Importance of Patches in Maintaining Forest Landscape Connectivity: A Case Study of Barluk, Xinjiang, China
Abstract
1. Introduction
2. Materials and Methods
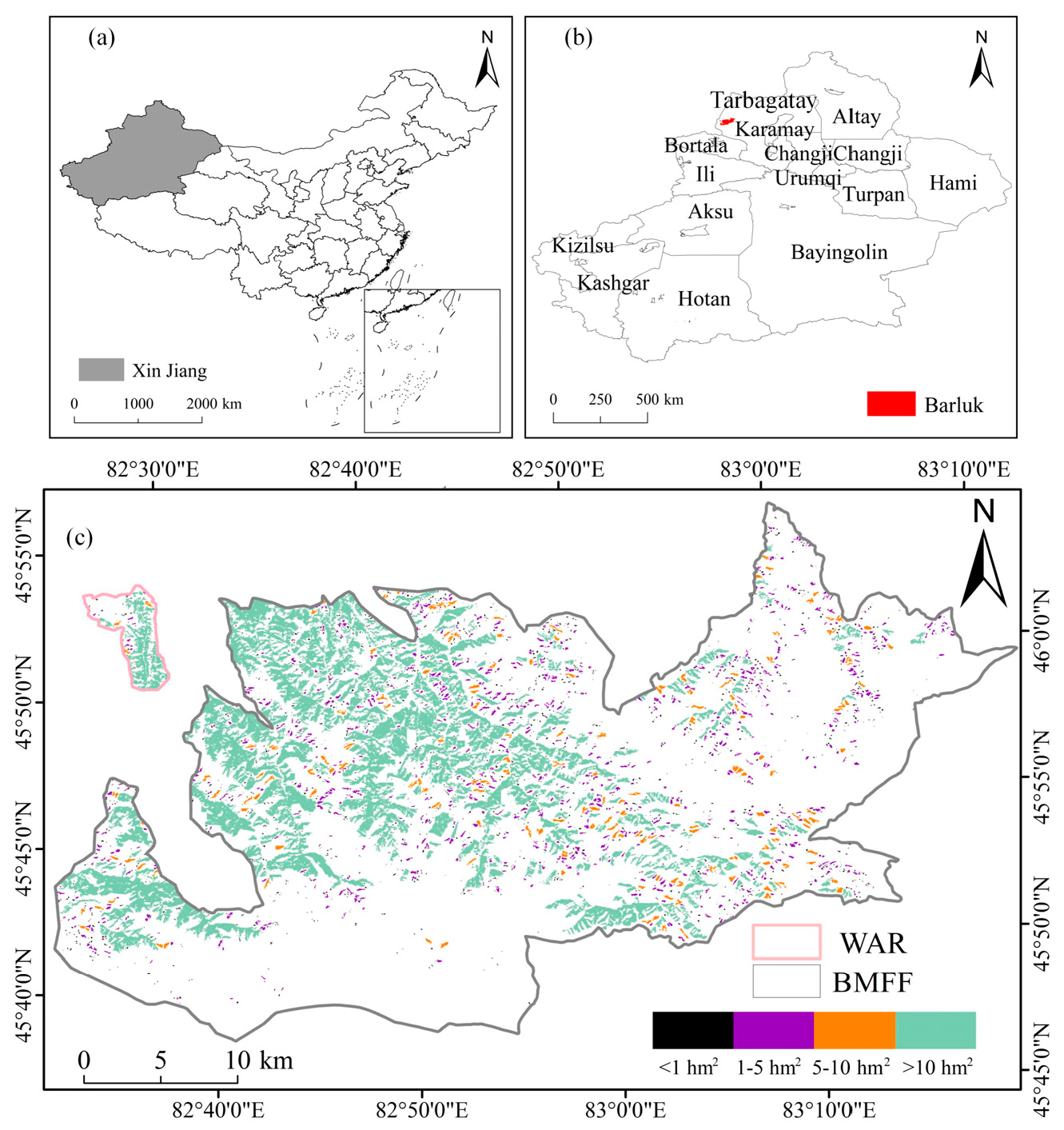
2.1. Study Area
2.2. Data Sources and Processing
2.3. Selection of Dispersal Distance for Landscape Patches
2.4. Selection of the Landscape Connectivity Evaluation Index
2.5. Selection of Important Patches
2.6. The Importance of Small Patches in Maintaining Landscape Connectivity
3. Results
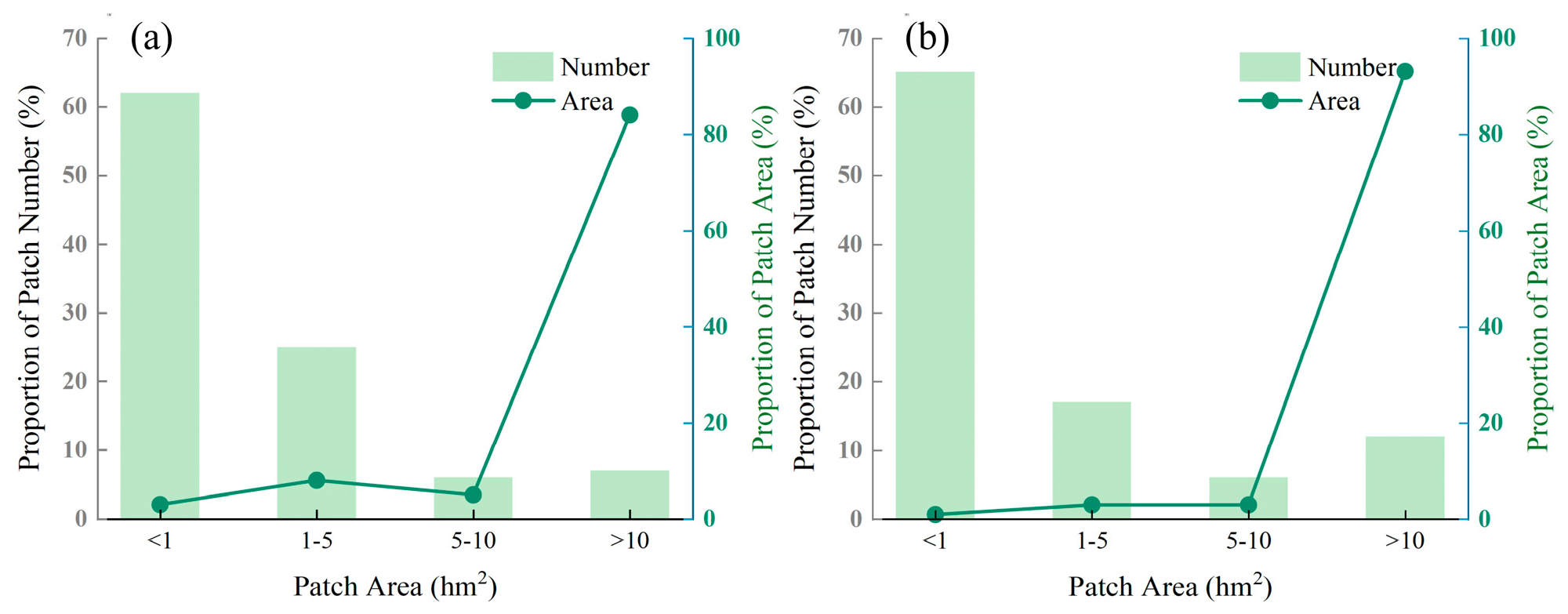
3.1. Patch Composition of Forest Landscapes
3.2. Overall Forest Landscape Connectivity
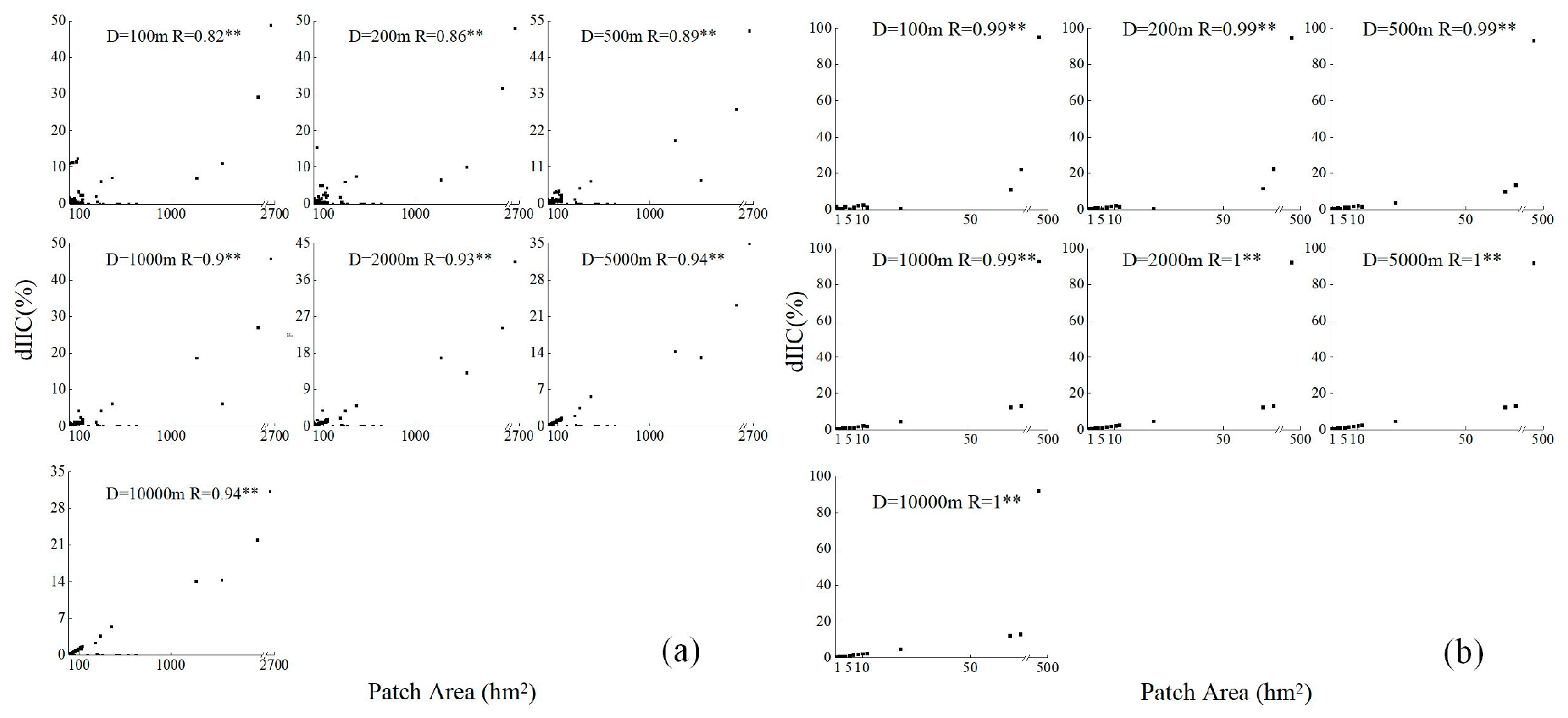
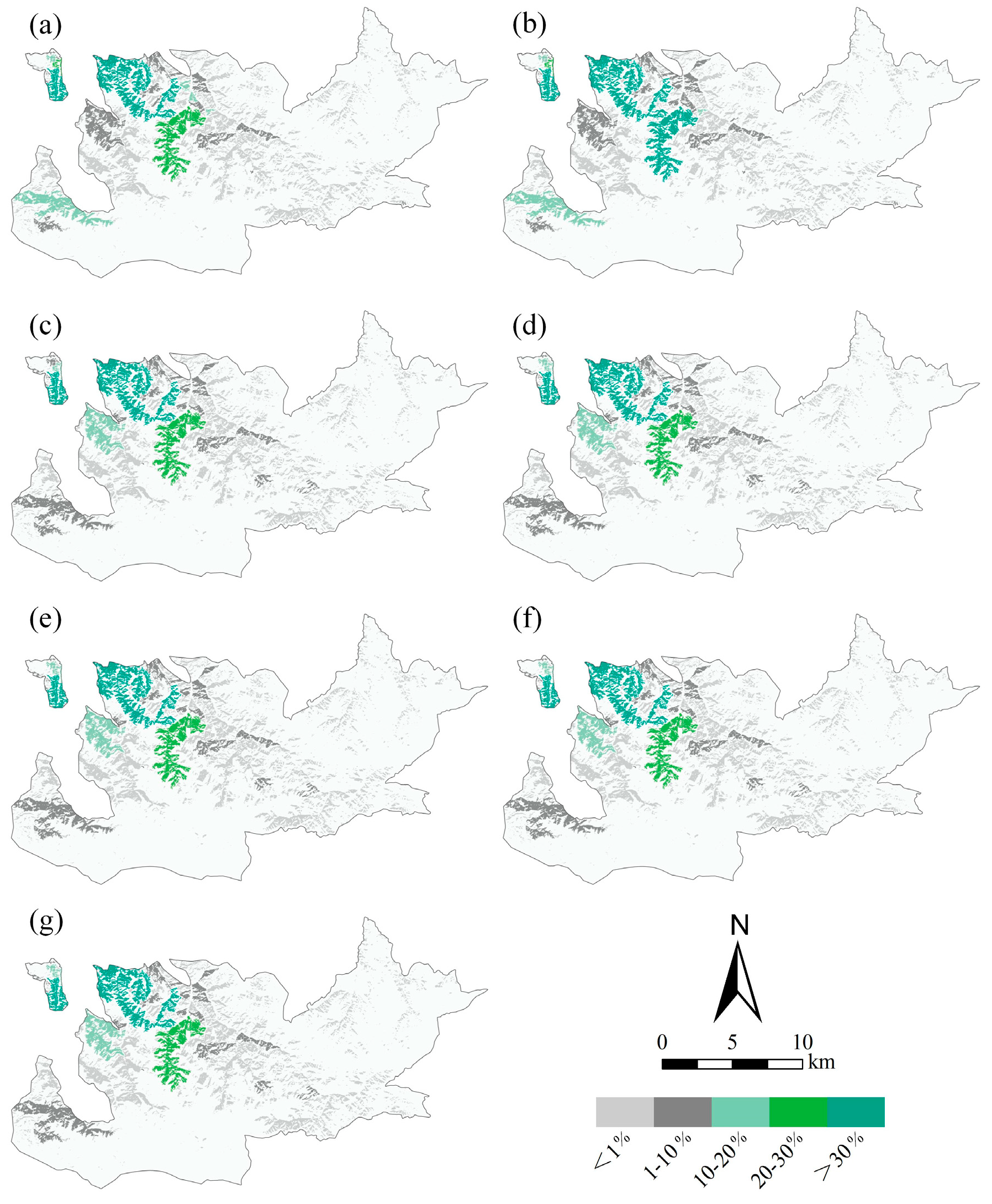
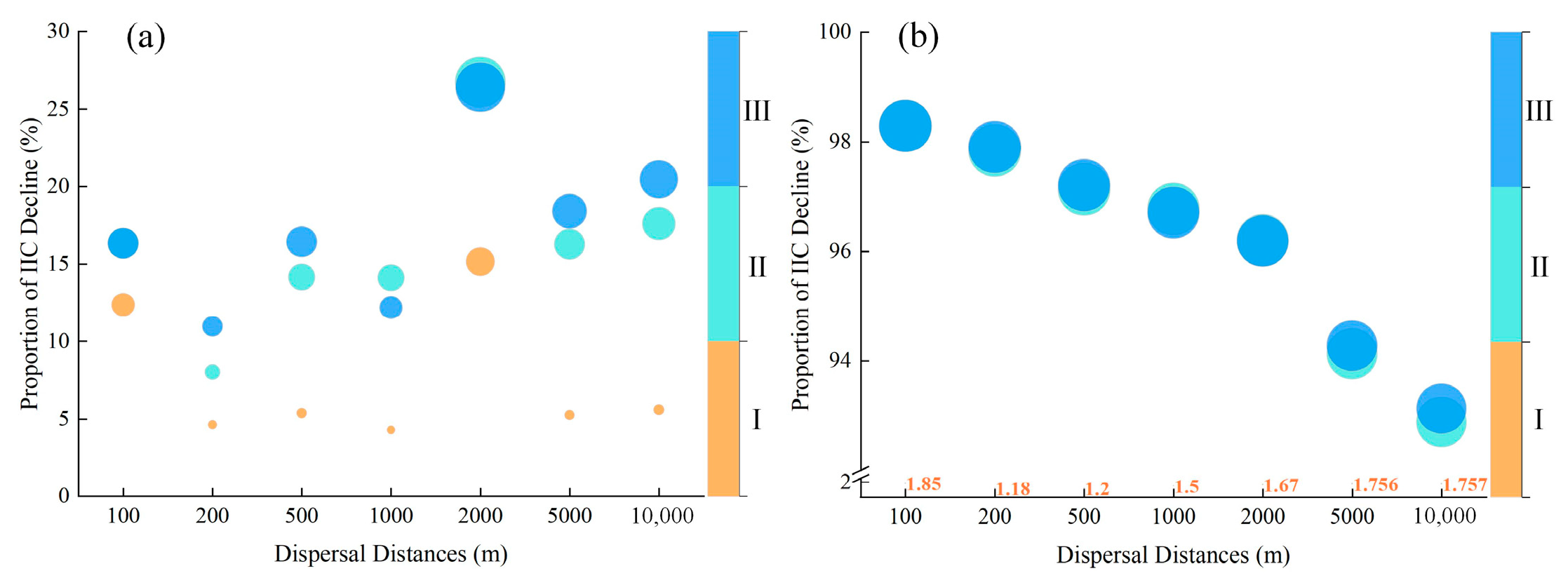
3.3. The Role of Individual Forest Patches on Overall Connectivity
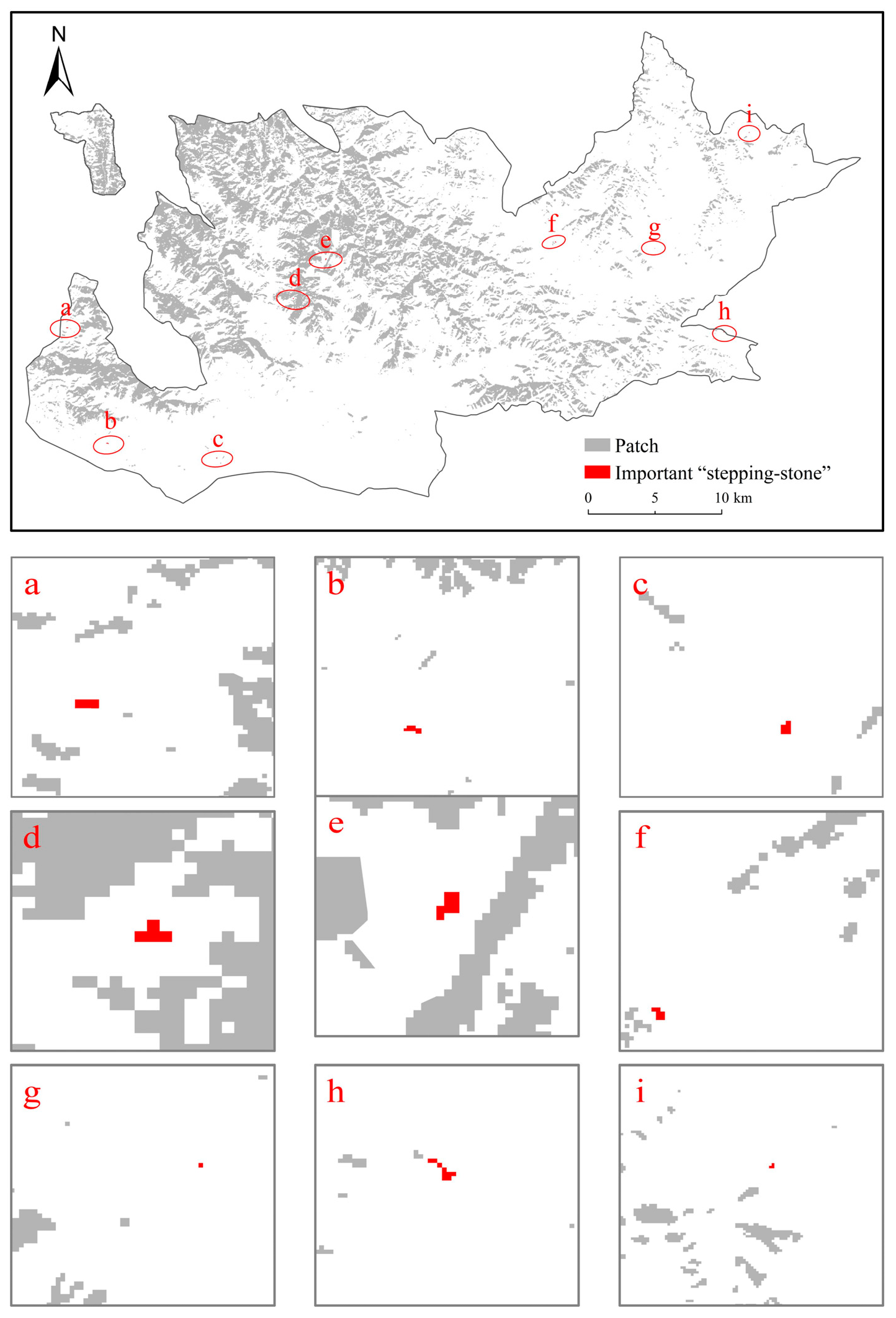
3.4. Identification of Significant Small Patches
4. Discussion
4.1. Forest Fragmentation and Overall Connectivity
4.2. Prioritizing Important Patches to Maintain Forest Landscape Connectivity
4.3. Small Patches Can Ensure and Enhance Landscape Connectivity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riva, F.; Martion, C.J.; Millard, K.; Fahrig, L. Loss of the world’s smallest forests. Glob. Change Biol. 2022, 28, 7164–7166. [Google Scholar] [CrossRef] [PubMed]
- Bao, R.; Li, T.; Zhang, X.; Fu, X.; Zhao, Y.; Tang, M.F.; Deng, H.B. Study on the assessment and management system of forest ecosystem damage. Ecology 2021, 41, 924–933. (In Chinese) [Google Scholar] [CrossRef]
- Grantham, H.S.; Duncan, A.; Evans, T.D.; Jones, K.R.; Beyer, H.L.; Schuster, R.; Walston, J.; Ray, J.C.; Robinson, J.G.; Callow, M.; et al. Anthropogenic modification of forests means only 40% of remaining forests have high ecosystem integrity. Nat. Commun. 2020, 11, 5978. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Lindenmayer, D.B.; Manning, A.D. Biodiversity, ecosystem function, and resilience: Ten guiding principles for commodity production landscapes. Front. Ecol. Environ. 2006, 4, 80–86. [Google Scholar] [CrossRef]
- Fischer, J.; Lindenmayer, D.B. Landscape modification and habitat fragmentation: A synthesis. Glob. Ecol. Biogeogr. 2007, 16, 265–280. [Google Scholar] [CrossRef]
- Dixo, M.; Metzger, J.P.; Morgante, J.S.; Zamudio, K.R. Habitat fragmentation reduces genetic diversity and connectivity among toad populations in the Brazilian Atlantic Coastal Forest. Biol. Conserv. 2009, 142, 1560–1569. [Google Scholar] [CrossRef]
