Early Thinning: A Promising Tool to Prevent Fistulina hepatica Heart Rot in Castanea sativa Coppice Stands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Data Collection
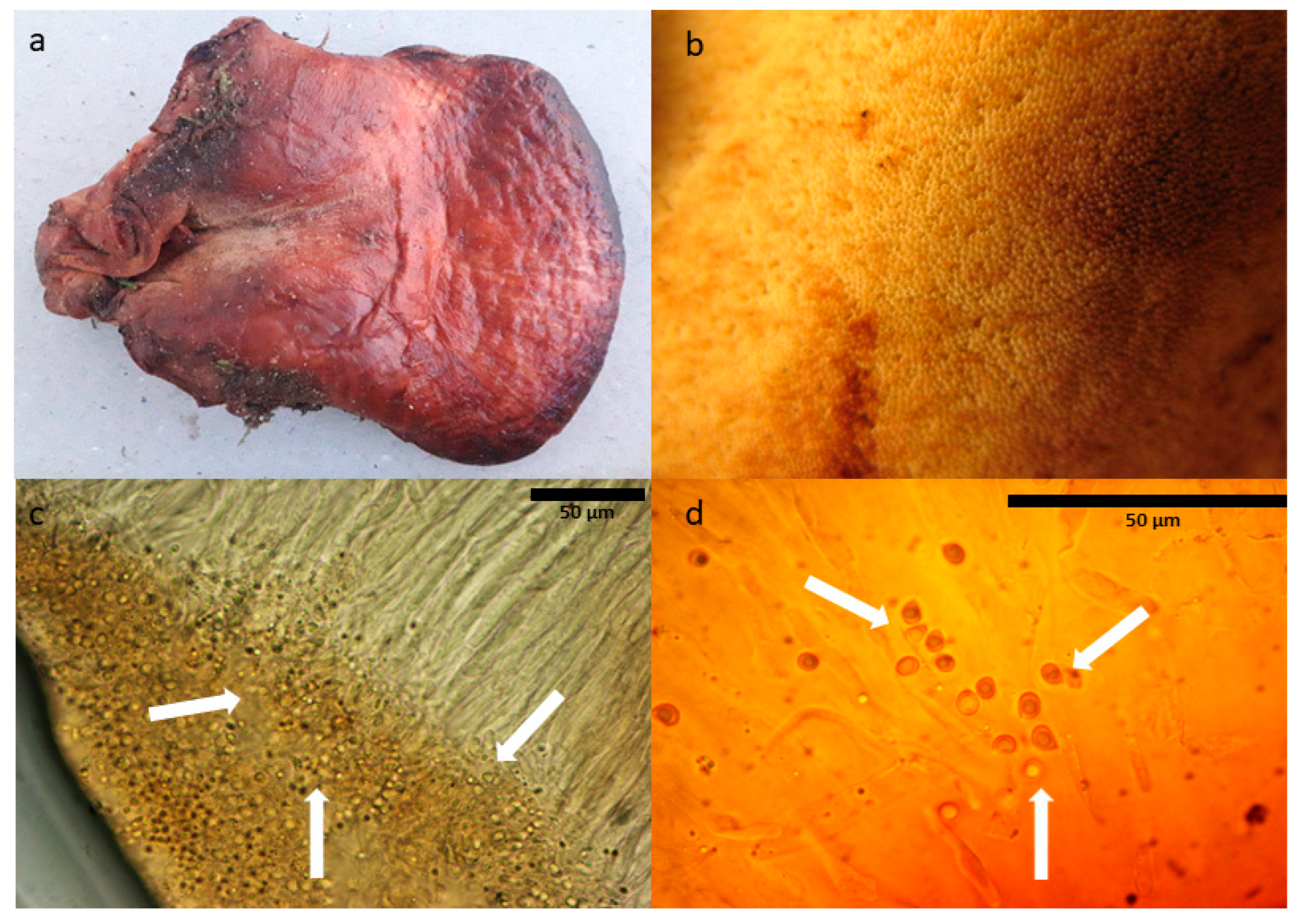
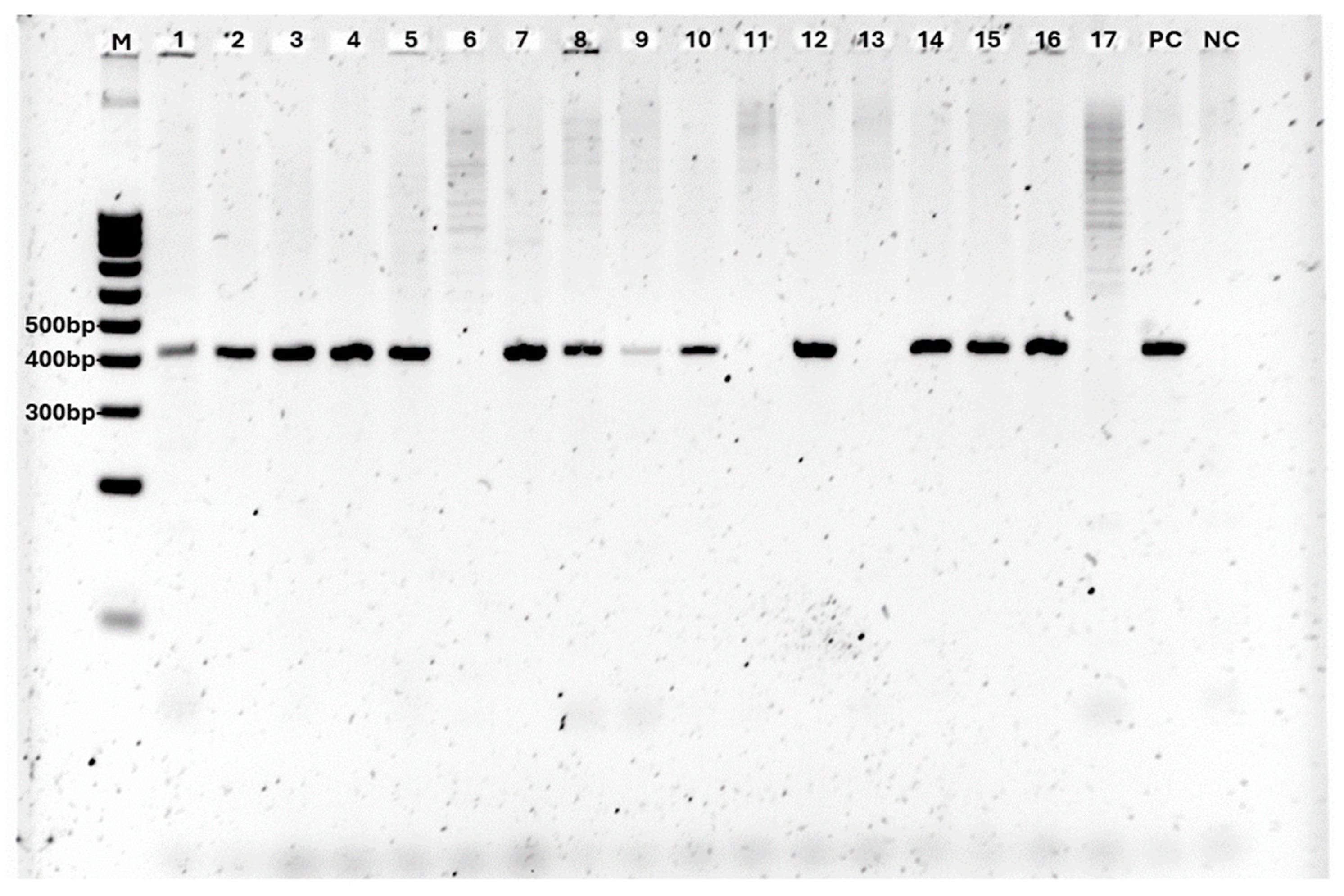
2.3. Molecular Diagnosis of Fistulina hepatica
2.4. Statistical Analysis
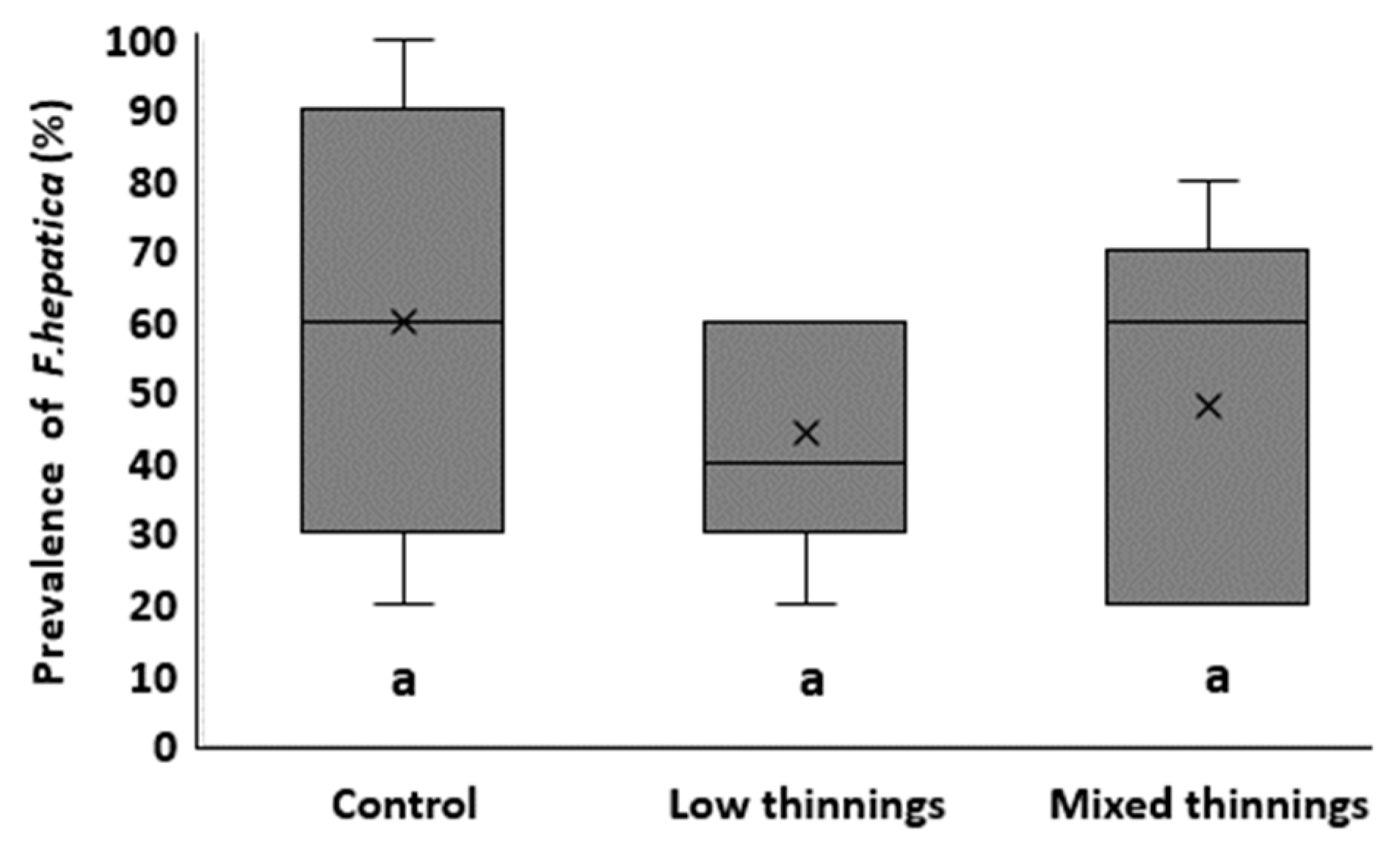
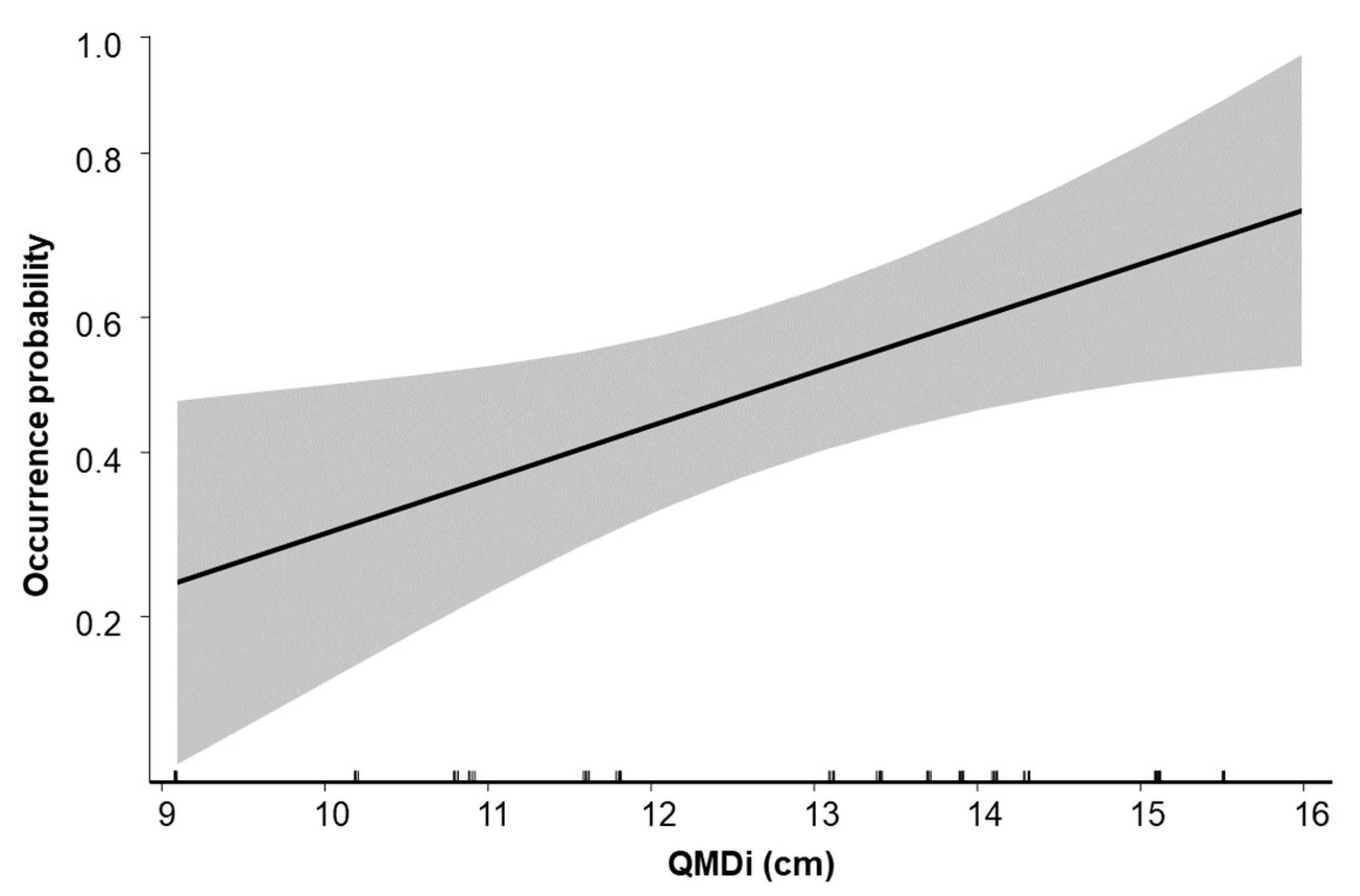
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Block | Plot ID | Coordinates (ETRS89) | Slope (%) | Elevation (m.a.s.l.) | NDVI (a.u.c.) | 2013 | 2021 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | 2012–2020 | Treatment Code | BAi (m2/ha) | Extracted BA2013 (%) | QMDi (cm) | C. parasitica (%) | BA2021 (m2/ha) | QMD2021 (cm) | ||||
| 1 | 1A | 455285.3 | 4,629,103.3 | 21 | 702 | 3.55 | Ta | 27.9 | 36 | 13.9 | 62 | 34.5 | 17.2 |
| 1B | 455252.8 | 4,629,067.3 | 43 | 708 | 3.6 | Tb | 25.6 | 28 | 15.1 | 73 | 34 | 20.5 | |
| 1C | 455228.5 | 4,629,040.7 | 65 | 708 | 3.63 | Tc | 30.4 | 0 | 14.3 | 64 | 41.5 | 18.8 | |
| 2 | 2A | 454280.1 | 4,631,892.6 | 60 | 647 | 3.65 | Ta | 33.2 | 42 | 15.1 | 71 | 40 | 21.1 |
| 2B | 454391.5 | 4,631,943.1 | 71 | 637 | 3.65 | Tb | 31.5 | 37 | 13.1 | 79 | 33.5 | 18.3 | |
| 2C | 454417.8 | 4,631,952.3 | 55 | 644 | 3.62 | Tc | 27.1 | 0 | 13.4 | 67 | 45 | 19.1 | |
| 3 | 3A | 456266.4 | 4,633,157.0 | 61 | 700 | 3.63 | Ta | 25.2 | 35 | 13.7 | 48 | 48 | 17.2 |
| 3B | 456167.8 | 4,633,171.5 | 61 | 705 | 3.75 | Tb | 31.5 | 37 | 14.1 | 40 | 44.5 | 16.6 | |
| 3C | 456107.6 | 4,633,147.7 | 74 | 732 | 3.71 | Tc | 29 | 0 | 15.5 | 10 | 50 | 19.7 | |
