Soil Carbon–Water Trade-Off Relationships and Driving Mechanisms in Different Forest Types on the Yunnan Plateau, China
Abstract
1. Introduction
2. Materials and Methods
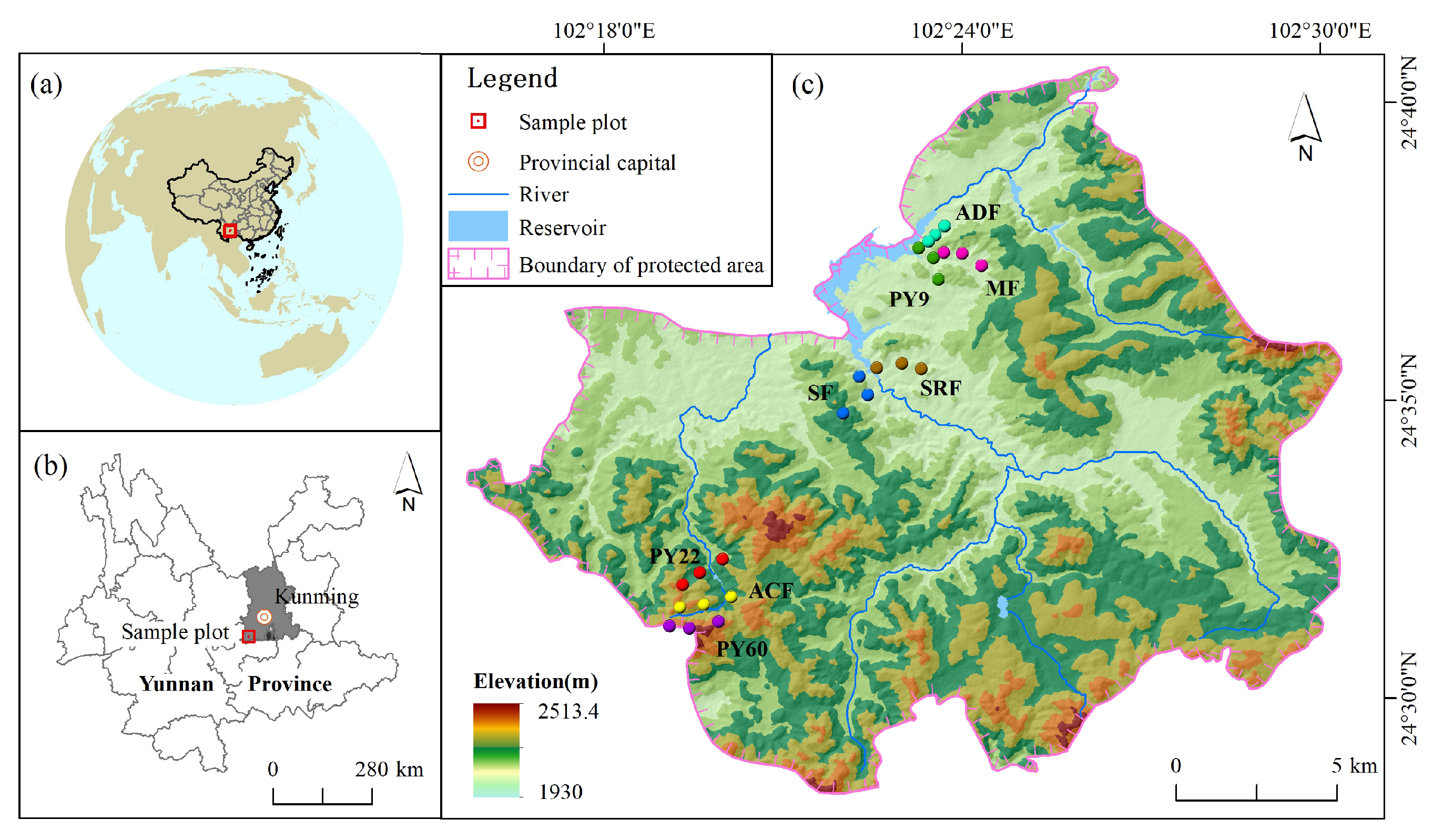
2.1. Study Site
2.2. Plot Establishment and Sampling
2.3. Litter Accumulation and Water Holding Capacity Measurements
2.4. Soil Parameter Measurement
2.5. Calculation of Coupled Coordination Degrees and Trade-Offs Between SOCS and SWS
2.6. Statistical Analysis
3. Results
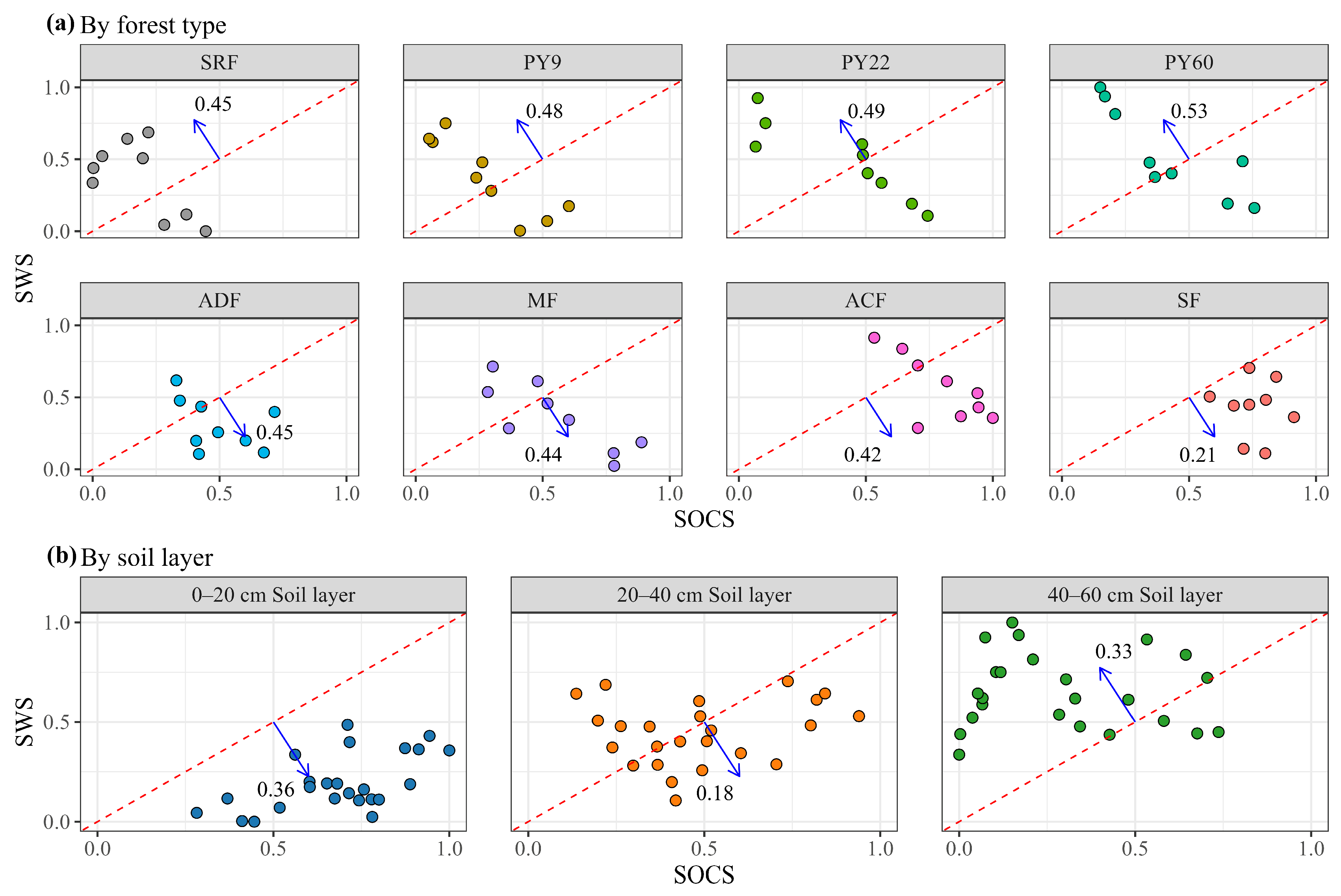
3.1. Distribution Patterns and Relative Gains of SOCS and SWS
3.2. Trade-Off Between SOCS and SWS
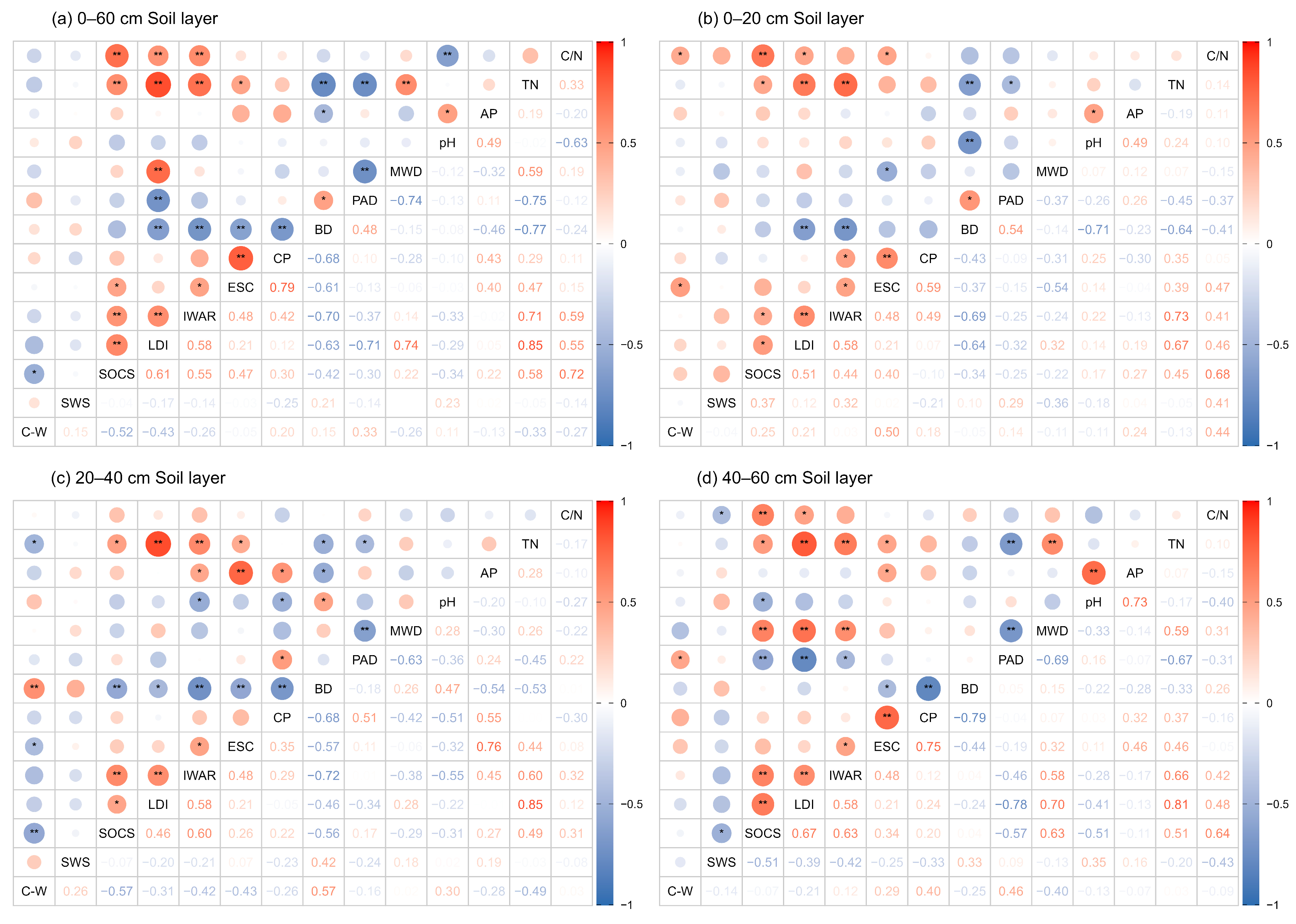
3.3. Controlling Factors of SOCS and SWS
4. Discussion
4.1. Forest Type Controls on Soil Organic Carbon and Water Storage
4.2. Effects of Litter and Soil Physicochemical Properties on Soil Carbon and Water Storage
4.3. Soil Carbon–Water Trade-Offs and Influencing Factors
4.4. Uncertainties and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SRF | Sloping rainfed farmland |
| PY9 | Young Pinus yunnanensis forest |
| PY22 | Near-mature Pinus yunnanensis forest |
| PY60 | Mature Pinus yunnanensis forest |
| ADF | Acacia dealbata forest |
| MF | Mixed conifer-broadleaf forest |
| ACF | Alnus cremastogyne forest |
| SF | Secondary evergreen broadleaf forest |
| LDI | Litter decomposition intensity |
| IWAR | Initial water absorption rate |
| ESC | Effective stock capacity |
| PAD | Percentage of aggregate destruction |
| MWD | Mean weight diameter of aggregates |
| Sand | Soil sand content |
| Clay | Soil clay content |
| BD | Soil bulk density |
| SOC | Soil organic carbon content |
| C:N | Soil carbon-to- nitrogen ratio |
| Silt | Soil Silt content |
| TN | Total nitrogen |
| AP | Available phosphorus |
| SWS | Soil water stock |
| SOCS | Soil organic carbon stock |
References
