Abstract
Fungi can be used as bioindicators to assess the biodiversity and conservation status of different habitats because of their high adaptability and sensitivity to changes in water, air, and soil quality. In this study, records of macrofungi and lichenised fungi were extracted from GBIF, surveyed using GIS software, and used to compare the fungal diversity of the Pyrenean oak and chestnut groves of Castilla y León, analysing the possible implications of their presence for the conservation of these forest habitats. In Quercus pyrenaica forests, a greater number of lichen and macrofungi species and records were recorded than in Castanea sativa forests, although the greater area occupied by the former could have influenced this diversity. The higher presence of ectomycorrhizal macrofungal species in chestnut groves, as well as the higher sensitivity to pollution of lichens in Pyrenean oak-dominated environments, showed the relevance of the analysis of these data for a better understanding of the conservation status of forest habitats. However, in order to obtain more accurate results, it would be necessary to carry out specific studies on a smaller scale.
1. Introduction
The fungi kingdom is highly diverse, both taxonomically and in terms of life forms [1]. Macrofungi, which are distinguished by having spore-bearing structures visible to the naked eye, belong mostly to the division Basidiomycota (although some may be Ascomycota or even zygomycetes fungi). These organisms are usually saprobes or mycorrhizal symbionts, but some are also pathogens of plants or other fungi [2]. Lichenised fungi are an ecologically diverse and successful group living in symbiosis with photoautotrophic organisms (algae or cyanobacteria) [3]. It is one of the most important life forms among the Ascomycota, although some Basidiomycota also live as lichens [4]. The study of fungal diversity is very valuable for assessing ecosystem quality because fungi participate in the recycling of organic matter and improve the living standards of plant species through symbiotic associations [5].
The detection of anthropogenic pollution impact is usually conducted through chemical tests, and physical parameters are measured directly from the environment [6]. However, in recent decades, bio-indicators have gained a lot of relevance and have been widely applied to assess alterations in the environment because they are cheaper than physical and chemical indicators of environmental quality and can provide additional information on the health status of ecosystems [7]. Biological indicators can be defined as species, groups of species, or communities of a wide range of organisms whose presence, quantity, and nature allow us to make inferences about the quality of the environment [8]. Usually, a group of organisms or even a species is selected as the most appropriate bio-indicator in each environment, depending on the studied habitats, the local conditions, and disturbances [9]. A good bioindicator must have several characteristics, such as being easily identifiable, being sensitive to small changes in the environment, or playing a relevant role in the dynamics of ecosystems, among others [10]. However, it must be taken into account that no taxon fits all these premises [11]. The main reflection of these biological indicators is manifested through the biodiversity observed in an established area [12], and they can also act as early warning signals as they provide information on measurable qualitative environmental aspects according to their presence, absence, abundance, activity, morphology, physiology, or behaviour [8]. This information could be used to identify negative or positive effects on the environment so that conservation strategies may be considered [12].
The most commonly used biological indicators are plants, algae, and animals [13], with fewer references to the use of fungi as bioindicators. Despite this, it is considered that fungi possess the potential to act as efficient bioindicators due to their life cycle characteristics, such as ubiquitous distribution, sensitivity, survivability, and tolerance to a changing environment [14]. Their adaptability and sensitivity enable them to show real-time responses to environmental changes and stressors in their habitats, making them versatile and reliable tools for assessing the quality of the environment [5].
Fungi, given their diversity and wide range of ecological adaptations, are suitable as bio-indicators for monitoring the following: water, air, and soil quality; the state of agricultural and forest habitats; and the impact of climate change [15]. For example, lichens are used as bioindicators of air quality because they acquire all their nutrients from direct exposure to the atmosphere, which promotes the accumulation of air pollutants [16]. On the other hand, the presence or absence of certain species of macrofungi can be used as an indicator of the degree of disturbance of forest habitats [17], with poorly growing trees producing fewer sporocarps from mycorrhizal species than healthy ones. In this context, both lichens [18] and macrofungi [19] have been used in forest governance to identify forest habitats in need of management measures.
The main objective of the present work is to analyse the diversity of macrofungi and lichenised fungi in the chestnut (Castanea sativa Mill.) and Pyrenean oak (Quercus pyrenaica Willd.) forests of the Castilla y León region (Central Spain) through the combined use of records in databases and Geographic Information Systems (GIS). This diversity analysis also makes it possible to assess the application of these organisms as bioindicators and to understand the implications for the conservation of forest habitats.
2. Materials and Methods
2.1. Study Area
The study area was limited to chestnut and oak forest formations in the Castilla y León region. For this purpose, the digital cartography of the Spanish Forest Map at a scale of 1:25,000 [20] was obtained. The forest areas located within the boundaries of Castilla y León were selected using the ArcGIS10.8 software package [21]. The main information related to the forests analysed is summarised in Table 1. Then, the two types of habitats studied were chosen from this area (Figure 1) and assigned to codes 9230 and 9260 within the codification of the Habitats of Community Interest (HCI) of the European Union [22].

Table 1.
Main characteristics of the analysed forests in Castilla y León (C-NW Iberian Peninsula). BioR: Biogeographic region; Nplot: number of plots; Has: number of hectares; Age: percentage of trees with the indicated age; Other trees: second most reported tree species. Source: MFE [20].
