engineering of Plant Volatile Organic Compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile Terpenoids: Multiple Functions, Biosynthesis, Modulation and Manipulation by Genetic Engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Ouyang, Q.; Li, Y.; Shi, T.; Li, L.; Yang, X.; Ji, K.; Wang, L.; Yue, Y. Genome-Wide Investigation of WRKY Transcription Factors in Sweet Osmanthus and Their Potential Regulation of Aroma Synthesis. Tree Physiol. 2020, 40, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, X.; Mostafa, S.; Noor, I.; Lin, X.; Ren, S.; Cui, J.; Jin, B. WRKY Transcription Factors in Jasminum sambac: An Insight into the Regulation of Aroma Synthesis. Biomolecules 2023, 13, 1679. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Wan, W.; Yin, D.; Deng, X.; Ma, Z.; Gao, T.; Cao, X. Genome-Wide Analysis of WRKY Transcription Factor Genes in Toona sinensis: An Insight into Evolutionary Characteristics and Terpene Synthesis. Front. Plant Sci. 2023, 13, 1063850. [Google Scholar] [CrossRef]
- Yu, Y.; Lyu, S.; Chen, D.; Lin, Y.; Chen, J.; Chen, G.; Ye, N. Volatiles Emitted at Different Flowering Stages of Jasminum sambac and Expression of Genes Related to α-Farnesene Biosynthesis. Molecules 2017, 22, 546. [Google Scholar] [CrossRef]
- Barman, M.; Mitra, A. Temporal Relationship between Emitted and Endogenous Floral Scent Volatiles in Summer- and Winter-blooming Jasminum Species. Physiol. Plant. 2019, 166, 946–959. [Google Scholar] [CrossRef]
- Johnson, C.S.; Kolevski, B.; Smyth, D.R. TRANSPARENT TESTA GLABRA2, a Trichome and Seed Coat Development Gene of Arabidopsis, Encodes a WRKY Transcription Factor. Plant Cell 2002, 14, 1359–1375. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, J.; Wang, X.; Han, X.; Wei, B.; Wang, J.; Li, B.; Yu, H.; Huang, Q.; Gu, H.; et al. The WRKY Transcription Factor WRKY71/EXB1 Controls Shoot Branching by Transcriptionally Regulating RAX Genes in Arabidopsis. Plant Cell 2015, 27, 3112–3127. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The Protein Families Database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The Conserved Domain Database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein Localization Predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef] [PubMed]
- Almagro Armenteros, J.J.; Salvatore, M.; Emanuelsson, O.; Winther, O.; von Heijne, G.; Elofsson, A.; Nielsen, H. Detecting Sequence Signals in Targeting Peptides Using Deep Learning. Life Sci. Alliance 2019, 2, e201900429. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Ba, A.N.; Pogoutse, A.; Provart, N.; Moses, A.M. NLStradamus: A Simple Hidden Markov Model for Nuclear Localization Signal Prediction. BMC Bioinform. 