Effects of Cold Acclimation on Morpho-Anatomical Traits of Heteroblastic Foliage in Pinus massoniana (Lamb.) Seedlings
Abstract
1. Introduction
2. Materials and Methods
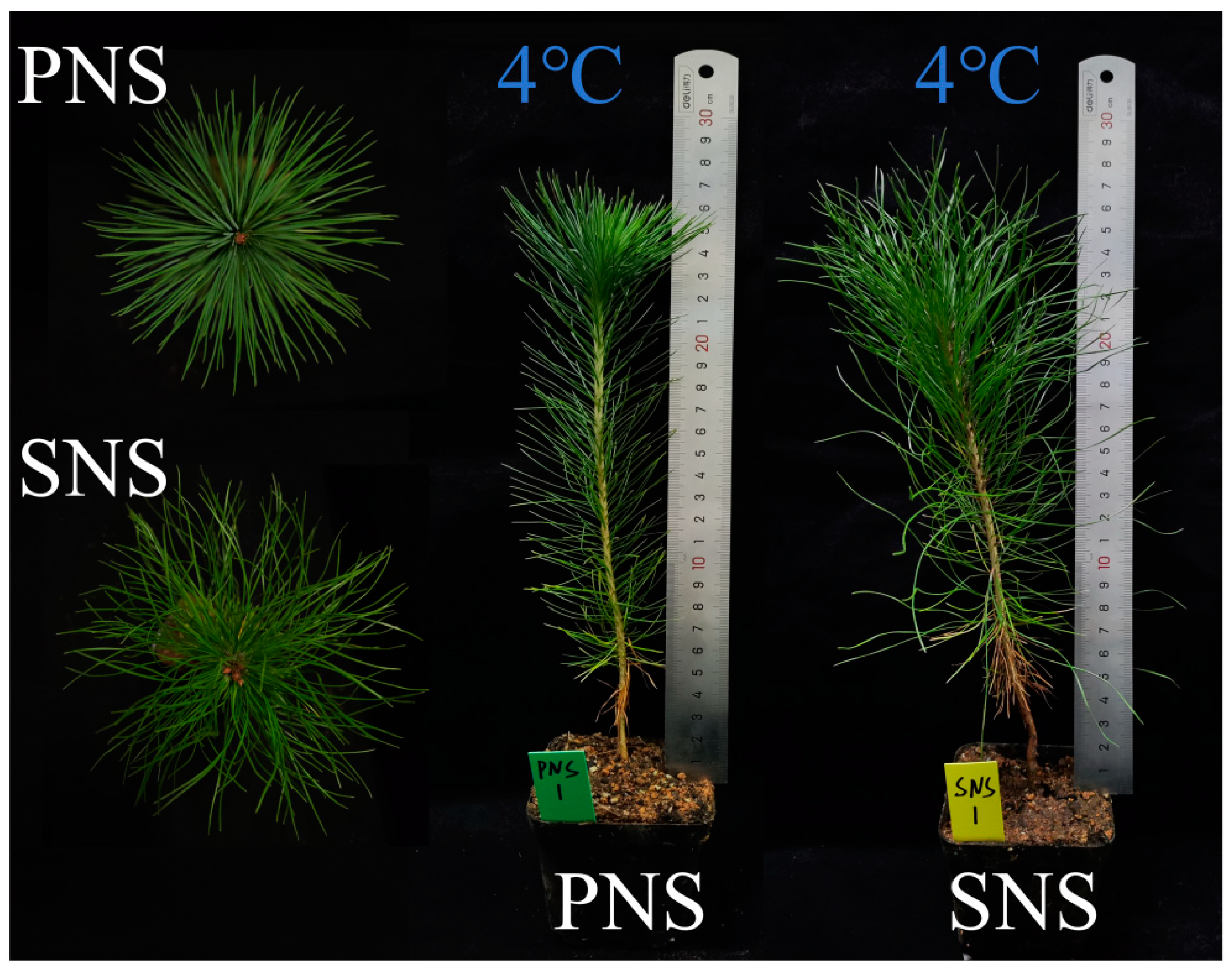
2.1. Seedling Culture and Temperature Treatment
2.2. Preparation and Staining of Paraffin Sections
3. Results
3.1. Response of Structure and Starch Grain Accumulation in Heteroblastic Foliage Seedlings to Cold Acclimation
3.1.1. Leaf Morphological Structure and Starch Grain Accumulation
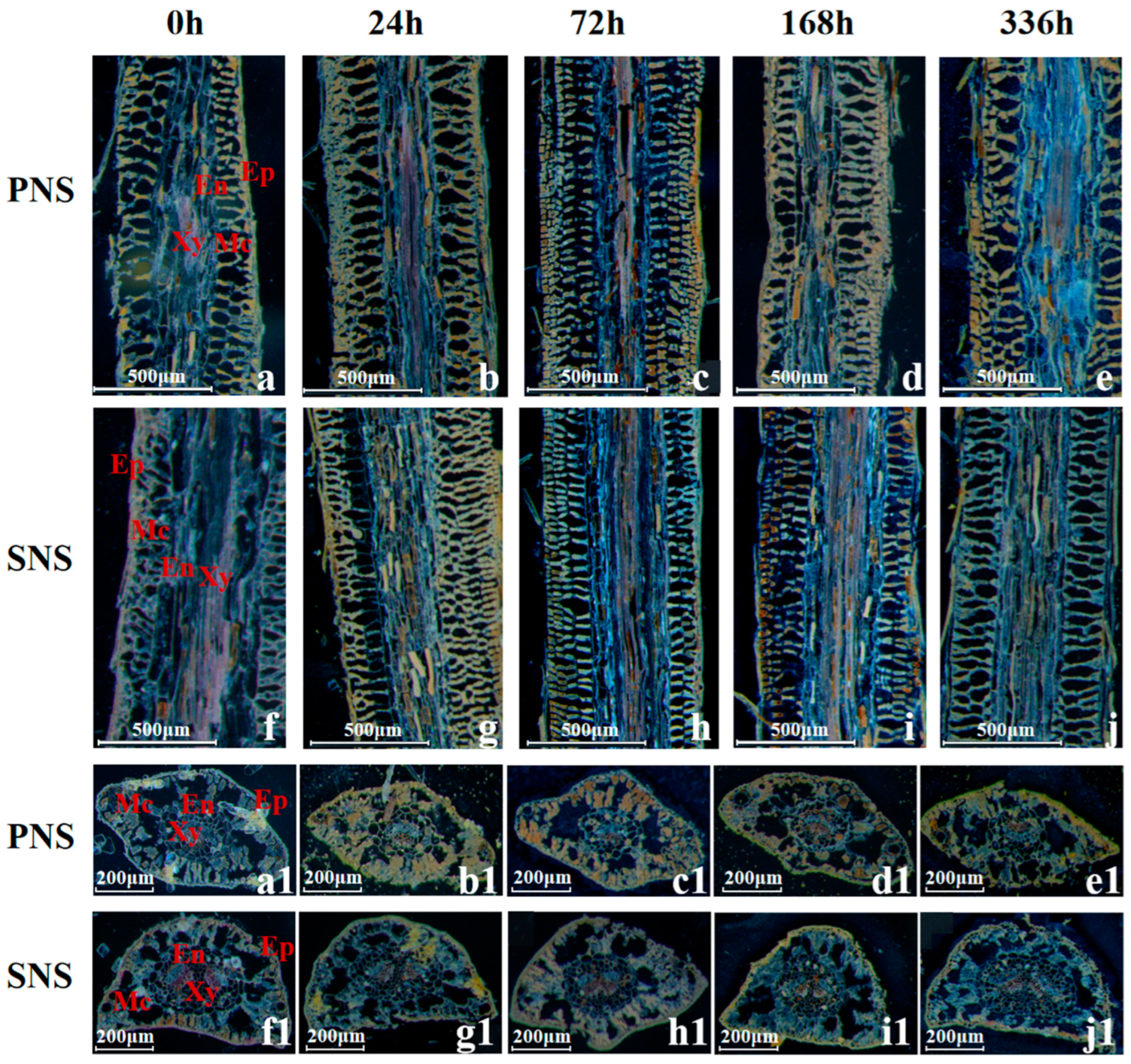
3.1.2. Stem Morphological Structure and Starch Grain Accumulation
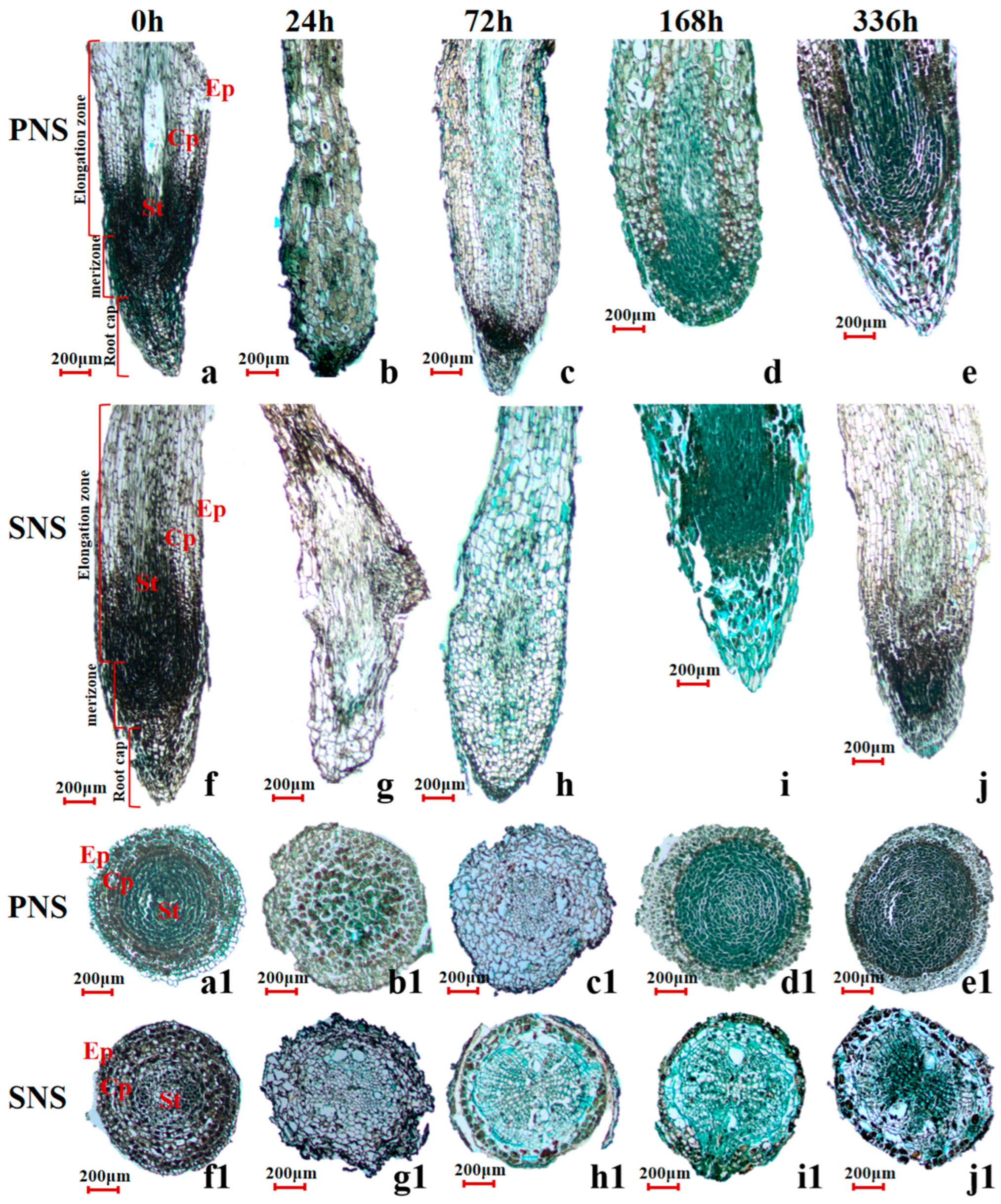
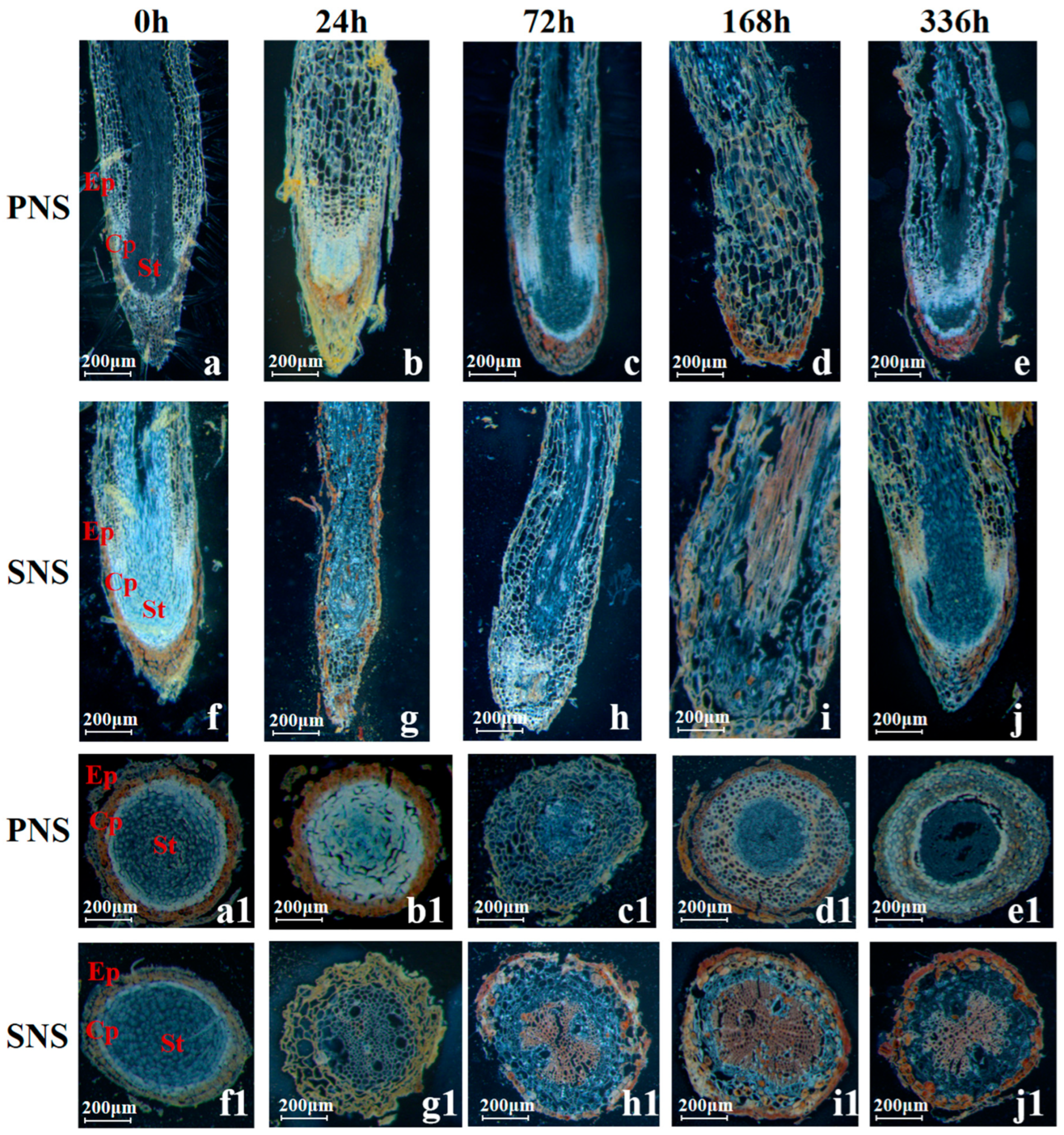
3.1.3. Root Morphological Structure and Starch Grain Accumulation
3.2. Response of Structure and Lignin in Heteroblastic Foliage Seedlings to Cold Acclimation
3.2.1. Leaf Morphological Structure and Lignin Accumulation
3.2.2. Stem Morphological Structure and Lignin Accumulation
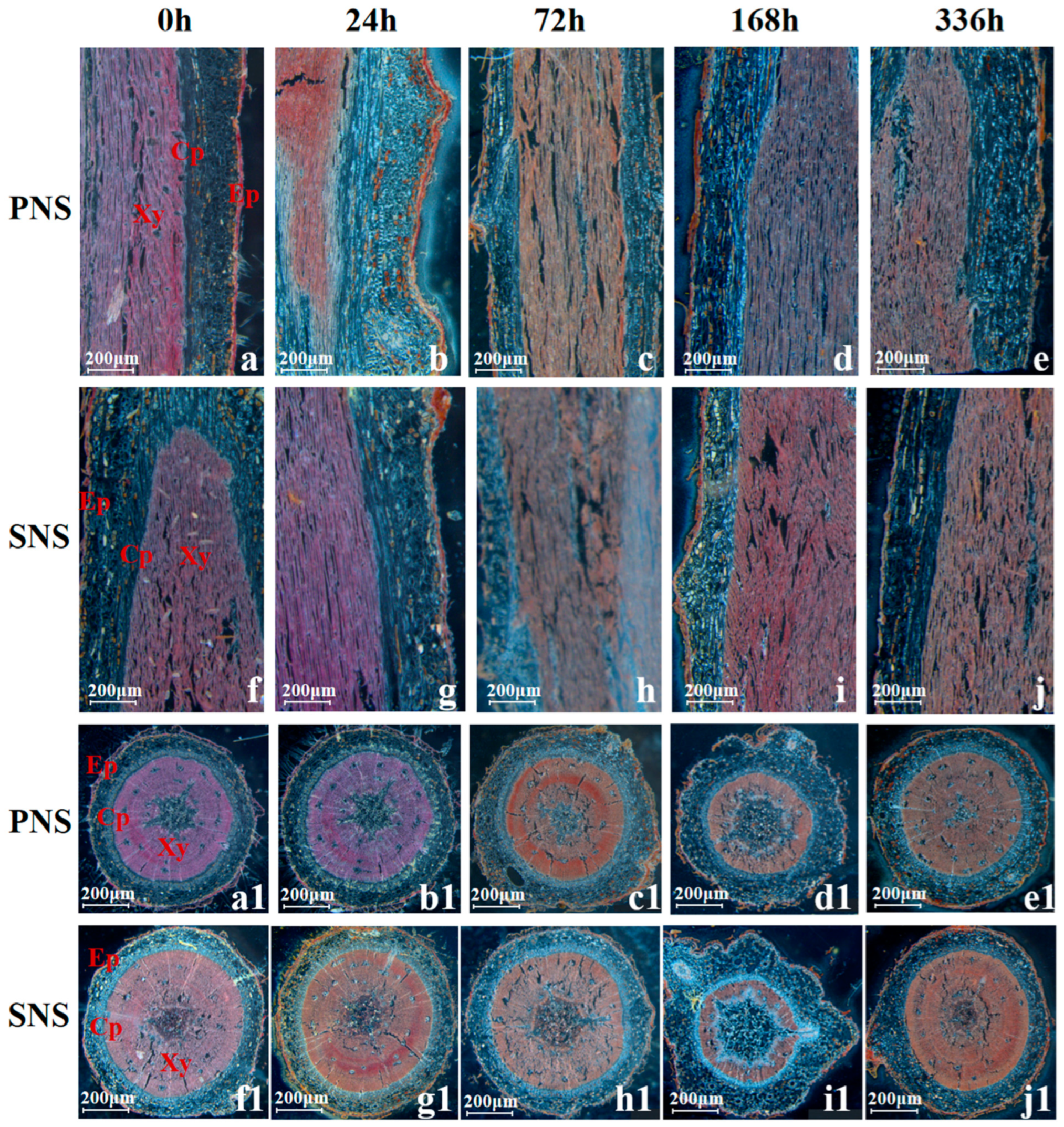
3.2.3. Root Morphological Structure and Lignin Accumulation
4. Discussion
4.1. Morpho-Anatomical Changes of Heteroblastic Foliage Seedlings during Cold Acclimation
4.2. Changes of Starch Grain in Heteroblastic Foliage Seedlings during Cold Acclimation
4.3. Changes of Lignin in Heteroblastic Foliage Seedling during Cold Acclimation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, C.Y.Y.; Bräutigam, K.; Hüner, N.P.; Ensminger, I. Champions of winter survival: Cold acclimation and molecular regulation of cold hardiness in evergreen conifers. New Phytol. 2021, 229, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Larran, A.S.; Pajoro, A.; Qüesta, J.I. Is winter coming? Impact of the changing climate on plant responses to cold temperature. Plant Cell Environ. 2023, 46, 3175–3193. [Google Scholar] [CrossRef] [PubMed]