| 4 | 4A | 462807.1 | 4,639,663.4 | 49 | 927 | 3.72 | Ta | 17.5 | 40 | 9.1 | 18 | 50 | 13 |
| 4B | 462769.5 | 4,639,672.9 | 44 | 921 | 3.75 | Tb | 32.7 | 58 | 11.6 | 32 | 29.5 | 17.8 | |
| 4C | 462736.7 | 4,639,651.2 | 60 | 938 | 3.74 | Tc | 41.8 | 0 | 11.8 | 28 | 59 | 18.3 | |
| 5 | 5A | 464176.7 | 4,639,881.4 | 57 | 857 | 3.63 | Ta | 30.2 | 50 | 10.8 | 38 | 44.5 | 13.8 |
| 5B | 464271.4 | 4,639,932.7 | 58 | 850 | 3.59 | Tb | 26.3 | 48 | 10.9 | 32 | 36 | 16.8 | |
| 5C | 464089.9 | 4,639,820.0 | 60 | 862 | 3.59 | Tc | 27.3 | 0 | 10.2 | 26 | 32 | 15.2 | |
References
- Clark, S.L.; Marcolin, E.; Patrício, M.S.; Loewe-Muñoz, V. A silvicultural synthesis of sweet (Castanea sativa) and American (C. dentata) chestnuts. For. Ecol. Manag. 2023, 539, 121041. [Google Scholar] [CrossRef]
- Zlatanov, T.; Schleppi, P.; Velichkov, I.; Hinkov, G.; Georgieva, M.; Eggertsson, O.; Zlatanova, M.; Vacik, H. Structural diversity of abandoned chestnut (Castanea sativa Mill.) dominated forests: Implications for forest management. For. Ecol. Manag. 2013, 291, 326–335. [Google Scholar] [CrossRef]
- Marcolin, E.; Pividori, M.; Colombari, F.; Manetti, M.C.; Pelleri, F.; Conedera, M.; Gehring, E. Impact of the Asian Gall Wasp Dryocosmus kuriphilus on the radial growth of the European chestnut Castanea sativa. J. Appl. Ecol. 2021, 58, 1212–1224. [Google Scholar] [CrossRef]
- Sartor, C.; Dini, F.; Torello-Marinoni, D.; Mellano, M.G.; Beccaro, G.L.; Alma, A.; Quacchia, A.; Botta, R. Impact of the Asian wasp Dryocosmus kuriphilus (Yasumatsu) on cultivated chestnut: Yield loss and cultivar susceptibility. Sci. Hortic. 2015, 197, 454–460. [Google Scholar] [CrossRef]
