The Role of the Soil Seed Bank in the Recovery and Restoration of a Burned Amazonian Terra Firme Forest
Abstract
1. Introduction
2. Methods
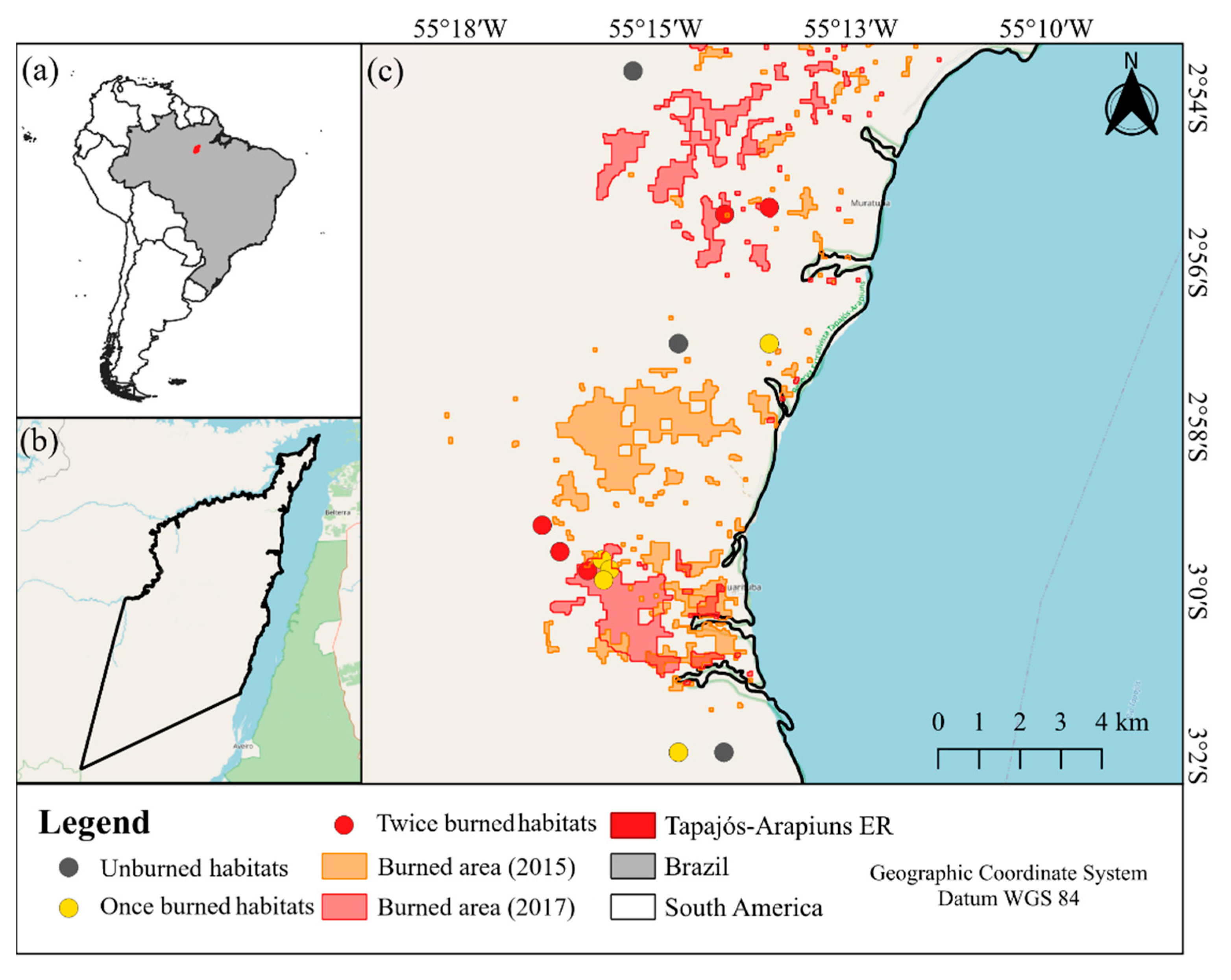
2.1. Study Area
2.2. Study Design and Soil Seed Bank
2.3. Functional Composition of the Soil Seed Bank
2.4. Data Analysis
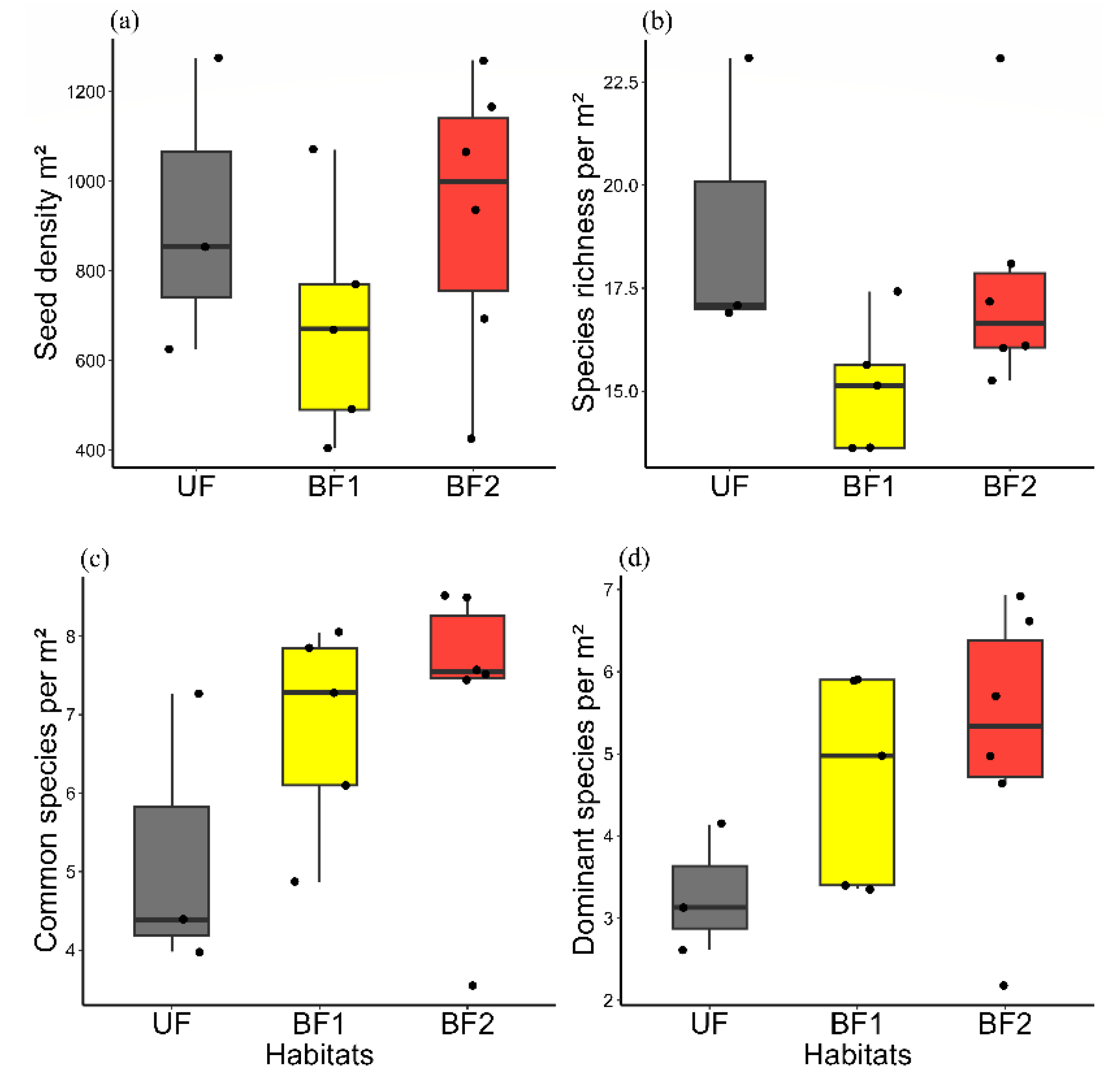
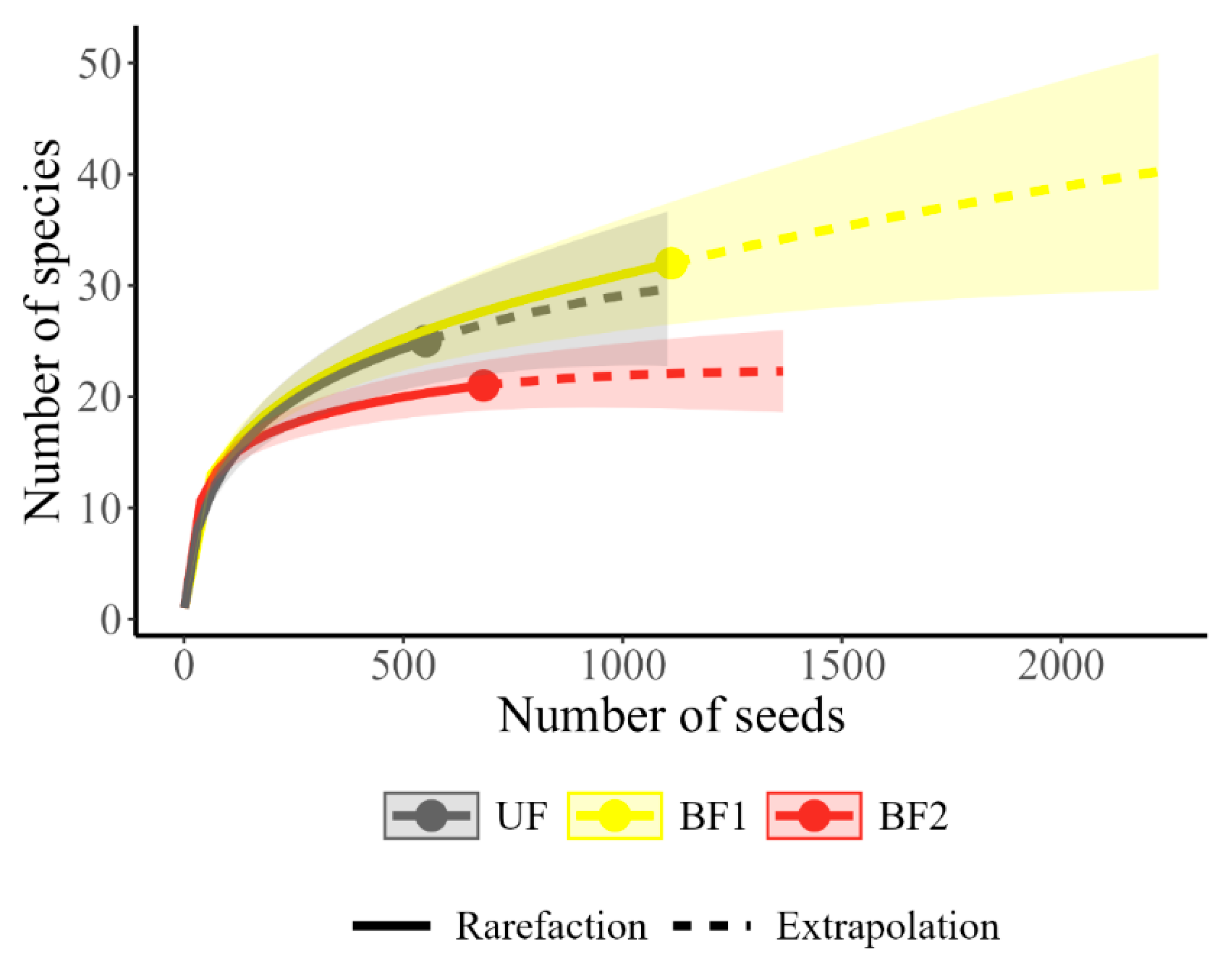
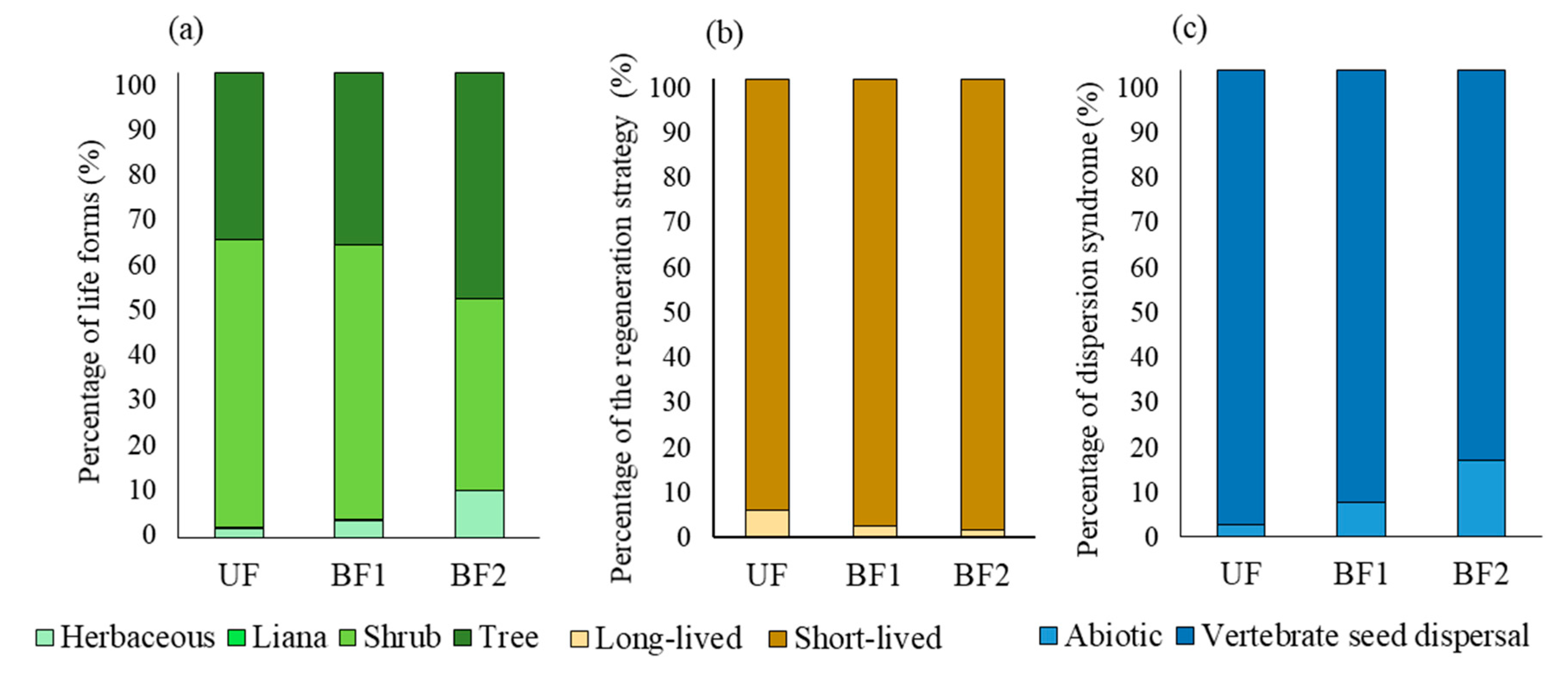
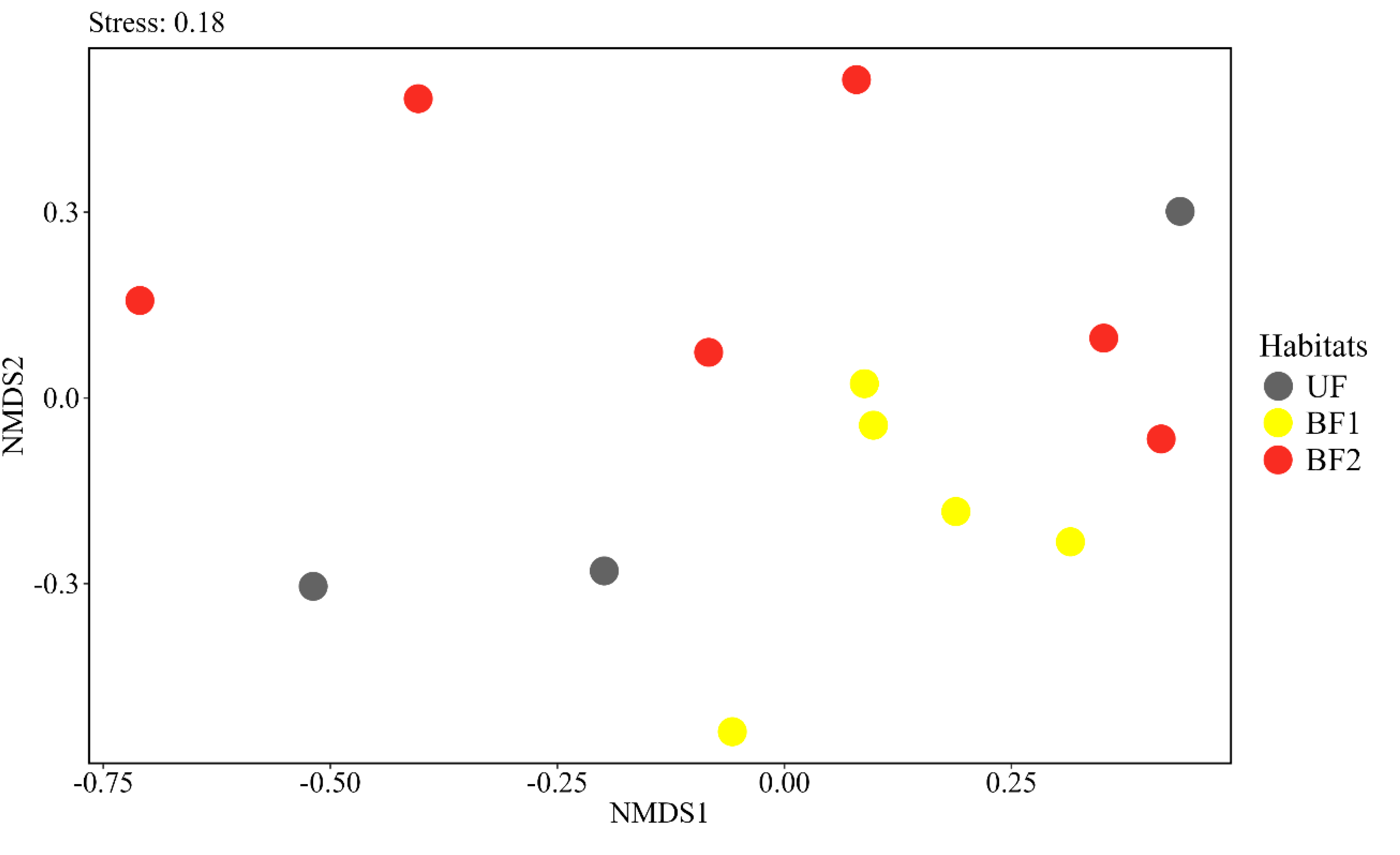
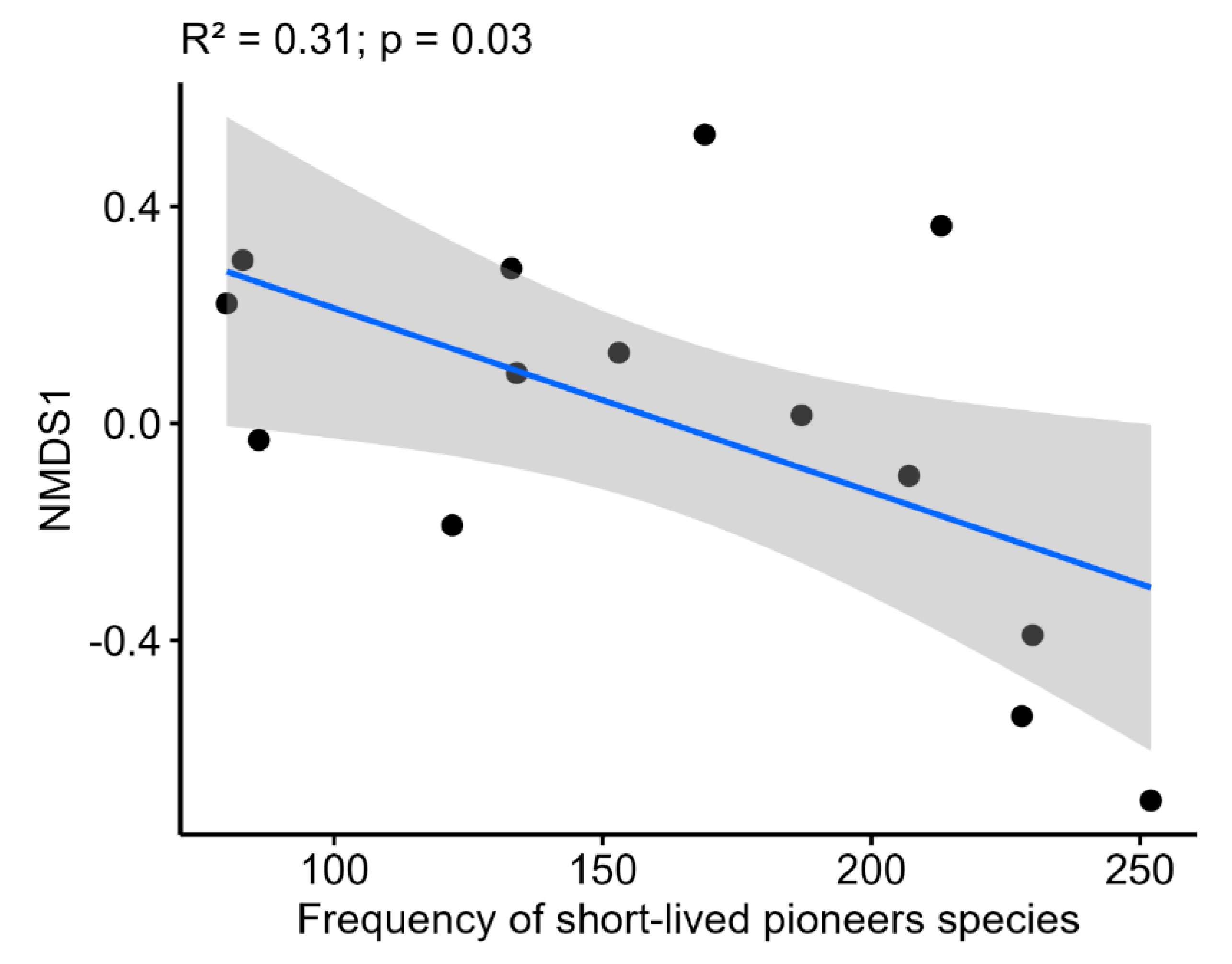
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Artaxo, P.; Hansson, H.C.; Machado, L.A.T.; Rizzo, L.V. Tropical forests are crucial in regulating the climate on Earth. PLOS Clim. 2022, 1, e0000054. [Google Scholar] [CrossRef]
- Pillay, R.; Venter, M.; Aragon-Osejo, J.; González-del-Pliego, P.; Hansen, A.J.; Watson, J.E.; Venter, O. Tropical forests are home to over half of the world’s vertebrate species. Front. Ecol. Environ. 2022, 20, 10–15. [Google Scholar] [CrossRef]
- Ghazoul, J.; Burivalova, Z.; Garcia-Ulloa, J.; King, L.A. Conceptualizing forest degradation. Trends Ecol. Evol. 2015, 30, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Saatchi, S.; Keller, M.; Bowman, K.; Ferraz, A.; Moorcroft, P.R.; Morton, D.C.; Bonal, D.; Brando, P.; Burban, B.; et al. Impacts of degradation on water, energy, and carbon cycling of the Amazon tropical forests. J. Geophys. Res. Biogeosci. 2020, 125, e2020JG005677. [Google Scholar] [CrossRef]
- Lapola, D.M.; Pinho, P.; Barlow, J.; Aragão, L.E.O.C.; Berenguer, E.; Carmenta, R.; Liddy, H.M.; Seixas, H.; Silva, C.V.J.; Silva-Junior, C.H.L.; et al. The drivers and impacts of Amazon forest degradation. Science 2023, 379, eabp8622. [Google Scholar] [CrossRef]
- Brandão, D.O.; Barata, L.E.S.; Nobre, C.A. The effects of environmental changes on plant species and forest dependent communities in the Amazon region. Forests 2022, 13, 466. [Google Scholar] [CrossRef]