- Osland, M.J.; Stevens, P.W.; Lamont, M.M.; Brusca, R.C.; Hart, K.M.; Waddle, J.H.; Langtimm, C.A.; Williams, C.M.; Keim, B.D.; Seminoff, J.A.; et al. Tropicalization of temperate ecosystems in North America: The northward range expansion of tropical organisms in response to warming winter temperatures. Glob. Chang. Biol. 2021, 27, 3006–3008. [Google Scholar] [CrossRef] [PubMed]
- Busch, F.; Huner, N.P.; Ensminger, I. Increased air temperature during simulated autumn conditions does not increase photosynthetic carbon gain but affects the dissipation of excess energy in seedlings of the evergreen conifer Jack pine. Plant Physiol. 2007, 143, 1242–1251. [Google Scholar] [CrossRef]
- Sofronova, V.; Dymova, O.; Golovko, T.; Chepalov, V.; Petrov, K. Adaptive changes in pigment complex of Pinus sylvestris needles upon cold acclimation. Russ. J. Plant Physiol. 2016, 63, 433–442. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Wen, X.; Yang, F.; Ma, Z.; Sun, X.; Yu, G. Freezing-induced loss of carbon uptake in a subtropical coniferous plantation in southern China. Ann. For. Sci. 2011, 68, 1151–1161. [Google Scholar] [CrossRef][Green Version]
- Jahed, K.R.; Saini, A.K.; Sherif, S.M. Coping with the cold: Unveiling cryoprotectants, molecular signaling pathways, and strategies for cold stress resilience. Front. Plant Sci. 2023, 14, 1246093. [Google Scholar] [CrossRef]
- Wu, J.; Nadeem, M.; Galagedara, L.; Thomas, R.; Cheema, M. Recent insights into cell responses to cold stress in plants: Signaling, defence, and potential functions of phosphatidic acid. Environ. Exp. Bot. 2022, 203, 105068. [Google Scholar] [CrossRef]
- Equiza, M.A.; Miravé, J.P.; Tognetti, J.A. Morphological, anatomical and physiological responses related to differential shoot vs. root growth inhibition at low temperature in spring and winter wheat. Ann. Bot. 2001, 87, 67–76. [Google Scholar] [CrossRef]