- van Langevelde, F. Modelling the negative effects of landscape fragmentation on habitat selection. Ecol. Inform. 2015, 30, 271–276. [Google Scholar] [CrossRef]
- Spanowicz, A.G.; Jaeger, J.A.G. Measuring landscape connectivity: On the importance of within-patch connectivity. Landsc. Ecol. 2019, 34, 2261–2278. [Google Scholar] [CrossRef]
- Liu, S.L.; Yin, Y.J.; Li, J.R.; Cheng, F.Y.; Dong, S.K.; Zhang, Y.Q. Using cross-scale landscape connectivity indices to identify key habitat resource patches for Asian elephants in Xishuangbanna, China. Landsc. Urban Plan. 2018, 171, 80–87. [Google Scholar] [CrossRef]
- Taylor, P.D.; Fahrig, L.; Henein, K.; Merriam, G. Connectivity is a vital element of landscape structure. Oikos 1993, 68, 571–573. [Google Scholar] [CrossRef]
- Forman, R.T.; Collinge, S.K. Nature conserved in changing landscapes with and without spatial planning. Landsc. Urban Plan. 1997, 37, 129–135. [Google Scholar] [CrossRef]
- Calabrese, J.M.; Fagan, W.F. A comparison-shopper’s guide to connectivity metrics. Front. Ecol. Environ. 2004, 2, 529–536. [Google Scholar] [CrossRef]
- Hanski, I. Metapopulation Ecology; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Urban, D.; Minor, E.S.; Treml, E.A.; Schick, R.S. Graph models of habitat mosaics. Ecol. Lett. 2009, 12, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Ayram, C.A.C.; Mendoza, M.E.; Salicrup, D.R.P.; Granados, E.L. Identifying potential conservation areas in the Cuitzeo Lake basin, Mexico by multitemporal analysis of landscape connectivity. J. Nat. Conserv. 2014, 22, 424–435. [Google Scholar] [CrossRef]
- Martello, F.; dos Santos, J.S.; Silva-Neto, C.M.; Cássia-Silva, C.; Siqueira, K.N.; de Ataide, M.V.R.; Ribeiro, M.C.; Collevatti, R.G. Landscape structure shapes the diversity of plant reproductive traits in agricultural landscapes in the Brazilian Cerrado. Agric. Ecosyst. Environ. 2023, 341, 108216. [Google Scholar] [CrossRef]
- Laita, A.; Kotiaho, J.S.; Mönkkönen, M. Graph-theoretic connectivity measures: What do they tell us about connectivity? Landsc. Ecol. 2011, 26, 951–967. [Google Scholar] [CrossRef]
- Segurado, P.; Branco, P.; Ferreira, M.T. Prioritizing restoration of structural connectivity in rivers: A graph based approach. Landsc. Ecol. 2013, 28, 1231–1238. [Google Scholar] [CrossRef]
