Abstract
Mixed-species plantations involving Eucalyptus and Acacia trees are an effective alternative for managing sustainable plantations. In this study, we evaluated the growth, productivity, nutrient return, and soil properties of a mixed Eucalyptus hybrid (Eucalyptus camaldulensis Dehnh. × E. urophylla S.T. Blake; E) and Acacia auriculiformis A. Cunn. ex Benth. plantation (A) and Eucalyptus hybrid and A. auriculiformis plantations. The mixed Eucalyptus hybrid and A. auriculiformis plantation included three ratios at E33:A67, E50:A50, and E67:A33, while the Eucalyptus (E100) and A. auriculiformis (A100) plantations were established on degraded lands in the Had Wanakorn Forestry Research and Student Training Station, Prachuap Khiri Khan province, Thailand. Three replications within a plot size of 20 × 20 m2 were designed to plant Eucalyptus hybrid and A. auriculiformis seedlings at a spacing of 2 × 3 m2. The diameters at breast height (DBH) and height (H) of the Eucalyptus hybrid and A. auriculiformis were measured and monitored after planting for five years. The aboveground biomass of the five-year-old mixed and monoculture plantations was then estimated. Litterfall production and nutrient return from the mixed and monoculture plantations were measured for three years. In addition, soil samples at depths of 0–5, 5–10, and 10–20 cm were collected to analyze the soil’s chemical properties. Differences in growth, aboveground biomass, litterfall production, nutrient return, and soil properties were analyzed and tested using Tukey’s HSD. The results indicated that both the DBH and H of the Eucalyptus hybrid in the mixed and monoculture plantations were not significantly different (p > 0.05). Similarly, the DBH and H of A. auriculiformis in each treatment were also not significantly different (p > 0.05). However, the DBH and H of the Eucalyptus hybrid were higher than those of A. auriculiformis. The aboveground biomass for the mixed plantation ratios E50:A50, E100, E67:A33, and E33:A67 was not significantly different, while the stem biomass was the highest in E100. Litterfall production was influenced by the proportion of the Eucalyptus hybrid relative to A. auriculiformis, but the monoculture A100 plantation had the highest litter production. The nitrogen return estimated for the mixed plantation was between A100 and E100. Similarly, the total nitrogen in the topsoil (0–5 cm) of the mixed plantation was higher than that in the monoculture E100 plantation. These results indicate that mixing A. auriculiformis with Eucalyptus can improve soil nutrients and nutrient cycling and increase nutrient returns, suggesting that mixed plantations are an effective option for sustainable plantation management and can mitigate the negative environmental impacts of Eucalyptus monocultures.
1. Introduction
Eucalyptus is a multi-purpose tree and plays an important role in the economics of the private sector and local communities. Due to its high growth rate, this tree has been widely planted across Thailand, especially in the northern and eastern regions [1]. Eucalyptus camaldulensis Dehnh. has also been reported to be drought-tolerant [2]. In Thailand, the area covered by Eucalyptus plantations is estimated to be approximately 846,708 ha [3]. However, several concerns related to its impact on the environment have arisen [4,5]. At the same time, fast-growing trees such as Acacia species can be planted in plantations owned by individual farmers and the private sector as an alternative. These nitrogen-fixing trees have been reported to improve soil properties and increase organic matter and soil nutrients, especially nitrogen [6,7]. Additionally, a high litter production of 5.94 to 10.38 Mg ha−1 has been reported from an Acacia plantation [8,9].
Fast-growing tree species are mostly planted in monoculture plantations. The rotation of woody species is between three and seven years. They are harvested for woodchips, plywood, and pulp for use in the paper industry. The wood production of plantations can vary depending on genetics, as was the case for the wood volume of Acacia mangium Willd. at approximately 73–106 m3 ha−1 and that of an Acacia hybrid at 138 m3 ha−1 [10]. Thus, commercial plantations have benefited from planting high-quality genetic clones to enhance their growth and productivity [10,11].
Mixed plantations of Eucalyptus and nitrogen-fixing trees have been planted in many areas. The growth and productivity of these plantations depend on the species being used [12] and the proportion of each planted species [13,14,15,16]. Mixed plantations of Eucalyptus globulus Labill. and Acacia mearnsii De Wild. can lead to an increase in the diameter at the breast height and the height of E. globulus. The productivity of mixed plantations can be comparable to that of Eucalyptus monoculture plantations [17]. In addition, the absorbed photosynthetically active radiation (APAR) and light use efficiency of mixed plantations (Eucalyptus and Acacia) have been reported to be greater than those of monoculture plantations [18].
The mixing of nitrogen-fixing trees and Eucalyptus has been reported to enhance the growth of Eucalyptus relative to monocultures [14,19]. Mixed plantations of E. globulus and A. mearnsii can increase soil nutrients, especially nitrogen, which is crucial for promoting the productivity of intercropped trees [20]. Fertilizers are important for increasing growth and productivity [21]. Soil nutrients in long-term agriculture and degraded lands are commonly very low. Adding fertilizer in plantations is needed to maintain adequate nutrient levels in the soil. In addition, mixed plantations of Eucalyptus and nitrogen-fixing trees can accelerate litter decomposition and improve litter quality [22]. Additionally, mixed plantations of Eucalyptus and Acacia can promote soil microbial diversity and activity and improve carbon and nitrogen cycling [23]. The nutrient status of the topsoil, especially N, tends to increase shortly after the establishment of a mixed plantation, in part due to higher nutrient return via litterfall [14]. From a long-term sustainability perspective, nutrient cycling in a mixed plantation can improve soil properties and maintain nutrient balance [24].
Generally, Eucalyptus monocultures are often of concern given their high water and nutrient demand for growth and nutrient removal after harvesting [24]. In contrast, mixed plantations of Eucalyptus and nitrogen-fixing species are less popular due to potential competition for resources, resulting in lower growth rates and productivity [25]. In addition, a mixed plantation is usually characterized by intra- and inter-specific competition. The competition between species is different depending on their interaction [26,27], and appropriate species matching can promote growth and reduce inter-specific competition in a mixed plantation. A better understanding of mixed plantations can be beneficial in managing fast-growing tree plantations. Therefore, this study aimed to investigate the growth, productivity, nutrient return, and soil nutrients of mixed (Eucalyptus hybrid and Acacia auriculiformis) and monoculture plantations. These results can provide an alternative approach for the sustainable management of such plantations.
2. Materials and Methods
2.1. Site Description and Plant Materials
The experimental plot was located at the Had Wanakorn Forestry Research and Student Training Station (11°37′11″ N; 99°41′08″ E), Thapsakae district, Prachuap Khiri Khan province, Thailand (Figure 1), which is a 15-year-old abandoned coconut plantation. The experimental plot was located approximately 2 km from the coast and had a sandy soil texture. The mean microclimatic conditions during the period of investigation (2018–2023), as measured by the maximum temperature, minimum temperature, rainfall, and humidity, were 35.8 °C, 21.9 °C, 1108 mm, and 76.8%, respectively. The rainy season usually spanned from May to November, and the dry season from December to April, as per the Prachuap Khiri Khan Meteorological Station, Thailand.