- Peres, C.A.; Gardner, T.A.; Barlow, J.; Zuanon, J.; Michalski, F.; Lees, A.C.; Vieira, I.C.; Moreira, F.M.; Feeley, K.J. Biodiversity conservation in human-modified Amazonian forest landscapes. Biol. Conserv. 2010, 143, 2314–2327. [Google Scholar] [CrossRef]
- Hawes, J.E.; Vieira, I.C.; Magnago, L.F.; Berenguer, E.; Ferreira, J.; Aragão, L.E.; Cardoso, A.; Lees, A.C.; Lennox, G.D.; Tobias, J.A.; et al. A large-scale assessment of plant dispersal mode and seed traits across human-modified Amazonian forests. J. Ecol. 2020, 108, 1373–1385. [Google Scholar] [CrossRef]
- Barlow, J.; Berenguer, E.; Carmenta, R.; França, F. Clarifying Amazonia’s burning crisis. Glob. Chang. Biol. 2020, 26, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, E.; Lennox, G.D.; Ferreira, J.; Malhi, Y.; Aragão, L.E.; Barreto, J.R.; Del Bon Espírito-Santo, F.; Figueiredo, A.E.S.; França, F.; Gardner, T.A.; et al. Tracking the impacts of El Niño drought and fire in human-modified Amazonian forests. Proc. Natl. Acad. Sci. USA 2021, 118, e2019377118. [Google Scholar] [CrossRef] [PubMed]
- Dwomoh, F.K.; Wimberly, M.C.; Cochrane, M.A.; Numata, I. Forest degradation promotes fire during drought in moist tropical forests of Ghana. For. Ecol. Manag. 2019, 440, 158–168. [Google Scholar] [CrossRef]
- Nepstad, D.; Lefebvre, P.; Lopes da Silva, U.; Tomasella, J.; Schlesinger, P.; Solórzano, L.; Guerreira Benito, J. Amazon drought and its implications for forest flammability and tree growth: A basin-wide analysis. Glob. Chang. Biol. 2004, 10, 704–717. [Google Scholar] [CrossRef]
- Reis, M.; Alencastro Graça, P.M.L.; Yanai, A.M.; Ramos, C.J.P.; Fearnside, P.M. Forest fires and deforestation in the central Amazon: Effects of landscape and climate on spatial and temporal dynamics. J. Environ. Manag. 2021, 288, 112310. [Google Scholar] [CrossRef]
- Nepstad, D.; Carvalho, G.; Barros, A.C.; Alencar, A.; Capobianco, J.P.; Bishop, J.; Moutinho, P.; Lefebvre, P.; Silva, U.L., Jr.; Prins, E. Road paving, fire regime feedbacks, and the future of Amazon forests. For. Ecol. Manag. 2001, 154, 395–407. [Google Scholar] [CrossRef]