- Dorado, F.J.; Alías, J.C.; Chaves, N.; Solla, A. Warming Scenarios and Phytophthora cinnamomi Infection in Chestnut (Castanea sativa Mill.). Plants 2023, 12, 556. [Google Scholar] [CrossRef]
- Vannini, A.; Vettraino, A.M. Ink disease in chestnuts: Impact on the European chestnut. For. Snow Landsc. Res. 2001, 76, 345–350. [Google Scholar]
- Rigling, D.; Prospero, S. Cryphonectria parasitica, the causal agent of chestnut blight: Invasion history, population biology and disease control. Mol. Plant Pathol. 2018, 19, 7–20. [Google Scholar] [CrossRef]
- Conedera, M.; Manetti, M.C.; Giudici, F.; Amorini, E. Distribution and economic potential of the sweet chestnut (Castanea sativa Mill.) in Europe. Ecol. Mediterr. 2004, 30, 179–193. [Google Scholar] [CrossRef]
- Conedera, M.; Tinner, W.; Krebs, P.; de Rigo, D.; Caudullo, G. Castanea sativa in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston-Durrant, T., Mauri, A., Eds.; Publications Office of the EU: Luxembourg, 2016; pp. 78–79. [Google Scholar]
- MAPA. Cuarto Inventario Forestal Nacional (IFN4); MAPA: Madrid, Spain, 2017. [Google Scholar]
- Regué, A.; Bassié, L.; De-Miguel, S.; Colinas, C. Environmental and stand conditions related to Fistulina hepatica heart rot attack on Castanea sativa. For. Pathol. 2019, 49, e12517. [Google Scholar] [CrossRef]
- Correal-Mòdol, E. Propietats Químiques, Físiques, Mecàniques i Resistents de la Fusta Massissa i Laminada de Castanyer del Sistema Mediterrani Català. PhD. Thesis, Universitat de Lleida, Lleida, Spain, 2013. [Google Scholar]
