Abstract
Considerable research has focused on gene silencing in tree-feeding insects, but how trees recognize and process double-stranded RNA (dsRNA) engineered to target plant pests is unknown. We performed transcriptomic assembly, preliminary differential expression analysis, and in silico annotation on loblolly pine (Pinus taeda, L.) seedlings exposed to southern pine beetle-specific dsRNA. This pilot study sought to elucidate the baseline response of seedlings challenged with insect-specific dsRNA. Treated and untreated seedlings were sequenced and following transcriptome assembly 20 RNAi-related proteins (RRPs) were annotated. Differential gene expression analysis conducted using DESeq2 followed by pathway enrichment revealed 7131 differentially expressed transcripts, of which 33% were upregulated and 67% were downregulated. Only two RRPs selected for analysis were upregulated in treated seedlings, showing a lack of detectable RNAi response with our methodology. Beyond RNAi-related proteins, pathway enrichment mapped to immune response systems and genetic and cellular processing. Upregulated transcripts included autophagy, amino sugar and nucleotide sugar metabolism, and plant hormone signal transduction. Downregulated transcripts included RNA degradation and fatty acid metabolism pathways. Multiple DICER-LIKE and ARGONAUTE proteins were also annotated in five other North American pines, revealing diversity among these crucial proteins. Understanding host plant response to RNAi-mediated pest control is essential to further develop this technology against tree pests.
1. Introduction
Climate change and shifting weather regimes are bringing changes in tree species distribution and altered pest and pathogen interactions [1]. The impacts of these effects on local vegetation are spatially variable, but predictions of hotter and dryer conditions will undoubtedly lead to shifts in the suitable habitats for numerous plant species and alter existing interspecific competitive interactions [2,3], which may be particularly threatening to species with specialized or stringent requirements or limited distributions [4,5]. Climate stress and altered geographic ranges can potentially expose trees to novel pests and pathogens while also reducing overall tree fitness and compromising defenses [6,7,8]. Additionally, insects are cyclically linked to the effects of weather on host plants; increasingly stressed hosts lead to high instances of pest outbreaks which in turn lead to tree mortality and the conversion of vital carbon sinks to carbon sources, as dead and dying trees no longer sequester carbon but rather act as a carbon source [9,10,11]. Pines (Pinus spp.) are important in urban landscapes and an integral component of forest ecosystems, driving community composition, biotic and abiotic interactions, and contributing substantially to ecosystem services; they represent one of the most diverse and widespread conifer divisions [12]. Pine forests are critically important components of the global carbon cycle, acting as essential carbon sinks that pull carbon from the air and sequester it in standing biomass [13,14,15]. Given the prevalence and importance of conifers, conservation and protection of these trees is vital. Protecting increasingly stressed trees while promoting overall forest health will require an integrative approach combining existing and novel technologies.
Insect pest outbreaks, coupled with abiotic stressors, are among the most significant threats to conifer forest health and sustainability. Extensive pest-specific management prescriptions are available, but given the increasing complexity of forest dynamics under the veil of climate change, anthropogenic stress, and invasive species, existing management infrastructure can no longer keep pace with eruptive insect outbreaks [16,17]. Molecular approaches to pest management that are increasingly in use in the agricultural sector are being explored to bolster current forest protection. One such technology, RNA-interference (RNAi), results in sequence-specific gene silencing and subsequent phenotypic effects based on the targeted sequence and can be applied as a pest management strategy [18,19]. First reported as “co-suppression” in plants [20] and “quelling” in fungi [21], RNAi is a biological pathway present across multiple eukaryote lineages that is responsible for sequence-specific post-transcriptional gene silencing (PTGS) [22], which evolved as a viral defense or as a means for intrinsic genome regulation and management [23,24]. While RNAi pathways can be induced by a variety of small RNA molecules, one of the most potent and effective methods for inducing gene silencing and subsequent mortality in insects is through the introduction of exogenous double-stranded RNA (dsRNA) [18,25].
Multiple conifer pests have demonstrated lethality or increased susceptibility to host plant resistance through RNAi-mediated gene silencing, including both southern pine beetle (SPB; Dendroctonus frontalis, Zimmermann) [26] and mountain pine beetle (MPB; D. ponderosae, Hopkins) [27], Chinese white pine beetle (D. armandi, Tsai & Li) [28], and six spined ips (Ips calligraphus, Germar) [29]. Effective and efficient dsRNA delivery remains a barrier and is essential to developing RNAi technology into useful forest protection products. Numerous techniques have been explored for delivering the trigger for RNAi to protect plants, including foliar sprays, trunk injections, and soaks and drenches [30,31,32,33,34,35], but little work has been conducted exploring the deployment of RNAi technology specifically within conifer tissue. Rapid uptake and whole seedling dissemination of hydroponically administered dsRNAs has been demonstrated in loblolly pine [36], but retention wanes rapidly over 7 days; data from further time points are lacking. Prior to internalization, mode of delivery and tissue-specific application may play a critical role in the uptake, subsequent processing, and longevity of exogenous dsRNAs.
Systemic spread of exogenously applied dsRNA has been demonstrated in various herbaceous and woody plants, but there has been less work evaluating in planta dsRNA longevity [30,32,37,38]. The mode of dsRNA uptake could affect in-plant localization and alter the course for dsRNA processing. Evidence suggests that dsRNAs applied to foliage enter via stomata; after crossing the plasma membrane, the dsRNA could access the silencing machinery which is located in the cytoplasm [39,40]. Less is known about the uptake and integration of dsRNAs applied to roots. However, confocal imaging reveals that, in deciduous ash (Fraxinus sp.) and apple (Malus sp.), dsRNA and hairpin RNA constructs persist within the xylem and intercellular spaces [32,41]. Confinement to the apoplast or xylem tissues, which lack DICER-LIKE proteins, may lead to reduced processing of exogenous dsRNAs introduced via root uptake, thereby maintaining dsRNA integrity for prolonged tree protection [42]. Delivering dsRNAs within plant material may provide intrinsic protection against pests, but since plants possess their own RNAi machinery, they may respond to and process these protective dsRNAs; understanding the interplay between host and construct will be crucial. Consequently, we set out to investigate the diversity of RNAi-related proteins in loblolly pine and their response to insect-specific dsRNA. We annotated proteins corresponding to 14 RNAi-related proteins (RRPs). Additionally, we exposed loblolly pine seedlings to a SPB-specific dsRNA targeted to the SHIBERE transcript (dsSHI), which has demonstrated lethality in adult beetles when orally ingested in a sucrose solution [26]. We then generated transcriptomes for loblolly pine seedlings under both dsRNA-challenged and unchallenged conditions and evaluated differential gene expression to investigate whether exogeneous dsRNA has an effect on the expression of key RNAi genes within pines. Finally, we annotated and compared a subset of DICER-LIKE and ARGONAUTE proteins in five additional pines to investigate differences in key proteins across Western and Eastern North American pine species and to establish a framework for future research into RNAi in pines. Ours is the first study to broadly investigate the effect of dsRNA on gene expression in loblolly pine, and despite the small sample size, these data are an important baseline for future work investigating the cross-kingdom effects of dsRNA.
2. Materials and Methods
2.1. dsRNA Selection and Synthesis
Southern pine beetle-specific dsSHI known to induce mortality [26] was synthesized. Briefly, RNA was extracted from adult SPB reared from infested bark. Following cDNA synthesis with SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) and both Random and Oligo(dT)12–18 primers (Invitrogen, Carlsbad, CA, USA), the target gene was amplified using specific primers [26], and OneTaq 2X Master Mix with Standard Buffer (New England Biolabs, Ipswich, MA, USA) prepared according to the manufacturer’s instructions. Following PCR, the amplicon was purified with a Qiagen purification kit (Qiagen, Germantown, MD, USA) and dsRNA was synthesized using a MEGAscript RNAi Kit (Thermo Scientific, Waltham, MA, USA) and purified according to the manufacturer’s instructions.