- Aragão, L.E.; Anderson, L.O.; Fonseca, M.G.; Rosan, T.M.; Vedovato, L.B.; Wagner, F.H.; Silva, C.V.J.; Junior, C.H.L.S.; Arai, E.; Aguiar, A.P.; et al. 21st Century drought-related fires counteract the decline of Amazon deforestation carbon emissions. Nat. Commun. 2018, 9, 536. [Google Scholar] [CrossRef] [PubMed]
- Balch, J.K.; Massad, T.J.; Brando, P.M.; Nepstad, D.C.; Curran, L.M. Effects of high-frequency understorey fires on woody plant regeneration in southeastern Amazonian forests. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120157. [Google Scholar] [CrossRef]
- Brando, P.; Macedo, M.; Silvério, D.; Rattis, L.; Paolucci, L.; Alencar, A.; Coe, M.; Amorim, C. Amazon wildfires: Scenes from a foreseeable disaster. Flora 2020, 268, 151609. [Google Scholar] [CrossRef]
- Barlow, J.; Peres, C.A.; Lagan, B.O.; Haugaasen, T. Large tree mortality and the decline of forest biomass following Amazonian wildfires. Ecol. Lett. 2003, 6, 6–8. [Google Scholar] [CrossRef]
- Liesenfeld, M.V.A.; Vieira, G.; Miranda, I.P.A. Fire ecology and the impact on Amazon vegetation. Braz. For. Res. 2016, 36, 505–517. [Google Scholar] [CrossRef]
- Melo, V.F.; Barros, L.S.; Silva, M.C.; Veloso, T.G.; Senwo, Z.N.; Matos, K.S.; Nunes, T.K. Soil bacterial diversities and response to deforestation, land use and burning in North Amazon, Brazil. Appl. Soil Ecol. 2021, 158, 103775. [Google Scholar] [CrossRef]
- Cochrane, M.A.; Schulze, M.D. Fire as a recurrent event in tropical forests of the eastern amazon: Effects on forest structure, biomass, and species composition 1. Biotropica 1999, 31, 2–16. [Google Scholar] [CrossRef]
- Faria, B.L.; Staal, A.; Silva, C.A.; Martin, P.A.; Panday, P.K.; Dantas, V.L. Climate change and deforestation increase the vulnerability of Amazonian forests to post-fire grass invasion. Glob. Ecol. Biogeogr. 2021, 30, 2368–2381. [Google Scholar] [CrossRef]