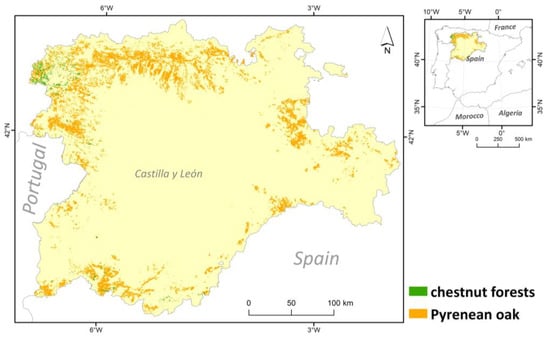
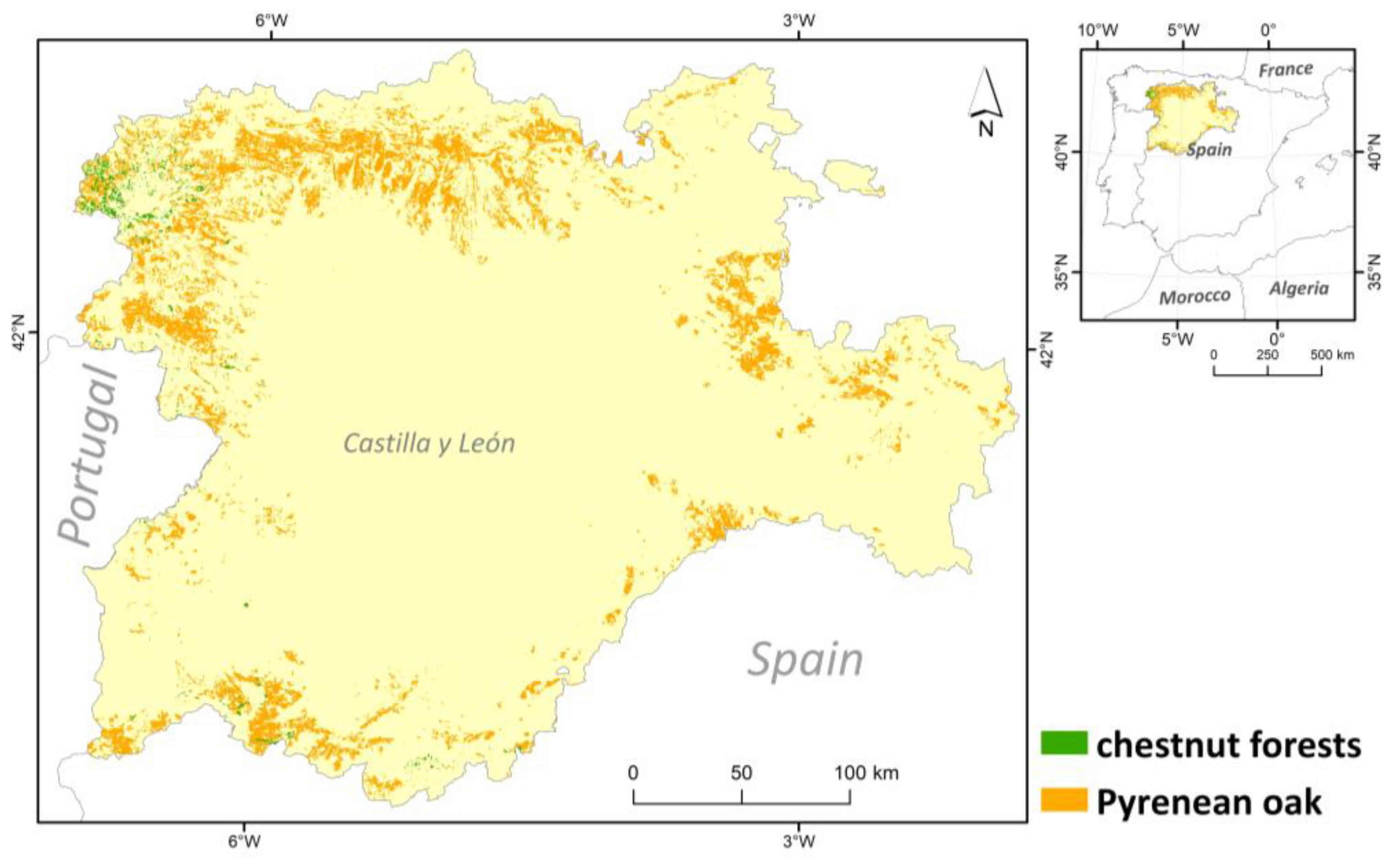
Figure 1.
Distribution of Pyrenean oak (Quercus pyrenaica) and chestnut (Castanea sativa) forests in Castilla y León region (C-NW Spain).
The territory of Castilla y León is spread over two different biogeographical regions, the Atlantic region and the Mediterranean region, although the former is restricted to a small area in the north of the region [23]. Precisely, the presence of these two biogeographical regions underlines the relevance of the selected territory, as these two biogeographical regions cover a large part of the European territory, with consequent climatic and ecological differences. In order to compare the data from the two regions, the hectares occupied by chestnut and Pyrenean oak in both biogeographical regions were calculated by GIS analysis.
2.2. Data Collection
The GBIF (Global Biodiversity Information Facility) database was used to obtain the records of macrofungi and lichens [24,25]. First, all records of the fungi kingdom and the phyla Ascomycota and Basidiomycota were downloaded. From this dataset, the citations involved in the analysed area were selected (16,684 records), checking that they were correctly georeferenced (non-georeferenced records and those with erroneous georeferencing were excluded: 2020 records, mainly due to confusion between zone 29 and the correct zone 30) as well as their correct taxonomic categorisation [26] (information was supplemented for some citations in which their Phylum, Class, Order, or Family was not specified). Once the dataset was reviewed, occurrence citations were separated into lichens (lichenised fungi) and macrofungi. In addition, the lichens and macrofungi present in the Pyrenean oak forests or chestnut groves of the studied area were selected separately, with a buffer of 200 metres to avoid data loss due to inaccurate georeferencing because of the orography. Thus, we worked with 5706 records (1484 records of macrofungi and 4222 records of lichens). Of these records, 93% were preserved records, and 7% were human observations. Regarding the contribution of collections or institutions in this study, 90% of the data came from the herbaria of the University of León and the Royal Botanical Garden of Madrid. A few fungal species were also eliminated from the records obtained (80 records), which could fall under the definition of macrofungi [2] but are pathogens of crop plants, e.g., Taphrina populina Fr.
2.3. Data Analysis
Different aspects such as species richness (alpha diversity), similarity between the two habitats (beta diversity), macrofungi lifestyles, as well as lichen habit and sensitivity to pollution were evaluated. The alpha diversity was calculated separately for both forest habitats through the elaboration of lists of lichen and macrofungi species, both at the general level for the whole study area and in the 10 × 10 km grids. In the latter case, differences in alpha diversity in the study territory can be assessed within each of the habitats and groups analysed. Beta diversity was assessed using the Sørensen Similarity Index to compare the likeness in species of each fungal group in both forest types [27,28].
The assessment of the conservation status of the forest habitats was carried out by analysing the life history of the macrofungi species and the sensitivity to pollution of the different species of lichenised fungi present in the two forest types. To determine the mode of life (mycorrhizal, saprophytic, and parasitic), basic literature was reviewed [29,30], while data from various sources were used to assess the habit (corticolous, epiphytic, lignicolous, muscicolous, saxicolous, and terricolous) and sensitivity to lichen contamination (low, medium and high), according to scientific literature [31,32].