- Devi, B.S.S.; Murthy, M.S.R.; Debnath, B.; Jha, C.S. Forest patch connectivity diagnostics and prioritization using graph theory. Ecol. Model. 2013, 251, 279–287. [Google Scholar] [CrossRef]
- Thompson, S.E.D.; Chisholm, R.A.; Rosindell, J. Characterising extinction debt following habitat fragmentation using neutral theory. Ecol. Lett. 2019, 22, 2087–2096. [Google Scholar] [CrossRef]
- Soares, R.M.V.; Lira, P.K.; Manes, S.; Vale, M.M. A methodological framework for prioritizing habitat patches in urban ecosystems based on landscape functional connectivity. Urban Ecosyst. 2024, 27, 147–157. [Google Scholar] [CrossRef]
- Fischer, J.; Peterson, G.D.; Gardner, T.A.; Gordon, L.J.; Fazey, I.; Elmqvist, T.; Felton, A.; Folke, C.; Dovers, S. Integrating resilience thinking and optimisation for conservation. Proc. Natl. Acad. Sci. USA 2009, 24, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, D. Small patches make critical contributions to biodiversity conservation. Proc. Natl. Acad. Sci. USA 2019, 116, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F. Hyperdynamism in fragmented habitats. J. Veg. Sci. 2002, 13, 595–602. [Google Scholar] [CrossRef]
- Herrera, L.P.; Sabatino, M.C.; Jaimes, F.R.; Saura, S. Landscape connectivity and the role of small habitat patches as stepping stones: An assessment of the grassland biome in South America. Biodivers. Conserv. 2017, 26, 3465–3479. [Google Scholar] [CrossRef]
- Wintle, B.A.; Kujala, H.; Whitehead, A.; Cameron, A.; Veloz, S.; Kukkala, A.; Moilanen, A.; Gordon, A.; Lentini, P.E.; Cadenhead, N.C.R.; et al. Global synthesis of conservation studies reveals the importance of small habitat patches for biodiversity. Proc. Natl. Acad. Sci. USA 2019, 116, 909–914. [Google Scholar] [CrossRef]
- Cadavid-Florez, L.; Laborde, J.; Mclean, D.J. Isolated trees and small woody patches greatly contribute to connectivity in highly fragmented tropical landscapes. Landsc. Urban Plan. 2020, 196, 103745. [Google Scholar] [CrossRef]
- Wang, P.J.; Liu, Y.Y.; Permanhan, J.; Liang, L.W.; Mamtimin, S. Ecological types and composition of bryophyte communities in the Barluk Mountain National Nature Reserve, Xinjiang. Resour. Environ. Arid Areas 2023, 37, 146–152. (In Chinese) [Google Scholar]
- Lander, T.A.; Boshier, D.H.; Harris, S.A. Fragmented but not isolated: Contribution of single trees, small patches and long-distance pollen flow to genetic connectivity for Gomortega keule, an endangered Chilean tree. Biol. Conserv. 2010, 143, 2583–2590. [Google Scholar] [CrossRef]