2009, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. TrimAl: A Tool for Automated Alignment Trimming in Large-Scale Phylogenetic Analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Y.; Cai, G.; Cai, R.; Hu, Z.; Wang, H. Tree Visualization By One Table (TvBOT): A Web Application for Visualizing, Modifying and Annotating Phylogenetic Trees. Nucleic Acids Res. 2023, 51, W587–W592. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.-M.; Brenner, S.E. WebLogo: A Sequence Logo Generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A Multiple Sequence Alignment Editor and Analysis Workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Madhusoodanan, N.; Lee, J.; Eusebi, A.; Niewielska, A.; Tivey, A.R.N.; Lopez, R.; Butcher, S. The EMBL-EBI Job Dispatcher Sequence Analysis Tools Framework in 2024. Nucleic Acids Res. 2024, 52, W521–W525. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Lescot, M. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Schroeder, A.; Mueller, O.; Stocker, S.; Salowsky, R.; Leiber, M.; Gassmann, M.; Lightfoot, S.; Menzel, W.; Granzow, M.; Ragg, T. The RIN: An RNA Integrity Number for Assigning Integrity Values to RNA Measurements. BMC Mol. Biol. 2006, 7, 3. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-Level Expression Analysis of RNA-Seq Experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent Prioritization and Exploratory Visualization of Biological Functions for Gene Enrichment Analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Medrano, J.F. Real-Time PCR for MRNA Quantitation. Biotechniques 2005, 39, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Koressaar, T.; Remm, M. Enhancements and Modifications of Primer Design Program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Gene Name | Gene ID | Amino Acids | Molecular Weight (Da) | Isoelectric Point (pI) | Instability Index (II) | Aliphatic Index | Grand Average of Hydropathicity | NLS |
|---|---|---|---|---|---|---|---|---|
| CcWRKY1 | Ccam01g01320 | 202 | 22,929.21 | 6.29 | 53.73 | 48.66 | −0.92 | no |
| CcWRKY2 | Ccam01g01332 | 279 | 31,328.84 | 8.23 | 70.14 | 47.96 | −1.02 | no |
| CcWRKY3 | Ccam01g01375 | 202 | 23,216.22 | 9.10 | 40.71 | 60.79 | −0.79 | yes |
| CcWRKY4 | Ccam01g03358 | 831 | 91,314.11 | 6.09 | 53.96 | 68.98 | −0.60 | no |
| CcWRKY5 | Ccam01g03535 | 183 | 20,884.51 | 9.58 | 34.00 | 53.22 | −0.92 | yes |
| CcWRKY6 | Ccam02g00068 | 305 | 32,859.34 | 9.67 | 49.90 | 66.95 | −0.37 | no |
| CcWRKY7 | Ccam02g00318 | 577 | 63,115.17 | 5.88 | 51.78 | 68.28 | −0.62 | no |
| CcWRKY8 | Ccam02g00335 | 309 | 33,954.07 | 6.46 | 34.75 | 68.19 | −0.69 | no |
| CcWRKY9 | Ccam02g00336 | 278 | 30,788.64 | 8.13 | 41.63 | 72.63 | −0.59 | no |
| CcWRKY10 | Ccam02g02058 | 204 | 22,130.84 | 5.35 | 30.09 | 72.21 | −0.41 | no |
| CcWRKY11 | Ccam02g03195 | 352 | 38,764.21 | 9.70 | 56.43 | 62.59 | −0.71 | no |
| CcWRKY12 | Ccam03g00297 | 447 | 49,627.64 | 4.81 | 48.17 | 60.49 | −0.82 | no |
| CcWRKY13 | Ccam03g00517 | 580 | 62,741.96 | 6.10 | 56.92 | 57.03 | −0.74 | no |