2.2. Plant Material and Sample Preparation
Loblolly pine seeds (7-56×OP, USDA Forest Service) were moist-chilled for 90 d at 4 °C and then individually seeded into a 2:1 ratio of pine bark fines soil conditioner (Barky Beaver, Moss, TN, USA) and Promix general purpose growing medium BX (Premier Tech Horticulture, Rivière-du-Loup, QC, Canada) during July 2021. Seedlings were maintained under ambient greenhouse conditions (~20–32 °C, 15:9 L:D, 50–75% RH) and watered daily through germination, and then as needed.
One hundred days post-planting, seedlings (N = 4) of comparable size and morphology were removed from potting medium and rinsed for 30 s in dd H2O to remove soil and expose as much root tissue as possible for the uptake of dsRNA. Seedlings were then transferred to 19 mL autoclaved cylindrical glass assay containers (1.6 cm × 15 cm) (Fisherbrand, Carlsbad, CA, USA) containing 15 mL of sterilized H2O [36]. Seedlings were randomly assigned to dsSHI (n = 2), which received 150 μg of dsSHI mixed into the water (10 μg/mL), while control seedlings (n = 2) received only water. Assay containers were sealed around the seedling stem with parafilm (Bemis Company, Inc, Neenah, WI, USA) to prevent evaporation, and the outer surface was wrapped in opaque brown paper to prevent light penetration to the water solution (Figure 1). Experimental seedlings were returned to the greenhouse following treatment and maintained under ambient conditions.

Figure 1.
Representation of experimental loblolly pine seedlings (n = 4) housed in sealed, covered assay containers. Seedlings were arranged in racks with one tube in each corner and maintained in a greenhouse.
After 48 h, each seedling was rinsed for 30 s in dd H2O; then, entire seedlings were individually ground into a fine powder using liquid nitrogen and a mortar and pestle. RNA was extracted using an adaptation of the work by Chang et al. [36,43]. RNA pellets were resuspended in RNase-free H2O, and concentration and purity were analyzed via absorbance measurements of 260/230 nm and 260/280 nm (NanoDrop Technologies, Wilmington, DE, USA) and then diluted to meet sequencing requirements (25–30 ng/μL). Each RNA sample was also assessed to determine the presence or absence of dsSHI using end-point PCR [36] (Figure S1).
2.3. RNA-Seq, Read Processing, and De Novo Assembly
Library preparation, which included mRNA enrichment via poly-A capture, and sequencing were conducted by Novogene Corporation Inc. (Sacramento, CA, USA). Libraries were sequenced with 2 × 150 paired-end reads using the Illumina NovaSeq 6000 platform. The RNA sequence data were deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) (BioProject: PRJNA993588). Additional reads were obtained for P. banksiana (Lamb.), P. contorta (Douglas), P. elliottii (Engelm.), P. flexilis (E.James), P. ponderosa (Douglas ex C.Lawson), and P. taeda from NCBI SRA (BioProject: PRJNA703422) (Table S1). To ensure that only high-quality reads were used for de novo assembly, raw reads were preprocessed. Erroneous k-mers were corrected using Rcorrector v1.0.5 default program settings; however, read pairs where at least one read was flagged as unfixable by Rcorrector were removed via a custom python script [44]. Low-quality reads with Phred score < 20, short reads (<50 bp), and adapter sequences were removed using TrimGalore v0.6.6 [45]. Read quality control, prior to and after processing, was performed using FastQC v0.11.9 [44,46].
To generate a composite transcriptome of the loblolly pine used here, processed reads from all four samples were assembled into a de novo transcriptome by Trinity v2.13.2 [47] using default paired-end read parameters. Following de novo assembly, CD-HIT v4.8.1 [48] was used to reduce the number of redundant sequences and produce a list of representative sequences for analysis with a sequence identity threshold of 0.9 (90%). Assembly completeness was quantified via Benchmarking Universal Single-Copy Orthologs (BUSCO) assessment v5.3.0 [49] against the embryophyte lineage. After assessment, TransDecoder v5.5.0 [50] extracted long open reading frames with a minimum length of 100 bp and conducted a homology search and retained highly similar hits (E-value < 1 × 10−5) to proteins from the SwissProt database [46,51] using default paired-end read parameters. Following de novo assembly, CD-HIT v4.8.1 [47] was used to reduce the number of redundant sequences and produce a list of representative sequences for analysis with a sequence identity threshold of 0.9 (90%). Assembly completeness was quantified via Benchmarking Universal Single-Copy Orthologs (BUSCO) assessment v5.3.0 [48] against the embryophyte lineage. After assessment, TransDecoder v5.5.0 [49] extracted long open reading frames with a minimum length of 100 aa from all assembled transcripts and conducted a homology search using BLASTp which retained highly similar hits (E-value < 1 × 10−5) to proteins from the SwissProt database [50]. Finally, coding region predictions were generated when sequences contained structural characteristics consistent with coding regions or demonstrated blast homology to proteins retained during the homology query.
2.4. Protein Selection and Identification
Fourteen RNAi-related proteins (RRPs) were selected for evaluation and annotation based on their relevance to one of the RNAi pathways in plants or involvement in pathogen response or defense. Some protein groups correspond to a single protein ortholog: DAWDLE (DDL), HYPONASTIC LEAVES 1 (HYL1), HEAT SHOCK PROTEIN 90 (HSP90), ALTERED MERISTEM PROGRAM 1 (AMP1), HUA ENHANCER 1 (HEN1), SILENCING DEFECTIVE 3 (SDE3), SUPPRESSOR OF GENE SILENCING 3 (SGS3), and SERRATE (SE); whereas some of the groups contain multiple proteins with similar or redundant function: DICER-LIKE PROTEINS (DCL), DSRNA BINDING PROTEINS (DRB), ARGONAUTE proteins (AGO), RNA-DEPENDENT RNA POLYMERASES (RdRp), AMINOPHOSPHOLIPID ATPases (ALA), and SOMATIC EMBRYOGENESIS RECEPTOR KINASES (SERK). Annotated gene sequences for each RRP were collected from NCBI’s Nucleotide database [52] and compiled into a database via the makeblastdb program (BLAST+ v2.12.0) [53]. Following compilation of the reference databases (Table S2), genomic sequences for predicted coding regions for each pine transcriptome were queried against the RRP databases using tBLASTx and hits (E value < 1 × 10−5) were retained as candidate RRPs, and then queried against NCBI’s RefSeq database [54] using BLASTx. BLASTx results with high homology (E value < 1 × 10−5) were retained and again sorted to retain only candidate RRPs where the BLASTx homology results matched the identity of the queried RRP database. Protein sequences that were identified with high homology to the target protein were validated with InterProScan v5.60-92.0 [55] via identification of domains retained in the PANTHER Classification System v17.0 [56].
2.5. Differential Expression Analysis
Each pair of experimental cleaned reads were aligned against the reference loblolly pine genome (Ptaeda2.0, GenBank: GCA_000404065.3, [57]) using HISAT2 v2.2.1 with default parameters [58]. Following alignment and preparation of output files, transcript assembly and quantification was conducted on each sample by StringTie v2.2.0 [59], after which a merged GTF file was created containing the composite set of transcripts across samples. Differential expression analyses were conducted in RStudio v2022.07.0+548 [60] running R Statistical Software v4.2.0 [61], utilizing DESeq2 v1.38.2 [62]. Counts were normalized using size factor normalization to account for differences in library sizes across samples. Significance of expression differences between treatment groups was assessed using the Wald test, and differentially expressed transcripts (DETs) were filtered based on an adjusted p-value threshold of 0.05, calculated with the Benjamini–Hochberg method to minimize the false discovery rate [63]. No specific fold change cut-off was applied for filtering DETs; instead, all DETs meeting the adjusted p-value threshold were included. All differentially expressed transcripts (DETs) were evaluated for homology to RRPs, but in cases where multiple isoforms of the same parent transcript were detected, only a single representative isoform was included in the final DE count for that RRP.
2.6. Functional Annotation and Pathway Enrichment
After the determination of DETs, coding regions were identified from each DET list as described previously. Predicted peptide sequences were then queried against the eggNOG 5 database using eggNOG-mapper v2.1.9 [64] with an E-value threshold of 0.001 to detect amino acid sequences homologous to previously identified protein sequences and Clusters of Orthologous Groups categories were assigned when applicable [65,66]. KEGG pathway analyses were conducted using assigned pathway terms from eggNOG-mapper v2 [64,67].