- Lorenzo, M.; Pinedo, M.L.; Equiza, M.A.; Fernández, P.V.; Ciancia, M.; Ganem, D.; Tognetti, J.A. Changes in apoplastic peroxidase activity and cell wall composition are associated with cold-induced morpho-anatomical plasticity of wheat leaves. Plant Biol. 2019, 21, 84–94. [Google Scholar] [CrossRef]
- Martínez-Berdeja, A.; Stitzer, M.C.; Taylor, M.A.; Okada, M.; Ezcurra, E.; Runcie, D.E.; Schmitt, J. Functional variants of DOG1 control seed chilling responses and variation in seasonal life-history strategies in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2020, 117, 2526–2534. [Google Scholar] [CrossRef]
- Ruelland, E.; Vaultier, M.-N.; Zachowski, A.; Hurry, V. Cold signalling and cold acclimation in plants. Adv. Bot. Res. 2009, 49, 35–150. [Google Scholar]
- Moura, J.C.M.S.; Bonine, C.A.V.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef]
- Harbol, S.C.; Long, R.W.; Medeiros, J.S. Juniperus virginiana sourced from colder climates maintain higher ratios of soluble sugars to starch during cold acclimation. Tree Physiol. 2023, tpad115. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Leus, L.; De Keyser, E.; Van Labeke, M.-C. Seasonal changes in cold hardiness and carbohydrate metabolism in four garden rose cultivars. J. Plant Physiol. 2019, 232, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Wang, Y.; Cloix, C.; Li, K.; Jenkins, G.I.; Wang, S.; Shang, Z.; Shi, Y.; Yang, S.; Li, X. The Arabidopsis RCC1 family protein TCF1 regulates freezing tolerance and cold acclimation through modulating lignin biosynthesis. PLoS Genet. 2015, 11, e1005471. [Google Scholar] [CrossRef]
- Wang, H.; Wu, F.; Li, M.; Zhu, X.; Shi, C.; Ding, G. Morphological and physiological responses of Pinus massoniana seedlings to different light gradients. Forests 2021, 12, 523. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, G.; Feng, Z.; Sun, L.; Zhou, Z.; Zheng, Y.; Yuan, C. Joint influence of genetic origin and climate on the growth of Masson pine (Pinus massoniana Lamb.) in China. Sci. Rep. 2020, 10, 4653. [Google Scholar] [CrossRef]