- Valdés, A.; Lenoir, J.; De Frenne, P.; Andrieu, E.; Brunet, J.; Chabrerie, O.; Cousins, S.A.O.; Deconchat, M.; De Smedt, P.; Diekmann, M.; et al. High ecosystem service delivery potential of small woodlands in agricultural landscapes. J. Appl. Ecol. 2020, 57, 4–16. [Google Scholar] [CrossRef]
- Yan, Y.Z.; Jarvie, S.; Zhang, Q.; Zhang, S.S.; Han, P.; Liu, Q.F.; Liu, P.T. Small patches are hotspots for biodiversity conservation in fragmented landscapes. Ecol. Indic. 2021, 130, 108086. [Google Scholar] [CrossRef]
- Saura, S.; Bodin, Ö.; Fortin, M. Stepping stones are crucial for species’ long-distance dispersal and range expansion through habitat networks. J. Appl. Ecol. 2014, 51, 171–182. [Google Scholar] [CrossRef]
- Diniz, M.F.; Coelho, M.T.P.; de Sousa, F.G.; Hasui, É.; Loyola, R. The underestimated role of small fragments for carnivore dispersal in the Atlantic Forest. Perspect. Ecol. Conserv. 2021, 19, 81–89. [Google Scholar] [CrossRef]
- Fahrig, L.; Arroyo-Rodrıguez, V.; Bennett, J.R.; Boucher-Lalonde, V.; Cazetta, E.; Currie, D.J.; Eigenbrod, F.; Ford, A.T.; Harrison, S.P.; Jaeger, J.A.G.; et al. Is habitat fragmentation bad for biodiversity? Biol. Conserv. 2019, 230, 179–186. [Google Scholar] [CrossRef]
- Sutherland, G.D.; Harestad, A.S.; Price, K.; Lertzman, K.P. Scaling of natal dispersal distances in terrestrial birds and mammals. Conserv. Ecol. 2020, 4, 16. Available online: http://www.consecol.org/vol4/iss1/art16/ (accessed on 25 October 2023). [CrossRef]
- Tarabon, S.; Bergès, L.; Dutoit, T.; Isselin-Nondedeu, F. Maximizing habitat connectivity in the mitigation hierarchy. A case study on three terrestrial mammals in an urban environment. J. Environ. Manag. 2019, 243, 340–349. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Z.P.; Shi, H.; Han, L. Effect of forest connectivity on the dispersal of species: A case study in the Bogda World Natural Heritage Site, Xinjiang, China. Ecol. Indic. 2021, 125, 1075–1076. [Google Scholar] [CrossRef]
- Liu, S.B.; Wang, X.G.; Zhang, W.F.; Liu, L.B.; Wen, Z.G.; Peng, Z.J. An analysis of the lithofacies sections and establishment of the phase model for the Lower-Middle Devonian Kulumudi Formation in Barluk Mountain area of Xinjiang. Geol. Bull. China 2016, 35, 1427–1440. (In Chinese) [Google Scholar]