2.7. Phylogenetic Comparison
To investigate the similarity of core RNAi genes across selected geographically distinct pines, we annotated DICER-LIKE and ARGONAUTE family proteins. Following de novo transcriptome assembly from reads obtained from NCBI SRA (Table S1), DCL and AGO proteins were annotated via BLAST homology and functional assessment as described previously. Following annotation, phylogenetic trees were constructed for each protein with Phylogeny.fr using the “one-click” phylogeny analysis setting [68].
3. Results
3.1. RNA-Seq, Read Processing, and De Novo Assembly
Experimental loblolly pine sample sequencing (N = 4) yielded 288,706,336 raw reads, with filtering and processing removing ~1.8% (5,179,698 reads). End-point PCR confirmed the presence of experimentally applied dsSHI in treated samples (N = 2) at time of sampling. Raw reads obtained from NCBI SRA (N = 15) (Table S1) for additional pine species totaled 614,279,324 with an average of 102,379,887 reads/sample and with ~5.0% removed during filtering (Table S3). These transcriptomes had an average of 82,049 assembled transcripts and an average N50 of 1882 (Table 1).

Table 1.
Descriptive statistics for representative transcripts identified with CD-HIT from Trinity-derived transcriptomes. Contig N50 statistics computed twice, once including all transcript contigs (all contigs), and again using a single representative contig, the longest isoform, per Trinity-defined ‘gene’ (longest isoform). “Experimental” denotes P. taeda transcriptome generated from reads collected in the present study, whereas all other entries denote transcriptome data from reads retrieved from NCBI.
BUSCO analysis demonstrated the relative completeness of transcriptomes generated for experimental and database-obtained reads with an average completeness score of 91.6% across all transcriptomes, where 61.6% of complete orthologs were represented by a single copy (Table S4).
3.2. Protein Identification
From the experimentally collected transcriptome data, at least one protein was identified for each of the 14 selected RRPs; however, the presence of multiple proteins, and/or isoforms, varied by protein. DICER-LIKE and ARGONAUTE represented the only protein groups with multiple unique proteins identified (four and three, respectively), confirmed by the subfamily information available in PANTHER Classification System; all other selected proteins were identified to a single PANTHER subfamily, or family-level identification could not be determined. For each unique family or subfamily, the transcript with both the highest similarity and bit score assignment was designated as the main sequence for this analysis (Table 2). DICER-LIKE and HSP90 were the only protein groups identified without a complete coding region; in these cases, the best scoring transcripts contained only a portion of the 5-prime or 3-prime flank of the coding region. A portion of the RRPs had multiple transcripts annotated to them, and these included AGO, ALA, DCL, SERK, and RdRp; for all other protein groups, only a single protein was identified with a complete coding region for its ascribed RRP.

Table 2.
Putative RNAi-related proteins identified in loblolly pine from experimental samples, including their similarity (E-value, bit score, and percent identity) to the top sequence when queried against the NCBI nr database and the corresponding PANTHER classification identified using InterProScan.
Annotation of RRPs for the additional pine species was limited to DICER-LIKE and ARGONAUTE. Across the six species, two DICER-LIKE proteins were identified: DCL3 and DCL4. Multiple transcripts, and sometimes multiple isoforms of each transcript, were identified to the DCL3 subfamily. However, DCL4 was represented by only a single transcript containing a complete coding region with a single isoform in all species except P. ponderosae, where two isoforms were assigned to the DCL4 subfamily, both of which were designated complete coding regions. ARGONAUTE proteins annotated from the pine species showed greater numbers of unique protein subfamilies identified from the PANTHER database as well as a greater number of isoforms containing coding regions identified to those same subfamilies. ARGONAUTE proteins annotated according to PANTHER subfamily designation included AGO1, AGO2, AGO4, and AGO7.
3.3. Differential Expression Analysis
Treated and untreated loblolly pine reads were aligned to the Ptaeda2.0 reference genome using HISAT2 (Table S5), yielding an average overall alignment rate of 93.48% across the sets. Differential expression analysis was conducted by DESeq2 on 85,443 assembled transcripts, revealing a total of 7131 DETs with an average length of ~52,426 bp. Of these transcripts, 2629 were upregulated (having a significantly higher transcript count in treated seedlings relative to untreated seedlings) and 4502 were downregulated (Table 3). From these DETs a total of 161,997 long open reading frames (longORF; ≥100 aa) were identified; each transcript contained ~22 longORF, demonstrating the large size of the transcripts assembled with HISAT2. In addition to having a greater number of DETs in the downregulated group, these transcripts were larger on average (~56,120 bp), yielding ~24 longORFs/transcript, compared to their upregulated counterparts (~46,102 bp, and ~20 longORFs/transcript). Subsequently, 113,087 possible peptide sequences were compiled, corresponding to a 69.8% prediction rate from longORF to a possible peptide sequence. Transcript identification tags for annotated RRPs were queried against all DET lists, revealing multiple differentially expressed RRPs. Some transcripts contained multiple complete coding regions of the same type of predicted peptide; however, only a single isoform was considered for the DE count. Of the 16 differentially expressed RRPs, two transcripts, an RdRp and an ALA, had higher mRNA levels in dsRNA-treated seedlings compared to controls. In contrast, five additional transcripts containing RdRp, three HSP90, two ALA, and one of each AGO, AMP1, SGS3, and SERK, were present in lower levels in treated seedlings (Supplementary File S1).

Table 3.
Statistics for differential expression analysis using HISAT, StringTie, and Ballgown. Including counts for the total number of transcripts assembled by HISAT with non-zero values across samples (total transcripts), differentially expressed transcripts (DETs) identified by DESeq2, long open reading frames (longORFs) and predicted peptide sequences identified from differentially expressed transcripts by TransDecoder, predicted peptide sequences that were assigned a functional annotation group by eggNOG mapper, and predicted peptide sequences which were assigned pathway identifiers during KEGG pathway enrichment. For “direction”, up corresponds to transcripts with significantly higher counts in dsRNA-treated loblolly, and down to transcripts with lower counts in treated seedlings.
3.4. Functional Annotation and Pathway Enrichment
Of the 113,087 possible peptide sequences compiled, 40,022 (35.2%) were assigned to a Cluster of Orthologous Groups (COG) category by eggNOG mapper. Excluding sequences that were assigned to unknown function (5444; 13.6%) or had no significant matches (1568; 3.9%), COG categories that accounted for >1% peptide sequences were consistent between the up- and downregulated transcript datasets. Between the two groups, the top nine categories were identical, with the ranked order varying in only two positions. An additional tenth category, “Transcription”, was present in the dataset representing increased gene expression (Figure 2). The most abundantly assigned category was “Replication and Repair”, accounting for ~47.6% of total assigned transcripts across both datasets. The next most abundantly assigned category was “Post-translational Modification and Chaperone Functions”, accounting for only 12.6% of assigned transcripts.
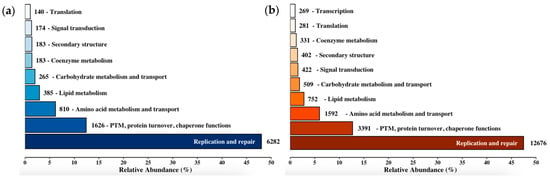
Figure 2.
Functional classification of predicted peptide sequences from differentially expressed (a) upregulated and (b) downregulated transcripts. Top Clusters of Ortholog Groups (COG) categories with each representing >1% of the total classified peptides. COG classification obtained from eggNOG-mapper v2.
Predicted peptide sequences were also subjected to pathway enrichment utilizing the Kyoto Encyclopedia of Genes and Genomes (KEGG) PATHWAY database through eggNOG mapper [64,67]. In total, 10,977 predicted peptide sequences (9.7%) were assigned to KEGG pathways (Table 3) with the up and down-expressed datasets containing 322 and 355 unique pathway identifiers, respectively.