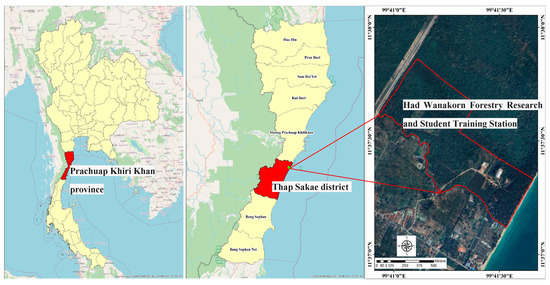
Figure 1.
Location of study site at Had Wanakorn Forestry Research and Student Training Station, Thap Sakae district, Prachuap Khiri Khan province, Thailand.
2.2. Experimental Design
Experimental plots of 20 × 20 m2 were established in an area of approximately 0.8 hectares, which was cleared out before planting the trees. The planted Eucalyptus hybrid (E) was a clone of E. camaldulensis Dehnh. × E. urophylla S.T. Blake, while the A. auriculiformis A. Cunn. ex Benth. (A) was a clone of Acacia hybrids (intra-specific hybrids of A. aulicuriformis). Seedlings of the Eucalyptus hybrid and A. auriculiformis were produced from clonal cutting and planted in October 2018.
The Eucalyptus hybrid and A. auriculiformis were planted in monoculture and mixed-species plots that contained 100% Eucalyptus (E100), 100% A. auriculiformis (A100), 67% E + 33% A (E67:A33), 50% E + 50% A (E50:A50), and 33% E + 67% A (E33:A67), with each plot containing 66 individuals (100%).
A randomized complete block design (RCBD) with three replications was implemented in plots of the Eucalyptus hybrid and A. auriculiformis planted at a spacing of 2 × 3 m2. Fertilizer application was not carried out in any treatment.
The layout of mixed-species and monoculture plantations of E33:A67, E50:A50, E67:A33, E100, and A100 is shown in Figure 2. Weeds were removed twice a year during the first two years. In addition, pruning was practiced on 50% of the stems for the 1-year-old A. auriculiformis and was repeated on the 2-year-old trees at a height of 2 m aboveground.
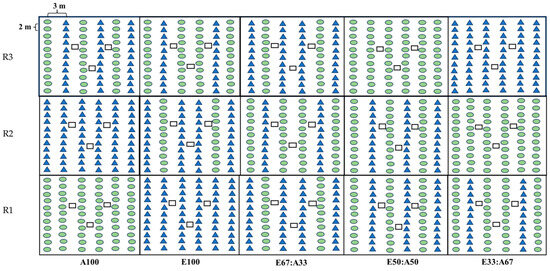
Figure 2.
The layout of the Eucalyptus hybrid ( ) and Acacia
auriculiformis (
) and Acacia
auriculiformis ( ) in the mixed-species and monoculture plantations, with
the location of litter traps (
) in the mixed-species and monoculture plantations, with
the location of litter traps ( ), at the ratios A100,
E100, E67:A33, E50:A50, and E33:A67 at the Had Wanakorn Forestry Research and
Student Training Station, Thap Sakae
district, Prachuap Khiri Khan province, Thailand.
), at the ratios A100,
E100, E67:A33, E50:A50, and E33:A67 at the Had Wanakorn Forestry Research and
Student Training Station, Thap Sakae
district, Prachuap Khiri Khan province, Thailand.
 ) and Acacia
auriculiformis (
) and Acacia
auriculiformis ( ) in the mixed-species and monoculture plantations, with
the location of litter traps (
) in the mixed-species and monoculture plantations, with
the location of litter traps ( ), at the ratios A100,
E100, E67:A33, E50:A50, and E33:A67 at the Had Wanakorn Forestry Research and
Student Training Station, Thap Sakae
district, Prachuap Khiri Khan province, Thailand.
), at the ratios A100,
E100, E67:A33, E50:A50, and E33:A67 at the Had Wanakorn Forestry Research and
Student Training Station, Thap Sakae
district, Prachuap Khiri Khan province, Thailand.
2.3. Tree Growth and Aboveground Biomass
The survival and growth of the Eucalyptus hybrid and A. auriculiformis in all treatments were monitored for the entire duration of plantation rotation (5 years). The crown diameter, diameter at breast height (DBH), and height (H) of all trees among the treatments were measured. The aboveground biomass of the 5-year-old Eucalyptus hybrid and A. auriculiformis was estimated using the methodology developed by Wongchai et al. (2020) [28]. The equations for Eucalyptus and Acacia species are given in Table 1.

Table 1.
Aboveground biomass equations of Eucalyptus and Acacia species in the mixed and monoculture plantations.
2.4. Litterfall Production
Three litter traps (constructed using a nylon mesh suspended inside a PVC frame) of 1 × 1 m2 were set up within the treatments (Figure 2) to estimate litter production. Weeds under the traps were removed, and litter for all treatments was collected monthly for three years, collecting the litterfall from the age of 2.5 years. Any damaged traps were repaired immediately. Once collected, the litter was separated by species into various components, i.e., leaves, branches, barks, reproductive parts (flowers, seeds, and their supporting structures), and miscellaneous items. These components were then sorted and oven-dried at 70 °C for 48 h or until they reached a constant weight, after which each of the samples was weighed individually.
The litter components were analyzed for their nutrient concentration. To reduce the cost of analyses, monthly litter samples were homogenized, and 50 g subsamples were pooled to make composites for the dry and rainy seasons. All of the samples were analyzed at the Forest Soil Laboratory, Faculty of Forestry, Kasetsart University. The N concentration was based on dry combustion using a CNHS analyzer (Perkin Elmer model 2400 Series II CHNS/O Elemental Analyzer), and the concentrations of P, K, Ca, and Mg were determined by wet washing the litter with NHO3-HClO4 acid (HNO3:HClO4; 5:2). The concentration of P was analyzed using the vanadomolybdate yellow color method with a spectrometer at a wavelength of 440 nm, while the concentrations of K, Ca, and Mg were analyzed using atomic absorption spectrometry.
2.5. Soil Nutrients
Samples at three soil depths (0–5, 5–10, and 10–20 cm) around five points in the monoculture plantations (A100 and E100) were collected. However, eight soil samples were collected in the mixed-species plantation (four samples were collected between Acacia rows and four samples between Eucalyptus rows). The soil samples from each soil depth were mixed to make one sample. The samples were then air-dried and sieved through a 2 mm sieve, and any root fragments were removed. The organic matter (OM) was determined as per the recommendations of Walkley and Black (1934) [29]. Total nitrogen (N) was measured using the Dumas method and a CHNS analyzer. Available phosphorus (P) was determined using the method proposed by Bray and Kurtz (1945) [30]. The levels of exchangeable potassium (K) were determined using atomic absorption spectrophotometry.
3. Data Analysis
Growth in terms of DBH and H, aboveground biomass, litterfall production, nutrient return, and soil nutrients, including total N, available P, exchangeable K, Ca, and Mg, was analyzed using a one-way analysis of variance (ANOVA), and the means were compared using Tukey’s HSD test at a 95% significance level.
4. Results
4.1. Growth Performance
The survival of the five-year-old Eucalyptus hybrid and A. auriculiformis in the monoculture and mixed plantations was not significantly different (p > 0.05). The survival percentages of the Eucalyptus hybrids in E100, E67:A33, E50:A50, and E33:A67 were 96.92, 97.04, 95.96, and 95.45, respectively, while those for Acacia were 96.41, 96.30, 94.95, and 93.94, respectively.