- Enright, N.J.; Fontaine, J.B.; Bowman, D.M.; Bradstock, R.A.; Williams, R.J. Interval squeeze: Altered fire regimes and demographic responses interact to threaten woody species persistence as climate changes. Front. Ecol. Environ. 2015, 13, 265–272. [Google Scholar] [CrossRef]
- Silva, R.M.; Lopes, A.G.; Santos, C.A.G. Deforestation and fires in the Brazilian Amazon from 2001 to 2020: Impacts on rainfall variability and land surface temperature. J. Environ. Manag. 2023, 326, 116664. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Guariguata, M.R. Natural regeneration as a tool for large-scale forest restoration in the tropics: Prospects and challenges. Biotropica 2016, 48, 716–730. [Google Scholar] [CrossRef]
- Warrier, R.R.; Kunhikannan, C. Significance of soil seed bank in forest vegetation a review. Seeds 2022, 1, 181–197. [Google Scholar] [CrossRef]
- Monaco, T.A.; MacKown, C.T.; Johnson, D.A.; Jones, T.A.; Norton, J.M.; Norton, J.B.; Redinbaugh, M.G. Nitrogen effects on seed germination and seedling growth. Rangel. Ecol. Manag. J. Range Manag. Arch. 2003, 56, 646–653. [Google Scholar] [CrossRef]
- Martins, A.M.; Engel, V.L. Soil seed banks in tropical forest fragments with different disturbance histories in southeastern Brazil. Ecol. Eng. 2007, 31, 165–174. [Google Scholar] [CrossRef]
- Bossuyt, B.; Honnay, O. Can the seed bank be used for ecological restoration? An overview of seed bank characteristics in European communities. J. Veg. Sci. 2008, 19, 875–884. [Google Scholar] [CrossRef]
- Cury, R.T.D.S.; Montibeller-Santos, C.; Balch, J.K.; Brando, P.M.; Torezan, J.M.D. Effects of fire frequency on seed sources and regeneration in southeastern Amazonia. Front. For. Glob. Chang. 2020, 3, 82. [Google Scholar] [CrossRef]
- Tangney, R.; Merrit, D.J.; Calloow, N.; Fontaine, J.B.; Miller, B.P. Seed traits determine species responses to fire under varying soil heating scenarios. Funct. Ecol. 2020, 34, 1967–1978. [Google Scholar] [CrossRef]