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Houghton, R.A.; Fang, J.; Kauppi, P.E.; Keith, H.; Kurz, W.A.; Ito, A.; Lewis, S.L. The enduring world forest carbon sink. Nature 2024, 631, 563–569. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Li, W.; Migliavacca, M.; Forkel, M.; Denissen, J.M.C.; Reichstein, M.; Yang, H.; Duveiller, G.; Weber, U.; Orth, R. Widespread increasing vegetation sensitivity to soil moisture. Nat. Commun. 2022, 13, 3959. [Google Scholar] [CrossRef]
- Wang, L.; Manzoni, S.; Ravi, S.; Riveros-Iregui, D.; Caylor, K. Dynamic interactions of ecohydrological and biogeochemical processes in water-limited systems. Ecosphere 2015, 6, 1–27. [Google Scholar] [CrossRef]
- Hua, F.; Bruijnzeel, L.A.; Meli, P.; Martin, P.A.; Zhang, J.; Nakagawa, S.; Miao, X.; Wang, W.; McEvoy, C.; Pena-Arancibia, J.L.; et al. The biodiversity and ecosystem service contributions and trade-offs of forest restoration approaches. Science 2022, 376, 839–843. [Google Scholar] [CrossRef]
- Jiao, W.; Wang, L.; Smith, W.K.; Chang, Q.; Wang, H.; D’Odorico, P. Observed increasing water constraint on vegetation growth over the last three decades. Nat. Commun. 2021, 12, 3777. [Google Scholar] [CrossRef]
- Jia, X.; Shao, M.a.; Zhu, Y.; Luo, Y. Soil moisture decline due to afforestation across the Loess Plateau, China. J. Hydrol. 2017, 546, 113–122. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, G.; Ran, L.; Wang, Y.; Fu, B. Afforestation Reduces Deep Soil Carbon Sequestration in Semiarid Regions: Lessons From Variations of Soil Water and Carbon Along Afforestation Stages in China’s Loess Plateau. J. Geophys. Res. Biogeosci. 2024, 129, e2024JG008287. [Google Scholar] [CrossRef]
- Manns, H.R.; Berg, A.A. Importance of soil organic carbon on surface soil water content variability among agricultural fields. J. Hydrol. 2014, 516, 297–303. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, T.; Ren, K.; Sha, G.; Guo, X.; Fu, Y.; Yu, H. The coupling interaction of soil organic carbon stock and water storage after vegetation restoration on the Loess Plateau, China. J. Environ. Manag. 2022, 306, 114481. [Google Scholar] [CrossRef]