- Schwarze, F.W.M.R.; Baum, S.; Fink, S. Dual modes of degradation by Fistulina hepatica in xylem cell walls of Quercus robur. Mycol. Res. 2000, 104, 846–852. [Google Scholar] [CrossRef]
- Yurkewich, J.I.; Castaño, C.; Colinas, C. Chestnut Red Stain: Identification of the fungi associated with the costly discolouration of Castanea sativa. For. Pathol. 2017, 21, e12335. [Google Scholar] [CrossRef]
- Vericat, P.; Navarro, P.; Correal-Mòdol, E.; Castaño, C.; Piqué, M.; Beltrán, M.; Óbón, B.; Rodriguez, J.; Colinas, C.; García, M.; et al. El castanyer a Catalunya: Manual de Gestió, Conservació i Valorització; Diputació de Barcelona i Obra Social “la Caixa”: Barcelona, Spain, 2012. [Google Scholar]
- Diamandis, S.; Perlerou, C. The mycoflora of the chestnut ecosystems in Greece. For. Snow Landsc. Res. 2001, 76, 499–504. [Google Scholar]
- Piętka, J.; Ciurzycki, W. The first record of Fistulina hepatica (Schaeff.) With. on Castanea sativa Mill. in Poland. Acta Mycol. 2018, 53, 1108. [Google Scholar] [CrossRef]
- González, G.C.; Barroetaveña, C.; Visnovsky, S.B.; Rajchenberg, M.; Pildain, M.B. A new species, phylogeny, and a worldwide key of the edible wood decay Fistulina (Agaricales). Mycol. Prog. 2021, 20, 733–746. [Google Scholar] [CrossRef]
- Cartwright, K.T.S.G. A reinvestigation into the cause of “brown oak”, Fistulina hepatica (Huds.) fr. Trans. Br. Mycol. Soc. 1937, 21, 68–83. [Google Scholar] [CrossRef]
- Vasaitis, R. Heart rots, sap rots and canker rots. In Infectious Forest Diseases; Gonthier, P., Nicolotti, G., Eds.; CABI: Wallingford, UK, 2013. [Google Scholar] [CrossRef]