- Wang, H.; Wu, F.; Li, M.; Liang, D.; Ding, G. Heteroblastic foliage affects the accumulation of non-structural carbohydrates and biomass in Pinus massoniana (Lamb.) seedlings. Forests 2021, 12, 1686. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Tu, J.; Liang, D.; Li, M.; Wu, F. Comparative analysis of differential gene expression reveals novel insights into the heteroblastic foliage functional traits of Pinus massoniana seedlings. Int. J. Biol. Macromol. 2024, 264, 130762. [Google Scholar] [CrossRef]
- Forster, M.A.; Bonser, S.P. Heteroblastic development and the optimal partitioning of traits among contrasting environments in Acacia implexa. Ann. Bot. 2009, 103, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, M.S.; Day, M.E.; Berlyn, G.P. Regulation of foliar plasticity in conifers: Developmental and environmental factors. J. Sustain. For. 2009, 28, 48–62. [Google Scholar] [CrossRef]
- Zotz, G.; Wilhelm, K.; Becker, A. Heteroblasty—A Review. Bot. Rev. 2011, 77, 109–151. [Google Scholar] [CrossRef]
- Wang, H.; Wu, F.; Li, M.; Zhu, X.; Shi, C.; Shao, C.; Ding, G. Structure and chlorophyll fluorescence of heteroblastic foliage affect first-year growth in Pinus massoniana Lamb. seedlings. Plant Physiol. Biochem. 2022, 170, 206–217. [Google Scholar] [CrossRef]
- Chen, T.; Xie, M.; Jiang, Y.; Yuan, T. Abortion occurs during double fertilization and ovule development in Paeonia ludlowii. J. Plant Res. 2022, 135, 295–310. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Ruiz-Medina, M.A.; Miranda, J.C.; González-Rodríguez, Á.M. Coexistent heteroblastic needles of adult Pinus canariensis C.Sm. ex DC. in buch trees differ structurally and physiologically. Forests 2021, 12, 341. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Liu, W.-J.; Wang, B.; Zhang, M.-H. Application of histochemical staining in detecting lignin structural units. Ind. Crops Prod. 2024, 217, 118838. [Google Scholar] [CrossRef]
