Thinning Improves Large Diameter Timber Cultivation but Undermines Ecosystem Multifunctionality in the Short Term
Abstract
1. Introduction
2. Materials and Methods
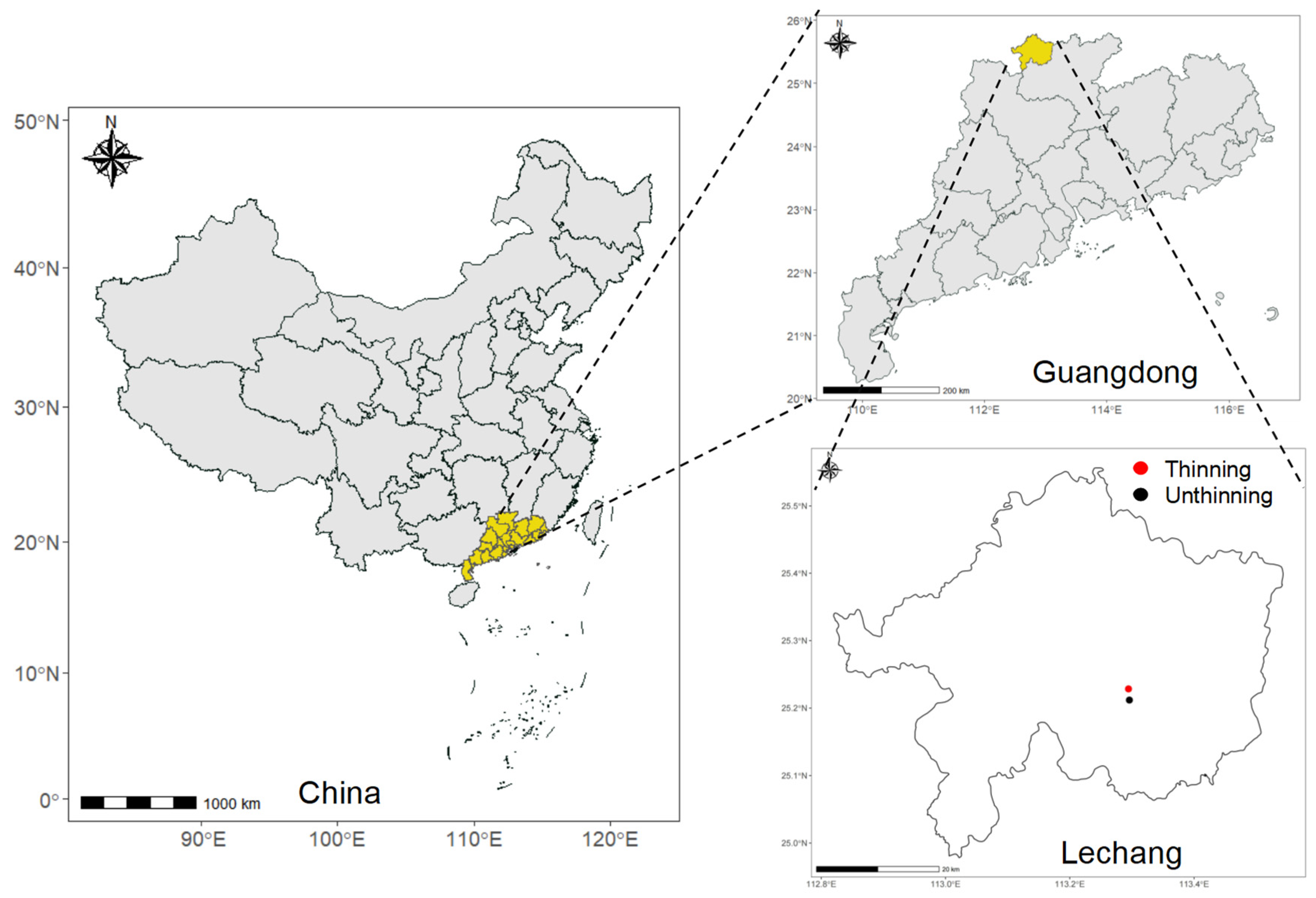
2.1. Study Area
2.2. Plot Design and Soil Sampling
2.3. Plant and Soil Characteristic Assessments
2.4. Nutrient Resorption and Utilization Efficiency
2.5. Ecosystem Multifunctionality Measurement
2.6. Statistical Analysis
3. Results
3.1. Tree Diameter at Breast Height and Understory Vegetation Characteristics
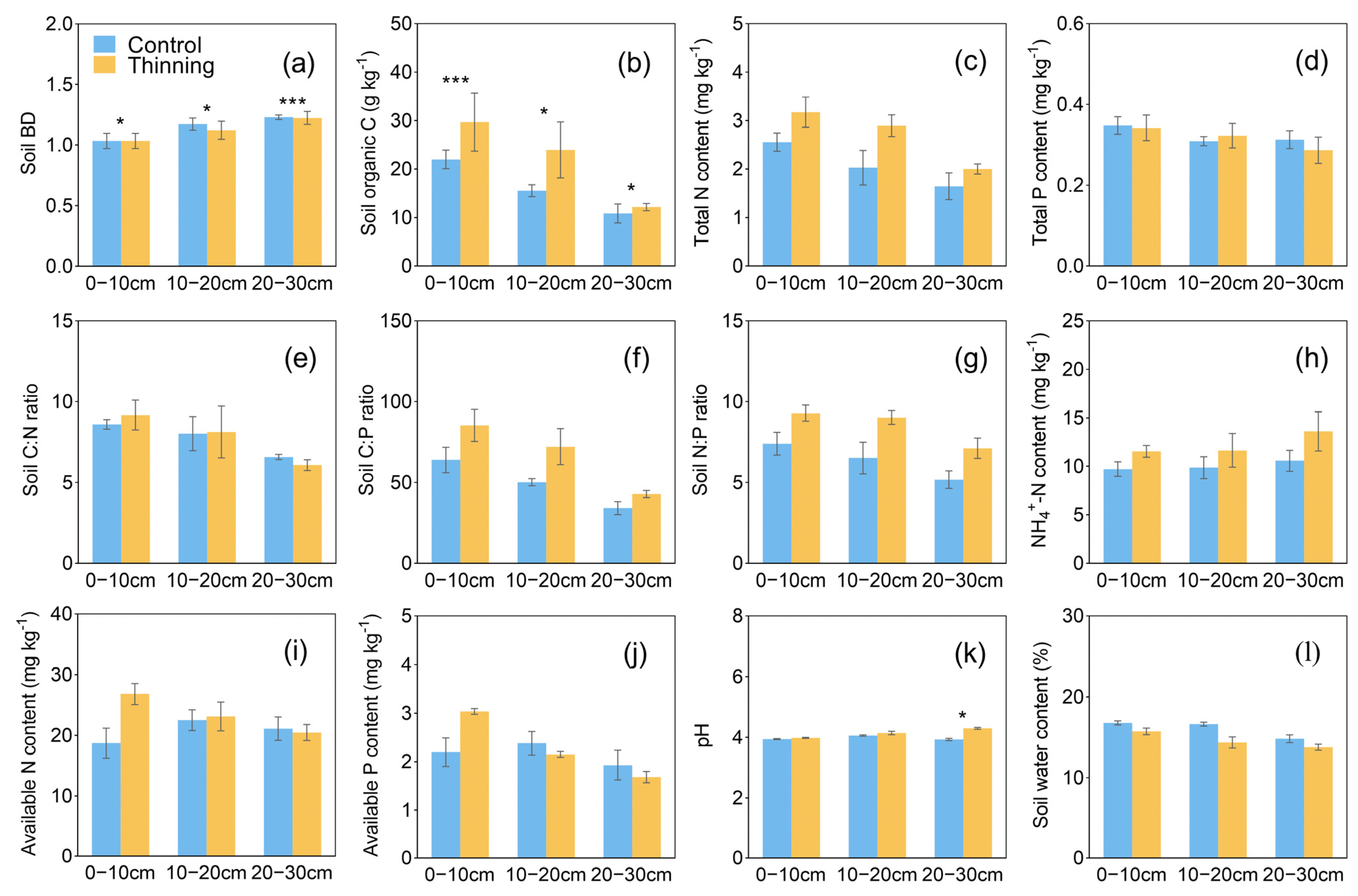
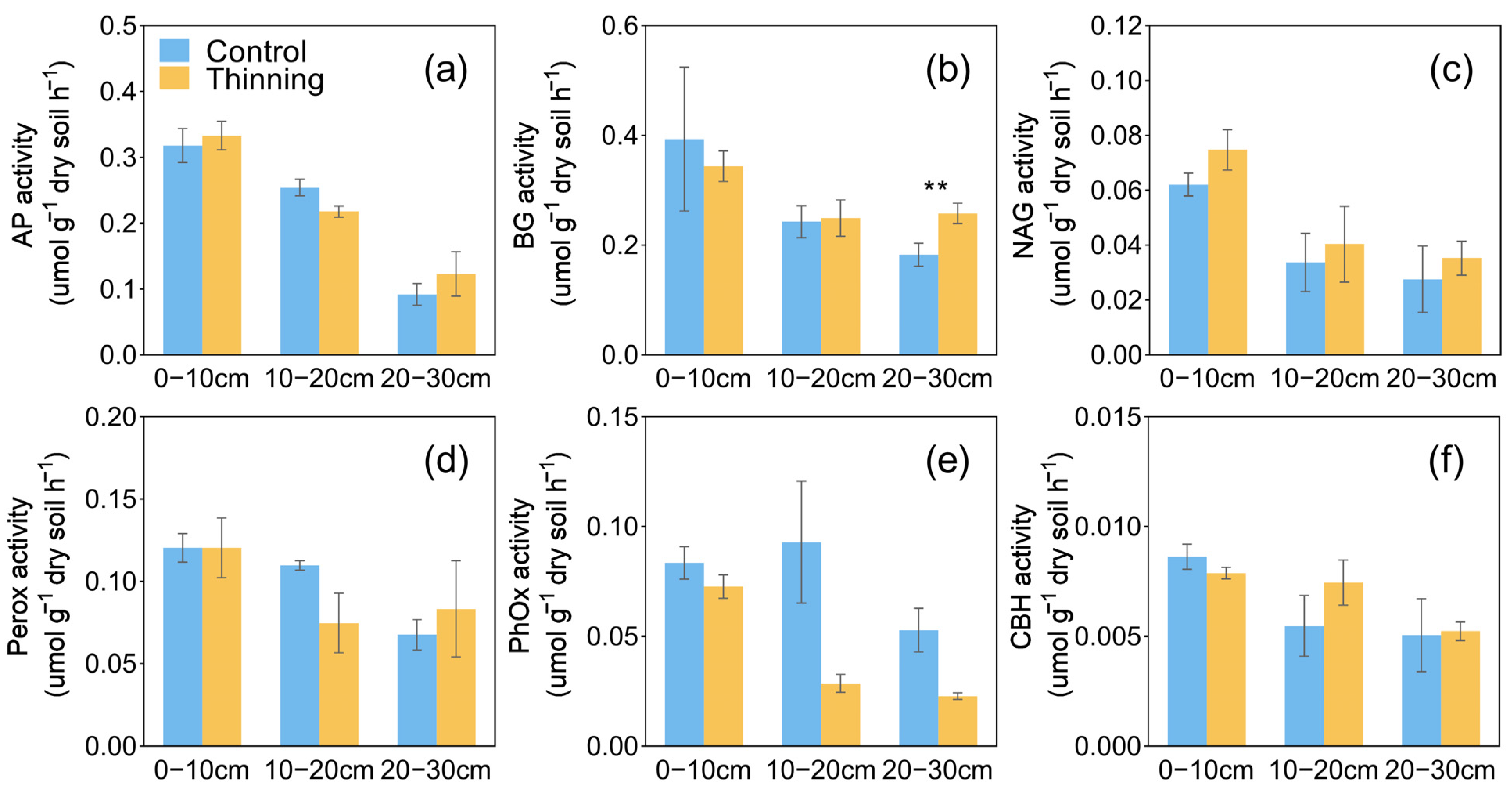
3.2. Soil Properties and Enzyme Activities
3.3. Soil Microbial Community and Microbial Biomass C, N and P
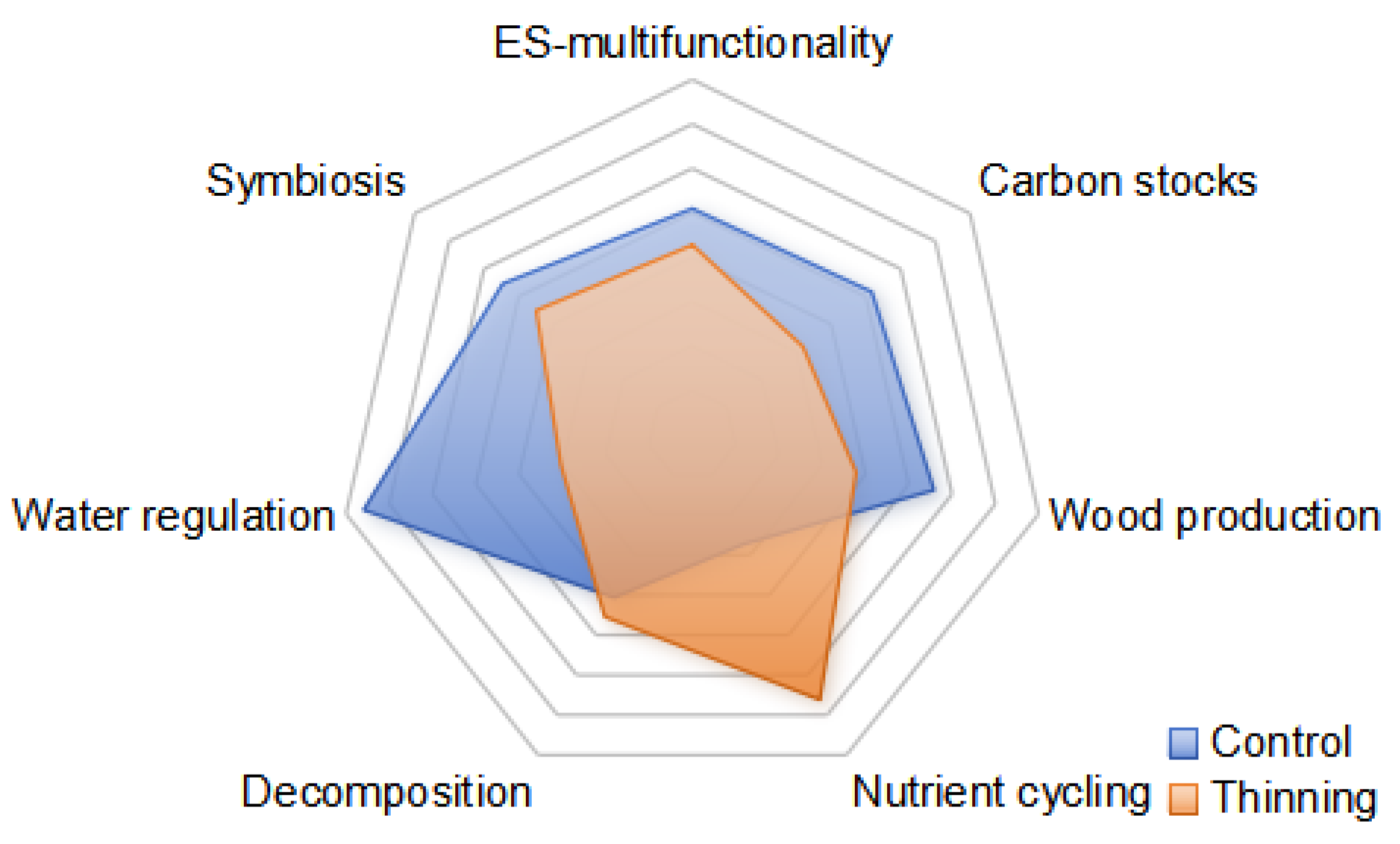
3.4. Ecosystem Multifunctionality
3.5. Correlation of Ecosystem Multifunctionality with Soil and Plant Properties
4. Discussion
4.1. Effects of Thinning on Plant and Soil Properties
4.2. Effects of Thinning on Ecosystem Multifunctionality
4.3. Impacts of Soil and Plant Properties on Ecosystem Multifunctionality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trumbore, S.; Brando, P.; Hartmann, H. Forest health and global change. Science 2015, 349, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wei, J.; Wang, W.; Huang, C.; Feng, C.; Xu, D.; Haider, F.U.; Li, X. Monitoring changes in composition and diversity of forest vegetation layers after the cessation of management for renaturalization. Forests 2024, 15, 907. [Google Scholar] [CrossRef]
- Baccini, A.; Walker, W.; Carvalho, L.; Farina, M.; Sulla-Menashe, D.; Houghton, R.A. Tropical forests are a net carbon source based on aboveground measurements of gain and loss. Science 2017, 358, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Giam, X. Global biodiversity loss from tropical deforestation. Proc. Natl. Acad. Sci. USA 2017, 114, 5775. [Google Scholar] [CrossRef] [PubMed]
- Menz, M.H.; Dixon, K.W.; Hobbs, R.J. Hurdles and opportunities for landscape-scale restoration. Science 2013, 339, 526–527. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Li, X.; Zhou, S.; Liu, X.; Lie, Z.; Aguila, L.C.R.; Xu, W.; Liu, J. Soil organic carbon sources exhibit different patterns with stand age in rhizosphere and non-rhizosphere soils. Catena 2025, 248, 108579. [Google Scholar] [CrossRef]