- Beltrán, M.; Vericat, P.; Piqué, M.; Farriol, R. Models de Gestió per als Boscos de Castanyer (Castanea sativa Mill.): Producció de Fusta i Fruit; Centre de la Propietat Forestal, Departament d’Agricultura, Ramaderia, Pesca, Alimentació i Medi Natural, Generalitat de Catalunya: Barcelona, Spain, 2013. [Google Scholar]
- Nilson, T.; Rautiainen, M.; Pisek, J.; Peterson, U. Seasonal Reflectance Courses of Forests. In New Advances and Contributions to Forestry Research; Oteng-Amoako, A.A., Ed.; InTech: London, UK, 2012; pp. 33–58. [Google Scholar] [CrossRef]
- Guglielmo, F.; Gonthier, P.; Garbelotto, M.; Nicolotti, G. Optimization of sampling procedures for DNA-based diagnosis of wood decay fungi in standing trees: Sampling for decay fungi diagnosis. Lett. Appl. Microbiol. 2010, 51, 90–97. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, J.T., Eds.; Academic Press: San Diego, CA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84). 2017. Available online: https://github.com/taiyun/corrplot (accessed on 11 April 2022).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Anderson, D.R. Model Based Inferences in the Life Sciences: A Primer on Evidence. Ann. Entomol. Soc. Am. 2008, 102, 737–738. [Google Scholar] [CrossRef]
- Mazerolle, M.J. Model Selection and Multimodel Inference Based on (Q)AIC(c) (2.2-1) [R]. 2019. Available online: https://rdrr.io/cran/AICcmodavg/ (accessed on 15 May 2022).
- Griffith, D.M.; Veech, J.A.; Marsh, C.J. Cooccur: Probabilistic species co-occurrence analysis in R. J. Stat. Softw. 2016, 69, 1–17. [Google Scholar] [CrossRef]
- Veech, J.A. A probabilistic model for analysing species co-occurrence. Glob. Ecol. Biogeogr. 2013, 22, 252–260. [Google Scholar] [CrossRef]