- Duan, X.B.; Nurbaye, A. Research on Wild Plant Resources in Barluk Mountain Natural Reserve of Xinjiang. J. Anhui Agric. Sci. 2011, 39, 5996–5999. (In Chinese) [Google Scholar]
- Yang, J.; Huang, X. The 30 m annual land cover dataset and its dynamics in China from 1990 to 2019. Earth Syst. Sci. Data 2021, 13, 3907–3925. [Google Scholar] [CrossRef]
- Saura, S.; Torné, J. Conefor Sensinode 2.2: A software package for quantifying the importance of habitat patches for landscape connectivity. Environ. Model. Softw. 2009, 24, 135–139. [Google Scholar] [CrossRef]
- Urban, D.; Keitt, T. Landscape Connectivity: A Graph-Theoretic Perspective. Ecology 2001, 82, 1205–1218. [Google Scholar] [CrossRef]
- Pascual-Hortal, L.; Saura, S. Comparison and development of new graph-based landscape connectivity indices: Towards the priorization of habitat patches and corridors for conservation. Landsc. Ecol. 2006, 21, 959–967. [Google Scholar] [CrossRef]
- Saura, S.; Pascual-Hortal, L. Conefor Sensinode 2.2 User’s Manual: Software for Quantifying the Importance of Habitat Patches for Maintaining Landscape Connectivity Through Graphs and Habitat Availability Indices; University of Lleida: Lleida, Spain, 2007. [Google Scholar]
- Flather, C.H.; Bevers, M. Patchy reaction-diffusion and population abundance: The relative importance of habitat amount and arrangement. Am. Nat. 2002, 159, 40–56. [Google Scholar] [CrossRef] [PubMed]
- King, A.W.; With, K.A. Dispersal success on spatially structured landscapes: When do spatial pattern and dispersal behavior really matter? Ecol. Model. 2002, 147, 23–39. [Google Scholar] [CrossRef]
- Oliver, T.H.; Powney, G.D.; Baguette, M.; Schtickzelle, N. Synchrony in population counts predicts butterfly movement frequencies. Ecol. Entomol. 2017, 42, 375–378. [Google Scholar] [CrossRef]
- Foster, E.; Love, J.; Rader, R.; Reid, N.; Drielsma, M.J. Integrating a generic focal species, metapopulation capacity, and connectivity to identify opportunities to link fragmented habitat. Landsc. Ecol. 2017, 32, 1837–1847. [Google Scholar] [CrossRef]
- Pietsch, M. Contribution of connectivity metrics to the assessment of biodiversity-Some methodological considerations to improve landscape planning. Ecol. Indic. 2018, 95, 116–127. [Google Scholar] [CrossRef]
- Minor, E.S.; Urban, D.L. A Graph-Theory Framework for Evaluating Landscape Connectivity and Conservation Planning. Conserv. Biol. 2008, 22, 297–307. [Google Scholar] [CrossRef]