- Mori, A.S.; Lertzman, K.P.; Gustafsson, L. Biodiversity and ecosystem services in forest ecosystems. J. Appl. Ecol. 2017, 54, 12–27. [Google Scholar] [CrossRef]
- Harrison, S.; Spasojevic, M.J.; Li, D. Climate and plant community diversity in space and time. Proc. Natl. Acad. Sci. USA 2020, 117, 4464. [Google Scholar] [CrossRef] [PubMed]
- López-Bedoya, P.A.; Bohada-Murillo, M.; Ángel-Vallejo, M.C.; Audino, L.D.; Davis, A.L.; Gurr, G.; Noriega, J.A. Primary forest loss and degradation reduces biodiversity and ecosystem functioning: A global meta-analysis using dung beetles as an indicator taxon. J App. Ecol. 2022, 59, 1572–1585. [Google Scholar] [CrossRef]
- Yan, P.; Fernández-Martínez, M.; Van Meerbeek, K.; Yu, G.; Migliavacca, M.; He, N. The essential role of biodiversity in the key axes of ecosystem function. Global Change Biol. 2023, 29, 4569–4585. [Google Scholar] [CrossRef]
- Chen, H.Y.H.; Biswas, S.R.; Sobey, T.M.; Brassard, B.W.; Bartels, S.F. Reclamation strategies for mined forest soils and overstory drive understorey vegetation. J. Appl. Ecol. 2018, 55, 926–936. [Google Scholar] [CrossRef]
- Fakhry, A.M.; Khazzan, M.M.; Aljedaani, G.S. Impact of disturbance on species diversity and composition of Cyperus conglomeratus plant community in southern Jeddah, Saudi Arabia. J. King Saud Univ.Sci. 2020, 32, 600–605. [Google Scholar] [CrossRef]
- Rembold, K.; Mangopo, H.; Tjitrosoedirdjo, S.S.; Kreft, H. Plant diversity, forest dependency, and alien plant invasions in tropical agricultural landscapes. Biol. Conserv. 2017, 213, 234–242. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Wang, Y.; Jiang, P.; Hu, Y.; Ouyang, S.; Wu, H.; Lei, P.; Kuzyakov, Y.; Xiang, W. Plantations thinning: A meta-analysis of consequences for soil properties and microbial functions. Sci. Total Environ. 2023, 877, 162894. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, S.; Lu, Y.; Froese, R.E.; Xu, X.; Zeng, J.; Ming, A.; Liu, X.; Xie, Y.; Li, Q. Thinning effects on forest evolution in Masson pine (Pinus massoniana Lamb.) conversion from pure plantations into mixed forests. For. Ecol. Manag. 2020, 477, 118503. [Google Scholar] [CrossRef]