- Muñoz-Adalia, E.J.; Sanz-Ros, A.V.; Flores-Pacheco, J.A.; Hantula, J.; Diez, J.J.; Vainio, E.J.; Fernández, M. Sydowia polyspora dominates fungal communities carried by two Tomicus species in pine plantations threatened by Fusarium circinatum. Forests 2017, 8, 127. [Google Scholar] [CrossRef]
- Anagnostakis, S.L. Chestnut Blight: The Classical Problem of an Introduced Pathogen. Mycologia 1987, 79, 23–37. [Google Scholar] [CrossRef]
- Lovat, C.A.; Donnelly, D.J. Mechanisms and metabolomics of the host-pathogen interactions between Chestnut (Castanea species) and Chestnut blight (Cryphonectria parasitica). For. Pathol. 2019, 49, e12562. [Google Scholar] [CrossRef]
- Meyer, J.B.; Gallien, L.; Prospero, S. Interaction between two invasive organisms on the European chestnut: Does the chestnut blight fungus benefit from the presence of the gall wasp? FEMS Microbiol. Ecol. 2015, 91, fiv122. [Google Scholar] [CrossRef][Green Version]
- Vannini, A.; Morales-Rodriguez, C.; Aleandri, M.; Bruni, N.; Dalla-Valle, M.; Mazzetto, T.; Martignoni, D.; Vettraino, A. Emerging new crown symptoms on Castanea sativa (Mill.): Attempting to model interactions among pests and fungal pathogens. Fungal Biol. 2018, 122, 911–917. [Google Scholar] [CrossRef]
- Roberts, M.; Gilligan, C.A.; Kleczkowski, A.; Hanley, N.; Whalley, A.E.; Healey, J.R. The effect of forest management options on forest resilience to pathogens. Front. For. Glob. Change 2020, 3, 7. [Google Scholar] [CrossRef]
- Aylor, D.E. Spread of plant disease on a continental scale: Role of aerial dispersal of pathogens. Ecology 2003, 84, 1989–1997. [Google Scholar] [CrossRef]