The DBH and H of the Eucalyptus hybrid in the mixed and monoculture plantations were not found to be significantly different (both p > 0.05). The DBH and H of the Eucalyptus hybrid and A. auriculiformis increased with the age of the plantations. The DBH and H of the five-year-old Eucalyptus hybrid in E100, E67:A33, E50:A50, and E33:A67 ranged from 11.19 to 12.08 cm and 16.75 to 17.41 m, respectively (Figure 3). Additionally, the DBH and H of A. auriculiformis among treatments were also found to be not significantly different (both p > 0.05). The DBH and H of the five-year-old A. auriculiformis in A100, E33:A67, E50:A50, and E67:A33 ranged from 9.61 to 9.89 cm and 11.87 to 12.00 m, respectively.
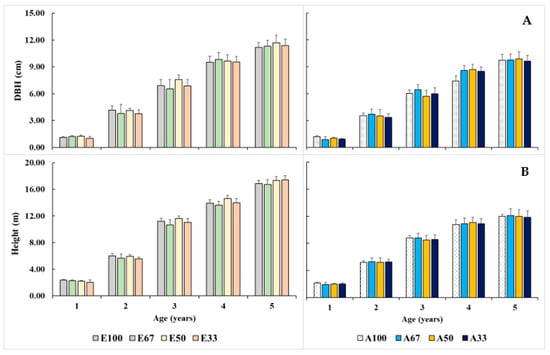
Figure 3.
DBH (A) and H (B) of the 1–5-year-old Eucalyptus hybrid (E) and A. auriculiformis (A) in the mixed and monoculture plantations.
The Eucalyptus hybrid was taller than the A. auriculiformis in all treatments. On the contrary, the crown diameter of the Eucalyptus hybrid was smaller than that of A. auriculiformis (Table 2). This corresponded with the canopy stratification observed between the Eucalyptus hybrid and A. auriculiformis in the mixed plantation.

Table 2.
Crown diameters of the 5-year-old Eucalyptus hybrid (E) and A. auriculiformis (A) in the mixed and monoculture plantations.
4.2. Aboveground Biomass
Statistically significant differences were observed in the stem biomass among the various treatments (p < 0.05), with E100 having the highest stem biomass (77.44 Mg ha−1), while that of A100 was the lowest (41.79 Mg ha−1) (Figure 4). The stem biomass of E67:A33, E50:A50, and E33:A67 was in between the above-mentioned levels (65.27, 67.23, and 56.44 Mg ha−1, respectively). The stem biomass of Eucalyptus was higher than that of A. auriculiformis because it grew well. Given its large and dense canopy, the amount of branch and leaf biomass produced by A. auriculiformis was higher than that of the Eucalyptus hybrid. The bark biomass of the Eucalyptus hybrid and A. auriculiformis in the E100, A100, E67:A33, E50:A50, and E33:A67 plantations was 8.33, 5.71, 7.02, 8.04, and 6.85 Mg ha−1, respectively. The aboveground biomass of the E100, A100, E67:A33, E50:A50, and E33:A67 plantations was found to be significantly different (p < 0.05) (74.31, 94.40, 86.17, 94.67, and 83.83 Mg ha−1, respectively), but that of E100, E67:A33, E50:A50, and E33:A67 was similar. In this case, the Eucalyptus hybrid was a major contributor to the biomass pool in E50:A50 and E67:A33, while A. auriculiformis was a major contributor to the biomass pool accumulated in A67:E33. Additionally, the aboveground biomass of the E67:A33 and E33:A67 plantations was proportional to the number of planted Eucalyptus hybrid and A. auriculiformis trees.
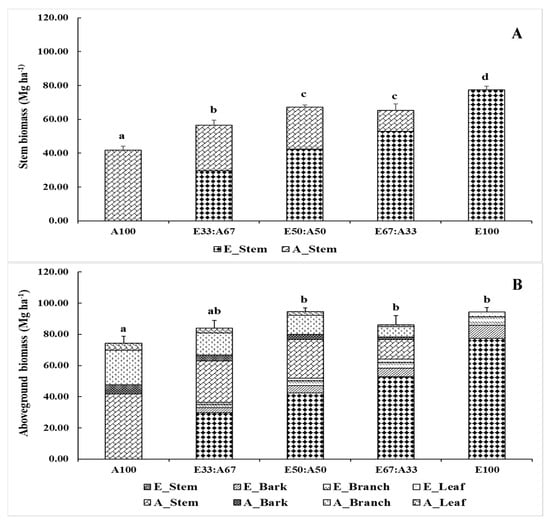
Figure 4.
Stem biomass (A) and aboveground biomass (B) of the 5-year-old Eucalyptus hybrid and A. auriculiformis in the mixed and monoculture plantations. The letters (a, b, c, and d) above the bars indicate statistical differences at p < 0.05, as determined by Tukey’s HSD.
4.3. Litterfall
The amount of litterfall in all plantations increased during the months of December and January, which corresponded with the early dry season (Figure 5). Leaves were the major litter component falling onto the forest floor, followed by branches, reproductive parts, barks, and miscellaneous components, respectively (Figure 6). Bark litter was exclusively found in the hybrid Eucalyptus plantation, while the reproductive parts were exclusive to the A. auriculiformis plantation. The litter production varied according to the age and proportion of the planted Eucalyptus hybrid and A. auriculiformis trees. The litter production of the 3.5-year-old Eucalyptus hybrid and A. auriculiformis in the mixed and monoculture plantations was similar, but no significant difference was observed in the 4.5- and 5.5-year-old plantations. The amount of litter produced by the 3.5- and 4.5-year-old Eucalyptus hybrid plantation was relatively high, but it reduced when the plantation reached an age of 5.5 years, as the canopy was lighter after natural pruning.
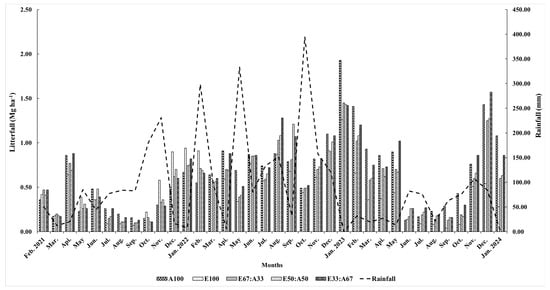
Figure 5.
Monthly litterfall of the Eucalyptus hybrid and A. auriculiformis in the mixed and monoculture plantations from February 2021 to January 2024.