- Li, S.; Lu, S.; Wang, J.; Chen, Z.; Zhang, Y.; Duan, J.; Liu, P.; Wang, X.; Guo, J. Responses of physiological, morphological and anatomical traits to abiotic stress in woody plants. Forests 2023, 14, 1784. [Google Scholar] [CrossRef]
- Yadav, V.; Arif, N.; Singh, V.P.; Guerriero, G.; Berni, R.; Shinde, S.; Raturi, G.; Deshmukh, R.; Sandalio, L.M.; Chauhan, D.K. Histochemical techniques in plant science: More than meets the eye. Plant Cell Physiol. 2021, 62, 1509–1527. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Noedoost, F.; Geuns, J.M.; Djalovic, I.; Siddique, K.H. Effect of cold stress on photosynthetic traits, carbohydrates, morphology, and anatomy in nine cultivars of Stevia rebaudiana. Front. Plant Sci. 2018, 9, 1430. [Google Scholar] [CrossRef]
- Lawrence, E.H.; Springer, C.J.; Helliker, B.R.; Poethig, R.S. MicroRNA156-mediated changes in leaf composition lead to altered photosynthetic traits during vegetative phase change. New Phytol. 2021, 231, 1008–1022. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Wang, F.; Ma, L.; Qi, Z.; Liu, S.; Chen, C.; Wu, J.; Wang, P.; Yang, C.; Wu, Y. Physiological and biochemical mechanisms and cytology of cold tolerance in Brassica napus. Front. Plant Sci. 2020, 11, 1241. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Gates, R.N.; Xu, Y.; Hu, T. Diffusion limitations and metabolic factors associated with inhibition and recovery of photosynthesis following cold stress in Elymus nutans Griseb. J. Photochem. Photobiol. B Biol. 2016, 163, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Leus, L.; De Keyser, E.; Van Labeke, M.-C. Cold acclimation and deacclimation of two garden rose cultivars under controlled daylength and temperature. Front. Plant Sci. 2020, 11, 327. [Google Scholar] [CrossRef]