| CcWRKY14 | Ccam03g00678 | 587 | 63,893.12 | 7.65 | 57.10 | 48.57 | −0.81 | no |
| CcWRKY15 | Ccam03g00785 | 318 | 35,629.67 | 5.88 | 62.85 | 56.16 | −0.77 | yes |
| CcWRKY16 | Ccam03g00786 | 369 | 40,928.02 | 6.64 | 48.93 | 63.20 | −0.58 | no |
| CcWRKY17 | Ccam03g01932 | 185 | 21,015.35 | 9.51 | 56.98 | 50.54 | −0.96 | yes |
| CcWRKY18 | Ccam03g02460 | 195 | 21,949.96 | 8.51 | 44.02 | 67.03 | −0.63 | no |
| CcWRKY19 | Ccam03g02482 | 344 | 37,433.49 | 6.33 | 62.54 | 56.69 | −0.67 | no |
| CcWRKY20 | Ccam03g02618 | 318 | 35,462.09 | 5.52 | 64.62 | 52.20 | −0.84 | no |
| CcWRKY21 | Ccam03g02853 | 314 | 34,696.04 | 6.70 | 51.94 | 68.06 | −0.56 | yes |
| CcWRKY22 | Ccam03g02855 | 397 | 43,406.46 | 5.59 | 54.76 | 63.58 | −0.61 | no |
| CcWRKY23 | Ccam03g03394 | 353 | 39,198.64 | 9.62 | 64.56 | 70.48 | −0.66 | no |
| CcWRKY24 | Ccam03g03468 | 206 | 23,872.61 | 8.16 | 56.49 | 45.73 | −0.97 | no |
| CcWRKY25 | Ccam03g03793 | 488 | 54,296.39 | 6.91 | 54.34 | 48.38 | −0.92 | no |
| CcWRKY26 | Ccam03g03866 | 539 | 58,720.89 | 7.66 | 53.06 | 56.81 | −0.81 | no |
| CcWRKY27 | Ccam04g00306 | 471 | 52,258.51 | 8.87 | 47.95 | 62.48 | −0.82 | no |
| CcWRKY28 | Ccam04g00410 | 430 | 47,039.08 | 5.09 | 42.63 | 56.05 | −0.76 | no |
| CcWRKY29 | Ccam04g00759 | 154 | 17,619.86 | 8.88 | 42.03 | 62.66 | −0.70 | no |
| CcWRKY30 | Ccam04g01225 | 310 | 34,539.94 | 6.60 | 57.52 | 73.90 | −0.61 | no |
| CcWRKY31 | Ccam04g01229 | 491 | 53,286.15 | 8.47 | 57.39 | 59.86 | −0.81 | no |
| CcWRKY32 | Ccam04g01721 | 261 | 28,875.12 | 7.74 | 30.20 | 65.40 | −0.79 | no |
| CcWRKY33 | Ccam04g01778 | 302 | 33,464.86 | 8.97 | 53.71 | 65.23 | −0.69 | no |
| CcWRKY34 | Ccam04g01779 | 208 | 23,301.12 | 5.61 | 56.75 | 66.59 | −0.81 | yes |
| CcWRKY35 | Ccam04g02102 | 654 | 71,102.38 | 5.68 | 52.74 | 64.36 | −0.74 | no |
| CcWRKY36 | Ccam05g00359 | 490 | 55,070.47 | 4.94 | 54.73 | 82.71 | −0.75 | no |
| CcWRKY37 | Ccam05g00959 | 740 | 80,083.45 | 6.09 | 50.14 | 56.27 | −0.73 | no |
| CcWRKY38 | Ccam05g02331 | 165 | 18,405.91 | 9.72 | 33.52 | 63.82 | −0.81 | no |
| CcWRKY39 | Ccam05g02771 | 415 | 45,104.08 | 5.48 | 42.52 | 59.71 | −0.70 | no |
| CcWRKY40 | Ccam05g03164 | 299 | 32,838.52 | 5.77 | 54.63 | 67.16 | −0.66 | no |
| CcWRKY41 | Ccam06g01064 | 182 | 20,838.10 | 9.37 | 40.19 | 51.32 | −1.04 | yes |
| CcWRKY42 | Ccam06g01432 | 208 | 24,184.94 | 8.41 | 61.09 | 42.12 | −1.05 | no |
| CcWRKY43 | Ccam06g01636 | 334 | 37,415.68 | 9.52 | 50.64 | 65.39 | −0.53 | no |
| CcWRKY44 | Ccam06g02136 | 352 | 39,633.05 | 9.73 | 62.27 | 61.19 | −0.74 | no |
| CcWRKY45 | Ccam07g00975 | 430 | 47,493.15 | 5.19 | 57.10 | 57.60 | −0.76 | no |
| CcWRKY46 | Ccam07g01469 | 596 | 64,053.71 | 5.73 | 52.90 | 59.51 | −0.70 | no |
| CcWRKY47 | Ccam07g01532 | 317 | 35,077.74 | 7.11 | 57.41 | 42.78 | −0.91 | yes |
| CcWRKY48 | Ccam07g01664 | 267 | 29,370.72 | 5.76 | 68.89 | 52.28 | −0.75 | no |