- McDermid, S.S.; Weng, E.; Puma, M.; Cook, B.; Hengl, T.; Sanderman, J.; De Lannoy, G.J.M.; Aleinov, I. Soil carbon losses reduce soil moisture in global climate model simulations. Earth Interact. 2022, 26, 195–208. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Ji, W.; Liu, W.; Li, Z. Water deficit limits soil organic carbon sequestration under old apple orchards in the loess-covered region. Agric. Ecosyst. Environ. 2024, 359, 108739. [Google Scholar] [CrossRef]
- Han, L.; Nan, G.; He, X.; Wang, J.; Zhao, J.; Zhang, X. Soil moisture and soil organic carbon coupled effects in apple orchards on the Loess Plateau, China. Sci. Rep. 2024, 14, 12281. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Si, B.; Ma, X.; Wu, P. Deep soil water extraction by apple sequesters organic carbon via root biomass rather than altering soil organic carbon content. Sci. Total Environ. 2019, 670, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-w.; Shangguan, Z.-p. The coupling interaction of soil water and organic carbon storage in the long vegetation restoration on the Loess Plateau. Ecol. Eng. 2016, 91, 574–581. [Google Scholar] [CrossRef]
- Liu, C.; Jia, X.; Ren, L.; Zhao, C.; Yao, Y.; Zhang, Y.; Shao, M.a. Cropland-to-shrubland conversion reduces soil water storage and contributes little to soil carbon sequestration in a dryland area. Agric. Ecosyst. Environ. 2023, 354, 108572. [Google Scholar] [CrossRef]
- Jin, Z.; Ji, W.; Li, R.; Li, Z. Conversion of Farmland to Apple Orchards Modifies Water–Carbon–Nitrogen Trade-Offs in Deep Loess Deposits. Land Degrad. Dev. 2025, 36, 2276–2288. [Google Scholar] [CrossRef]