3. Results
3.1. Species Richness
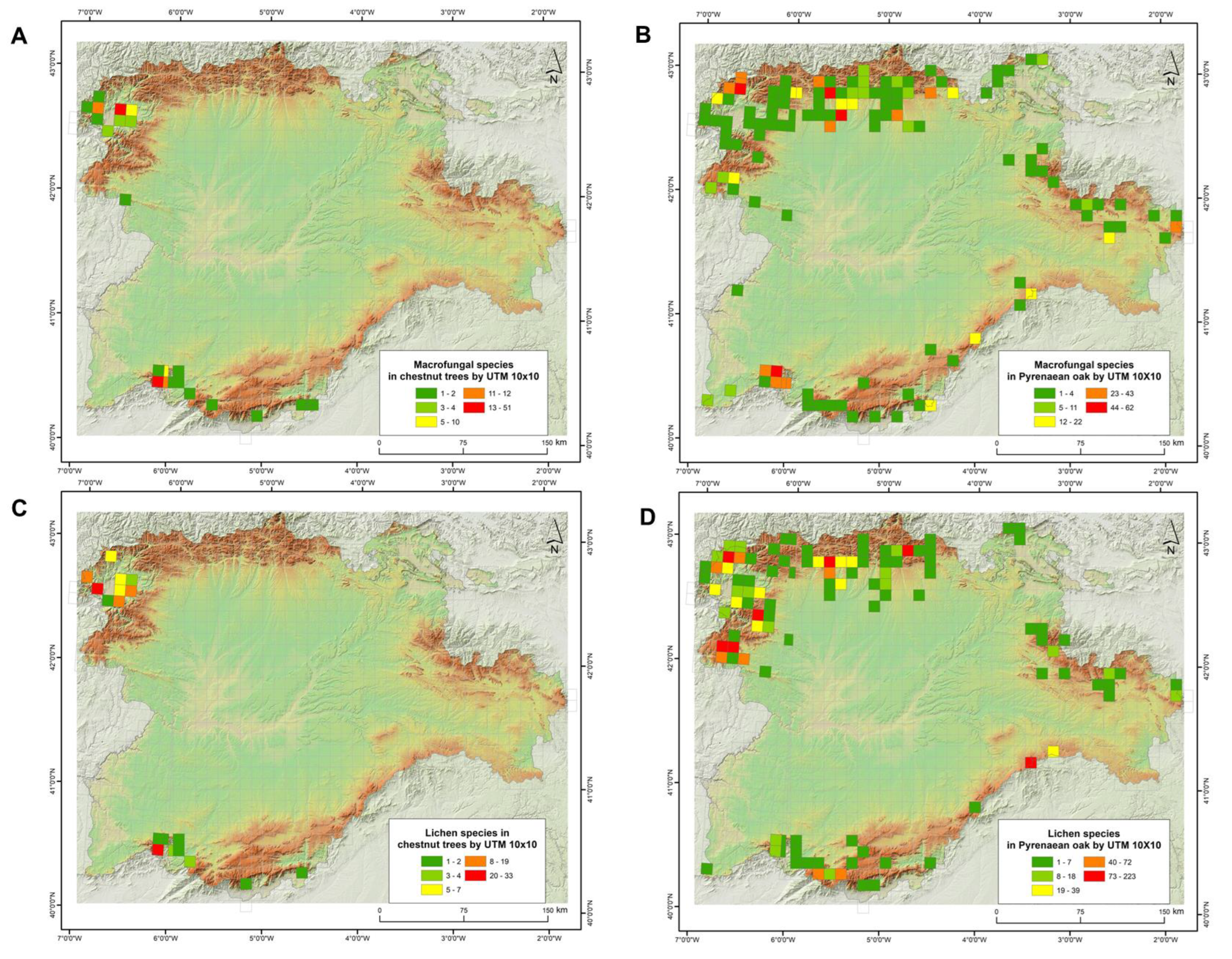
The number of macrofungal species present in Quercus pyrenaica forests was 560 and 127 in Castanea sativa forests, while the number of lichen species was 526 in the former and 85 in the latter (Table S1). Therefore, fungal composition showed a higher alpha diversity in Pyrenean oak forest formations for the two fungal groups, about 4.5 times higher in the case of macrofungi and up to 6 times higher for lichenised fungi. In any case, these data must be contextualised in relation to the surface area of each forest formation. The spatial analysis showed that the hectares occupied by this species of oak in Castilla and León region were about 545,390 ha, which is 5.78% of the total area of this region. Chestnut groves covered about 19,512 ha, i.e., 0.21% of the study area. In relation to the number of species per UTM 10 × 10 grid squares (Figure 2), in Castanea sativa forests, the highest number of species for lichens and macrofungi corresponded to the distribution of these forest ecosystems (NW and SW of the region analysed). In the case of environments dominated by Quercus pyrenaica, differences were detected for both groups of fungal organisms. There were more species of macrofungi in the north and south-west of the study area, while lichenised fungi were more numerous in the north-west and some south-eastern squares.
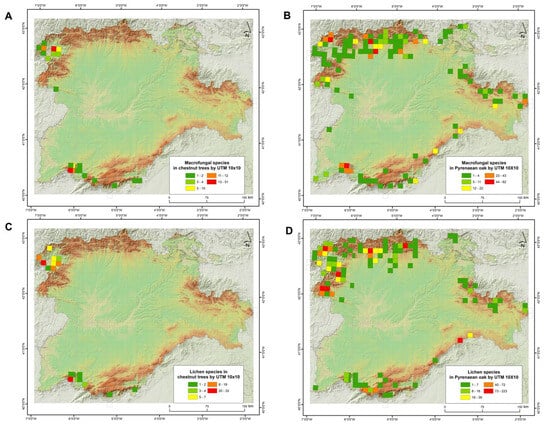
Figure 2.
Number of species of macrofungi (upper part) and lichenised fungi (lower part) in forest formations of Castanea sativa (A,C) and Quercus pyrenaica (B,D) in Castilla y León (C–NW Spain).
In both forest environments, species of macrofungi belonging to the phylum Basidiomycota predominated, with percentages above 90%, although the presence of species belonging to Ascomycota was higher in the ecosystems dominated by Pyrenean oak, where they accounted for 7.5% of the species recorded. As for the diversity of macrofungal orders, 10 orders were present in chestnut groves (one of Ascomycota), while 21 were present in Quercus pyrenaica communities (three belonging to Ascomycota). The most represented orders were the same in both plant formations: Agaricales, Boletales, Polyporales, and Russulales, except for the order Pezizales, which was not present in Castanea sativa forests. The first four orders mentioned accounted for 71.5% and 78.7% of the macrofungal species recorded in Pyrenean oak and chestnut groves, respectively, with the order Agaricales being the most diverse, hosting just over 43% of the species in both ecosystems (Table S2).
In lichenised fungi, all belonging to the phylum Ascomycota, this pattern of diversity was repeated in relation to the orders, with 19 in Pyrenean oak and 11 in chestnut woods. The four most represented orders were the same in both environments (Caliciales, Lecanorales, Peltigerales, and Pertusariales), accounting for 69.1% and 83.6% of the lichens recorded. Among these orders, Lecanorales was notable, as it included 38.6% of lichenicolous species in oak communities and 48.2% in chestnut communities.
The fifteen most abundant species of macrofungi and lichens were identified according to the records assigned to Pyrenean oak and chestnut forests, and it was observed that only five species coincide in both environments. These species are shown in Table 2 with grey background. For lichens, among the fifteen most cited species in the two forest habitats, twelve species coincided, as also shown in Table 2. Therefore, when comparing the two environments, there was greater overlap between the dominant species of lichens than of macrofungi.

Table 2.
Most frequent species of lichenised fungal macrofungi on Pyrenean oak and chestnut forest formations in the study area. In brackets, number of records. In grey background, species identified in both ecosystems.
When comparing species richness between biogeographical regions, both in Quercus pyrenaica and Castanea sativa communities, it was observed that the species richness of macrofungi and lichens is much higher in the Mediterranean region than in the Atlantic region (Table 3).

Table 3.
Beta diversity between the two biogeographical regions of Castilla y León, both of lichenised fungi and macrofungi in Castanea sativa and Quercus pyrenaica forests. SpAt: number of species in the Atlantic region; SpMed: number of species in the Mediterranean region; SC: Species in common; Is: Sørensen similarity index.
3.2. Beta Diversity
Macrofungi and lichenised fungi shared 87 and 79 species, respectively, between the two forests. In the case of chestnut groves, they accounted for 68.5% and 92.9% of the total number of species referenced for both groups of organisms. The results obtained for Sørensen’s index in these two fungal groups were almost identical, 0.25 and 0.26, respectively. The results obtained for Sørensen’s index were higher for lichens in Pyrenean oak forests and showed very low values for macrofungi in chestnut forests (Table 3).