| CcWRKY49 | Ccam07g01903 | 325 | 36,564.64 | 9.71 | 55.60 | 65.11 | −0.72 | no |
| CcWRKY50 | Ccam08g01789 | 252 | 28,300.28 | 9.16 | 47.47 | 68.85 | −0.62 | no |
| CcWRKY51 | Ccam08g01792 | 317 | 35,249.97 | 8.74 | 57.54 | 63.38 | −0.80 | no |
| CcWRKY52 | Ccam08g01826 | 555 | 59,846.56 | 6.33 | 55.48 | 64.20 | −0.74 | no |
| CcWRKY53 | Ccam08g02171 | 308 | 33,376.94 | 9.56 | 43.95 | 67.86 | −0.51 | yes |
| CcWRKY54 | Ccam09g01851 | 474 | 52,618.54 | 5.55 | 46.69 | 64.85 | −0.76 | no |
| CcWRKY55 | Ccam10g00062 | 193 | 21,995.86 | 9.49 | 49.33 | 58.65 | −0.81 | no |
| CcWRKY56 | Ccam10g00088 | 336 | 37,554.78 | 5.84 | 63.41 | 54.88 | −0.74 | no |
| CcWRKY57 | Ccam10g01821 | 240 | 27,430.30 | 5.53 | 58.25 | 53.12 | −1.03 | no |
| CcWRKY58 | Ccam10g01879 | 313 | 34,489.17 | 5.95 | 66.23 | 45.18 | −0.87 | yes |
| CcWRKY59 | Ccam10g02021 | 512 | 55,821.95 | 8.30 | 51.92 | 62.97 | −0.64 | no |
| CcWRKY60 | Ccam11g00131 | 303 | 33,627.60 | 5.64 | 60.15 | 64.39 | −0.68 | yes |
| CcWRKY61 | Ccam11g00149 | 236 | 26,917.31 | 6.40 | 54.36 | 61.48 | −0.84 | no |
| CcWRKY62 | Ccam11g01089 | 797 | 87,554.96 | 6.35 | 54.73 | 62.15 | −0.72 | no |
| CcWRKY63 | Ccam11g01090 | 706 | 76,678.53 | 5.86 | 56.37 | 61.60 | −0.71 | no |
| CcWRKY64 | Ccam11g01170 | 503 | 54,849.97 | 8.34 | 56.59 | 63.92 | −0.60 | no |
| CcWRKY65 | Ccam11g01283 | 242 | 27,323.35 | 5.88 | 61.00 | 51.07 | −0.88 | yes |
| CcWRKY66 | Ccam11g01643 | 472 | 52,370.07 | 8.97 | 48.45 | 62.75 | −0.92 | no |
| CcWRKY67 | Ccam11g01901 | 742 | 79,958.11 | 5.70 | 50.01 | 56.99 | −0.71 | no |
| CcWRKY68 | Ccam12g00109 | 557 | 61,024.06 | 6.23 | 53.05 | 61.38 | −0.69 | no |
| CcWRKY69 | Ccam12g00173 | 336 | 36,891.77 | 8.67 | 60.18 | 45.86 | −0.91 | yes |
| CcWRKY70 | Ccam12g00336 | 276 | 30,345.67 | 5.12 | 63.80 | 52.03 | −0.75 | no |
| CcWRKY71 | Ccam00g00092 | 572 | 62,699.88 | 6.85 | 49.20 | 58.72 | −0.72 | no |
| CcWRKY72 | Ccam00g00207 | 287 | 31,649.25 | 4.83 | 47.47 | 57.07 | −0.53 | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, S.; Jiang, J.; Lin, H. Whole-Genome Identification and Expression Profiles of WRKY Genes Related to the Leaf Expansion Period in the Camphor Tree. Forests 2025, 16, 266. https://doi.org/10.3390/f16020266
Long S, Jiang J, Lin H. Whole-Genome Identification and Expression Profiles of WRKY Genes Related to the Leaf Expansion Period in the Camphor Tree. Forests. 2025; 16(2):266. https://doi.org/10.3390/f16020266
Chicago/Turabian StyleLong, Shilin, Jingyao Jiang, and Hanyang Lin. 2025. "Whole-Genome Identification and Expression Profiles of WRKY Genes Related to the Leaf Expansion Period in the Camphor Tree" Forests 16, no. 2: 266. https://doi.org/10.3390/f16020266
APA StyleLong, S., Jiang, J., & Lin, H. (2025). Whole-Genome Identification and Expression Profiles of WRKY Genes Related to the Leaf Expansion Period in the Camphor Tree. Forests, 16(2), 266. https://doi.org/10.3390/f16020266