- Zhang, J.; Lan, Z.; Li, H.; Jaffar, M.T.; Li, X.; Cui, L.; Han, J. Coupling effects of soil organic carbon and moisture under different land use types, seasons and slope positions in the Loess Plateau. CATENA 2023, 233, 107520. [Google Scholar] [CrossRef]
- Li, R.; Zhang, X.; Ji, W.; Li, B.; Li, Z. Multivariate Controls of Water–Carbon Coupling Relationship Under Various Land Use Types in the Thick Loess Deposits. Land Degrad. Dev. 2025, 36, 2289–2302. [Google Scholar] [CrossRef]
- Yang, F.; Huang, M.; Li, C.; Wu, X.; Guo, T.; Zhu, M. Changes in soil moisture and organic carbon under deep-rooted trees of different stand ages on the Chinese Loess Plateau. Agric. Ecosyst. Environ. 2022, 328, 107855. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, W.; Tao, F.; Shi, X.; Fu, B. A Global Synthesis of Multi-Factors Affecting Water Storage Capacity in Forest Canopy, Litter and Soil Layers. Geophys. Res. Lett. 2023, 50, e2022GL099888. [Google Scholar] [CrossRef]
- Su, S.; Liu, X. The Water Storage Function of Litters and Soil in Five Typical Plantations in the Northern and Southern Mountains of Lanzhou, Northwest China. Sustainability 2022, 14, 8231. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Ni, X.; Lin, C.; Chen, G.; Xie, J.; Yang, Z.; Liu, X.; Xiong, D.; Xu, C.; Yue, K.; Wu, F.; et al. Decline in nutrient inputs from litterfall following forest plantation in subtropical China. For. Ecol. Manag. 2021, 496, 445–456. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Peng, X.; Wu, Q.; Peñuelas, J.; Peng, Y.; Li, Z.; Heděnec, P.; Yuan, C.; Wu, F.; et al. A synthesis on the spatial patterns and driving factors of water-holding capacity of forest litter layer across China. J. Hydrol. 2025, 659, 133272. [Google Scholar] [CrossRef]
- Merino-Martín, L.; Stokes, A.; Gweon, H.S.; Moragues-Saitua, L.; Staunton, S.; Plassard, C.; Oliver, A.; Le Bissonnais, Y.; Griffiths, R.I. Interacting effects of land use type, microbes and plant traits on soil aggregate stability. Soil Biol. Biochem. 2021, 154, 108072. [Google Scholar] [CrossRef]