- Zhang, W.; Swarup, R.; Bennett, M.; Schaller, G.E.; Kieber, J.J. Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical meristem. Curr. Biol. 2013, 23, 1979–1989. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Peng, X.; Teng, L.; Yan, X.; Zhao, M.; Shen, S. The cold responsive mechanism of the paper mulberry: Decreased photosynthesis capacity and increased starch accumulation. BMC Genom. 2015, 16, 898. [Google Scholar] [CrossRef]
- Nagelmüller, S.; Hiltbrunner, E.; Körner, C. Low temperature limits for root growth in alpine species are set by cell differentiation. AoB Plants 2017, 9, plx054. [Google Scholar] [CrossRef]
- Climent, J.; Silva, F.C.E.; Chambel, M.R.; Pardos, M.; Almeida, M.H. Freezing injury in primary and secondary needles of Mediterranean pine species of contrasting ecological niches. Ann. For. Sci. 2009, 66, 407. [Google Scholar] [CrossRef]
- Bhat, K.A.; Mahajan, R.; Pakhtoon, M.M.; Urwat, U.; Bashir, Z.; Shah, A.A.; Agrawal, A.; Bhat, B.; Sofi, P.A.; Masi, A. Low temperature stress tolerance: An insight into the omics approaches for legume crops. Front. Plant Sci. 2022, 13, 888710. [Google Scholar] [CrossRef]
- Zang, Y.; Wu, G.; Li, Q.; Xu, Y.; Xue, M.; Chen, X.; Wei, H.; Zhang, W.; Zhang, H.; Liu, L. Irrigation regimes modulate non-structural carbohydrate remobilization and improve grain filling in rice (Oryza sativa L.) by regulating starch metabolism. J. Integr. Agric. 2024, 23, 1507–1522. [Google Scholar] [CrossRef]