- Silva, K.A.; Martins, S.V.; Neto, A.M.; Lopes, A.T. Soil Seed Banks in a Forest Under Restoration and in a Reference Ecosystem in Southeastern Brazil. Floresta Ambiente 2019, 26, e20190047. [Google Scholar] [CrossRef]
- Le Page, Y.; Morton, D.; Hartin, C.; Bond-Lamberty, B.; Pereira JM, C.; Hurtt, G.; Asrar, G. Synergy between land use and climate change increases future fire risk in Amazon forests. Earth Syst. Dyn. 2017, 8, 1237–1246. [Google Scholar] [CrossRef]
- Marengo, J.A.; Souza, C.M., Jr.; Thonicke, K.; Burton, C.; Halladay, K.; Betts, R.A.; Alves, L.M.; Soares, W.R. Changes in climate and land use over the Amazon region: Current and future variability and trends. Front. Earth Sci. 2018, 6, 228. [Google Scholar] [CrossRef]
- Projeto MapBiomas—Mapeamento das Áreas Queimadas no Brasil. Available online: https://brasil.mapbiomas.org/2023/08/09/amazonia-ja-perdeu-17-de-sua-cobertura-nativa/ (accessed on 6 January 2023).
- Alencar, A.A.; Brando, P.M.; Asner, G.P.; Putz, F.E. Landscape fragmentation, severe drought, and the new Amazon forestfire regime. Ecol. Appl. 2015, 25, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Spínola, J.N.; Silva, M.J.S.; Silva, J.R.A.; Barlow, J.; Ferreira, J. A shared perspective on managing Amazonian sustainable-use reserves in an era of megafires. J. Appl. Ecol. 2020, 57, 2132–2138. [Google Scholar] [CrossRef]
- Barlow, J.; Peres, C.A. Fire-mediated dieback and compositional cascade in an Amazonian forest. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1787–1794. [Google Scholar] [CrossRef]
- Withey, K.; Berenguer, E.; Palmeira, A.F.; Espírito-Santo, F.D.B.; Lennox, G.D.; Silva, C.V.J.; Aragão, L.E.O.C.; Ferreira, J.; França, F.; Malhi, Y.; et al. Quantifying immediate carbon emissions from El Niño-mediated wildfires in humid tropical forests. Philos. Trans. R. Soc. 2018, 373, 20170312. [Google Scholar] [CrossRef]
- Paolucci, L.N.; Pereira, R.L.; Rattis, L.; Silvério, D.V.; Marques, N.C.; Macedo, M.N.; Brando, P.M. Lowland tapirs facilitate seed dispersal in degraded Amazonian forests. Biotropica 2019, 52, 245–252. [Google Scholar] [CrossRef]