3.3. Macrofungi Lifestyle, Habit and Environmental Sensitivity of Lichenised Fungi
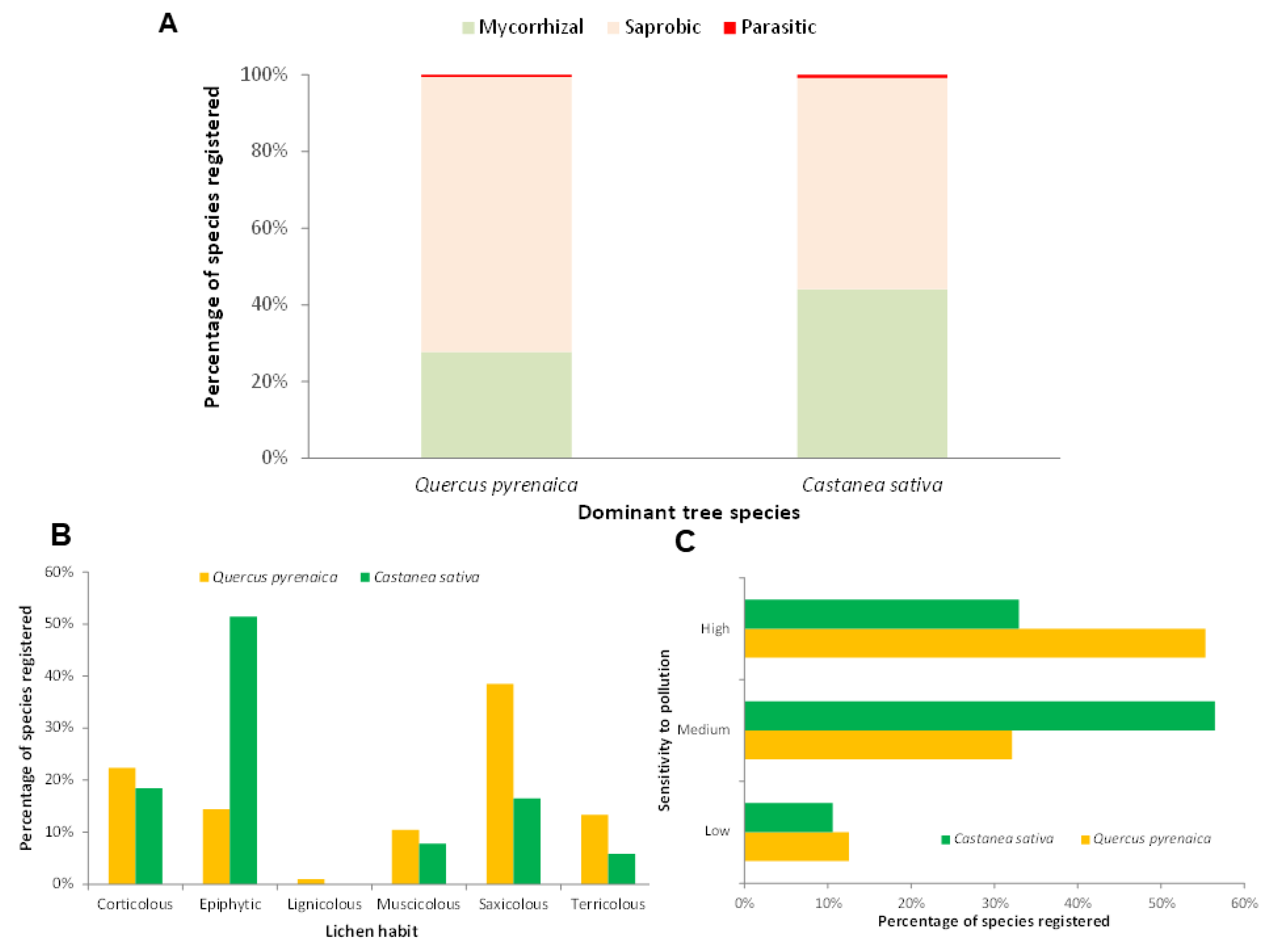
Most of the macrofungal species were found to be saprophytic in both studied forest formations. However, the proportion of saprophytic species was higher in Pyrenean oak forests, with 71.8% compared to 55.1% in chestnut forests (Figure 3A). Similarly, chestnut groves showed a higher proportion of mycorrhizal species (44.1% vs. 27.7%), while the representation of parasitic species was less than 1%.
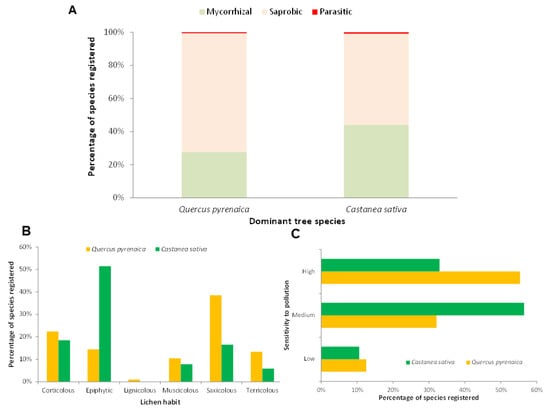
Figure 3.
Lifestyle percentage of macrofungi registered species (A), percentage of lichen reported species habit (B) and sensitivity to contamination of lichen registered species (in %) (C) in Castanea sativa and Quercus pyrenaica forest ecosystems of Castilla y León region (C-NW Spain).
When comparing the habits of the lichenised fungi present in the two forest formations, it was observed that the three most common habits were epiphytic, saxicolous, and corticolous (Figure 3B). However, in Pyrenean oak, saxicolous species dominated, whereas in Castanea sativa trees, most were epiphytic. In addition, differences were also observed in terricolous species, which were twice as common in Quercus pyrenaica. When analysing the sensitivity to pollution of the lichen species present in both forest habitats, it was observed that most of the Pyrenean oak species had a high sensitivity, in contrast to the chestnut species, which mostly had a medium sensitivity (Figure 3C).
4. Discussion
The higher number of species of macrofungi and lichenised fungi in Quercus pyrenaica forests compared to those recorded in Castanea sativa forests seems to be related to the extent of these ecosystems in the studied region [2]. However, when assessing the relationship between the species number and hectares of each forest formation, the chestnut groves revealed higher species richness per hectare for both fungal groups. Similar results were obtained in field works carried out in the NW Spain for epiphytic lichens [33] and in the CW of the Iberian Peninsula for macrofungi [34]. Other studies carried out in smaller plots occupied by the analysed forest formations revealed higher alpha diversity associated with Quercus pyrenaica than with Castanea sativa [35]. These results showed the importance of local studies for a more accurate understanding of fungal diversity [36], but larger-scale studies may have greater applicability for analysing phenomena such as global change [37]. In any case, both forest types had a higher specific fungal richness than other forest environments, such as pine forests of different species [33,38], although in this case, greater human intervention in the management of these formations must be taken into account [39]. Differences in the most frequent species might have been caused by specific habitat characteristics such as resource availability, soil composition, or tree species characteristics such as pH and bark texture [40].