- Condeso, T.E.; Meentemeyer, R.K. Effects of landscape heterogeneity on the emerging forest disease sudden oak death. J. Ecol. 2007, 95, 364–375. [Google Scholar] [CrossRef]
- Vacek, Z.; Vacek, S.; Bulušek, D.; Podrázský, V.; Remeš, J.; Král, J.; Putalová, T. Effect of fungal pathogens and climatic factors on production, biodiversity and health status of ash mountain forests. Dendrobiology 2017, 77, 161–175. [Google Scholar] [CrossRef]
- Muñoz-Adalia, E.J.; Colinas, C. Susceptibility of cork oak (Quercus suber) to canker disease caused by Diplodia corticola: When time is of the essence. New For. 2021, 52, 863–873. [Google Scholar] [CrossRef]
- Muñoz-Adalia, E.J.; Uppara, A.B.; Albó, D.; Meijer, A.; Colinas, C. Cork harvest planning and climate: High air humidity favors availability of airborne inoculum of Diplodia corticola. For. Ecol. Manag. 2023, 536, 120935. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.L.; Marçais, B.; Nageleisen, L.M.; Piou, D.; Vannini, A. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar] [CrossRef]
- Wargo, P. Consequences of environmental stress on oak: Predisposition to pathogens. Ann. Sci. Forest. 1996, 53, 359–368. [Google Scholar] [CrossRef]
- Waring, R.H. Estimating Forest growth and efficiency in relation to canopy leaf area. Adv. Ecol. Res. 1983, 13, 327–354. [Google Scholar] [CrossRef]
- Calamita, F.; Imran, H.A.; Vescovo, L.; Mekhalfi, M.L.; La Porta, N. Early Identification of Root Rot Disease by Using Hyperspectral Reflectance: The Case of Pathosystem Grapevine/Armillaria. Remote Sens. 2021, 13, 2436. [Google Scholar] [CrossRef]