- Takayama, N.; Saito, H.; Fujiwara, A.; Horiuchi, M. The effect of slight thinning of managed coniferous forest on landscape appreciation and psychological restoration. Prog. Earth Planet. Sci. 2017, 4, 17. [Google Scholar] [CrossRef]
- Kitagawa, R.; Ueno, M.; Masaki, T. Thinning affects understorey tree community assembly in monoculture plantations by facilitating stochastic immigration from the landscape. App. Veg. Sci. 2017, 20, 673–682. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, J.; Peng, S.; Chen, Y.; Cao, Y. The effects of forest thinning on understory diversity in China: A meta-analysis. Land Degrad. Dev. 2020, 31, 1225–1240. [Google Scholar] [CrossRef]
- Moreau, G.; Auty, D.; Pothier, D.; Shi, J.; Lu, J.; Achim, A.; Xiang, W. Long-term tree and stand growth dynamics after thinning of various intensities in a temperate mixed forest. For. Ecol. Manag. 2020, 473, 118311. [Google Scholar] [CrossRef]
- Keenan, R.J.; Weston, C.J.; Volkova, L. Potential for forest thinning to reduce risk and increase resilience to wildfire in Australian temperate Eucalyptus forests. Curr. Opin. Environ. Sci. Health 2021, 23, 100280. [Google Scholar] [CrossRef]
- Settineri, G.; Mallamaci, C.; Mitrović, M.; Sidari, M.; Muscolo, A. Effects of different thinning intensities on soil carbon storage in Pinus laricio forest of Apennine South Italy. Eur. J. Forest Res. 2018, 137, 131–141. [Google Scholar] [CrossRef]
- Moreau, G.; Chagnon, C.; Achim, A.; Caspersen, J.; D’Orangeville, L.; Sánchez-Pinillos, M.; Thiffault, N. Opportunities and limitations of thinning to increase resistance and resilience of trees and forests to global change. Forestry 2020, 95, 595–615. [Google Scholar] [CrossRef]
- Witzell, J.; Bergström, D.; Bergsten, U. Variable corridor thinning-a cost-effective key to provision of multiple ecosystem services from young boreal conifer forests? Scand. J. Forest Res. 2019, 34, 497–507. [Google Scholar] [CrossRef]