The analysis of the species with the highest number of records in the consulted databases led to the presence of typical species in other forest environments than that of these two trees of the family Fagaceae. In Quercus pyrenaica forests, Auriscalpium vulgare or Rhizopogon roseolus were frequently cited, while in chestnut forests, Hygrophoropsis aurantiaca was registered (Table 2). All of these species are typical of coniferous forests [39]. The presence of macrofungi associated with various pine species can be explained by the composition of Pyrenean oak forests in many areas of the Iberian Peninsula, where some species, such as Pinus sylvestris L. may occur, sometimes to a remarkable extent [41]. The choice of a 200 m buffer in the delimitation of mapped areas for Quercus pyrenaica habitats may also have influenced a higher number of records of pine-associated macrofungal species, although this type of buffer is commonly used in geographic vegetation analysis [42]. It should be noted that other fungal species were also recorded in association with other deciduous forest species, e.g., Xylaria carpophila (Pers.) Fr. on beechnuts of Fagus sylvatica L. The growth of certain macrofungi associated with dominant tree species, such as Peniophora quercina or Lanzia echinophila (Bull.) Korf indicates their ecosystemic role as recyclers of plant material and their influence on the proper functioning of both environments [17].
The assessment of the most diverse macrofungal genera on chestnut groves revealed that Amanita Pers., Cortinarius (Pers.) Gray., and Lactarius Pers., all essentially mycorrhizal [30], had the highest number of species. Other studies carried out in the same environments in the northwest of the Iberian Peninsula [43] also showed a great diversity of mycorrhizal genera. The higher species richness of the genus Cortinarius has been highlighted as an indicator of mature forests [44], which for Castanea sativa forests in the studied area could indicate a better conservation status compared to those in northeastern Portugal [43]. The presence of ectomycorrhizal macrofungi also seems to have a relevant role in the prevention of forest pathologies in chestnut groves, such as those caused by various species of the genus Phytophthora de Bary [45]. In Pyrenean oak forest formations, mycorrhizal genera such as Cortinarius, Russula Pers., and Lactarius also stood out for their specific richness, although other saprophytic genera appeared, such as Mycena (Pers.) Roussel and Marasmius Fr. The presence of saprophytic genera among the most diverse has already been reported in other macrofungal analyses carried out in Quercus pyrenaica forests in the Iberian Peninsula [46], which may indicate a greater use of these formations for livestock [47] in relation to the smaller areas occupied by chestnut forests. A high stocking rate could lead to a lower degree of conservation of Pyrenean oak ecosystems [48] and a higher presence of saprophytic species [49].
The dominance of Basidiomycota taxa has already been reported in other studies [49,50], although a higher diversity of Ascomycota associated with Pyrenean oak was observed. This could be influenced by habitat-specific factors or particular ecological interactions, as Ascomycota decrease with stand age and are displaced by Basidiomycota at those developmental stages where mycorrhizal fungi are more abundant [50,51]. The lower richness of Ascomycota in chestnut groves coincides with the findings of other studies [52] and could, according to these records, be related to a higher degree of maturity of chestnut groves compared to Pyrenean oak forests in the Iberian Peninsula.
Some studies revealed a greater interest in fungal diversity in peninsular forest formations in the Mediterranean region [53], which may explain the results obtained in the analysis of records for the studied area, although the large surface area represented by the Mediterranean region compared to the Atlantic region must also be taken into account. The species number per hectare, however, was higher in the Atlantic region, except for the case of the macrofungi that developed their fruiting bodies in Castanea sativa forests. These data could be biased by the low number of citations in the Atlantic region, probably due to lower sampling in that area and perhaps conditioned by the locations of chestnut groves in these areas [54]. The higher species richness per unit area in the Atlantic biogeographic region is probably due to the existence of more favourable climatic conditions for the development of favourable habitats, as has been shown by comparing fungal diversity between the two biogeographic regions [55] in other geographical areas where they coexist.
Beta diversity between the two forest environments showed a low similarity when using any of the two fungal groups considered. The values obtained are similar to those presented in other peninsular studies comparing macrofungal diversity in tree habitats of various species of the genus Quercus L. [56] or even other wooded ecosystems in several areas of the Mediterranean Basin [28]. In the case of lichenised fungi, some studies suggest a greater species similarity between native trees in relation to other exotic species [57], although the presence of many taxa is conditioned by factors other than the species acting as a phorophyte, such as the age of the trees [58]. The use of other biological indicators assessing the influence of bioclimatic regions on species similarity or divergence also revealed that species distribution is influenced by other factors [59], such as the dominant tree species [38]. Forest structure and dynamics, with circumstances such as forest age or tree age, promote forests with more diverse structures, different substrate types and microhabitats, which positively influences species diversity, especially rare, threatened, or late successional species [40].
The analysis of macrofungal life forms in these two forest formations was carried out in order to contribute to the assessment of the conservation status of the Pyrenean oak and chestnut forests of the studied area. The predominance of saprophytic taxa in Pyrenean oak ecosystems, as well as that of mycorrhizal species in chestnut forests, coincided with analyses carried out in NE Portugal [35], although the percentage of saprophytic species present in Quercus pyrenaica formations was higher in the studied area. An optimal conservation status in Mediterranean forest environments estimates an approximate presence of 51% of saprophytic species, 47% of symbiotic species, and 2% of parasitic species [60], which would indicate that the Pyrenean oak formations in the administrative region of Castilla y León did not have an optimal conservation status [19]. The high percentage of saprobes in the Pyrenean oak habitats was very similar to that obtained in studies carried out in Mediterranean mixed Quercus forests, in which livestock use is considered to be the main cause that may have conditioned the increase in macrofungal species with this lifestyle [48,56]. The poor state of conservation of Pyrenean oak forest formations could be due to the fact that many of them have been configured as pastures and a high stocking rate, which would increase the level of organic matter and thus the amount of saprophytic fungi, as pointed out in the analysis of the diversity of macrofungal genera. In contrast, similar levels of saprophytic and mycorrhizal species were observed in the chestnut groves of the study area, as was the case in a study carried out in a mature Mediterranean forest dominated by holm oak in which the percentage of saprophytes decreased because the forests were more closed with a diverse shrub substrate [49]. These factors, together with the orography and other issues [54], could have influenced Castanea sativa forests to show a better degree of conservation than Quercus pyrenaica forests [19].
The most common habit of the lichenicolous species differed according to the forest environment studied. This difference in the dominant habit might be conditioned by the nature of each of these habitats since the distribution pattern of epiphytes and saxicolous is very different, with saxicolous being more abundant in exposed rocky substrates of mountainous habitats and epiphytes in mixed-use landscapes with a variety of substrates [61,62]. Thus, the dominance of saxicolous lichens in Pyrenean oak could be related to more rocky substrates that favour these preferences [33]. Moreover, epiphytes need a certain degree of shade and humidity to live, so in Mediterranean areas characterised by low rainfall, high temperatures, and seasonal droughts, they would be more dependent on the nature of the forest canopy and shrubs that provide shade and higher humidity to tree trunks [61,63]. Forests of both trees are able to create distinct microclimates under their canopy [64], so the abundance of epiphytic lichens in chestnut groves could be due to the more suitable environmental conditions for this habit, with sufficient light and less water deficit [65].