- Loehle, C. Tree life history strategies: The role of defenses. Can. J. For. Res. 1988, 18, 209–222. [Google Scholar] [CrossRef]


| Model | Description | Df | logLik | Deviance | AIC | ΔAIC |
|---|---|---|---|---|---|---|
| M2 | R~QMDi + (B) | 1 | −48.70 | 97.40 | 103.41 | 0.00 |
| M1 | R~QMDi + BAf + (B) | 2 | −48.40 | 96.81 | 104.82 | 1.41 |
| M3 | R~QMDi + BA2021 + (B) | 2 | −48.43 | 96.87 | 104.88 | 1.42 |
| M4 | R~QMDi + N + (B) | 2 | −48.67 | 97.35 | 105.35 | 1.94 |
| M6 | R~QMDi + T + (B) | 2 | −48.20 | 96.40 | 107.24 | 3.83 |
| M5 | R~QMDi + N + BAi + BA2021 + (B) | 4 | −48.22 | 96.45 | 108.45 | 5.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meijer, A.; Muñoz-Adalia, E.J.; Colinas, C. Early Thinning: A Promising Tool to Prevent Fistulina hepatica Heart Rot in Castanea sativa Coppice Stands. Forests 2024, 15, 1639. https://doi.org/10.3390/f15091639
Meijer A, Muñoz-Adalia EJ, Colinas C. Early Thinning: A Promising Tool to Prevent Fistulina hepatica Heart Rot in Castanea sativa Coppice Stands. Forests. 2024; 15(9):1639. https://doi.org/10.3390/f15091639
Chicago/Turabian StyleMeijer, Andreu, Emigdio Jordán Muñoz-Adalia, and Carlos Colinas. 2024. "Early Thinning: A Promising Tool to Prevent Fistulina hepatica Heart Rot in Castanea sativa Coppice Stands" Forests 15, no. 9: 1639. https://doi.org/10.3390/f15091639
APA StyleMeijer, A., Muñoz-Adalia, E. J., & Colinas, C. (2024). Early Thinning: A Promising Tool to Prevent Fistulina hepatica Heart Rot in Castanea sativa Coppice Stands. Forests, 15(9), 1639. https://doi.org/10.3390/f15091639