- Rothley, K.D.; Rae, C. Working backwards to move forwards: Graph-based connectivity metrics for reserve network selection. Environ. Model. Assess. 2005, 10, 107–113. [Google Scholar] [CrossRef]
- Pascual-Hortal, L.; Saura, S. Integrating landscape connectivity in broad-scale forest planning through a new graph-based habitat availability methodology: Application to capercaillie (Tetrao urogallus) in Catalonia (NE Spain). Eur. J. For. Res. 2008, 127, 23–31. [Google Scholar] [CrossRef]
- Jordán, F.; Báldi, A.; Orci, K.M.; Rácz, I.; Varga, Z. Characterizing the importance of habitat patches and corridors in maintaining the landscape connectivity of a Pholidoptera transsylvanica (Orthoptera) metapopulation. Landsc. Ecol. 2003, 18, 83–92. [Google Scholar] [CrossRef]
- Latorre-Cárdenas, M.C.; González-Rodríguez, A.; Godínez-Gómez, O.; Arima, E.Y.; Young, K.R.; Denvir, A.; García-Oliva, F.; Ghilardi, A. Estimating Fragmentation and Connectivity Patterns of the Temperate Forest in an Avocado-Dominated Landscape to Propose Conservation Strategies. Land 2023, 12, 631. [Google Scholar] [CrossRef]
- Woodroffe, R.; Ginsberg, J.R. Edge effects and the extinction of populations inside protected areas. Science 1998, 280, 2126–2128. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, Z.; Wei, M.M.; Wang, M.Y.; Shi, H.; Ruckstuhl, K.; Yang, W.K.; Alves, J. Small patches play a critical role in the connectivity of the Western Tianshan landscape, Xinjiang, China. Ecol. Indic. 2022, 144, 109542. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Corlett, R.T. Seed dispersal distances and plant migration potential in tropical East Asia. Biotropica 2009, 41, 592–598. [Google Scholar] [CrossRef]
- Huang, P.Y.; Pan, W.B.; Li, X.P. Investigation of biological and ecological characteristics on Amygdalus ledebouriana. J. Xinjiang Univ. (Nat. Sci. Ed. Chin. Engl.) 1985, 2, 65–70. (In Chinese) [Google Scholar]
- Wang, Z.J.; Jin, K.Y. Analysis of Xinjiang Distribution of Amygdalus ledebouriana and the reason of becoming vulnerable. North. Hortic. 2014, 23, 43–45. (In Chinese) [Google Scholar]
- Liu, W.J.; Hughes, A.C.; Bai, Y.; Li, Z.J.; Mei, C.C.; Ma, Y.X. Using landscape connectivity tools to identify conservation priorities in forested areas and potential restoration priorities in rubber plantation in Xishuangbanna, Southwest China. Landsc. Ecol. 2020, 35, 389–402. [Google Scholar] [CrossRef]