Pathways representing <0.5% of the total mapped peptides were excluded from subsequent analyses, resulting in 37 unique pathways representing the top differentially expressed transcripts in both the up- and downregulated datasets. Twenty-eight pathways appeared in both datasets and the remaining nine pathways were unique to the corresponding dataset (Figure 3). Considering both the number of unique pathways and the total number of peptides assigned to those pathways, the largest portion of sequences were mapped to pathways under the metabolism category (54.6%), followed by organismal systems (16.5%) and genetic information processing (14.8%).
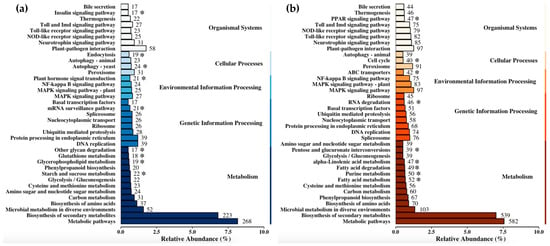
Figure 3.
KEGG pathway enrichment for predicted peptide sequences from differentially expressed (a) upregulated and (b) downregulated transcripts (eggNOG-mapper v2). Pathways that mapped to less than 0.5% of annotated transcripts were excluded. Asterisks denote pathways unique to each list.
3.5. Phylogenetic Comparison
Multiple DICER-LIKE and ARGONAUTE proteins (two and four, respectively) were annotated across pine species (Table 4).

Table 4.
Overview of putative RNAi machinery sequences in selected pine species. Amino acid length (AA length) of protein sequence and corresponding classification in the PANTHER system identified by InterProScan.
Analysis of DICER-LIKE orthologs across six pine species revealed similar phylogenetic patterns as previous genus-wide investigations [69] (Figure 4).
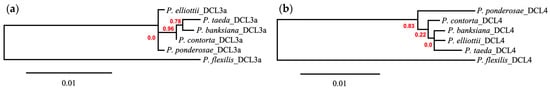
Figure 4.
Phylogram including (a) DICER-LIKE 3a and (b) DICER-LIKE 4 homologs with branch support values shown in red. Generated by Phylogeny.fr.
In all of the evaluated pines, DCL4 was represented by a single complete coding region, whereas multiple complete coding regions were predicted as ENDORIBONUCLEASE DICER HOMOLOG 3. Following alignment to previously annotated proteins, a subset of proteins predicted as DCL3 were identified as DCL3a. In both DCL analyses, P. flexilis, the only member analyzed belonging to the subgenus Strobus, was the most divergent of the protein sequences. Within subgenus Pinus section Trifoliae, to which the remaining five pines belong, analysis of DCL4 showed support for P. ponderosae as distinct from the remaining members; however, there was little support for disambiguation of the remaining proteins. Analysis of DCL3a proteins lacked sufficient diversity to support the three subsections of Trifoliae, as P. taeda and P. banksiana, members of subsection Australes and Contortae, respectively, were the most related within this analysis; however, P. contorta is the next most similar protein and is the closest relative of P. banksiana.
Similar to the DICER-LIKE proteins, ARGONAUTE proteins generally adhered to the broad phylogenetic patterns of pines, although between proteins there was diversity in clade formation. Analysis of ARGONAUTE 1 (AGO1) revealed a near reconstruction of the pattern observed in the reference phylogeny, with strong support for subsection Contortae and Australes, with P. ponderosae most dissimilar to the other Trifoliae and P. flexilis the most dissimilar from the Pinus subgenus (Figure 5). While AGO1 demonstrated high levels of support for the clades established in the work by Jin et al. (2021), other ARGONAUTE proteins did not retain this similarity. P. flexilis again was placed as the most divergent protein for ARGONAUTE 2 (AGO2) and ARGONAUTE 7 (AGO7). However, despite being classified in a different subgenus, for ARGONAUTE 4 (AGO4), P. flexilis showed more relatedness to the Contortae and Australes pines, with P. elliottii and P. ponderosae forming a unique clade in this phylogenic reconstruction. AGO2, similarly to DCL4, was represented by a single complete coding region, except for in P. elliottii and P. taeda, both of which contained two isoforms for AGO2. AGO7 had the lowest overall bootstrapping support, but this was likely due to the near-identical nature of the protein sequences.
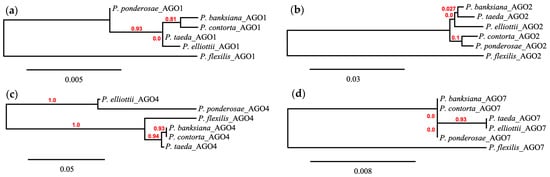
Figure 5.
Phylogram including (a) Argonaute 1, (b) Argonaute 2, (c) Argonaute 4, and (d) Argonaute 7 homologs with branch support values shown in red. Generated by Phylogeny.fr.
4. Discussion
Our work represents the first collection of transcriptomic data from conifer seedlings exposed to insect-specific dsRNA and lays groundwork for further work delving into interactions relevant to RNAi deployment. Deploying in planta dsRNA-mediated gene silencing against tree pests will require an in-depth understanding of the compatibility of the technology with the host tree, as host response may impact delivery and RNAi efficacy. Understanding species-specific differences in tree response to dsRNA biopesticides will be a critical aspect of wide-range application of this technology. Here, we demonstrate that DICER-LIKE and ARGONAUTE, key proteins in the RNAi pathway, are highly conserved across pine species, with a greater diversity of ARGONAUTE proteins due to their redundant functionalities. Due to their roles in plant development, stress response, and defense [70], we expect less divergence from ancestral phylogenetic patterns in DICER-LIKE proteins across pine species; this was supported in the DCL4 protein phylogeny. Some ARGONAUTE proteins, like AGO1A, adhered closely to the reference phylogeny, while others, like AGO7, showed little to no genetic differences between species, suggesting that some of these proteins experience little evolutionary change, possibly due to the redundant function of some AGO [71]. Providing protection and enhancing compatibility with the host plant will be crucial for developing practical dsRNA-based tree protection products. With efforts for developing and optimizing dsRNA delivery in loblolly pine underway [36], understanding the composition and similarities of RRPs across pine species will aid in the adaptation of this technology across the genus. Exploring how dsRNAs will behave in other pine species will be necessary for the useful deployment of this technology for widespread tree protection.
The loblolly pine genome is one of the largest sequenced and assembled [57,72], yet portions of its genetic content remain unknown. Some proteins related to sRNA biogenesis and silencing pathways have been identified in other Pinus, but very few annotated sequences for RNAi-related genes in pines are available [73,74]. We identified at least one ortholog of 14 different RNAi-related proteins in loblolly pine, providing foundational information to facilitate future RNAi-related work in this species. Plants possess multiple RNAi pathways that can result in PTGS beyond dsRNA mediated, or virus-induced gene silencing. Micro-RNA (miRNA)-mediated gene silencing relies on encoded miRNA genes that are preprocessed and cleaved by a suite of proteins, including DICER-LIKE proteins, from the virus-induced gene silencing pathway, in conjunction with accessory proteins SE, a zinc finger, and HYL1, a double-stranded RNA binding protein. Following preprocessing, the intermediate miRNA is again cleaved by a DICER-LIKE protein and methylated by the RNA methyltransferase HEN1, after which the mature miRNA is exported from the nucleus to the cytoplasm, where it binds to RISC and is guided to complementary messenger RNA to complete the gene-silencing process [75]. The natural-antisense siRNA (nat-siRNA) pathway is initiated following the transcription of multiple genes whose complementary mRNA products anneal to each other, resulting in dsRNA structures. These novel dsRNA structures are cleaved by a DICER-LIKE protein, in combination with SGS3 and RNA-DEPENDENT RNA POLYMERASE 6, resulting in 24 nt natural-antisense siRNAs which are incorporated into RISC and result in degradation of cis-antisense mRNA molecules [76,77]. Finally, the transacting siRNA (ta-siRNA) pathway operates similarly to and in conjunction with the miRNA pathway, in that following the transcription of specially encoded transacting siRNA genes, these mRNA products are bound by complementary miRNA sequences housed in RISC and cleaved [78]. These single-stranded RNAs are protected from degradation by SGS3 and subsequently converted to dsRNA by RDR6 by synthesizing the complementary strand. Once double-stranded, a DICER-LIKE protein cleaves these molecules into mature 21 nt transacting siRNAs [78]. Exogenous and endogenous RNA processing pathways are complex; understanding their interplay and their effects on longevity and bioactivity of insect-specific dsRNA will be crucial to fine-tuning this technology for tree pest management.