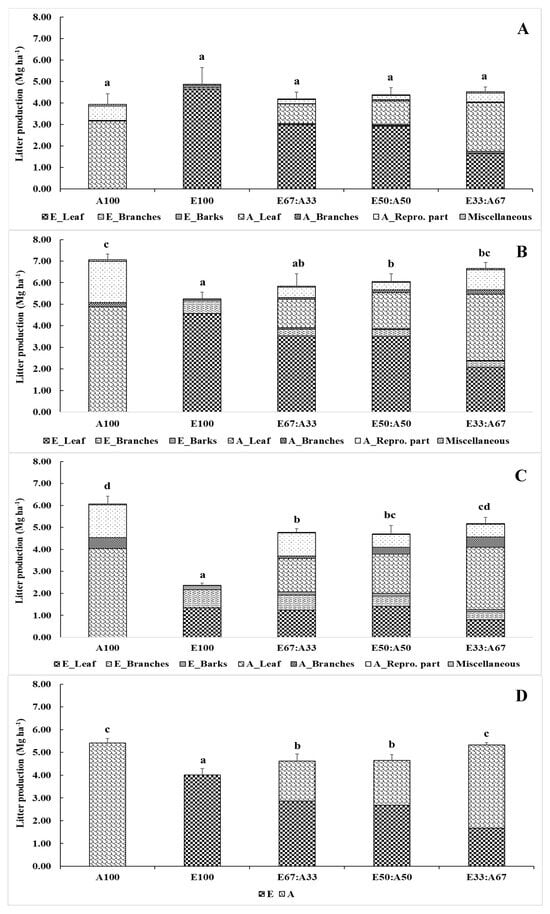
Figure 6.
Litterfall separated by components, including leaves, branches, barks, reproductive parts, and miscellaneous parts, of the Eucalyptus hybrid and A. auriculiformis at ages of 3.5 (A), 4.5 (B), and 5.5 years (C). The mean litterfall (D) estimated for the mixed and monoculture plantations. The letters (a, b, c, and d) above the bars indicate statistical differences at p < 0.05, as determined by Tukey’s HSD.
The mean litterfall of A100 was the highest, followed by E33:A67, E50:E50, E67:A33, and E100 (5.42, 5.32, 4.65, 4.61, and 4.01 Mg ha−1, respectively). The litterfall of the mixed plantation was estimated to be at average levels, with the majority of litter produced by A. auriculiformis.
4.4. Nutrient Return
The mean nutrient return in the mixed plantation varied according to the proportion of the Eucalyptus hybrid and A. auriculiformis. The nitrogen return was the highest in A100 and the lowest in E100 (Table 3). The nitrogen return of the mixed plantation increased according to the proportion of A. auriculiformis. On the contrary, calcium return was relatively high in the E100, E33:A67, E50:A50, and E67:A33 plantations. The nutrient return in A100 was in the order N > Ca > K > Mg > P, while that of the E100, E67:A33, E50:A50, and E33:A67 plantations was in the order Ca > N > K > Mg > P, respectively.

Table 3.
Mean nutrient return of the Eucalyptus hybrid and A. auriculiformis growing in the mixed and monoculture plantations.
4.5. Soil Nutrients
Soil nutrients, including total nitrogen, available phosphorus, and potassium, in the topsoil (0–5 cm) of the mixed and monoculture plantations were significantly different, except for calcium and magnesium. The A100 plantation had the highest total estimated nitrogen at 0.90 g kg−1, with E50:A50 and E33:A67 having similar levels (0.82 and 0.83 g kg−1, respectively) (Table 4). In the mixed plantation, the total nitrogen, available phosphorus, organic matter, and potassium levels in the topsoil were between the values measured for A100 and E100. In the subsoil (5–10 and 10–20 cm), the levels of total nitrogen, available phosphorus, organic matter, potassium, and magnesium among treatments were not different (p > 0.05). In addition, the soil nutrients decreased with soil depth.

Table 4.
Soil nutrients of the 5-year-old Eucalyptus hybrid and A. auriculiformis in the mixed and monoculture plantations.
5. Discussion
5.1. Growth Performance
The Eucalyptus hybrid and A. auriculiformis have been reported to have a high survival rate as they can adapt well to poor soil conditions [31,32]. In addition, A. auriculiformis is an intra-specific hybrid between two distinct provenances, which is capable of tolerating drought conditions [33]. On the contrary, the Eucalyptus hybrid, which is a hybrid of E. camaldulensis and E. urophylla, can grow well on degraded lands with low rainfall [31,34].
Mixed Eucalyptus hybrid and A. auriculiformis in the E33:A67, E50:A50, and E67:A33 plantations had no effect on the growth of Acacia or Eucalyptus compared to the monoculture plantations. The measured DBH and height of the Eucalyptus hybrid and A. auriculiformis in the mixed plantation exhibited good growth. Previously, the DBH and H of a four-year-old Eucalyptus hybrid in E100 (9.50 cm and 13.94 m, respectively) were reported to be similar to the Eucalyptus hybrid (K62) (8.80 cm and 12.99 m, respectively) in the eastern part of Thailand [35]. However, the DBH and H of the five-year-old Eucalyptus hybrid in this study were higher than those of E. camaldulensis planted in northern Thailand (10.06 cm and 15.34 m, respectively) [28].
In the A. auriculiformis plantation, the DBH and H among treatments were also similar, with A. auriculiformis experiencing good growth compared to other sites. Additionally, genetic improvement of the Eucalyptus hybrid and A. auriculiformis has been reported to enhance the growth rate and productivity [11,36,37,38]. The DBH and H of four-year-old A. auriculiformis were similar to the Acacia hybrid in BaVi (9.20 cm and 10.70 m, respectively) [39] and A. auriculiformis in Vietnam (8.75 cm and 7.84 m, respectively) [11]. In addition, the estimated growth was also similar to an Acacia hybrid (5.7 years old) plantation in northern Thailand (9.54 cm and 11.83 m, respectively). However, the growth of the five-year-old A. auriculiformis in this study was lower than that of A. auriculiformis of the same age planted in Vietnam (12.0 cm and 18.8 m, respectively). Higher levels of nitrogen are important for growth [40] but are a critical limiting factor [41]. The differences observed in DBH and H may be due to higher soil nitrogen in the A. auriculiformis plantation, which was in its third rotation [40].
Besides nitrogen supply, environmental factors such as rainfall, light, and soil properties are equally important for growth. The growth of Eucalyptus facilitated through a mixed plantation of Eucalyptus and nitrogen-fixing trees has been reported in numerous planting sites [16,42,43]. In contrast, this study found that the growth of the Eucalyptus hybrid was not affected by the introduction of A. auriculiformis in a mixed plantation setting. This result may be attributed to relatively high rainfall after planting, as evidenced by the site’s total rainfall of 1443.6 and 1812.0 mm in 2018 and 2022, respectively. Additionally, the 30-year annual average of rainfall in Prachuap Khiri Khan province has been reported to be between 965.7 and 1112.60 mm [44].
The Eucalyptus tree has a high water use efficiency, which promotes its growth and productivity [45,46]. In addition, the sandy soil texture provides good drainage, further supporting the growth of Eucalyptus hybrids. Nutrients may not be a key factor influencing the growth of Eucalyptus hybrids in this area. Other resources, such as available water and light and soil conditions, can play a significant role in promoting growth [25,47]. Thus, rainfall likely had a substantial impact on the growth of the Eucalyptus hybrid in this study.
Mixing a Eucalyptus hybrid and A. auriculiformis can also change the vertical structure of plantations. The mean height of the five-year-old Eucalyptus hybrid was 17.35 m, while that of A. auriculiformis was 12.36 m. The Eucalyptus hybrid dominated A. auriculiformis, leading to a stratified canopy. This clearly occurred in the mixed plantation and has been reported previously [48,49]. The canopy stratification of mixed plantations can positively affect the light use efficiency [17,18], with mixed plantations demonstrating a higher efficiency compared to Eucalyptus monocultures. In addition, the E. camaldulensis hybrid undergoes natural pruning easily, which facilitates higher light penetration for A. auriculiformis. As a consequence, mixed plantations have been reported to have relatively higher photosynthesis compared to Eucalyptus monocultures [50].