- Xu, Y.J.; Si, Y.L.; Takekawa, J.; Liu, Q.; Prins, H.H.T.; Yin, S.L.; Prosser, D.J.; Gong, P.; de Boer, W.F. A network approach to prioritize conservation efforts for migratory birds. Conserv. Biol. 2020, 34, 416–426. [Google Scholar] [CrossRef]
- Qi, K.; Fan, Z.; Xie, Y. The influences of habitat proportion and patch-level structural factors in the spatial habitat importance ranking for connectivity and implications for habitat conservation. Urban For. Urban Green. 2021, 64, 127239. [Google Scholar] [CrossRef]
- Roberts, H.P.; King, D.I. Area requirements and landscape-level factors influencing shrubland birds. J. Wildl. Manag. 2017, 81, 1298–1307. [Google Scholar] [CrossRef]
- Laurance, W.F.; Camargo, J.L.C.; Luizão, R.C.C.; Laurance, S.G.; Pimm, S.L.; Bruna, E.M.; Stouffer, P.C.; Williamson, G.B.; Benítez-Malvido, J.; Vasconcelos, H.L.; et al. The fate of Amazonian forest fragments: A 32-year investigation. Biol. Conserv. 2011, 144, 56–67. [Google Scholar] [CrossRef]
- Perino, A.; Pereira, H.M.; Navarro, L.M.; Fernández, N.; Bullock, J.M.; Ceausu, S.; Cortés-Avizanda, A.; van Klink, R.; Kuemmerle, T.; Lomba, A.; et al. Rewilding complex ecosystems. Science 2019, 364, eaav5570. [Google Scholar] [CrossRef] [PubMed]
- Hannah, L.; Roehrdanz, P.R.; Marquet, P.A.; Enquist, B.J.; Midgley, G.; Foden, W.; Lovett, J.C.; Corlett, R.T.; Corcoran, D.; Butchart, S.H.M.; et al. 30% land conservation and climate action reduces tropical extinction risk by more than 50%. Ecography 2020, 43, 943–953. [Google Scholar] [CrossRef]
- Hall, M.; Nimmo, D.; Watson, S.; Bennett, A.F. Linear habitats in rural landscapes have complementary roles in bird conservation. Biodivers. Conserv. 2018, 27, 2605–2623. [Google Scholar] [CrossRef]
- Saura, S.; Rubio, L. A common currency for the different ways in which patches and links can contribute to habitat availability and connectivity in the landscape. Ecography 2010, 33, 523–537. [Google Scholar] [CrossRef]
- Fahrig, L.; Watling, J.I.; Arnillas, C.A.; Arroyo-Rodríguez, V.; Jörger-Hickfang, T.; Müller, J.; Pereira, H.M.; Riva, F.; Rösch, V.; Seibold, S.; et al. Resolving the SLOSS dilemma for biodiversity conservation: A research agenda. Biol. Rev. 2022, 97, 99–114. [Google Scholar] [CrossRef]
- da Rocha, É.G.; Brigatti, E.; Niebuhr, B.B.; Ribeiro, M.C.; Vieira, M.V. Dispersal through fragmented landscapes: The role of stepping stones and perceptual range. Landsc. Ecol. 2021, 36, 3249–3267. [Google Scholar] [CrossRef]