- Zhao, B.; Ballantyne, A.P.; Meng, S.; Zhao, G.; Zheng, Z.; Zhu, J.; Cao, J.; Zhang, Y.; Zhao, X. Understory plant removal counteracts tree thinning effect on soil respiration in a temperate forest. Global Change Biol. 2020, 28, 6102–6113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, C.; Jin, Y.; Sun, Z. Impacts of thinning on soil carbon and nutrients and related extracellular enzymes in a larch plantation. For. Ecol. Manag. 2019, 450, 117523. [Google Scholar] [CrossRef]
- Yang, L.; Qin, J.; Geng, Y.; Zhang, C.; Pan, J.; Niu, S.; Tian, D.; Zhao, X.; Wang, J. Long-term effects of forest thinning on soil respiration and its components in a pine plantation. For. Ecol. Manag. 2022, 513, 120189. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Wang, D.; Zhao, Z. Response of height growth of regenerating trees in a Pinus tabulaeformis Carr. plantation to different thinning intensities. For. Ecol. Manag. 2019, 444, 280–289. [Google Scholar] [CrossRef]
- Wang, D.; Olatunji, O.A.; Xiao, J. Thinning increased fine root production, biomass, turnover rate and understory vegetation yield in a Chinese fir plantation. For. Ecol. Manag. 2019, 440, 92–100. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Qi, S.; Fan, Z.; Xue, Y.; Tang, Q.; Liu, Z.; Zheng, X.; Wu, C.; Xi, B.; et al. Thinning vs. pruning: Impacts on sap flow density and water use efficiency in young populus tomentosa plantations in Northern China. Forests 2024, 15, 536. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Y.; Chen, B.; Ming, A.; Pang, L. Nutrient resorption and C: N: P stoichiometry responses of a Pinus massoniana plantation to various thinning intensities in Southern China. Forests 2022, 13, 1699. [Google Scholar] [CrossRef]
- Caihong, Z.; Nier, S.; Hao, W.; Honglin, X.; Hailong, S.; Ling, Y. Effects of thinning on soil nutrient availability and fungal community composition in a plantation medium-aged pure forest of Picea koraiensis. Sci. Rep. 2023, 13, 2492. [Google Scholar] [CrossRef]