After annotating a suite of RRPs in loblolly pine and assessing the differential expression of transcripts corresponding to these proteins, we found a surprising absence of a measurable RNAi response. We note that a small sample size due to limitations in available plant material could explain the lack of measurable differences in the expression of RRPs in treated seedlings. In insects, exposure to dsRNA increases the expression of certain RRPs, including ARGONAUTE and DICER-LIKE [79]. However, only three RRPs were upregulated in dsRNA-treated seedlings in the current study, despite dsRNA presence in the tissue at the time of sampling. Our methodology may have muted any changes in gene expression; our sampling was not tissue-specific and we collected RNA from total seedlings. Previous studies demonstrated tissue-specific differences in dsRNA persistence in loblolly pine [36] and in deciduous white oak, Quercus alba (L.) [80]. Had a similar methodology been used here, tissue-specific transcriptomic differences in RRPs may have become evident. Additionally, the RNAi response may be minimal, possibly relegated to certain cell types within exposed seedlings, or it may be ephemeral, expressed only briefly. The 48 h time scale used here was selected based on previous work showing that dsSHI recovery in loblolly pine declined from 24 to 72 h, suggesting that some of the dsRNA was cleared in that time [36]. Finally, since the RNAi response is critical to cellular processing and defense, the baseline RNAi response may be expressed constitutively and would not require a drastic increase in expression in response to exogenous dsRNA. More sensitive techniques, such as single-cell RNA sequencing or quantitative reverse-transcription PCR (RT-qPCR), could be utilized to investigate the response of these genes on a much finer scale, and a time series analysis will be vital to characterizing the onset of an RNAi response.
The current study delivered dsRNA hydroponically, relying on systemic in planta spread of the dsRNA treatment. Our earlier study with white oak seedlings, which terminated at 7 days, showed the consistent recovery of dsRNA when delivered hydroponically [80]. In contrast, hydroponic delivery of dsRNAs in loblolly pine showed rapid dissemination but also steady decline over 7 days [36], suggesting inherent differences in the longevity of dsRNAs between deciduous and coniferous plants. Differences in response to topically applied dsRNA have also been documented in herbaceous plants. In Nicotiana benthamiana (Domin), high-pressure sprays of two dsRNAs of differing length were not sufficient to silence a transgene (GFP), and subsequent sRNA sequencing revealed that dsRNA was not being processed into siRNAs [81]. Conversely, siRNA biogenesis and reduced transcript accumulation of target genes was confirmed in Arabidopsis thaliana (L.) Heynh. following simple foliar application [82,83]. Pampolini and Rieske [84] demonstrated that topical foliar application was sufficient to allow for the dsRNA penetration of the leaf surface and subsequent translocation to covered, untreated leaves in Fraxinus pennsylvanica (Marshall). Moreover, this dsRNA retained its bioactivity, leading to a reduction in total larval gallery area of emerald ash borer (Agrilus planipennis, Fairmaire) reared on experimental trees. Hunter, Glick, Paldi, and Bextine [30] demonstrated the continued persistence of dsRNA targeting Asian citrus psyllid (Diaphorina citri, Kuwayama) in 3 m tall Citrus sp. trees for 57 days after only a single application. While the mechanism for sRNA transduction in plants is believed to be facilitated by intercellular plasmodesmata and the vascular system over short and long distances, respectively, less is known regarding the mechanism for long RNA translocation [85,86]. Mechanisms of dsRNA uptake have been determined in insects, fungi, and nematodes, including both clathrin-mediated endocytosis and SID-1 sensitization [87,88,89,90], but there is scarce data documenting the mechanism of dsRNA uptake in plant cells. Multiple factors complicate the efficient uptake of dsRNAs into plant tissue, including waxy leaf cuticles, extracellular nucleases, and incompatibility with plasma membrane uptake mechanisms [91]. Despite dsRNA being present in our seedlings, our methodology did not detect an increase in RNAi machinery.
Although our preliminary differential expression analysis revealed only three upregulated RRPs in treated plants, other pathways showed higher rates of expression when exposed to dsRNA. When comparing the most prominently represented pathways from the up and downregulated DETs, nine pathways were unique to each list. Autophagy plays a critical role in plant survival and stress responses by facilitating the bulk decomposition of cytoplasmic materials such as proteins [92]. While understudied in plants, upregulation of autophagy in treated seedlings may be due to viral defense mechanisms, as selective autophagy can regulate the degradation of viral dsRNA in Arabidopsis thaliana [93]. The presence of dsRNA in plants not only induces the RNAi response but can also induce pattern-triggered immunity defense responses, demonstrating that dsRNAs serve as a pathogen-associated molecular pattern, initiating other defense pathways after recognition [94]. Upregulation of transcripts mapped to plant hormone signal transduction in treated seedlings may be a result of pattern-triggered immunity, which can lead to a cascade of various hormone-mediated processes, sometimes at the cost of plant growth and development [95]. Future work investigating the vigor and growth-related impacts of insecticidal dsRNA on treated plants will be necessary to understand the nuances of host-delivered deployment. Upregulation of the mRNA surveillance pathway may be indicative of cellular mRNA degradation, which can manifest to reduce the unnecessary intervention of the RNAi pathway to manage native transcription [96]. Crosstalk between RNA surveillance and RNA silencing may determine the regulation of these pathways in response to endogenously and exogenously derived RNA molecules, with surveillance acting as a first line defense for the destruction of aberrant, endogenous mRNAs [97]. Interestingly, in the presence of excess exogenous dsRNA, the interference pathway can override the surveillance pathway [96]. However, despite dsRNA being present at the time of sampling in the current study, mRNA surveillance pathway-related transcripts were present in higher quantities in treated seedlings. Due to the complexity of plant immunity and defense response combined with the lack of information relating to both the fate and downstream impacts of exogenous dsRNA in woody plants, future work should focus on elucidating the effects of this technology on plant processes to optimize plant-delivered RNAi protection. This is the first study to investigate the molecular response of a woody plant to exogenous dsRNA targeting an insect pest, providing foundational information for the deployment of RNAi technology against tree pests.
Despite their long evolutionary history with North American conifers, our rapidly changing climate and limited management have altered the frequency, intensity, and location of bark beetle outbreaks [98,99]. In addition to altered outbreak dynamics, beetles on both sides of the continent are experiencing northward range expansion facilitated by warmer temperatures and suitable historic and novel hosts [100,101,102,103]. The boreal forests of North America represent a convergence point for expanding ranges of these bark beetles, demonstrating an urgent need for multifaceted approaches to management. Integrating molecular tools to combat forest pests in tandem with traditional management approaches will bolster existing integrated pest management strategies and may open avenues for innovative applications. dsRNA-mediated gene silencing is effective in southern and mountain pine beetles, and a single dsRNA has been reported to have congeneric impacts [104], opening the possibilities of broad-scale pest suppression using genus-specific approaches. With careful design, it may be possible to target and control multiple forest pests with a single dsRNA construct, which may prove particularly useful in areas experiencing simultaneous attacks from multiple bark beetle species.
The foundational information generated here shows a similarity of core RNAi machinery among pines. Combining nimbly designed RNAi constructs that can target conserved regions across multiple target pest species with compatibility in diverse tree hosts may be the key to the widespread, efficient deployment of this technology for the protection of forest trees.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15060938/s1.
Author Contributions
Conceptualization, Z.B. and L.K.R.; methodology, Z.B.; software, Z.B.; validation, Z.B.; formal analysis, Z.B.; investigation, Z.B. and L.K.R.; resources, L.K.R.; data curation, Z.B.; writing—original draft preparation, Z.B.; writing—review and editing, L.K.R.; visualization, Z.B.; supervision, L.K.R.; project administration, L.K.R.; funding acquisition, L.K.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the United States Department of Agriculture Animal and Plant Health Inspection Service AP20PPQS&T00C061, the University of Kentucky, and the Kentucky Agricultural Experiment Station under McIntire-Stennis 2352657000 and is published with the approval of the director.