- Zanotto, S.; Bertrand, A.; Purves, R.W.; Olsen, J.E.; Elessawy, F.M.; Ergon, Å. Biochemical changes after cold acclimation in Nordic red clover (Trifolium pratense L.) accessions with contrasting levels of freezing tolerance. Physiol. Plant. 2023, 175, e13953. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Wang, D.; Wu, J.; Xu, H.; Xiao, Y.; Xie, H.; Shi, W. The deterioration of starch physiochemical and minerals in high-quality indica rice under low-temperature stress during grain filling. Front. Plant Sci. 2024, 14, 1295003. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Y.; Liu, X.; Huang, J.; Wang, Q.; Gu, J.; Lu, Y. Transcriptome profiling of the cold response and signaling pathways in Lilium lancifolium. BMC Genom. 2014, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Doğru, A.; Çakirlar, H. Effects of leaf age on chlorophyll fluorescence and antioxidant enzymes activity in winter rapeseed leaves under cold acclimation conditions. Braz. J. Bot. 2020, 43, 11–20. [Google Scholar] [CrossRef]
- Kitashova, A.; Adler, S.O.; Richter, A.S.; Eberlein, S.; Dziubek, D.; Klipp, E.; Nägele, T. Limitation of sucrose biosynthesis shapes carbon partitioning during plant cold acclimation. Plant Cell Environ. 2023, 46, 464–478. [Google Scholar] [CrossRef]
- Geng, P.; Zhang, S.; Liu, J.; Zhao, C.; Wu, J.; Cao, Y.; Fu, C.; Han, X.; He, H.; Zhao, Q. MYB20, MYB42, MYB43, and MYB85 regulate phenylalanine and lignin biosynthesis during secondary cell wall formation. Plant Physiol. 2020, 182, 1272–1283. [Google Scholar] [CrossRef]
- Vanholme, R.; De Meester, B.; Ralph, J.; Boerjan, W. Lignin biosynthesis and its integration into metabolism. Curr. Opin. Biotechnol. 2019, 56, 230–239. [Google Scholar] [CrossRef]
- Shafi, A.; Gill, T.; Zahoor, I.; Ahuja, P.S.; Sreenivasulu, Y.; Kumar, S.; Singh, A.K. Ectopic expression of SOD and APX genes in Arabidopsis alters metabolic pools and genes related to secondary cell wall cellulose biosynthesis and improve salt tolerance. Mol. Biol. Rep. 2019, 46, 1985–2002. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.-W. Oxidation treatment on wood cell walls affects gas permeability and sound absorption capacity. Carbohydr. Polym. 2022, 276, 118874. [Google Scholar] [CrossRef]