- Li, X.; Aguila, L.C.R.; Luo, J.; Liu, Y.; Wu, T.; Lie, Z.; Liu, X.; Cheng, Y.; Jiang, F.; Liu, J. Carbon storage capacity of Castanopsis hystrix plantations at different stand-ages in South China. Sci. Total Environ. 2023, 894, 164974. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Aguila, L.C.R.; Wu, D.; Lie, Z.; Xu, W.; Tang, X.; Liu, J. Carbon sequestration and storage capacity of Chinese fir at different stand ages. Sci. Total Environ. 2023, 904, 166962. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Wu, G.; Lie, Z.; Sheng, H.; Aguila, L.C.R.; Khan, M.S.; Liu, X.; Zhou, S.; Wu, T.; et al. Mixed plantations do not necessarily provide higher ecosystem multifunctionality than monoculture plantations. Sci. Total Environ. 2024, 914, 170156. [Google Scholar] [CrossRef]
- Li, X.; Wu, T.; Wu, G.; Aguila, L.C.R.; Liu, X.; Liu, Y.; Cheng, Y.; Jiang, F.; Lie, Z.; Liu, J. Increasing stand age increases N deficiency but alleviates relative P limitations in Castanopsis hystrix plantations in southern China. Land Degrad. Dev. 2024, 35, 2173–2183. [Google Scholar] [CrossRef]
- See, C.R.; Yanai, R.D.; Fisk, M.C.; Vadeboncoeur, M.A.; Quintero, B.A.; Fahey, T.J. Soil nitrogen affects phosphorus recycling: Foliar resorption and plant-soil feedbacks in a northern hardwood forest. Ecology 2015, 96, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; Volume 9, pp. 595–624. [Google Scholar]
- Han, W.; Tang, L.; Chen, Y.; Fang, J. Relationship between the relative limitation and resorption efficiency of nitrogen vs phosphorus in woody plants. PLoS ONE 2013, 8, e83366. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P. Nutrient cycling and nutrient use efficiency. Am. Nat. 1982, 119, 553–572. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Lucas-Borja, M.E.; Wang, Z.; Li, X.; Huang, Z. Microbial diversity regulates ecosystem multifunctionality during natural secondary succession. J. Appl. Ecol. 2021, 58, 2833–2842. [Google Scholar] [CrossRef]
- Ulvcrona, K.A.; Karlsson, K.; Ulvcrona, T. Identifying the biological effects of pre-commercial thinning on diameter growth in young Scots pine stands. Scand. J. Forest Res. 2014, 29, 427–435. [Google Scholar] [CrossRef]
- Aldea, J.; del Río, M.; Cattaneo, N.; Riofrío, J.; Ordóñez, C.; Uzquiano, S.; Bravo, F. Short-term effect of thinning on inter-and intra-annual radial increment in Mediterranean Scots pine-oak mixed forests. For. Ecol. Manag. 2023, 549, 121462. [Google Scholar] [CrossRef]