- Barbedo, C.J.; Centeno, D.C.; Ribeiro, R.C.L.F. Do recalcitrant seeds really exist? Hoehnea 2013, 40, 583–593. [Google Scholar] [CrossRef]
- Chaves, R.S.; Junqueira, A.B.; Clement, C.R. The Influence of Soil Quality and Market Orientation on Manioc (Manihot esculenta) Varietal Choice by Smallholder Farmers along the Lower Tapajós River, Pará, Brazil. Human Ecology 2018, 46, 229–239. [Google Scholar] [CrossRef]
- Barlow, J.; Peres, C.A. Avifaunal responses to single and recurrent wildfires in Amazonian forests. Ecol. Appl. 2004, 14, 1358–1373. [Google Scholar] [CrossRef]
- Andrade, D.F.; Gama, J.R.V.; Melo, L.O.; Ruschel, A.R. Inventário florestal de grandes áreas na Floresta Nacional do Tapajós, Pará, Amazônia, Brasil. Biota Amaz. 2015, 5, 109–115. [Google Scholar] [CrossRef]
- Pereira, C.A.; Tabarelli, M.; Barros, M.F.; Vieira, I.C.G. Restoring fire-degraded social forests via biocultural approaches: A key strategy to safeguard the Amazon legacy. Restor. Ecol. 2023, 31, e13976. [Google Scholar] [CrossRef]
- Vieira, I.C.G.; Proctor, J. Mechanisms of plant regeneration during succession after shifting cultivation in eastern Amazonia. Plant Ecol. 2007, 192, 303–315. [Google Scholar] [CrossRef]
- Sousa, T.R.; Costa, F.R.C.; Bentos, T.V.; Leal Filho, N.; Mesquita, R.C.G.; Ribeiro, I.O. The effect of forest fragmentation on the soil seed bank of Central Amazonia. For. Ecol. Manag. 2017, 393, 105–112. [Google Scholar] [CrossRef]
- Ramirez, N.; Barrios, Y.; Briceno, H. Correlations between morphological fruit types, fruit and seed colors, and functional groups. Biota Neotrop. 2021, 21, e20211238. [Google Scholar] [CrossRef]
- Muller-Landau, H.C.; Wright, S.J.; Calderón, O.; Condit, R.; Hubbell, S.P. Interspecific variation in primary seed dispersal in a tropical forest. J. Ecol. 2008, 96, 653–667. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org (accessed on 12 February 2021).