Data Availability Statement
Raw RNA sequencing reads have been deposited into the NCBI Sequence Read Archive (SRA) under the accession numbers SRR25238440-SRR 25238443 in association with BioProject PRJNA993588. Each Transcriptome Shotgun Assembly (TSA) project has been deposited at DDBJ/EMBL/GenBank under the accession GKVD00000000- GKVG00000000. The versions described in this paper are the first versions, GKVD01000000- GKVG01000000 (Table S5). Putative RNAi-related proteins annotated from experimentally derived reads for Pinus taeda have been deposited in NCBI GenBank under the accession numbers OR466146-OR466165; putative RNAi-related proteins annotated using available reads from NCBI SRA in additional pines are archived in NCBI Third Party Annotation database (P. banksiana: BK063876- BK063881; P. contorta: BK063882- BK063887; P. elliottii: BK063888- BK063893; P. flexilis: BK063894- BK063899; P. ponderosae: BK063900- BK063905; P. taeda: BK063906- BK063911).
Acknowledgments
The authors thank C. Dana Nelson (USDA Forest Service Forest Health Research and Education Center) for plant material, Julian R. Dupuis and Jeramiah Smith (University of Kentucky) for computational consultation, Morgan Knutsen, Beth Kyre, Flávia Pampolini, and Mary Wallace for laboratory and technical assistance, and Sebastien Santini (CNRS/AMU IGS UMR7256) and the PACA Bioinfo platform for the availability and management of the phylogeny.fr website used to construct phylograms for RNAi-related proteins across selected pine species. Additionally, the authors thank the University of Kentucky Center for Computational Sciences and Information Technology Services Research Computing for their support and use of the Lipscomb and Morgan Compute Clusters and associated research computing resources.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Linnakoski, R.; Kasanen, R.; Dounavi, A.; Forbes, K.M. Editorial: Forest Health Under Climate Change: Effects on Tree Resilience, and Pest and Pathogen Dynamics. Front. Plant Sci. 2019, 10, 1157. [Google Scholar] [CrossRef] [PubMed]
- Sicard, P.; Dalstein-Richier, L. Health and vitality assessment of two common pine species in the context of climate change in southern Europe. Environ. Res. 2015, 137, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Han, H.; Chen, S.; Li, M. A fragile soil moisture environment exacerbates the climate change-related impacts on the water use by Mongolian Scots pine (Pinus sylvestris var. mongolica) in northern China: Long-term observations. Agric. Water Manag. 2021, 251, 106857. [Google Scholar] [CrossRef]
- Lin, L.; He, J.; Xie, L.; Cui, G. Prediction of the Suitable Area of the Chinese White Pines (Pinus subsect. Strobus) under Climate Changes and Implications for Their Conservation. Forests 2020, 11, 996. [Google Scholar] [CrossRef]
- Hallingbäck, H.R.; Burton, V.; Vizcaíno-Palomar, N.; Trotter, F.; Liziniewicz, M.; Marchi, M.; Berlin, M.; Ray, D.; Benito Garzón, M. Managing Uncertainty in Scots Pine Range-Wide Adaptation Under Climate Change. Front. Ecol. Evol. 2021, 9, 724051. [Google Scholar] [CrossRef]
- Jactel, H.; Koricheva, J.; Castagneyrol, B. Responses of forest insect pests to climate change: Not so simple. Curr. Opin. Insect Sci. 2019, 35, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yuan, Y.; Li, X.; Zhang, J. Maximum Entropy Modeling to Predict the Impact of Climate Change on Pine Wilt Disease in China. Front. Plant Sci. 2021, 12, 652500. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gallego, M.; Galiano, L.; Martínez-Vilalta, J.; Stenlid, J.; Capador-Barreto, H.D.; Elfstrand, M.; Camarero, J.J.; Oliva, J. Interaction of drought- and pathogen-induced mortality in Norway spruce and Scots pine. Plant Cell Environ. 2022, 45, 2292–2305. [Google Scholar] [CrossRef] [PubMed]
- Kurz, W.A.; Dymond, C.C.; Stinson, G.; Rampley, G.J.; Neilson, E.T.; Carroll, A.L.; Ebata, T.; Safranyik, L. Mountain pine beetle and forest carbon feedback to climate change. Nature 2008, 452, 987–990. [Google Scholar] [CrossRef]
- Clark, K.L.; Skowronski, N.; Hom, J. Invasive insects impact forest carbon dynamics. Glob. Change Biol. 2010, 16, 88–101. [Google Scholar] [CrossRef]
- Ghimire, B.; Williams, C.A.; Collatz, G.J.; Vanderhoof, M.; Rogan, J.; Kulakowski, D.; Masek, J.G. Large carbon release legacy from bark beetle outbreaks across Western United States. Glob. Change Biol. 2015, 21, 3087–3101. [Google Scholar] [CrossRef]
- Richardson, D.M. Ecology and Biogeography of Pinus; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Johnsen, K.H.; Wear, D.; Oren, R.; Teskey, R.O.; Sanchez, F.; Will, R.; Butnor, J.; Markewitz, D.; Richter, D.; Rials, T.; et al. Meeting Global Policy Commitments: Carbon Sequestration and Southern Pine Forests. J. For. 2001, 99, 14–21. [Google Scholar] [CrossRef]
- Laclau, P. Biomass and carbon sequestration of ponderosa pine plantations and native cypress forests in northwest Patagonia. For. Ecol. Manag. 2003, 180, 317–333. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Sullivan, D.S.; Lindgren, P.M.F.; Ransome, D.B.; Zabek, L. Twenty-Five Years after Stand Thinning and Repeated Fertilization in Lodgepole Pine Forest: Implications for Tree Growth, Stand Structure, and Carbon Sequestration. Forests 2020, 11, 337. [Google Scholar] [CrossRef]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale Drivers of Natural Disturbances Prone to Anthropogenic Amplification: The Dynamics of Bark Beetle Eruptions. BioScience 2008, 58, 501–517. [Google Scholar] [CrossRef]
- Fettig, C.J.; Egan, J.M.; Delb, H.; Hilszczański, J.; Kautz, M.; Munson, A.S.; Nowak, J.T.; Negrón, J.F. 11—Management tactics to reduce bark beetle impacts in North America and Europe under altered forest and climatic conditions. In Bark Beetle Management, Ecology, and Climate Change; Gandhi, K.J.K., Hofstetter, R.W., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 345–394. [Google Scholar]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Sen, G.L.; Blau, H.M. A brief history of RNAi: The silence of the genes. FASEB J. 2006, 20, 1293–1299. [Google Scholar] [CrossRef]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef]
- Romano, N.; Macino, G. Quelling: Transient inactivation of gene expression in Neurospora crassa by transformation with homologous sequences. Mol. Microbiol. 1992, 6, 3343–3353. [Google Scholar] [CrossRef]
- Cogoni, C.; Macino, G. Post-transcriptional gene silencing across kingdoms. Curr. Opin. Genet. Dev. 2000, 10, 638–643. [Google Scholar] [CrossRef]