- Haider, F.U.; Coulter, J.A.; Cheema, S.A.; Farooq, M.; Wu, J.; Zhang, R.; Shuaijie, G.; Cai, L. Co-application of biochar and microorganisms improves soybean performance and remediate cadmium-contaminated soil. Ecotox. Environ. Safe. 2021, 214, 112112. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Yu, J.; Li, J.; Shangguan, Z.; Deng, L. Thinning increases forest ecosystem carbon stocks. For. Ecol. Manag. 2024, 555, 121702. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Q.; Gao, D.; Zhang, B.; Zuo, H.; Jiang, J. Effects of thinning and understory removal on the soil water-holding capacity in Pinus massoniana plantations. Sci. Rep. 2021, 11, 13029. [Google Scholar] [CrossRef]
- Meng, L.; Cui, Z.; Huang, Z.; Lucas-Borja, M.E.; Dunkerley, D.; Wu, G. Thinning effects on forest production and rainfall redistribution: Reduced soil water deficit and improved sustainability of semiarid plantation forestlands. Land Degrad. Dev. 2022, 33, 3163–3173. [Google Scholar] [CrossRef]
- Bai, S.; Dempsey, R.; Reverchon, F.; Blumfield, T.J.; Ryan, S.; Cernusak, L.A. Effects of forest thinning on soil-plant carbon and nitrogen dynamics. Plant Soil 2017, 411, 437–449. [Google Scholar] [CrossRef]
- Vesterdal, L.; Dalsgaard, M.; Felby, C.; Raulund-Rasmussen, K.; Jørgensen, B.B. Effects of thinning and soil properties on accumulation of carbon, nitrogen and phosphorus in the forest floor of Norway spruce stands. For. Ecol. Manag. 1995, 77, 1–10. [Google Scholar] [CrossRef]
- Huang, W.; Song, B.; Liu, Y.; Liu, J.; Wang, X. Multifunctional evaluation of spruce-fir forest based on different thinning intensities. Forests 2024, 15, 1703. [Google Scholar] [CrossRef]
- Tebenkova, D.N.; Lukina, N.V.; Chumachenko, S.I.; Danilova, M.A.; Kuznetsova, A.I.; Gornov, A.V.; Shevchenko, N.E.; Kataev, A.D.; Gagarin, Y.N. Multifunctionality and biodiversity of forest ecosystems. Contemp. Probl. Ecol. 2020, 13, 709–719. [Google Scholar] [CrossRef]