- Garwood, N.C. Tropical soil seed banks: A review. In Ecology of Soil Seed Bank; Leck, M.A., Parker, T., Simpson, R.L., Eds.; Academic Press: Cambridge, MA, USA, 1989; Volume 1, pp. 149–209. [Google Scholar] [CrossRef]
- Bordon, N.G.; Leal Filho, N.; Bentos, T.V. Ecology of the seed bank in the amazon rainforest. In Ecosystem and Biodiversity of Amazonia, 1st ed.; Mikkola, H., Ed.; University of Eastern Finland: Kuopio, Finland, 2021; Volume 1, pp. 67–88. [Google Scholar] [CrossRef]
- Dupuy, J.M.; Chazdon, R.L. Long-Term Effects of Forest Regrowth and Selective Logging on the Seed Bank of Tropical Forests in NE Costa Rica 1. Biotropica 1988, 30, 223–237. [Google Scholar] [CrossRef]
- Yang, X.; Baskin, C.C.; Baskin, J.M.; Pakeman, R.J.; Huang, Z.; Gao, R.; Cornelissen, J.H. Global patterns of potential future plant diversity hidden in soil seed banks. Nat. Commun. 2021, 12, 7023. [Google Scholar] [CrossRef] [PubMed]
- Douh, C.; Daïnou, K.; Loumeto, J.J.; Moutsambote, J.M.; Fayolle, A.; Tosso, F.; Forni, E.; Gourlet-Fleury, S.; Doucet, J.-L. Soil seed bank characteristics in two central African forest types and implications for forest restoration. For. Ecol. Manag. 2018, 409, 766–776. [Google Scholar] [CrossRef]
- Hopfensperger, K.N. A review of similarity between seed bank and standing vegetation across ecosystems. Oikos 2007, 116, 1438–1448. [Google Scholar] [CrossRef]
- Botelho, A.L.M.; Borges, L.H.M.; McFarland, B. Abundance and composition of the medium to large-sized mammals in a private área of a REDD+ project in Acre, Brazil. Biota Neotrop. 2018, 18, e20170487. [Google Scholar] [CrossRef]
- Bowd, E.; Blanchard, W.; McBurney, L.; Lindenmayer, D. Direct and indirect disturbance impacts on forest biodiversity. Ecosphere 2021, 12, e03823. [Google Scholar] [CrossRef]
- Clark, D.B.; Ferraz, A.; Clark, D.A.; Kellner, J.R.; Letcher, S.G.; Saatchi, S. Diversity, distribution and dynamics of large trees across an old-growth lowland tropical rain forest landscape. PLoS ONE 2019, 14, e0224896. [Google Scholar] [CrossRef]
- Cochrane, M.A. Fire science for rainforests. Nature 2003, 421, 913–919. [Google Scholar] [CrossRef]
- Carvalho, L.Z.G.; Massi, K.G.; Coutinho, M.P.; Magalhães, V.D. Fire effects on Atlantic Forest sites from a composition, structure and functional perspective. Braz. J. Biol. 2022, 82, e268185. [Google Scholar] [CrossRef]
- Lent, J.V.; Hernández-Barrios, J.C.; Anten, N.P.R.; Martinez-Ramos, M. Defoliation effects on seed dispersal and seedling recruitment in a tropical rain forest understorey palm. J. Ecol. 2014, 102, 709–720. [Google Scholar] [CrossRef]
- Carvalho, A.L.; d’Oliveira, M.V.N.; Putz, F.E.; Oliveira, L.C. Natural regeneration of trees in selectively logged forest in western Amazonia. For. Ecol. Manag. 2017, 392, 36–44. [Google Scholar] [CrossRef]
- Wieland, L.M.; Mesquita, R.C.; Bobrowiec, P.E.D.; Bentos, T.V.; Williamson, G.B. Seed rain and advance regeneration in secondary succession in the Brazilian Amazon. Trop. Conserv. Sci. 2011, 4, 300–316. [Google Scholar] [CrossRef]
- Fuzessy, L.; Sobral, G.; Carreira, D.; Rother, D.C.; Barbosa, G.; Landis, M.; Galetti, M.; Dallas, T.; Cláudio, V.C.; Culot, L.; et al. Functional roles of frugivores and plants shape hyper-diverse mutualistic interactions under two antagonistic conservation scenarios. Biotropica 2022, 54, 444–454. [Google Scholar] [CrossRef]
- Tunes, P.; Alves, V.N.; Valentin-Silva, A.; Batalha, M.A.; Guimarães, E. Does fire affect the temporal pattern of trophic resource supply to pollinators and seed-dispersing frugivores in a Brazilian savanna community? Plant Ecol. 2017, 218, 345–357. Available online: https://link.springer.com/article/10.1007/s11258-016-0695-5 (accessed on 3 February 2024). [CrossRef]
- Flores, B.M.; Holmgren, M. Why forest fails to recover after repeated wildfires in Amazonian floodplains? Experimental evidence on tree recruitment limitation. J. Ecol. 2021, 109, 3473–3486. [Google Scholar] [CrossRef]
- Norgrove, L.; Hauser, S. Estimating the consequences of fire exclusion for food crop production, soil fertility, and fallow recovery in shifting cultivation landscapes in the humid tropics. Environ. Manag. 2015, 55, 536–549. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Germination ecology of seeds in the persistent seed bank. In Seeds, 2nd ed.; Baskin, C.C., Baskin, J.M., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 1, pp. 187–276. [Google Scholar] [CrossRef]
| Family | Species | LF | DS | RS | Number of Germinated Seeds (m2) | ||
|---|---|---|---|---|---|---|---|
| UF | BF1 | BF2 | |||||
| Fabaceae | Amphiodon effusus Huber | Tree | L. pioneer | Abiotica | 0 | 0 | 1 |