- Wang, X.; Huang, P.; Ma, M.; Shan, K.; Wu, S. Effects of riparian pioneer plants on soil aggregate stability: Roles of root traits and rhizosphere microorganisms. Sci. Total Environ. 2024, 940, 173584. [Google Scholar] [CrossRef]
- Blankinship, J.C.; Fonte, S.J.; Six, J.; Schimel, J.P. Plant versus microbial controls on soil aggregate stability in a seasonally dry ecosystem. Geoderma 2016, 272, 39–50. [Google Scholar] [CrossRef]
- Schweizer, S.A.; Bucka, F.B.; Graf-Rosenfellner, M.; Kögel-Knabner, I. Soil microaggregate size composition and organic matter distribution as affected by clay content. Geoderma 2019, 355, 113901. [Google Scholar] [CrossRef]
- Li, H.; Zhu, H.; Liang, C.; Wei, X.; Yao, Y. Soil erosion significantly decreases aggregate-associated OC and N in agricultural soils of Northeast China. Agric. Ecosyst. Environ. 2022, 323, 107677. [Google Scholar] [CrossRef]
- Liang, H.; Xue, Y.; Li, Z.; Gao, G.; Liu, G. Afforestation may accelerate the depletion of deep soil moisture on the Loess Plateau: Evidence from a meta-analysis. Land Degrad. Dev. 2022, 33, 3829–3840. [Google Scholar] [CrossRef]
- Wu, X.; Fu, D.; Duan, C.; Huang, G.; Shang, H. Distributions and influencing factors of soil organic carbon fractions under different vegetation restoration conditions in a subtropical mountainous area, SW China. Forests 2022, 13, 629. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Cao, Z. Superior broad-leaved species alnus cremastogyne, its habitat, life history, special uses and genetics. J. Trop. For. Sci. 2023, 35, 322–330. [Google Scholar] [CrossRef]
- Liu, W. Studies in biomass and productivity of Acacia dealbata plantation in the protected district of water sources in north Kunming. Guangxi Zhiwu 1995, 15, 327–334. Available online: https://europepmc.org/article/cba/283230 (accessed on 24 September 2025).