5.2. Aboveground Biomass
In the present study, the stem biomass of E100 was the highest, while that of A100 was the lowest, and the stem biomasses of E67:A33, E50:A50, and E33:A67 were in between. The mixing of the Eucalyptus hybrid with A. auriculiformis trees did not increase the wood production relative to the Eucalyptus hybrid monoculture, mainly because the stem diameter of the Eucalyptus hybrid in the mixed plantation did not increase significantly. In addition, it has been observed that the growth of A. auriculiformis in mixed plantations is lower than that of the Eucalyptus hybrid, leading to low stem production in a mixed plantation setting [48]. The stem biomass of the mixed plantation in this study largely depended on the proportion and growth of the Eucalyptus hybrid. The stem biomass of the Eucalyptus hybrid was approximately 46% higher than that of the A. auriculiformis monoculture.
The aboveground biomass of E100, E50:A50, E67:A33, and E33:A67 was found to be similar, indicating that the nitrogen supply from A. auriculiformis litterfall in the mixed plantation did not influence the production of biomass. The Eucalyptus hybrid exhibited favorable growth and majorly contributed to the biomass produced in the mixed plantation, except for E33:A67. Litter from leaf and branch components was contributed by A. auriculiformis. A. auriculiformis had a relatively larger canopy than that of the Eucalyptus hybrid, leading to higher litter biomass. This result effectively increased the aboveground biomass of the mixed plantation. Previously, a less dense canopy was reported for a five-year-old Eucalyptus hybrid, leading to lower leaf and branch biomass compared to E. deglupta and E. urophylla [51].
Statistically similar amounts of aboveground biomass in the mixed plantation and the Eucalyptus hybrid monoculture indicated that A. auriculiformis could be intercropped with the Eucalyptus hybrid to increase the aboveground biomass and carbon allocation [43,48]. Santos et al. (2017) [24] indicated that mixed stands can reduce the rate of nutrient removal from a short rotation plantation. The residual of Acacia in mixed plantations can increase the nitrogen stock, thereby improving the nitrogen status of the plantation [52]. The density of A. auriculiformis in the Eucalyptus plantation was important for the overall productivity of the plantation, with higher numbers of A. auriculiformis trees leading to lower production in the mixed plantation. In contrast, the high stem production in E100 resulted in maximum economic returns, meaning that the mixed plantation would have a lower income. Although the economic return of wood in a mixed plantation could be lower than that of a Eucalyptus plantation, mixed plantations can reduce the cost of fertilizer during plantation management. As reported previously, the nitrogen fixation in mixed plantations of E. grandis and A. mangium (E50:A50) was 20% higher than that of E100, leading to high N mineralization in soil [42]. The N content in the biomass of the mixed plantation was higher than that of the Eucalyptus plantation, which can improve the soil N status of the plantation [52]. In addition, the large crown of Acacia in the mixed plantation can negatively impact the growth and number of weeds, leading to a lower cost for weeding.
The aboveground biomass produced by the mixed plantation ratios E50:A50 and E67:A33 was between that of E100 and A100 and was not significantly different from E100. This suggests that such mixing ratios should be promoted for sustainable plantation management. A mixed plantation can enhance the diversity and composition of understory vegetation and increase the levels of soil nutrients and the diversity of the bacterial community [42,53], as well as enhance nutrient cycling [25,52,54].
5.3. Litterfall and Nutrient Return
The overall litterfall peaked in December–January during the early dry season. Fast-growing trees such as the Eucalyptus hybrid in this study are very sensitive to water stress [9]. However, the high litterfall amounts measured in 2022 were probably a result of heavy rains and windy storms [55].
A major objective of mixed plantations involving Eucalyptus and nitrogen-fixing trees is to improve the aboveground nutrient status and nutrient cycling [22,47,52]. The litterfall produced by the mixed plantation in this area was intermediate. A. auriculiformis can promote litterfall production in mixed plantations with a large canopy, especially for ages five years and beyond. In contrast, the Eucalyptus hybrid had a lower litterfall production because of a small and light canopy. As such, mixed plantations can positively influence litter production [24,52].
Nutrient return has a significant relationship with litterfall production and nutrient concentration in the litter [12,22]. The nitrogen content can be particularly high in Acacia, a nitrogen-fixing tree. A100 had the highest nitrogen return because of higher litterfall production and nitrogen concentration. In the present study, the nitrogen return in the mixed plantation varied according to the proportion of A. auriculiformis and the Eucalyptus hybrid (E33:A67 > E50:A50 > E67:A33), canopy structure, and age. Overall, the nutrient return in the 3.5- and 4.5-year-old Eucalyptus hybrid monocultures was relatively high, which was probably influenced by a large canopy and crown competition, leading to high litterfall on the forest floor. Calcium was the major nutrient that returned to the Eucalyptus hybrid plantation because of its high concentration in Eucalyptus [24]. Meanwhile, the litter produced by A. auriculiformis gradually increased for plantations aged 3.5 years and then stabilized when the canopy closed at plantation ages of 4.5 and 5.5 years.
The higher decomposition of nitrogen in the mixed plantation was clearly influenced by the presence of A. auriculiformis compared to the monoculture plantations. This indicates that A. auriculiformis plays an important role in improving nutrient levels and promoting nutrient cycling in mixed plantations. Mixed plantations can enhance the aboveground nutrient levels, with soil nutrients being largely extracted by fast-growing trees [24]. In this study, the nitrogen return in the mixed plantation ratios E33:A67, E50:A50, and E67:A33 (57.50, 47.84, and 42.92 kg ha−1 yr−1, respectively) was higher than that of E100 (22.36 Mg ha−1 yr−1). This is a significant observation, as mixed plantations can reduce the cost of fertilizers in fast-growing tree plantations.
5.4. Soil Nutrients
Soil nutrients, including total nitrogen, available phosphorus, potassium, and organic matter, increased with the proportion of A. auriculiformis, particularly in the topsoil. Nitrogen levels remarkably increased in A100, E50:A50, and E33:A67 compared to E100. Previously, Tang et al. (2013) [22] similarly indicated that mixing Eucalyptus and nitrogen-fixing trees positively affects the soil organic carbon, total nitrogen, and available phosphorus, potassium, and magnesium compared to Eucalyptus monocultures. Additionally, a mixed plantation consisting of nitrogen-fixing trees can improve litter quality [9], promote soil microbial activity, and increase microbial density and diversity. These factors can improve nutrient release and nutrient cycling in such plantations [56]. The decomposition rate of litter in the mixed plantation (Eucalyptus and nitrogen-fixing trees) was higher than that of the Eucalyptus monoculture [22], leading to a higher nutrient release into the soil.
Nitrogen-fixing trees have been reported to improve soil development and soil quality in degraded lands, especially nitrogen levels [7,57]. In this area, soil nutrients increased following the establishment of a mixed plantation, as was the case for A. auriculiformis. A100 had the highest total nitrogen in the soil, while the mixed plantation had intermediate levels, indicating the role of A. auriculiformis in increasing the total nitrogen content. The lower soil nutrients in the Eucalyptus monoculture suggest that intercropping with A. auriculiformis can be considered with Eucalyptus. This is because the nutrient uptake by Eucalyptus plantations is relatively higher [58], while the nutrient return via litter is lower [52].