| Malvaceae | Apeiba echinata Gaertn. | Tree | L. pioneer | Abiotica | 3 | 4 | 5 |
| Poaceae | Axonopus compressus (Sw.) P. Beauv. | Herbaceous | S. pioneer | Abiotica | 0 | 0 | 50 |
| Axonopus purpusii (Mez) Chase | Herbaceous | S. pioneer | Abiotica | 0 | 0 | 10 | |
| Salicaceae | Banara guianensis Aubl. | Shrub | S. pioneer | Vertebrate | 0 | 2 | 0 |
| Rubiaceae | Borreria latifolia (Aubl.) K.Schum. | Herbaceous | S. pioneer | Abiotica | 0 | 0 | 1 |
| Borreria verticillata (L.) G.Mey. | Herbaceous | S. pioneer | Abiotica | 0 | 0 | 1 | |
| Malpighiaceae | Byrsonima densa (Poir.) DC. | Tree | S. pioneer | Vertebrate | 5 | 7 | 4 |
| Urticaceae | Cecropia distachya Huber | Tree | S. pioneer | Vertebrate | 228 | 66 | 93 |
| Asteraceae | Chromolaena odorata (L.) RMKing & H.Rob. | Shrub | S. pioneer | Abiotica | 2 | 19 | 45 |
| Menispermaceae | Cissampelos pareira L. | Liana | L. pioneer | Vertebrate | 0 | 0 | 1 |
| Melastomataceae | Clidemia hirta (L.) D. Don | Shrub | S. pioneer | Vertebrate | 0 | 0 | 2 |
| Polygonaceae | Coccoloba mollis Casar. | Tree | S. pioneer | Vertebrate | 0 | 0 | 1 |
| Plantaginaceae | Conobea aquatica Aubl. | Herbaceous | S. pioneer | Abiotica | 3 | 0 | 16 |
| Dilleniaceae | Doliocarpus dentatus (Aubl.) Standl. | Liana | L. pioneer | Vertebrate | 0 | 2 | 0 |
| Asteraceae | Emilia sonchifolia (L.) DC. | Herbaceous | S. pioneer | Abiotica | 0 | 0 | 1 |
| Myrtaceae | Eugenia patrisii Vahl | Tree | S. pioneer | Vertebrate | 17 | 8 | 1 |
| Moraceae | Ficus maxima Mill. | Tree | L. pioneer | Vertebrate | 3 | 0 | 1 |
| Heliconiaceae | Heliconia psittacorum L.f. | Herbaceous | S. pioneer | Vertebrate | 5 | 0 | 0 |
| Convolvulaceae | Ipomoea batatas (L.) Lam. | Herbaceous | S. pioneer | Abiotica | 0 | 6 | 0 |
| Bignoniaceae | Jacaranda copaia (Aubl.) D. Don | Tree | L. pioneer | Abiotica | 0 | 1 | 3 |
| Euphorbiaceae | Maprounea guianensis Aubl. | Tree | S. pioneer | Abiotica | 2 | 2 | 3 |
| Melastomataceae | Miconia ceramicarpa (DC.) Cogn. | Shrub | S. pioneer | Vertebrate | 17 | 35 | 83 |
| Miconia prasina (Sw.) DC. | Shrub | S. pioneer | Vertebrate | 413 | 264 | 129 | |
| Rubiaceae | Palicourea colorata (Willd. ex Roem. & Schult.) Delprete & J.H.Kirkbr. | Shrub | S. pioneer | Vertebrate | 8 | 1 | 0 |
| Palicourea guianensis Aubl. | Tree | S. pioneer | Vertebrate | 13 | 49 | 39 | |
| Poaceae | Panicum repens L. | Herbaceous | S. pioneer | Abiotica | 5 | 0 | 4 |
| Piperaceae | Piper carniconnectivum C. DC. | Shrub | S. pioneer | Vertebrate | 0 | 0 | 1 |
| Piper hostmannianum (Miq.) C. DC. | Shrub | S. pioneer | Vertebrate | 73 | 23 | 70 | |
| Piper marginatum Jacq. | Shrub | S. pioneer | Vertebrate | 35 | 34 | 3 | |
| Fabaceae | Pseudopiptadenia suaveolens (Miq.) J.W.Grimes | Tree | L. pioneer | Abiotica | 2 | 0 | 0 |
| Rubiaceae | Psychotria poeppigiana Mull. Arg. | Shrub | S. pioneer | Vertebrate | 12 | 0 | 3 |
| Fabaceae | Schnella guianensis (Aubl.) Wunderlin | Liana | L. pioneer | Abiotica | 2 | 0 | 0 |
| Cyperaceae | Scleria gaertneri Raddi | Herbaceous | S. pioneer | Abiotica | 2 | 18 | 8 |
| Solanaceae | Solanum schlechtendalianum Walp. | Shrub | S. pioneer | Vertebrate | 8 | 25 | 45 |
| Poaceae | Steinchisma laxum (Sw.) Zuloaga | Herbaceous | S. pioneer | Abiotica | 2 | 0 | 1 |
| Eriocaulaceae | Tonina fluviatilis Aubl. | Herbaceous | S. pioneer | Vertebrate | 2 | 1 | 2 |
| Cannabaceae | Trema micrantha (L.) Blume | Tree | S. pioneer | Vertebrate | 13 | 106 | 296 |
| Rutaceae | Zanthoxylum rhoifolium Lam. | Tree | L. pioneer | Vertebrate | 43 | 10 | 5 |
| Total | 918 | 683 | 926 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, V.B.; Jardim, M.A.G.; Barros, M.F.; Silva, D.S.; Vieira, I.C.G.; Tabarelli, M. The Role of the Soil Seed Bank in the Recovery and Restoration of a Burned Amazonian Terra Firme Forest. Forests 2024, 15, 1513. https://doi.org/10.3390/f15091513
Oliveira VB, Jardim MAG, Barros MF, Silva DS, Vieira ICG, Tabarelli M. The Role of the Soil Seed Bank in the Recovery and Restoration of a Burned Amazonian Terra Firme Forest. Forests. 2024; 15(9):1513. https://doi.org/10.3390/f15091513
Chicago/Turabian StyleOliveira, Vynicius B., Mário A. G. Jardim, Maria Fabíola Barros, Danilo S. Silva, Ima C. G. Vieira, and Marcelo Tabarelli. 2024. "The Role of the Soil Seed Bank in the Recovery and Restoration of a Burned Amazonian Terra Firme Forest" Forests 15, no. 9: 1513. https://doi.org/10.3390/f15091513
APA StyleOliveira, V. B., Jardim, M. A. G., Barros, M. F., Silva, D. S., Vieira, I. C. G., & Tabarelli, M. (2024). The Role of the Soil Seed Bank in the Recovery and Restoration of a Burned Amazonian Terra Firme Forest. Forests, 15(9), 1513. https://doi.org/10.3390/f15091513