- Obbard, D.J.; Gordon, K.H.J.; Buck, A.H.; Jiggins, F.M. The evolution of RNAi as a defence against viruses and transposable elements. Philos. Trans. R. Soc. B Biol. Sci. 2008, 364, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Shabalina, S.A.; Koonin, E.V. Origins and evolution of eukaryotic RNA interference. Trends Ecol. Evol. 2008, 23, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Whyard, S.; Singh, A.; Wong, S. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem. Mol. Biol. 2009, 39, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Kyre, B.R.; Rodrigues, T.B.; Rieske, L.K. RNA interference and validation of reference genes for gene expression analyses using qPCR in southern pine beetle, Dendroctonus frontalis. Sci. Rep. 2019, 9, 5640. [Google Scholar] [CrossRef] [PubMed]
- Kyre, B.R.; Bentz, B.J.; Rieske, L.K. Susceptibility of mountain pine beetle (Dendroctonus ponderosae Hopkins) to gene silencing through RNAi provides potential as a novel management tool. For. Ecol. Manag. 2020, 473, 118322. [Google Scholar] [CrossRef]
- Liu, B.; Fu, D.; Ning, H.; Tang, M.; Chen, H. Knockdown of CYP6CR2 and CYP6DE5 reduces tolerance to host plant allelochemicals in the Chinese white pine beetle Dendroctonus armandi. Pestic. Biochem. Physiol. 2022, 187, 105180. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.; Rieske, L.K. Ingestion of Species-Specific dsRNA Alters Gene Expression and Can Cause Mortality in the Forest Pest, Ips calligraphus. Forests 2023, 14, 422. [Google Scholar] [CrossRef]
- Hunter, W.B.; Glick, E.; Paldi, N.; Bextine, B.R. Advances in RNA interference: dsRNA Treatment in Trees and Grapevines for Insect Pest Suppression. Southwest. Entomol. 2012, 37, 85–87. [Google Scholar] [CrossRef]
- Miguel, K.; Scott, J. The next generation of insecticides: dsRNA is stable as a foliar-applied insecticide. Pest Manag. Sci. 2015, 72, 801–809. [Google Scholar] [CrossRef]
- Dalakouras, A.; Jarausch, W.; Buchholz, G.; Bassler, A.; Braun, M.; Manthey, T.; Krczal, G.; Wassenegger, M. Delivery of Hairpin RNAs and Small RNAs Into Woody and Herbaceous Plants by Trunk Injection and Petiole Absorption. Front. Plant Sci. 2018, 9, 1253. [Google Scholar] [CrossRef]
- Ghosh, S.K.B.; Hunter, W.B.; Park, A.L.; Gundersen-Rindal, D.E. Double-stranded RNA Oral Delivery Methods to Induce RNA Interference in Phloem and Plant-sap-feeding Hemipteran Insects. J. Vis. Exp. 2018, 12, e57390. [Google Scholar] [CrossRef] [PubMed]
- Cagliari, D.; Dias, N.P.; Galdeano, D.M.; dos Santos, E.Á.; Smagghe, G.; Zotti, M.J. Management of Pest Insects and Plant Diseases by Non-Transformative RNAi. Front. Plant Sci. 2019, 10, 1319. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Mishra, S.K.; Sridharan, K.; Barnes, E.R.; Alyokhin, A.; Tuttle, R.; Kokulapalan, W.; Garby, D.; Skizim, N.J.; Tang, Y.-w.; et al. First Sprayable Double-Stranded RNA-Based Biopesticide Product Targets Proteasome Subunit Beta Type-5 in Colorado Potato Beetle (Leptinotarsa decemlineata). Front. Plant Sci. 2021, 12, 728652. [Google Scholar] [CrossRef] [PubMed]
- Bragg, Z.; Rieske, L.K. Spatial Distribution and Retention in Loblolly Pine Seedlings of Exogenous dsRNAs Applied through Roots. Int. J. Mol. Sci. 2022, 23, 9167. [Google Scholar] [CrossRef]
- Kaldis, A.; Berbati, M.; Melita, O.; Reppa, C.; Holeva, M.; Otten, P.; Voloudakis, A. Exogenously applied dsRNA molecules deriving from the Zucchini yellow mosaic virus (ZYMV) genome move systemically and protect cucurbits against ZYMV. Mol. Plant Pathol. 2018, 19, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Biedenkopf, D.; Will, T.; Knauer, T.; Jelonek, L.; Furch, A.C.U.; Busche, T.; Koch, A. Systemic spreading of exogenous applied RNA biopesticides in the crop plant Hordeum vulgare. ExRNA 2020, 2, 12. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Suprun, A.R.; Aleynova, O.A.; Ogneva, Z.V.; Dubrovina, A.S. Physiological Conditions and dsRNA Application Approaches for Exogenously induced RNA Interference in Arabidopsis thaliana. Plants 2021, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Hoang, B.T.L.; Fletcher, S.J.; Brosnan, C.A.; Ghodke, A.B.; Manzie, N.; Mitter, N. RNAi as a Foliar Spray: Efficiency and Challenges to Field Applications. Int. J. Mol. Sci. 2022, 23, 6639. [Google Scholar] [CrossRef]
- Pampolini, F.; Rodrigues, T.B.; Leelesh, R.S.; Kawashima, T.; Rieske, L.K. Confocal microscopy provides visual evidence and confirms the feasibility of dsRNA delivery to emerald ash borer through plant tissues. J. Pest Sci. 2020, 93, 1143–1153. [Google Scholar] [CrossRef]
- Pumplin, N.; Sarazin, A.; Jullien, P.E.; Bologna, N.G.; Oberlin, S.; Voinnet, O. DNA Methylation Influences the Expression of DICER-LIKE4 Isoforms, Which Encode Proteins of Alternative Localization and Function. Plant Cell 2016, 28, 2786–2804. [Google Scholar] [CrossRef]
- Chang, S.; Puryear, J.; Cairney, J. A Simple and Efficient Method for Isolating RNA from Pine Trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Freedman, A.H.; Gaunt, L. TranscriptomeAssemblyTools. GitHub Repository. 2020. Available online: https://github.com/harvardinformatics/TranscriptomeAssemblyTools (accessed on 29 November 2021).
- Krueger, F.; James, F.; Ewels, P.; Afyounian, E.; Weinstein, M.; Schuster-Boeckler, B.; Hulselmans, G.; Sclamons. TrimGalore; v0.6.6; GitHub Repository; GitHub: San Francisco, CA, USA, 2020; Available online: https://github.com/FelixKrueger/TrimGalore/releases/tag/0.6.6 (accessed on 29 November 2021).
- Andrews, S.; Mareq; Mahé, F.; Yi, H.; Brokamp, J.; Reimer, N. FastQC, v0.11.19. GitHub Repository. GitHub: San Francisco, CA, USA, 2020. Available online: https://github.com/s-andrews/FastQC/releases/tag/v0.11.9 (accessed on 15 November 2021).
- Grabherr, M.; Haas, B.; Yassour, M.; Levin, J.; Thompson, D.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef] [PubMed]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J. TransDecoder, v5.5.0. GitHub Repository. GitHub: San Francisco, CA, USA, 2018. Available online: https://github.com/TransDecoder/TransDecoder/releases/tag/TransDecoder-v5.5.0 (accessed on 29 November 2021).
- Bairoch, A.; Apweiler, R. The SWISS-PROT Protein Sequence Database: Its Relevance to Human Molecular Medical Research. J. Mol. Med. 1997, 75, 312–316. Available online: https://pubmed.ncbi.nlm.nih.gov/9181472/ (accessed on 12 June 2023). [PubMed]
- NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018, 46, D8–D13. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Thomas, P.D.; Ebert, D.; Muruganujan, A.; Mushayahama, T.; Albou, L.-P.; Mi, H. PANTHER: Making genome-scale phylogenetics accessible to all. Protein Sci. 2021, 31, 8–22. [Google Scholar] [CrossRef]
- Zimin, A.V.; Stevens, K.A.; Crepeau, M.W.; Puiu, D.; Wegrzyn, J.L.; Yorke, J.A.; Langley, C.H.; Neale, D.B.; Salzberg, S.L. An improved assembly of the loblolly pine mega-genome using long-read single-molecule sequencing. GigaScience 2017, 6, giw016. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Posit Team. RStudio: Integrated Development Environment for R. 2022. Available online: http://www.posit.co/ (accessed on 20 April 2022).
- R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.R-project.org/ (accessed on 20 April 2022).