The low soil nutrient levels in the mixed and A. auriculiformis monoculture plantations measured in this study may be attributed to it being the first rotation of the plantations. Soil development can be slow, particularly in sandy soils with a low nutrient absorption capacity. According to Formaglio et al. (2023) [59], mixed Eucalyptus and Acacia plantations increase the nitrogen availability in the topsoil compared to Eucalyptus monocultures, which helps to meet the nitrogen requirements of the trees. This can effectively reduce the need for nitrogen fertilization in Eucalyptus plantations. However, subsequent rotations of plantations would be beneficial for accelerating growth and increasing productivity. Soil nutrients can be accumulated through soil amendments in a mixed plantation. The coppicing potential of Eucalyptus hybrids from stumps is characterized by a high number of shoots and robust growth. According to da Silva et al. (2020) [21], enhancing the nutrient availability in Eucalyptus plantations managed under a coppicing system can significantly stimulate growth and increase productivity. However, introducing nitrogen-fixing trees into other plantations, such as Tectona grandis plantations and agroforestry, should be recommended for improving soil nutrients, maintaining nutrient balance, and enhancing the growth and yield of intercropping trees.
Long-term monitoring in mixed plantations is necessary to better understand soil properties and productivity. Huong et al. (2015) [40] indicated that planting Acacia hybrids on degraded lands can improve soil fertility, especially nitrogen levels, and increase plantation productivity in subsequent rotations. The increased availability of nitrogen can also contribute to a higher production of wood biomass under poor soil conditions [14].
6. Conclusions
In this study, we reported the growth, productivity, nutrient return, and soil properties of a mixed Eucalyptus hybrid (E. camaldulensis × E. urophylla; E) and A. auriculiformis (A) plantation and monocultures of the Eucalyptus hybrid and A. auriculiformis. The results indicated that the DBH and H of the five-year-old Eucalyptus hybrid were higher than those of A. auriculiformis. However, the DBH and H of the Eucalyptus hybrid in the mixed and monoculture plantations were not different, and they were similar to those of A. auriculiformis. The Eucalyptus hybrid dominated A. auriculiformis in both the monoculture and mixed plantations. The aboveground biomass of the mixed plantation ratios E100, E67:A33, E50:A50, and E33:A67 was similar. However, the stem biomass of E100 was the highest, while the stem biomasses of E67:A33 and E50:A50 were intermediate. The nitrogen supplied by A. auriculiformis in the mixed plantation did not influence the growth or productivity of the Eucalyptus hybrid. Environmental factors such as rainfall might majorly influence the growth and productivity of Eucalyptus hybrids in this area. The mean litterfall and nutrient return of the mixed plantation were higher than those of E100. The topsoil soil nutrient levels improved significantly, especially nitrogen in A100, E67:A33, and E50:A50. Mixed Eucalyptus hybrid and A. auriculiformis plantations can be a valuable alternative to Eucalyptus monocultures. Such plantations can benefit from the positive influence of nitrogen-fixing trees, which can reduce the need for nitrogen fertilizers in plantations and improve nutrient cycling. Moreover, mixed plantations can mitigate the negative environmental impacts of Eucalyptus monocultures.
Author Contributions
Conceptualization, J.W.; Methodology, J.W., N.J. and L.-o.T.; Formal analysis, J.W. and L.-o.T.; Investigation, J.W., N.J., P.S. and L.-o.T.; Writing—original draft, J.W.; Writing—review & editing, S.D., J.W. and L.-o.T.; Supervision, S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Kasetsart University Research and Development Institute (RS (KU) 1.65).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors are grateful to the staff at the Had Wanakorn Forestry Research and Student Training Station for their assistance during the fieldwork.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thaiutsa, B. Silvicultural management of private Eucalyptus plantation for wood chips in Thailand. In Proceedings of the International Symposium on Eucalyptus Plantations Research, Management and Development; Wei, R., Xu, D., Eds.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2003; pp. 404–413. [Google Scholar]
- Doran, J.C.; Turnbull, J.W. Australian Trees and Shrubs: Species for Land Rehabilitation and Farm Planting in the Tropics; ACIAR Monograph No. 24; CABI: Wallingford, UK, 1997. [Google Scholar]
- GISDA. Eucalyptus Data from Satellites and Ground Image Sensors; Geo-Informatics and Space Technology Development Agency (Public Organization): Bangkok, Thailand, 2023. [Google Scholar]
- Vanguelova, E.; Pitman, R. Impacts of short rotation forestry on soil sustainability. In Short Rotation Forestry: Review of Growth and Environmental Impacts: Forest Research Monograph 2; McKay, H., Ed.; Forest Research: UK, 2009; pp. 37–78. [Google Scholar]
- Yimam, M.M.; Hailu, L. Effects of eucalyptus plantation on environment and water resource. Int. J. Adv. Res. Biol. Sci. 2022, 9, 156–163. [Google Scholar]
- Koutika, L.S.; Richardson, D.M. Acacia mangium Willd: Benefits and threats associated with its increasing use around the world. For. Ecosyst. 2019, 6. [Google Scholar] [CrossRef]
- Wongprom, J.; Poolsiri, R.; Diloksumpun, S.; Ngernsaengsaruay, C. Soil properties and tree composition in a 27-year-old Acacia mangium Willd. plantation on abandoned mining area at Phangnga Forestry Research Station. Biotropia 2020, 27, 125–133. [Google Scholar] [CrossRef]
- Saharjo, B.H.; Watanabe, H. Estimation of litter fall and seed production of Acacia mangium in a forest plantation in South Sumatra, Indonesia. For. Ecol. Manag. 2000, 130, 265–268. [Google Scholar] [CrossRef]
- Wongprom, J.; Poolsiri, R.; Diloksumpun, S.; Ngernsaengsaruay, C.; Tansakul, S.; Chandaeng, W. Litterfall, litter decomposition and nutrient return of rehabilitated mining areas and natural forest in Phangnga Forestry Research Station, southern Thailand. Biotropia 2022, 29, 74–85. [Google Scholar] [CrossRef]