The known sensitivity of lichenised fungi to air quality, mainly sulphur and nitrogen dioxide deposition, influences the richness and composition of forest lichens [66]. The occurrence of species more vulnerable to pollution in Pyrenean oak might suggest that these areas have better air quality in the study area. Air quality limits the possibility of recolonisation of highly sensitive species characteristic of unpolluted environments [67], which could have an impact on lower air quality in environments linked to chestnut forests. In a study carried out in chestnut groves in southern Italy [68], it was observed that lichen species less tolerant to environmental pollution were found further away from agroforestry areas. Part of these tree formations in the study area were related to various human uses of this tree (e.g., chestnut harvesting, basketry, etc.), as shown in a study carried out in the northwest of the Iberian Peninsula [69].
Studies combining the use of databases and GIS are very rare, although this work aims to show that databases with records of presence could be very useful for diversity studies and for assessing the degree of conservation of forest formations, at least for initial or preliminary evaluations. It should be noted that further studies and fieldwork on this group of organisms are needed to better understand their ecology and their efficient use in the management of the plant communities in which they develop their life cycles.
5. Conclusions
The use of biodiversity databases or repositories could be very useful for preliminary analyses of the diversity of a given group of organisms over a large area. This type of analysis with data taken in the field would be extremely complex to obtain for large territories, so the use of these databases combined with the management of geographic information systems would be a valuable tool.
Similarly, the use of these tools to use a group of organisms as assessors of the conservation status of forest ecosystems could be interesting as a preliminary step in more comprehensive studies in more localised areas. More focused work planning in specific areas could then be carried out in a more systematised and effective way.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f16010009/s1, Table S1. Species of macrofungi and lichens occurring in Quercus pyrenaica and Castanea sativa forests of Castilla y León (C-NW Spain; in alphabetical order). Source: GBIF (2023). Table S2. Main fungal Orders (in number of species) registered in Quercus pyrenaica and Castanea sativa forests of the Castilla y León region (Spain). Source: GBIF (2023).
Author Contributions
Conceptualization, D.R.-d.l.C. and L.D.-S.; methodology, D.R.-d.l.C. and L.D.-S.; software, L.D.-S. and S.P.-A.; validation, D.R.-d.l.C., S.P.-A. and L.D.-S.; formal analysis, D.R.-d.l.C., S.P.-A. and L.D.-S.; investigation, D.R.-d.l.C., S.P.-A. and L.D.-S.; resources, D.R.-d.l.C. and L.D.-S.; data curation, D.R.-d.l.C., S.P.-A. and L.D.-S.; writing—original draft preparation, D.R.-d.l.C., S.P.-A. and L.D.-S.; writing—review and editing, D.R.-d.l.C., S.P.-A. and L.D.-S.; visualization, D.R.-d.l.C. and L.D.-S.; supervision, D.R.-d.l.C. and L.D.-S.; project administration, D.R.-d.l.C. and L.D.-S.; funding acquisition, D.R.-d.l.C. and L.D.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data used for this article are available at https://www.gbif.org/occurrence/download/0088666-230530130749713.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Diversity, taxonomy and phylogeny of the Fungi. Biol. Rev. 2019, 94, 2101–2137. [Google Scholar] [CrossRef]
- Mueller, G.M.; Schmit, J.P.; Leacock, P.R.; Buyck, B.; Cifuentes, J.; Desjardin, D.E.; Halling, R.E.; Hjortstam, K.; Iturriaga, T.; Larsson, K.H.; et al. Global diversity and distribution of macrofungi. Biodivers. Conserv. 2007, 16, 37–48. [Google Scholar] [CrossRef]
- Lumbsch, H.T.; Leavitt, S.D. Goodbye morphology? A paradigm shift in the delimitation of species in lichenized fungi. Fungal Divers. 2011, 50, 59–72. [Google Scholar] [CrossRef]
- Sipman, H.J.; Aptroot, A. Where are the missing lichens? Mycol. Res. 2002, 105, 1433–1439. [Google Scholar] [CrossRef]
- Warnasuriya, S.D.; Udayanga, D.; Manamgoda, D.S.; Biles, C. Fungi as environmental bioindicators. Sci. Total Environ. 2023, 892, 164583. [Google Scholar] [CrossRef] [PubMed]
- Joimel, S.; Cortet, J.; Jolivet, C.C.; Saby, N.P.A.; Chenot, E.D.; Branchu, P.; Consalès, J.N.; Lefort, C.; Morel, J.L.; Schwartz, C. Physico-chemical characteristics of topsoil for contrasted forest, agricultural, urban and industrial land uses in France. Sci. Total Environ. 2016, 545, 40–47. [Google Scholar] [CrossRef]
- Zaghloul, A.; Saber, M.; Gadow, S. Biological indicators for pollution detection in terrestrial and aquatic ecosystems. Bull. Natl. Res. Cent. 2020, 44, 127. [Google Scholar] [CrossRef]
- Gerhardt, A. Bioindicator species and their use in biomonitoring. Environ. Monit. Assess. 2002, 1, 77–123. [Google Scholar]
- Holt, E.A.; Miller, S.W. Bioindicators: Using organisms to measure. Nature 2011, 3, 8–13. [Google Scholar]
- McGeoch, M.A. The selection, testing and application of terrestrial insects as bioindicators. Biol. Rev. 1998, 73, 181–201. [Google Scholar] [CrossRef]
- Puig-Gironès, R.; Real, J. A comprehensive but practical methodology for selecting biological indicators for long-term monitoring. PLoS ONE 2022, 17, e0265246. [Google Scholar] [CrossRef] [PubMed]
- Parmar, T.K.; Rawtani, D.; Agrawal, Y.K. Bioindicators: The natural indicator of environmental pollution. Front. Life Sci. 2016, 9, 110–118. [Google Scholar] [CrossRef]
- Stankovic, S.; Kalaba, P.; Stankovic, A.R. Biota as toxic metal indicators. Environ. Chem. Lett. 2014, 12, 63–84. [Google Scholar] [CrossRef]
- Soares, D.M.; Procópio, D.P.; Zamuner, C.K.; Nóbrega, B.B.; Bettim, M.R.; de Rezende, G.; Lopes, P.M.; Pereira, A.B.D.; Bechara, E.J.H.; Oliveira, A.G.; et al. Fungal bioassays for environmental monitoring. Front. Bioeng. Biotechnol. 2022, 10, 954579. [Google Scholar] [CrossRef]
- Lelli, C.; Bruun, H.H.; Chiarucci, A.; Donati, D.; Frascaroli, F.; Fritz, Ö.; Goldberg, I.; Nascimbene, J.; Tøttrup, A.P.; Rahbek, C.; et al. Biodiversity response to forest structure and management: Comparing species richness, conservation relevant species and functional diversity as metrics in forest conservation. For. Ecol. Manag. 2019, 432, 707–717. [Google Scholar] [CrossRef]
- Ristić, S.; Šajn, R.; Stamenković, S. Lichens as the Main Indicator in Biological Monitoring of Air Quality. In Contaminant Levels and Ecological Effects. Emerging Contaminants and Associated Treatment Technologies; Balabanova, B., Stafilov, T., Eds.; Springer: Cham, Switzerland, 2021; pp. 121–129. ISSN 2524-6410. [Google Scholar]
- Egli, S. Mycorrhizal mushroom diversity and productivity—An indicator of forest health? Ann. For. Sci. 2011, 68, 81–88. [Google Scholar] [CrossRef]
- Will-Wolf, S. Analyzing Lichen Indicator Data in the Forest Inventory and Analysis Program; US Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2011; 61p, ISBN 978-1-5059-0726-1.