- Liu, Y.; Wu, J.; Wu, D.; Li, S.; Wang, L. Seasonal variation in δ13C of Pinus. yunnanensis and Pinus. armandii at different stand ages. Sci. Rep. 2023, 13, 7938. [Google Scholar] [CrossRef]
- Fu, D.; Wu, X.; Duan, C.; Guan, Q.; Huang, N. Changes in functional structure characteristics mediate ecosystem functions during human-induced land-cover alteration: A case study in southwest China. J. Soil Water Conserv. 2018, 73, 461–468. [Google Scholar] [CrossRef]
- Zhou, H.; Yan, Y.; Dai, Q.; He, Z.; Yi, X. Latitudinal and altitudinal patterns and influencing factors of soil humus carbon in the low-latitude plateau regions. Forests 2023, 14, 344. [Google Scholar] [CrossRef]
- Li, L.; Ren, W.; Tian, L. Temporal patterns in root water uptake and intrinsic water-use efficiency of overstory and understory tree species in a subtropical humid pine forest. Agric. For. Meteorol. 2025, 371, e110626. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Xu, M.; Wang, X.-D.; Zhang, J. Tree species composition affects litter eco-hydrological function in Pinus massoniana conifer-broadleaf mixed forest stands in southwest China. Hydrol. Process. 2024, 38, e15104. [Google Scholar] [CrossRef]
- Bradford, J.B.; D’Amato, A.W. Recognizing trade-offs in multi-objective land management. Front. Ecol. Environ. 2012, 10, 210–216. [Google Scholar] [CrossRef]
- Mao, S.; Lv, J.; Li, M.; Li, L.; Xue, J. Trade-off and driving factors of water-energy-food nexus in Mu Us sandy land, China. J. Clean. Prod. 2024, 434, 139852. [Google Scholar] [CrossRef]
- Nan, G.; Wang, J.; Han, L.; He, X.; Jiang, W.; Ma, J. Does slope cropland to natural and artificial conversion change patterns of soil moisture–carbon trade-offs in time and depth on the water-scarce Loess Plateau, China? Agric. Ecosyst. Environ. 2025, 385, 109583. [Google Scholar] [CrossRef]
- Tao, S.; Chang, N.-j. Estimation of soil organic carbon stock and its controlling factors in cropland of Yunnan Province, China. J. Integr. Agric. 2022, 21, 1475–1487. [Google Scholar] [CrossRef]
- Balocchi, F.; Galleguillos, M.; Rivera, D.; Stehr, A.; Arumi, J.L.; Pizarro, R.; Garcia-Chevesich, P.; Iroumé, A.; Armesto, J.J.; Hervé-Fernández, P. Forest hydrology in Chile: Past, present, and future. J. Hydrol. 2023, 616, e128681. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Yang, X.; Li, T.; Shao, M.a. Factors controlling deep-profile soil organic carbon and water storage following Robinia pseudoacacia afforestation of the Loess Plateau in China. For. Ecosyst. 2022, 9, 100079. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, Y.; Yue, X.; Yao, B.; Zhang, L.; He, J.; Luo, Y.; Xu, X.; Zong, J. Factors controlling soil organic carbon and total nitrogen stocks following afforestation with Robinia pseudoacacia on cropland across China. For. Ecol. Manag. 2021, 494, e119274. [Google Scholar] [CrossRef]
- Wu, Q.; Ni, X.; Sun, X.; Chen, Z.; Hong, S.; Berg, B.; Zheng, M.; Chen, J.; Zhu, J.; Ai, L. Substrate and climate determine terrestrial litter decomposition. Proc. Natl. Acad. Sci. USA 2025, 122, 4122–4130. [Google Scholar] [CrossRef]
- Yang, L.; Wei, W.; Chen, L.; Mo, B. Response of deep soil moisture to land use and afforestation in the semi-arid Loess Plateau, China. J. Hydrol. 2012, 475, 111–122. [Google Scholar] [CrossRef]
- Jia, X.; Shao, M.; Yu, D.; Zhang, Y.; Binley, A. Spatial variations in soil-water carrying capacity of three typical revegetation species on the Loess Plateau, China. Agric. Ecosyst. Environ. 2019, 273, 25–35. [Google Scholar] [CrossRef]