- Tebenkova, D.N.; Lukina, N.V.; Kataev, A.D.; Chumachenko, S.I.; Kiselyova, V.V.; Kolycheva, A.A.; Shanin, V.N.; Gagarin, Y.N.; Kuznetsova, A.I. Scenario development for the imitation modelling of forest ecosystem services. For. Sci. Iss. 2023, 6, 136. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, H.; Lei, X.; Zeng, J.; Lan, J.; Guo, X.; Gao, D.; Liu, X.; Zhang, H. Impact of management measures on multiple ecosystem function trade-offs and their dynamics in subtropic Pinus massoniana plantations. Forests 2024, 15, 1224. [Google Scholar] [CrossRef]
- Schjønning, P.; Thomsen, I.K.; Petersen, S.O.; Kristensen, K.; Christensen, B.T. Relating soil microbial activity to water content and tillage-induced differences in soil structure. Geoderma 2011, 163, 256–264. [Google Scholar] [CrossRef]
- Zhou, S.; Lie, Z.; Liu, X.; Zhu, Y.; Josep, P.; Roy, N.; Su, X.; Liu, Z.; Chu, G.; Meng, Z.; et al. Distinct patterns of soil bacterial and fungal community assemblages in subtropical forest ecosystems under warming. Global Chang. Biol. 2023, 29, 1501–1513. [Google Scholar] [CrossRef]
- Simon, D.C.; Ameztegui, A. Modelling the influence of thinning intensity and frequency on the future provision of ecosystem services in Mediterranean mountain pine forests. Eur. J. Forest Res. 2023, 142, 521–535. [Google Scholar] [CrossRef]

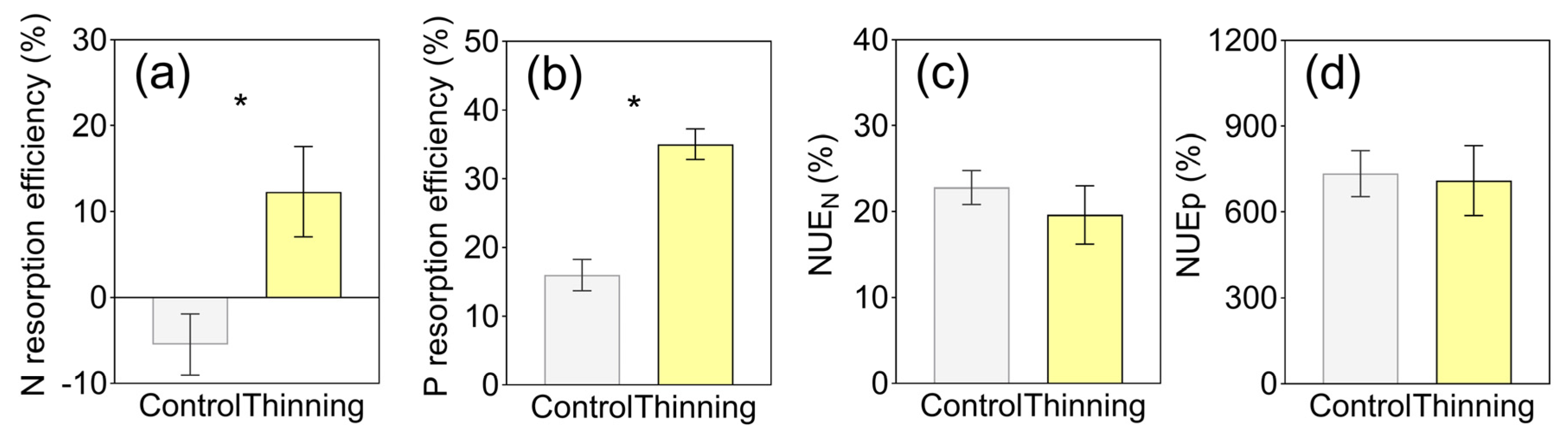
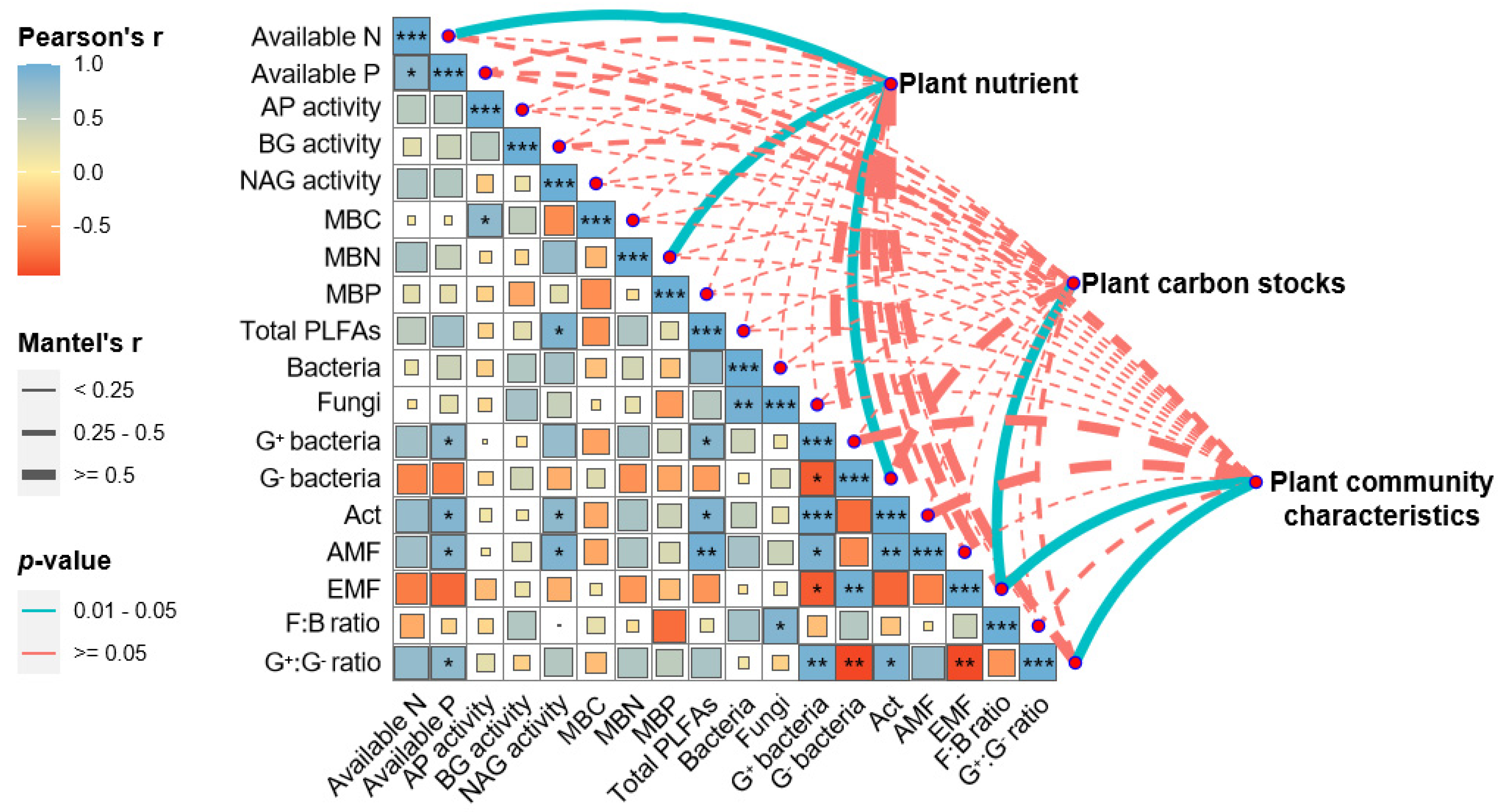

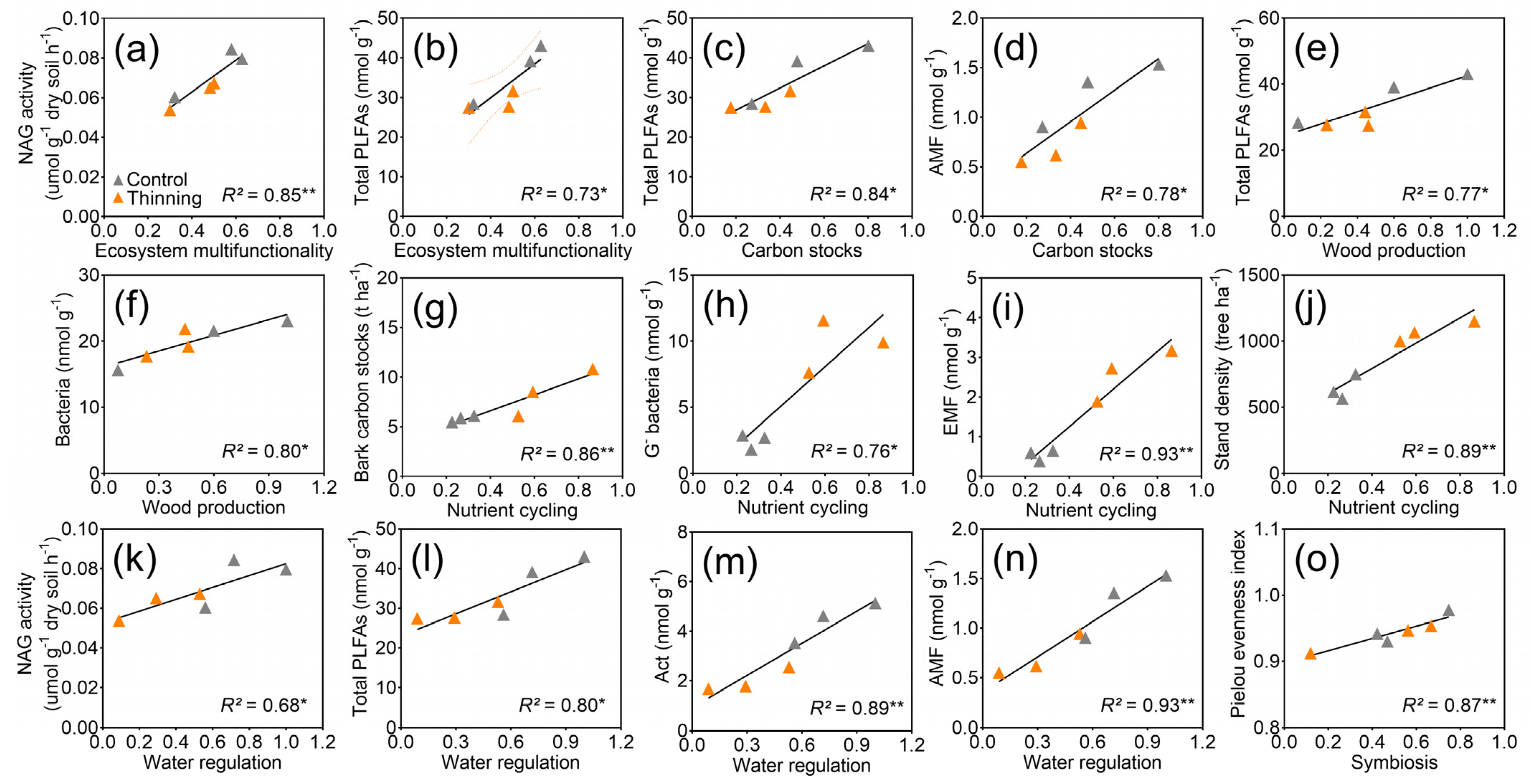
| Treatments | Proportion of Species Individuals in Different Diameter Classes (%) | ||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| Control | 9.34 ± 7.83 a | 81.93 ± 8.67 a | 8.73 ± 2.03 b | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Thinning | 0.00 ± 0.00 a | 51.42 ± 8.22 b | 45.87 ± 6.84 a | 2.70 ± 1.71 a | 0.00 ± 0.00 a |
| Components | Treatments | |
|---|---|---|
| Control | Thinning | |
| Tree layer | ||
| Tree height | 17.67 ± 0.13 a | 12.01 ± 0.19 b |
| BA | 20.47 ± 3.94 a | 22.30 ± 1.32 a |
| Tree density | 1072.22 ± 43.39 a | 644.44 ± 54.72 b |
| Understory layer | ||
| Species richness | 15.00 ± 8.66 a | 15.33 ± 8.85 a |
| Simpson dominance index | 0.91 ± 0.52 a | 0.91 ± 0.53 a |
| Shannon–Wiener diversity index | 2.53 ± 1.46 a | 2.58 ± 1.49 a |
| Pielou dominance index | 0.94 ± 0.54 a | 0.95 ± 0.55 a |
| Component | C Content (g kg−1) | N Content (g kg−1) | P Content (g kg−1) | |||