- Sánchez-Martínez, C.; Benito-Peñil, D.; García de Enterría, S.; Barajas-Castro, I.; Martín-Herrero, N.; Pérez-Ruiz, C.; Sánchez-Sánchez, J.; Sánchez-Agudo, J.A.; Rodríguez-de la Cruz, D.; Galante-Patiño, E. Manual de Gestión Sostenible de Bosques Abiertos Mediterráneos; Castilla Tradicional: Valladolid, Spain, 2012; pp. 68–79. ISBN 978-84-940714-0-9. [Google Scholar]
- Forestry Map of Spain (MFE), Spanish Ministry for the Ecological Transition and the Demographic Challenge. Available online: https://www.miteco.gob.es/es/cartografia-y-sig/ide/descargas/biodiversidad/mfe.aspx (accessed on 27 January 2023).
- ESRI. ArcGIS Desktop: Release 10.8; Environmental Systems Research Institute: Redlands, CA, USA, 2023. [Google Scholar]
- European Commission. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Union 1992, L206, 50. [Google Scholar]
- European Commission. Council Directive 2007/2/EC of the European Parliament and of the Council of 14 March 2007 establishing an Infrastructure for Spatial Information in the European Community (INSPIRE). Off. J. Eur. Union 2007, L108, 1. [Google Scholar]
- GBIF.org. GBIF Occurrence Download. Available online: https://www.gbif.org/occurrence/download/0088666-230530130749713 (accessed on 5 January 2023).
- GBIF Data Standards. Available online: https://www.gbif.org/standards (accessed on 5 January 2023).
- CABI Index Fungorum. Available online: http://www.indexfungorum.org/names/names.asp (accessed on 8 May 2023).
- Belguidoum, A.; Lograda, T.; Ramdani, M. Diversity and distribution of epiphytic lichens on Cedrus atlantica and Quercus faginea in Mount Babor Forest, Algeria. Biodiversitas 2021, 22, 887–899. [Google Scholar] [CrossRef]
- Fernández Ruiz, A.; Rodríguez de la Cruz, D.; Vicente Villardón, J.L.; Sánchez Durán, S.; García Jiménez, P.; Sánchez Sánchez, J. Considerations on Field Methodology for Macrofungi Studies in Fragmented Forests of Mediterranean Agricultural Landscapes. Agronomy 2022, 12, 528. [Google Scholar] [CrossRef]
- Eyssartier, G.; Roux, P. Le Guide des Champignons France et Europe; Éditions Belin: Paris, France, 2017; 1152p, ISBN 978-2-410-01042-8. [Google Scholar]
- Salcedo, I. Diversidad taxonómica y funcional de los hongos. Artikutza 2019, 100, 151–175. [Google Scholar]
- Barreno Rodríguez, E.; Pérez-Ortega, S. Líquenes de la Reserva Natural Integral de Muniellos, Asturias; KRK: Oviedo, Spain, 2003; 595p, ISBN 84-96119-36-X. [Google Scholar]
- Martellos, S.; Conti, M.; Nimis, P.L. Agregación de datos de líquenes italianos en ITALIC 7.0. Rev. De Hongos 2023, 9, 556. [Google Scholar]
- Maceda-Veiga, A.; Gómez-Bolea, A. Small, fragmented native oak forests have better preserved epiphytic lichen communities than tree plantations in a temperate sub-oceanic Mediterranean climate region. Bryologist 2017, 120, 191–201. [Google Scholar] [CrossRef]
- Santos-Silva, C.; Natário, B.; Andrade, J.; Louro, R. Serra de São Mamede Natural Park, a macrofungal diversity hotspot in the Mediterranean region. Check List 2022, 18, 109–137. [Google Scholar] [CrossRef]
- Baptista, P.; Sousa, M.J.; Dias, R.; Matos, M.; Rodrigues, P.; Martins, A. Macrofungi from Castanea sativa Mill. and Quercus pyrenaica Wild.: Evaluation of mycorrhizal vs nonmycorrhizal fungi biodiversity—Project AGRO 689. In Proceedings of the 5th International Conference on Mycorrhiza (ICOM5), Granada, Spain, 23–27 July 2006. [Google Scholar]
- Toljander, J.F.; Eberhardt, U.; Toljander, Y.K.; Paul, L.R.; Taylor, A.F. Species composition of an ectomycorrhizal fungal community along a local nutrient gradient in a boreal forest. New Phytol. 2006, 170, 873–884. [Google Scholar] [CrossRef]
- Yu, H.; Wang, T.; Skidmore, A.; Heurich, M.; Bässler, C. How future climate and tree distribution changes shape the biodiversity of macrofungi across Europe. Divers. Distrib. 2023, 29, 666–682. [Google Scholar] [CrossRef]
- González-Montelongo, C.; Pérez-Vargas, I. Is an invasive alien tree able to sustain a similar lichen diversity as the native forest? The case of the sweet chestnut (Castanea sativa Mill.) and the laurel forest in Macaronesia. For. Ecol. Manag. 2021, 488, 119009. [Google Scholar] [CrossRef]
- Collado, E.; Bonet, J.A.; Alday, J.G.; de Aragón, J.M.; De-Miguel, S. Impact of forest thinning on aboveground macrofungal community composition and diversity in Mediterranean pine stands. Ecol. Indic. 2021, 133, 108340. [Google Scholar] [CrossRef]
- Nascimbene, J.; Thor, G.; Nimis, P.L. Effects of forest management on epiphytic lichens in temperate deciduous forests of Europe–A review. For. Ecol. Manag. 2013, 298, 27–38. [Google Scholar] [CrossRef]
- Vilches de la Serna, B.; Sánchez-Mata, D.; Gavilán, R.G. Marcescent Quercus pyrenaica forest on the Iberian Peninsula. In Vegetation Structure and Function at Multiple Spatial, Temporal and Conceptual Scales; Box, E.O., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 257–283. ISSN 2198-2562. [Google Scholar]
- Fernandes, M.R.; Aguiar, F.C.; Ferreira, M.T. Assessing riparian vegetation structure and the influence of land use using landscape metrics and geostatistical tools. Landsc. Urban Plan. 2011, 99, 166–177. [Google Scholar] [CrossRef]
- Baptista, P.; Martins, A.; Tavares, R.M.; Lino-Neto, T. Diversity and fruiting pattern of macrofungi associated with chestnut (Castanea sativa) in the Trás-os-Montes region (Northeast Portugal). Fungal Ecol. 2010, 3, 9–19. [Google Scholar] [CrossRef]
- Richard, F.; Moreau, P.A.; Selosse, M.A.; Gardes, M. Diversity and fruiting patterns of ectomycorrhizal and saprobic fungi in an old-growth Mediterranean forest dominated by Quercus ilex L. Can. J. Bot. 2004, 82, 1711–1729. [Google Scholar] [CrossRef]
- Blom, J.M.; Vannini, A.; Vettraino, A.M.; Hale, M.D.; Godbold, D.L. Ectomycorrhizal community structure in a healthy and a Phytophthora-infected chestnut (Castanea sativa Mill.) stand in central Italy. Mycorrhiza 2009, 20, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Branco, S.; Baptista, P.; Martins, A.; Rodrigues, A.P. Estudo da comunidade macrofúngica associada a Quercus pyrenaica Willd. no Parque Natural de Montesinho. In Proceedings of the VII Congresso Luso-Galaico de Macromicologia. Macrofungos: Diversidade e Biotecnología, Vila Real, Portugal, 13–15 October 2005. [Google Scholar]