- Sartori, F.; Lal, R.; Ebinger, M.H.; Eaton, J.A. Changes in soil carbon and nutrient pools along a chronosequence of poplar plantations in the Columbia Plateau, Oregon, USA. Agric. Ecosyst. Environ. 2007, 122, 325–339. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Yue, F.; Li, D. Dynamics of carbon and nitrogen storage in two typical plantation ecosystems of different stand ages on the Loess Plateau of China. PeerJ 2019, 7, e7708. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, R.; Joshi, R.K.; Garkoti, S.C. Rhizosphere soil properties and microbial biomass changes with the chronosequence of stand age of broadleaf banj oak and coniferous deodar forests in the central Himalaya, India. Rhizosphere 2023, 27, e100761. [Google Scholar] [CrossRef]
- Chen, L.-C.; Wang, H.; Yu, X.; Zhang, W.-d.; Lü, X.-T.; Wang, S.-L. Recovery time of soil carbon pools of conversional Chinese fir plantations from broadleaved forests in subtropical regions, China. Sci. Total Environ. 2017, 587–588, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Brantley, S.; Ford, C.R.; Vose, J.M. Future species composition will affect forest water use after loss of eastern hemlock from southern Appalachian forests. Ecol. Appl. 2013, 23, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Prescott, C.E.; Zabek, L.M.; Staley, C.L.; Kabzems, R. Decomposition of broadleaf and needle litter in forests of British Columbia: Influences of litter type, forest type, and litter mixtures. Can. J. For. Res. 2000, 30, 1742–1750. [Google Scholar] [CrossRef]
- Hou, G.; Delang, C.O.; Lu, X.; Gao, L. A meta-analysis of changes in soil organic carbon stocks after afforestation with deciduous broadleaved, sempervirent broadleaved, and conifer tree species. Ann. For. Sci. 2020, 77, e92. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Pan, C.; Ma, L.; Xue, M. Identifying the efficacy of undecomposed litter mulch to mitigate runoff and sediment under simulated rainfall conditions. CATENA 2025, 250, 108719. [Google Scholar] [CrossRef]
- He, X.; Abs, E.; Allison, S.D.; Tao, F.; Huang, Y.; Manzoni, S.; Abramoff, R.; Bruni, E.; Bowring, S.P.K.; Chakrawal, A.; et al. Emerging multiscale insights on microbial carbon use efficiency in the land carbon cycle. Nat. Commun. 2024, 15, 8010. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, X.; Liao, Z.; Wang, S.; Huang, J.; Luo, Y.; Jiang, L.; Wang, G.G.; Wang, H.; Chen, F.-S. Physical rather than chemical protection determines soil organic carbon accumulation in a subtropical Chinese fir plantation treated by litter manipulation. Plant Soil 2025, 512, 1–15. [Google Scholar] [CrossRef]
- Lehmann, J.; Hansel, C.M.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, M.; Schimel, J.P.; Torn, M.S. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Franklin, S.M.; Kravchenko, A.N.; Vargas, R.; Vasilas, B.; Fuhrmann, J.J.; Jin, Y. The unexplored role of preferential flow in soil carbon dynamics. Soil Biol. Biochem. 2021, 161, 108398. [Google Scholar] [CrossRef]
- Obour, P.B.; Xia, Y.; Ugarte, C.M.; Grift, T.E.; Wander, M.M. Soil physical properties and water dynamics under contrasting management regimes at the Morrow Plots. Soil Tillage Res. 2025, 248, 106422. [Google Scholar] [CrossRef]
- Panchal, P.; Preece, C.; Peñuelas, J.; Giri, J. Soil carbon sequestration by root exudates. Trends Plant Sci. 2022, 27, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Castellano, M.J.; Mueller, K.E.; Olk, D.C.; Sawyer, J.E.; Six, J. Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob. Change Biol. 2015, 21, 3200–3209. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, X.; Li, Z. Plants extend root deeper rather than increase root biomass triggered by critical age and soil water depletion. Sci. Total Environ. 2024, 914, 169689. [Google Scholar] [CrossRef]