|---|---|---|---|---|---|---|
| Control | Thinning | Control | Thinning | Control | Thinning | |
| Leaf | 492.46 ± 7.85 a | 502.06 ± 5.00 a | 16.18 ± 0.64 a | 17.99 ± 0.27 a | 0.63 ± 0.02 a | 0.67 ± 0.03 a |
| Litter | 470.94 ± 3.39 a | 473.64 ± 7.61 a | 17.02 ± 0.15 a | 15.79 ± 1.12 a | 0.53 ± 0.02 a | 0.43 ± 0.03 a |
| Fine root | 476.55 ± 6.47 a | 486.83 ± 7.65 a | 9.22 ± 0.28 a | 8.79 ± 0.50 a | 0.40 ± 0.06 a | 0.35 ± 0.01 a |
| Shrub leaf | 429.68 ± 5.62 a | 453.90 ± 19.46 a | 12.14 ± 0.13 b | 15.73 ± 0.96 a | 0.58 ± 0.05 a | 1.78 ± 0.29 a |
| Shrub stem | 425.09 ± 3.36 a | 420.73 ± 7.34 a | 10.5 ± 0.31 a | 10.18 ± 0.10 a | 1.68 ± 0.76 a | 1.01 ± 0.16 a |
| Shrub root | 391.53 ± 7.62 a | 420.66 ± 17.10 a | 9.45 ± 0.60 b | 15.63 ± 1.84 a | 0.79 ± 0.05 a | 1.34 ± 0.12 a |
| Herb aboveground | 446.23 ± 5.23 a | 422.74 ± 9.32 a | 13.89 ± 0.15 b | 20.09 ± 0.47 a | 0.72 ± 0.04 a | 1.67 ± 0.25 a |
| Herb belowground | 418.37 ± 5.26 a | 413.15 ± 3.23 a | 10.39 ± 0.32 a | 11.40 ± 0.21 a | 0.88 ± 0.18 a | 0.90 ± 0.15 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, H.; Long, F.; Li, X.; Haider, F.U.; Shi, Z.; Xian, L.; Meng, C.; Li, H. Thinning Improves Large Diameter Timber Cultivation but Undermines Ecosystem Multifunctionality in the Short Term. Forests 2025, 16, 134. https://doi.org/10.3390/f16010134
Sheng H, Long F, Li X, Haider FU, Shi Z, Xian L, Meng C, Li H. Thinning Improves Large Diameter Timber Cultivation but Undermines Ecosystem Multifunctionality in the Short Term. Forests. 2025; 16(1):134. https://doi.org/10.3390/f16010134
Chicago/Turabian StyleSheng, Han, Fengling Long, Xu Li, Fasih Ullah Haider, Zhiyuan Shi, Lihua Xian, Chushu Meng, and Hui Li. 2025. "Thinning Improves Large Diameter Timber Cultivation but Undermines Ecosystem Multifunctionality in the Short Term" Forests 16, no. 1: 134. https://doi.org/10.3390/f16010134
APA StyleSheng, H., Long, F., Li, X., Haider, F. U., Shi, Z., Xian, L., Meng, C., & Li, H. (2025). Thinning Improves Large Diameter Timber Cultivation but Undermines Ecosystem Multifunctionality in the Short Term. Forests, 16(1), 134. https://doi.org/10.3390/f16010134