- Núñez, V.; Hernando, A.; Velázquez, J.; Tejera, R. Livestock management in Natura 2000: A case study in a Quercus pyrenaica neglected coppice forest. J. Nat. Conserv. 2012, 20, 1–9. [Google Scholar] [CrossRef]
- García Jiménez, P.; Fernández Ruiz, A.; Sánchez Sánchez, J.; Rodríguez de la Cruz, D. Mycological Indicators in Evaluating Conservation Status: The Case of Quercus spp. Dehesas in the Middle-West of the Iberian Peninsula (Spain). Sustainability 2020, 12, 10442. [Google Scholar] [CrossRef]
- Fernández, A.; Sánchez, S.; García, P.; Sánchez, J. Macrofungal diversity in an isolated and fragmented Mediterranean Forest ecosystem. Plant Biosyst. 2020, 154, 139–148. [Google Scholar] [CrossRef]
- Martin-Pinto, P.; Sanz-Benito, I.; Santos, M.; Oria-de-Rueda, J.A.; Geml, J. Anthropological impacts determine the soil fungal distribution of Mediterranean oak stands. Ecol. Indic. 2021, 132, 108343. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, X.; Shu, X.; Liu, Y.; Zhang, Q. Linking soil bacterial and fungal communities to vegetation succession following agricultural abandonment. Plant Soil 2018, 431, 19–36. [Google Scholar] [CrossRef]
- Anthony, M.A.; Crowther, T.W.; Van Der Linde, S.; Suz, L.M.; Bidartondo, M.I.; Cox, F.; Schaub, M.; Rautio, P.; Ferretti, M.; Vesterdal, L.; et al. Forest tree growth is linked to mycorrhizal fungal composition and function across Europe. ISME J. 2022, 16, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Núñez, J.A.; Oliet, J.A. Management of mushroom resources in Spanish forests: A review. Forestry 2023, 96, 135–154. [Google Scholar] [CrossRef]
- Rubio, A. 9260 Bosques de Castanea sativa. In Bases Ecológicas Preliminares Para la Conservación de los Tipos de Hábitat de Interés Comunitario en España; Dirección General de Medio Natural y Política Forestal, Ministerio de Medio Ambiente, y Medio Rural y Marino, Ed.; Ministerio de Medio Ambiente, y Medio Rural y Marino: Madrid, Spain.
- Djemiel, C.; Dequiedt, S.; Horrigue, W.; Bailly, A.; Lelièvre, M.; Tripied, J.; Guilland, C.; Perrin, S.; Comment, G.; Saby, N.P.A.; et al. Unraveling biogeographical patterns and environmental drivers of soil fungal diversity at the French national scale. SOIL 2023, 10, 251–273. [Google Scholar] [CrossRef]
- Sanz, A.; Fernández Ruiz, A.; García Jiménez, P.; Sánchez Sánchez, J.; Rodríguez de la Cruz, D. Estudio preliminar de la diversidad de macrohongos en El Monte de Villoria (Salamanca, España). Bot. Compl. 2022, 46, e80421. [Google Scholar] [CrossRef]
- Nascimbene, J.; Lazzaro, L.; Benesperi, R. Patterns of β-diversity and similarity reveal biotic homogenization of epiphytic lichen communities associated with the spread of black locust forests. Fungal Ecol. 2015, 14, 1–7. [Google Scholar] [CrossRef]
- Brunialti, G.; Giordani, P.; Ravera, S.; Frati, L. The reproductive strategy as an important trait for the distribution of lower-trunk epiphytic lichens in old-growth vs. non-old growth forests. Forests 2020, 12, 27. [Google Scholar] [CrossRef]
- Baselga, A.; Jiménez-Valverde, A. Environmental and geographical determinants of beta diversity of leaf beetles (Coleoptera: Chrysomelidae) in the Iberian Peninsula. Ecol. Entomol. 2007, 32, 312–318. [Google Scholar] [CrossRef]
- Moreno, G. Setas Micorrrizógenas, Parásitas y Saprófitas; Una Forma de Valorar el Impacto Ambiental en Nuestros Bosques; Congress Communication: Laredo, Spain, 1996. [Google Scholar]
- Aragón, G.; Martínez, I.; Izquierdo, P.; Belinchón, R.; Escudero, A. Effects of forest management on epiphytic lichen diversity in Mediterranean forests. Appl. Veg. Sci. 2010, 13, 183–194. [Google Scholar] [CrossRef]
- Wolseley, P.A.; Stofer, S.; Mitchell, R.; Truscott, A.M.; Vanbergen, A.; Chimonides, J.; Scheidegger, C. Variation of lichen communities with landuse in Aberdeenshire, UK. Lichenologist 2006, 38, 307–322. [Google Scholar] [CrossRef]
- Király, I.; Nascimbene, J.; Tinya, F.; Ódor, P. Factors influencing epiphytic bryophyte and lichen species richness at different spatial scales in managed temperate forests. Biodivers. Conserv. 2013, 22, 209–223. [Google Scholar] [CrossRef]
- Belinchón, R.; Martínez, I.; Escudero, A.; Aragón, G.; Valladares, F. Edge effects on epiphytic communities in a Mediterranean Quercus pyrenaica forest. J. Veg. Sci. 2007, 18, 81–90. [Google Scholar] [CrossRef]
- Tzenkova, A.; Ivancheva, J. Climate and microclimate in the region of the chestnut forests along the northern slopes of Belasitza Mountain. In Proceedings of the Sustainable Management of Sweet Chestnut Ecosystems-CAST Bul, Blagoevgrad, Bulgaria, 2–5 November 2005; pp. 37–44. [Google Scholar]
- Nascimbene, J.; Ylisirniö, A.L.; Pykälä, J.; Giordani, P. Lichens: Sensitive indicators of changes in the forest environment. In Integrative approaches as an opportunity for the conservation of forest biodiversity; Kraus, D., Krumm, F., Eds.; European Forest Institute: Joensuu, Finland, 2013; pp. 180–185. [Google Scholar]
- Paoli, L.; Fačkovcová, Z.; Lackovičová, A.; Guttová, A. Air pollution in Slovakia (Central Europe): A story told by lichens (1960–2020). Biologia 2021, 76, 3235–3255. [Google Scholar] [CrossRef]
- Aprile, G.G.; Catalano, I.; Migliozzi, A.; Mingo, A. Monitoring epiphytic lichen biodiversity to detect environmental quality and air pollution: The case study of Roccamonfina Park (Campania Region–Italy). Air Pollut. New Dev. 2011, 10, 179007. [Google Scholar]
- Roces-Diaz, J.V.; Díaz-Varela, E.R.; Barrio-Anta, M.; Álvarez-Álvarez, P. Sweet chestnut agroforestry systems in North-western Spain: Classification, spatial distribution and an ecosystem services assessment. For. Syst. 2018, 27, 10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).