- Wang, L.; Ali, G.; Wang, Z. Deep soil water depletion and soil organic carbon and total nitrogen accumulation in a long-term alfalfa pasture. Land Degrad. Dev. 2023, 34, 2164–2176. [Google Scholar] [CrossRef]
- Li, J.T.; Zhang, Y.; Chen, H.; Sun, H.; Tian, W.; Li, J.; Liu, X.; Zhou, S.; Fang, C.; Li, B. Low soil moisture suppresses the thermal compensatory response of microbial respiration. Glob. Change Biol. 2023, 29, 874–889. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Y.; Wang, Y.; Sun, Y.; Chen, Y. High stand density promotes soil organic carbon sequestration in Robinia pseudoacacia plantations in the hilly and gully region of the Loess Plateau in China. Agric. Ecosyst. Environ. 2023, 343, 108256. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lützow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N. Soil organic carbon storage as a key function of soils-A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Ding, S.J.; Zhang, X.F.; Yang, W.L.; Xin, X.L.; Zhu, A.N.; Huang, S.M. Soil nutrients and aggregate composition of four soils with contrasting textures in a long-term experiment. Eurasian Soil Sci. 2021, 54, 1746–1755. [Google Scholar] [CrossRef]
- McKenna, M.D.; Grams, S.E.; Barasha, M.; Antoninka, A.J.; Johnson, N.C. Organic and inorganic soil carbon in a semi-arid rangeland is primarily related to abiotic factors and not livestock grazing. Geoderma 2022, 419, 115844. [Google Scholar] [CrossRef]
- Wang, S.; Yang, M.; Gao, X.; Hu, Q.; Song, J.; Ma, N.; Song, X.; Siddique, K.H.M.; Wu, P.; Zhao, X. Divergent responses of deep SOC sequestration to large-scale revegetation on China’s Loess Plateau. Agric. Ecosyst. Environ. 2023, 349, 108433. [Google Scholar] [CrossRef]
- Miguez-Macho, G.; Fan, Y. Spatiotemporal origin of soil water taken up by vegetation. Nature 2021, 598, 624–628. [Google Scholar] [CrossRef]
- Quan, W.; Ding, G. Root tip structure and volatile organic compound responses to drought stress in Masson pine (Pinus massoniana Lamb.). Acta Physiol. Plant. 2017, 39, 258. [Google Scholar] [CrossRef]

| Effect | NumDF | DenDF | SOCS | SWS | C-W | |||
|---|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |||
| Forest type | 7 | 14 | 34.19 | <0.001 | 1.24 | 0.31 | 0.91 | 0.51 |
| Soil depth | 2 | 30 | 79.31 | <0.001 | 56.82 | <0.001 | 33.69 | <0.001 |
| Elevation | 1 | 14 | 2.29 | 0.14 | 0.82 | 0.37 | 0.17 | 0.68 |
| Slope | 1 | 14 | 1.49 | 0.23 | 1.64 | 0.21 | 0.69 | 0.41 |
| Sand content | 1 | 30 | 0.00 | 0.997 | 0.25 | 0.62 | 0.37 | 0.54 |
| Silt content | 1 | 30 | 1.98 | 0.17 | 3.22 | 0.08 | 0.03 | 0.85 |
| Forest type × Depth | 14 | 30 | 4.36 | <0.001 | 3.57 | <0.001 | 7.38 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Z.; Wang, P.; Fu, L.; Chen, S. Soil Carbon–Water Trade-Off Relationships and Driving Mechanisms in Different Forest Types on the Yunnan Plateau, China. Forests 2025, 16, 1548. https://doi.org/10.3390/f16101548
Ding Z, Wang P, Fu L, Chen S. Soil Carbon–Water Trade-Off Relationships and Driving Mechanisms in Different Forest Types on the Yunnan Plateau, China. Forests. 2025; 16(10):1548. https://doi.org/10.3390/f16101548
Chicago/Turabian StyleDing, Zhiqiang, Ping Wang, Lei Fu, and Shidong Chen. 2025. "Soil Carbon–Water Trade-Off Relationships and Driving Mechanisms in Different Forest Types on the Yunnan Plateau, China" Forests 16, no. 10: 1548. https://doi.org/10.3390/f16101548
APA StyleDing, Z., Wang, P., Fu, L., & Chen, S. (2025). Soil Carbon–Water Trade-Off Relationships and Driving Mechanisms in Different Forest Types on the Yunnan Plateau, China. Forests, 16(10), 1548. https://doi.org/10.3390/f16101548