- Shen, X.; Liu, Y.; Zeng, Y.; Zhao, Y.; Bao, Y.; Wu, Z.; Zheng, Y.; Jin, P. Hydrogen sulfide alleviates the chilling-induced lignification in loquat fruit by regulating shikimate, phenylpropanoid and cell wall metabolisms. Postharvest Biol. Technol. 2024, 214, 113012. [Google Scholar] [CrossRef]
- Zheng, J.; Li, S.E.; Maratab, A.L.I.; Huang, Q.H.; Zhneg, X.L.; Pang, L.J. Effects of UV-B treatment on controlling lignification and quality of bamboo (Phyllostachys prominens) shoots without sheaths during cold storage. J. Integr. Agric. 2020, 19, 1387–1395. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Q.; Chen, W.; Guo, Q.; Xia, Y.; Wu, D.; Jing, D.; Liang, G. Melatonin treatment maintains quality and delays lignification in loquat fruit during cold storage. Sci. Hortic. 2021, 284, 110126. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Wang, H.; He, H.; Wu, F. Effects of Cold Acclimation on Morpho-Anatomical Traits of Heteroblastic Foliage in Pinus massoniana (Lamb.) Seedlings. Forests 2024, 15, 1560. https://doi.org/10.3390/f15091560
Xu Y, Wang H, He H, Wu F. Effects of Cold Acclimation on Morpho-Anatomical Traits of Heteroblastic Foliage in Pinus massoniana (Lamb.) Seedlings. Forests. 2024; 15(9):1560. https://doi.org/10.3390/f15091560
Chicago/Turabian StyleXu, Yingying, Haoyun Wang, Hongyang He, and Feng Wu. 2024. "Effects of Cold Acclimation on Morpho-Anatomical Traits of Heteroblastic Foliage in Pinus massoniana (Lamb.) Seedlings" Forests 15, no. 9: 1560. https://doi.org/10.3390/f15091560
APA StyleXu, Y., Wang, H., He, H., & Wu, F. (2024). Effects of Cold Acclimation on Morpho-Anatomical Traits of Heteroblastic Foliage in Pinus massoniana (Lamb.) Seedlings. Forests, 15(9), 1560. https://doi.org/10.3390/f15091560