- Nambiar, E.K.S.; Harwood, C.E. Productivity of acacia and eucalypt plantations in Southeast Asia. 1. Bio-physical determinants of production: Opportunities and challenges. Int. For. Rev. 2014, 16, 225–248. [Google Scholar] [CrossRef]
- Hai, P.H.; Harwood, C.; Kha, L.D.; Pinyopusarerk, K.; Thinh, H.H. Genetic gain from breeding Acacia auriculiformis in Vietnam. J. Trop. For. Sci. 2008, 20, 313–327. [Google Scholar]
- Parrotta, J.A. Productivity, nutrient cycling, and succession in single- and mixed-species plantations of Casuarina equisetifolia, Eucalyptus robusta, and Leucaena leucocephala in Puerto Rico. For. Ecol. Manag. 1999, 124, 45–77. [Google Scholar] [CrossRef]
- Bouillet, J.P.; Laclau, J.P.; de Moraes Gonçalves, J.L.; Moreira, M.Z.; Trivelin, P.; Jourdan, C.; Galiana, A. Mixed-species Plantations of Acacia mangium and Eucalyptus grandis in Brazil. In Proceedings of the Site Management and Productivity in Tropical Plantation Forests, Proceedings of Workshops in Piracicaba (Brazil) 22–26 November 2004 and Bogor (Indonesia) 6–9 November 2006, Bogor, Indonesia; Nambiar, E.K.S., Ed.; Center for International Forestry Research (CIFOR): Bogor, Indonesia, 2008; pp. 157–172. [Google Scholar]
- Bouillet, J.P.; Laclau, J.P.; de Moraes Gonçalves, J.L.; Voigtlaender, M.; Gava, J.L.; Leite, F.; Hakamada, R.; Mareschal, L.; Mabiala, A.; Tardy, F.; et al. Eucalyptus and Acacia tree growth over entire rotation in single and mixed-species plantations across five sites in Brazil and Congo. For. Ecol. Manag. 2013, 301, 89–101. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J.; Khanna, P.K. Growth dynamics in a mixed-species plantation of Eucalyptus globulus and Acacia mearnsii. For. Ecol. Manag. 2004, 193, 81–95. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J.; Cowie, A.L.; Mitchell, P.A.; Brockwell, J. Productivity of three young mixed-species plantation containing N2-fixing Acacia and non-N2-fixing Eucalyptus and Pinus trees in Southern Australia. For. Sci. 2007, 53, 426–434. [Google Scholar] [CrossRef]
- Bauhus, J.; van Winden, A.P.; Nicotra, A.B. Aboveground interactions and productivity in mixed-species plantations of Acacia mearnsii and Eucalyptus globulus. Can. J. Res. 2004, 34, 686–694. [Google Scholar] [CrossRef]
- Forrester, D.I.; Lancaster, K.; Collopy, J.J.; Warren, C.R.; Tausz, M. Photosynthetic capacity of Eucalyptus globulus is higher when grown in mixture with Acacia mearnsii. Trees 2012, 26, 1203–1213. [Google Scholar] [CrossRef]
- Debell, D.S.; Whitesell, C.D.; Schubert, T.H. Mixed Plantation of Eucalyptus and Leguminous Trees Enhance Biomass Production; Pacific Southwest Forest and Range Experiment Station, Forest Service: Berkeley, CA, USA, 1985. [Google Scholar]
- Forrester, D.I.; Schortemeyer, M.; Stock, W.D.; Bauhus, J.; Khanna, P.K.; Cowie, A. Assessing nitrogen fixation in mixed-and single -species plantations of Eucalyptus globulus and Acacia mearnsii. Tree Physiol. 2007, 27, 1319–1328. [Google Scholar] [CrossRef]
- da Silva, N.F.; de Barros, N.F.; Neves, J.C.L.; Schulthais, F.; de Novais, R.F.; Mattiello, E.M. Yield and nutrient demand and efficiency of eucalyptus under coppicing regime. Forests 2020, 11, 852. [Google Scholar] [CrossRef]
- Tang, G.; Li, K.; Zhang, C.; Gao, C.; Li, B. Accelerated nutrient cycling via leaf litter, and not root interaction, increases growth of Eucalyptus in mixed-species plantations with Leucaena. For. Ecol. Manag. 2013, 310, 45–53. [Google Scholar] [CrossRef]
- Pereira, A.P.A.; Durrer, A.; Gumiere, T.; Gonçalves, J.L.M.; Robin, A.; Bouillet, J.P.; Wang, J.; Verma, J.P.; Singh, B.K.; Cardoso, E.J.B.N. Mixed Eucalyptus plantations induce changes in microbial communities and increase biological functions in the soil and litter layers. For. Ecol. Manag. 2019, 433, 332–342. [Google Scholar] [CrossRef]
- Santos, F.M.; Chaer, G.M.; Diniz, A.R.; Balieiro, F.d.C. Nutrient cycling over five years of mixed-species plantations of Eucalyptus and Acacia on a sandy tropical soil. For. Ecol. Manag. 2017, 384, 110–121. [Google Scholar] [CrossRef]
- Marron, N.; Epron, D. Are mixed-tree plantations including a nitrogen-fixing species more productive than monocultures? For. Ecol. Manag. 2019, 441, 242–252. [Google Scholar] [CrossRef]
- Thomas, A.; Priault, P.; Piutti, S.; Dallé, E.; Marron, N. Growth dynamics of fast-growing tree species in mixed forestry and agroforestry plantations. For. Ecol. Manag. 2021, 480, 118672. [Google Scholar] [CrossRef]
- Vanclay, J.K.; Gregorio, N.O.; Herbohn, J.L. Competition in a mixed-species planting with four contrasting tree species. Small-Scale For. 2023, 22, 351–369. [Google Scholar] [CrossRef]
- Wongchai, W.; Promwungkwa, A.; Insuan, W. Above-ground biomass allometric equation and dynamics accumulation of Eucalyptus camaldulensis and Acacia hybrid plantations in northern Thailand. Int. J. Renew. Energy Res. 2020, 10, 1664–1673. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–37. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Sahunalu, S.; Dhanmanonda, P.; Prachaiyo, B.; Muangnil, K. Growth and yield for various uses of Eucalyptus camaldulensis Dehnh. planted in an agroforestry system on the degraded land. Thai J. For. 1993, 12, 144–158. [Google Scholar]
- Suraj, P.G.; Hegde, R.; Varghese, M.; Kamalakannan, R.; Sahoo, D.R.; Bush, D.J.; Harwood, C.E. Growth and pulpwood traits of Leucaena leucocephala and Eucalyptus camaldulensis at rainfed and irrigated sites in southern India. Trees For. People 2024, 15, 100482. [Google Scholar] [CrossRef]
- Royampaeng, S. Physiology of Intraspecific and Interspecific Hybrids of Acacia auriculiformis A. Cunn. ex Benth. Ph.D. Thesis, Northern Territory University, Australia, 2001. [Google Scholar]
- Watanabe, Y.; Masunaga, T.; Fashola, O.O.; Agboola, A.; Oviasuyi, P.K.; Wakatsuki, T. Eucalyptus camaldulensis and Pinus caribaea growth in relation to soil physico-chemical properties in plantation forests in Northern Nigeria. Soil Sci. Plant Nutr. 2009, 55, 132–141. [Google Scholar] [CrossRef]
- Klangprapan, A.; Maelim, S.; Meunpong, P. Growth, yields and financial return of 4-year-old Eucalyptus clone K58 and K62 planted in paired rows at Sa Kaeo Plantation, Sa Kaeo Province. KKU Res. J. Grad. Stud. 2019, 19, 22–34. [Google Scholar]