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Koonin, E.V.; Lipman, D.J. A Genomic Perspective on Protein Families. Science 1997, 278, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Galperin, M.Y.; Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Res. 2015, 43, D261–D269. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef]
- Jin, W.-T.; Gernandt, D.S.; Wehenkel, C.; Xia, X.-M.; Wei, X.-X.; Wang, X.-Q. Phylogenomic and ecological analyses reveal the spatiotemporal evolution of global pines. Proc. Natl. Acad. Sci. USA 2021, 118, e2022302118. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Feng, Y.; Zhu, Z. Dicer-like (DCL) proteins in plants. Funct. Integr. Genom. 2009, 9, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Qi, Y. RNAi in Plants: An Argonaute-Centered View. Plant Cell 2016, 28, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Zimin, A.; Stevens, K.A.; Crepeau, M.W.; Holtz-Morris, A.; Koriabine, M.; Marçais, G.; Puiu, D.; Roberts, M.; Wegrzyn, J.L.; de Jong, P.J.; et al. Sequencing and Assembly of the 22-Gb Loblolly Pine Genome. Genetics 2014, 196, 875–890. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kendall, T.; Forsythe, E.S.; Dorantes-Acosta, A.; Li, S.; Caballero-Pérez, J.; Chen, X.; Arteaga-Vázquez, M.; Beilstein, M.A.; Mosher, R.A. Ancient Origin and Recent Innovations of RNA Polymerase IV and V. Mol. Biol. Evol. 2015, 32, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.-H.; Liu, C.; Yuan, H.-W.; Li, P.; Li, Y.; Li, W. Identification and expression profiles of sRNAs and their biogenesis and action-related genes in male and female cones of Pinus tabuliformis. BMC Genom. 2015, 16, 693. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mei, J.; Ren, G. Plant microRNAs: Biogenesis, Homeostasis, and Degradation. Front. Plant Sci. 2019, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Borsani, O.; Zhu, J.; Verslues, P.E.; Sunkar, R.; Zhu, J.-K. Endogenous siRNAs Derived from a Pair of Natural cis-Antisense Transcripts Regulate Salt Tolerance in Arabidopsis. Cell 2005, 123, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- El-Sappah, A.H.; Yan, K.; Huang, Q.; Islam, M.M.; Li, Q.; Wang, Y.; Khan, M.S.; Zhao, X.; Mir, R.R.; Li, J.; et al. Comprehensive Mechanism of Gene Silencing and Its Role in Plant Growth and Development. Front. Plant Sci. 2021, 12, 705249. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M. Biogenesis of trans-acting siRNAs, endogenous secondary siRNAs in plants. Genes Genet. Syst. 2013, 88, 77–84. [Google Scholar] [CrossRef]
- Garbutt, J.S.; Reynolds, S.E. Induction of RNA interference genes by double-stranded RNA; implications for susceptibility to RNA interference. Insect Biochem. Mol. Biol. 2012, 42, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Bragg, Z.; Rieske, L.K. Feasibility of Systemically Applied dsRNAs for Pest-Specific RNAi-Induced Gene Silencing in White Oak. Front. Plant Sci. 2022, 13, 830226. [Google Scholar] [CrossRef]
- Uslu, V.V.; Bassler, A.; Krczal, G.; Wassenegger, M. High-Pressure-Sprayed Double Stranded RNA Does Not Induce RNA Interference of a Reporter Gene. Front. Plant Sci. 2020, 11, 534391. [Google Scholar] [CrossRef] [PubMed]
- Dubrovina, A.S.; Aleynova, O.A.; Kalachev, A.V.; Suprun, A.R.; Ogneva, Z.V.; Kiselev, K.V. Induction of Transgene Suppression in Plants via External Application of Synthetic dsRNA. Int. J. Mol. Sci. 2019, 20, 1585. [Google Scholar] [CrossRef] [PubMed]
- Nityagovsky, N.N.; Kiselev, K.V.; Suprun, A.R.; Dubrovina, A.S. Exogenous dsRNA Induces RNA Interference of a Chalcone Synthase Gene in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 5325. [Google Scholar] [CrossRef] [PubMed]
- Pampolini, F.; Rieske, L.K. Foliar Application of dsRNA to Induce Gene Silencing in Emerald Ash Borer: Systemic Distribution, Persistence, and Bioactivity. Forests 2023, 14, 1853. [Google Scholar] [CrossRef]
- Kehr, J.; Kragler, F. Long distance RNA movement. New Phytol. 2018, 218, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Mermigka, G.; Verret, F.; Kalantidis, K. RNA silencing movement in plants. J. Integr. Plant Biol. 2016, 58, 328–342. [Google Scholar] [CrossRef]
- Feinberg, E.H.; Hunter, C.P. Transport of dsRNA into Cells by the Transmembrane Protein SID-1. Science 2003, 301, 1545–1547. [Google Scholar] [CrossRef]
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J. Insect Physiol. 2009, 56, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Gao, X.; Xu, J.; Liang, X.; Li, Q.; Yao, J.; Zhu, K.Y. Clathrin-dependent endocytosis plays a predominant role in cellular uptake of double-stranded RNA in the red flour beetle. Insect Biochem. Mol. Biol. 2015, 60, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Wytinck, N.; Sullivan, D.S.; Biggar, K.T.; Crisostomo, L.; Pelka, P.; Belmonte, M.F.; Whyard, S. Clathrin mediated endocytosis is involved in the uptake of exogenous double-stranded RNA in the white mold phytopathogen Sclerotinia sclerotiorum. Sci. Rep. 2020, 10, 12773. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.; Deikman, J.; Hendrix, B.; Iandolino, A. Barriers to Efficient Foliar Uptake of dsRNA and Molecular Barriers to dsRNA Activity in Plant Cells. Front. Plant Sci. 2020, 11, 816. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Li, X.; Yang, M.; Shao, Q.; Zhao, Y.; Ma, C.; Wang, P. Autophagy: An Intracellular Degradation Pathway Regulating Plant Survival and Stress Response. Front. Plant Sci. 2020, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Hafrén, A.; Macia, J.-L.; Love, A.J.; Milner, J.J.; Drucker, M.; Hofius, D. Selective autophagy limits cauliflower mosaic virus infection by NBR1-mediated targeting of viral capsid protein and particles. Proc. Natl. Acad. Sci. USA 2017, 114, E2026–E2035. [Google Scholar] [CrossRef]
- Niehl, A.; Wyrsch, I.; Boller, T.; Heinlein, M. Double-stranded RNAs induce a pattern-triggered immune signaling pathway in plants. New Phytol. 2016, 211, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, H. mRNA decay in plants: Both quantity and quality matter. Curr. Opin. Plant Biol. 2017, 35, 138–144. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X. RNA quality control as a key to suppressing RNA silencing of endogenous genes in plants. Mol. Plant 2016, 9, 826–836. [Google Scholar] [CrossRef]
- Williams, D.W.; Liebhold, A.M. Climate change and the outbreak ranges of two North American bark beetles. Agric. For. Entomol. 2002, 4, 87–99. [Google Scholar] [CrossRef]
- Biedermann, P.H.W.; Müller, J.; Grégoire, J.-C.; Gruppe, A.; Hagge, J.; Hammerbacher, A.; Hofstetter, R.W.; Kandasamy, D.; Kolarik, M.; Kostovcik, M.; et al. Bark Beetle Population Dynamics in the Anthropocene: Challenges and Solutions. Trends Ecol. Evol. 2019, 34, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Cullingham, C.I.; Cooke, J.E.K.; Dang, S.; Davis, C.S.; Cooke, B.J.; Coltman, D.W. Mountain pine beetle host-range expansion threatens the boreal forest. Mol. Ecol. 2011, 20, 2157–2171. [Google Scholar] [CrossRef] [PubMed]
- Dodds, K.J.; Aoki, C.F.; Arango-Velez, A.; Cancelliere, J.; D’Amato, A.W.; DiGirolomo, M.F.; Rabaglia, R.J. Expansion of Southern Pine Beetle into Northeastern Forests: Management and Impact of a Primary Bark Beetle in a New Region. J. For. 2018, 116, 178–191. [Google Scholar] [CrossRef]
- Sambaraju, K.R.; Carroll, A.L.; Aukema, B.H. Multiyear weather anomalies associated with range shifts by the mountain pine beetle preceding large epidemics. For. Ecol. Manag. 2019, 438, 86–95. [Google Scholar] [CrossRef]
- Aoki, C.F.; Munro, H.L.; Gandhi, K.J.K. 3—Responses and modeling of southern pine beetle and its host pines to climate change. In Bark Beetle Management, Ecology, and Climate Change; Gandhi, K.J.K., Hofstetter, R.W., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 55–85. [Google Scholar]
- Kyre, B.R.; Rieske, L.K. Using RNAi to silence heat shock protein has congeneric effects in North America’s Dendroctonus bark beetles. For. Ecol. Manag. 2022, 520, 120367. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).