- Kramer-Schadt, S.; Kaiser, T.S.; Frank, K.; Wiegand, T. Analyzing the effect of stepping stones on target patch colonisation in structured landscapes for Eurasian lynx. Landsc. Ecol. 2011, 26, 501–513. [Google Scholar] [CrossRef]
- Yan, Y.Z.; Jarvie, S.; Liu, Q.F.; Zhang, Q. Effects of fragmentation on grassland plant diversity depend on the habitat specialization of species. Biol. Conserv. 2022, 275, 109773. [Google Scholar] [CrossRef]
- Verena, R.; Teja, T.; Christoph, S.; Batáry, P. Biodiversity conservation across taxa and landscapes requires many small as well as single large habitat fragments. Oecologia 2015, 179, 209–222. [Google Scholar] [CrossRef]
- Phalan, B.T. What have we learned from the land sparing-sharing model? Sustainability 2018, 10, 1760. [Google Scholar] [CrossRef]
- Riva, F.; Fahrig, L. The disproportionately high value of small patches for biodiversity conservation. Conserv. Lett. 2022, 15, e12881. [Google Scholar] [CrossRef]
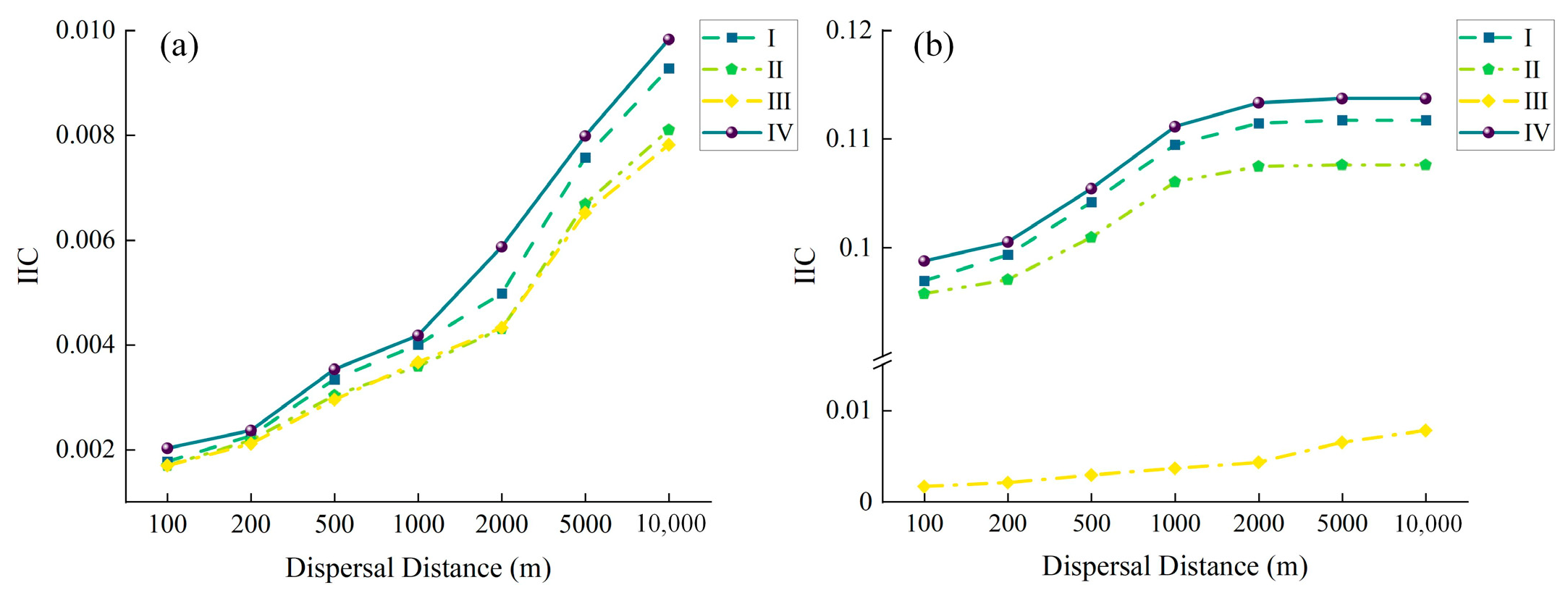

| Dispersal Distance (m) | 100 | 200 | 500 | 1000 | 2000 | 5000 | 10,000 | |
|---|---|---|---|---|---|---|---|---|
| NC | BMFF | 1167 | 564 | 157 | 82 | 66 | 60 | 60 |
| WAR | 15 | 7 | 3 | 1 | 1 | 1 | 1 | |
| IIC | BMFF | 0.0020 | 0.0024 | 0.0035 | 0.0042 | 0.0059 | 0.0080 | 0.0098 |
| WAR | 0.0988 | 0.1005 | 0.1054 | 0.1111 | 0.1133 | 0.1137 | 0.1137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Luo, Y.; Han, L.; Chen, K.; Wang, Z.; Yang, Q. Importance of Patches in Maintaining Forest Landscape Connectivity: A Case Study of Barluk, Xinjiang, China. Forests 2025, 16, 74. https://doi.org/10.3390/f16010074
Zhang Y, Luo Y, Han L, Chen K, Wang Z, Yang Q. Importance of Patches in Maintaining Forest Landscape Connectivity: A Case Study of Barluk, Xinjiang, China. Forests. 2025; 16(1):74. https://doi.org/10.3390/f16010074
Chicago/Turabian StyleZhang, Yujie, Yan Luo, Lei Han, Keyu Chen, Zhi Wang, and Qifan Yang. 2025. "Importance of Patches in Maintaining Forest Landscape Connectivity: A Case Study of Barluk, Xinjiang, China" Forests 16, no. 1: 74. https://doi.org/10.3390/f16010074
APA StyleZhang, Y., Luo, Y., Han, L., Chen, K., Wang, Z., & Yang, Q. (2025). Importance of Patches in Maintaining Forest Landscape Connectivity: A Case Study of Barluk, Xinjiang, China. Forests, 16(1), 74. https://doi.org/10.3390/f16010074