- Diloksumpun, S.; Wongprom, J.; A-kakhun, S. Growth and phyllode function traits of Acacia hybrid clones planted for post mining rehabilitation site in southern Thailand. In Proceedings of the SEAMEO BIOTROP 2nd International Conference on Tropical Biology: “Biology Ecological Restoration in Southeast Asia: Challenges, Gains, and Future Directions”, Held at Bogor, Indonesia, 12–13 October 2015; Damayanti, E.K., Fernandez, J.C., Eds.; Seameo Biotrop: Bogor, Indonesia, 2016; pp. 98–112. [Google Scholar]
- Hnukaew, M.; Poolsiri, R.; Haruthaithanasan, M.; Chompoowiset, P. Yield and nutrient contents of various 5-year-old Eucalypt clones in the upper northeast. Thai J. For. 2015, 34, 11–21. [Google Scholar]
- Luangviriyasaeng, V. Growth of different eucalypt clones planted on paddy bunds. Thai J. For. 2009, 28, 1–12. [Google Scholar]
- Kha, L.D.; Hardwood, C.E.; Kien, N.D.; Baltunis, B.S.; Hai, N.D.; Thinh, H.H. Growth and wood basic density of acacia hybrid clones at three locations in Vietnam. New For. 2012, 43, 13–29. [Google Scholar] [CrossRef]
- Huong, V.D.; Nambiar, E.K.S.; Quang, L.T.; Mendham, D.S.; Dung, P.T. Improving productivity and sustainability of successive rotations of Acacia auriculiformis plantations in south Vietnam. South. For. 2015, 77, 51–58. [Google Scholar] [CrossRef]
- Fisher, R.F.; Binkley, D. Ecology and Management of Forest Soils, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Paula, R.R.; Bouillet, J.P.; de Moraes Gonçalves, J.L.; Trivelin, P.C.; de Carvalho Balieiro, F.; Nouvellon, Y.; Oliveira, J.d.C.; de Deus Júnior, J.C.; Bordron, B.; Laclau, J.P. Nitrogen fixation rate of Acacia mangium Willd at mid rotation in Brazil is higher in mixed plantations with Eucalyptus grandis Hill ex Maiden than in monocultures. Ann. For. Sci. 2018, 75, 14. [Google Scholar] [CrossRef]
- Tchichelle, S.V.; Mareschal, L.; Koutika, L.S.; Epron, D. Biomass production, nitrogen accumulation and symbiotic nitrogen fixation in a mixed-species plantation of eucalyptus and acacia on a nutrient-poor tropical soil. For. Ecol. Manag. 2017, 403, 103–111. [Google Scholar] [CrossRef]
- Amatayakula, P.; Chomtha, T. Agricultural Meteorology to Know for Prachuap Khiri Khan; Agrometeorological Division, Meteorological Development Bureau: Bangkok, Thailand, 2017. [Google Scholar]
- Stape, J.L.; Binkley, D.; Ryan, M.G. Eucalyptus production and the supply, use and efficiency of use of water, light and nitrogen across a geographic gradient in Brazil. For. Ecol. Manag. 2004, 193, 17–31. [Google Scholar] [CrossRef]
- White, D.A.; Silberstein, R.P.; Balocchi-Contreras, F.; Quiroga, J.J.; Palma, J.H.N.; de Arellana, P.R. Growth, water use, and water use efficiency of Eucalyptus globulus and Pinus radiata plantations compared with natural stands of Roble-Hualo forest in the coastal mountains of central Chile. For. Ecol. Manag. 2021, 501, 119676. [Google Scholar] [CrossRef]
- Richards, A.E.; Forrester, D.I.; Bauhus, J.; Lorenzen, M.S. The influence of mixed tree plantations on the nutrition of individual species: A review. Tree Physiol. 2010, 30, 1192–1208. [Google Scholar] [CrossRef] [PubMed]
- Nouvellon, Y.; Laclau, J.P.; Epron, D.; Maire, G.L.; Bonnefond, J.M.; Gonçalves, J.L.M.; Bouillet, J.P. Production and carbon allocation in monocultures and mixed-species plantations of Eucalyptus grandis and Acacia mangium in Brazil. Tree Physiol. 2012, 32, 680–695. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.M.; Balieiro, F.d.C.; dos SAtaíde, D.H.; Diniz, A.R.; Chaer, G.M. Dynamics of aboveground biomass accumulation in monospecific and mixed-species plantations of Eucalyptus and Acacia on a Brazilian sandy soil. For. Ecol. Manag. 2016, 363, 86–97. [Google Scholar] [CrossRef]
- Maire Gl Nouvellon, Y.; Christina, M.; Ponzoni, F.J.; de Moraes Gonçalves, J.L.; Bouillet, J.B.; Laclau, J.P. Tree and stand light use efficiencies over a full rotation of single-and mixed-species Eucalyptus grandis and Acacia mangium plantations. For. Ecol. Manag. 2013, 288, 31–42. [Google Scholar] [CrossRef]
- Wongprom, J.; Wachrinrat, C.; Srigongpan, R.; Klangsap, N. Water use and water use efficiency of eucalypt clones planted on paddy bunds in Phanom Sarakham District, Chachoengsao Province. Thai J. For. 2009, 28, 38–46. [Google Scholar]
- Koutika, L.S.; Tchichelle, S.V.; Mareschal, L.; Epron, D. Nitrogen dynamics in a nutrient-poor soil under mixed-species plantations of eucalyptus and acacias. Soil Biol. Biochem. 2017, 108, 84–90. [Google Scholar] [CrossRef]
- Li, T.; Sun, Y.; Wang, L.; Xu, R.; Tigabu, M.; Li, M.; Wang, D. Effects of species mixture on understory vegetation, soil properties and bacterial diversity of Acacia cincinnata, Eucalyptus robusta and Acacia mangium plantations in Southeastern China. Plant Stress 2023, 10, 100278. [Google Scholar] [CrossRef]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-species versus monocultures in plantation forestry: Development, benefits ecosystem services and perspectives for the future. Glob. Ecol. Conserv. 2018, 15, e00419. [Google Scholar] [CrossRef]
- Wang, H.C.; Wang, S.F.; Lin, K.C.; Shaner, P.J.L.; Lin, T.C. Litterfall and element fluxes in a natural hardwood forest and a Chinese-fir plantation experiencing frequent typhoon disturbance in central Taiwan. Biotropica 2013, 45, 541–548. [Google Scholar] [CrossRef]
- Rachid, C.T.C.C.; Balieiro, F.C.; Peixoto, R.S.; Pinheiro, Y.A.S.; Piccolo, M.C.; Chaer, G.M.; Rosado, A.S. Mixed plantations can promote microbial integration and soil nitrate increases with changes in the N cycling genes. Soil Biol. Biochem. 2013, 66, 146–153. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Xia, H.; Zou, B.; Li, N.; Liu, J.; Zhu, W. Effects of nitrogen-fixing and non-nitrogen-fixing tree species on soil properties and nitrogen transformation during forest restoration in southern China. Soil Sci. Plant Nutr. 2010, 56, 297–306. [Google Scholar] [CrossRef]
- Laclau, J.P.; Deleporte, P.; Ranger, J.; Bouillet, J.P.; Kazotti, G. Nutrient dynamics throughout the rotation of Eucalyptus clonal stands in Congo. Ann. Bot. 2003, 91, 879–892. [Google Scholar] [CrossRef]
- Formaglio, G.; Krusche, A.V.; Mareschal, L.; Bouillet, J.P.; de Moraes Goncalves, J.L.; Nouvellon, Y.; Delgado-Rojas, J.S.; Montebelo, A.; Ranger, J. Planting nitrogen-fixing trees in tropical Eucalyptus plantations does not increase nutrient losses through drainage. For. Ecol. Manag. 2023, 537, 120940. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).