Climate–Growth Relationships of Mongolian Pine (Pinussylvestris var. mongholica) along an Altitudinal Gradient of Northeast China
Abstract
1. Introduction
2. Materials and Methods
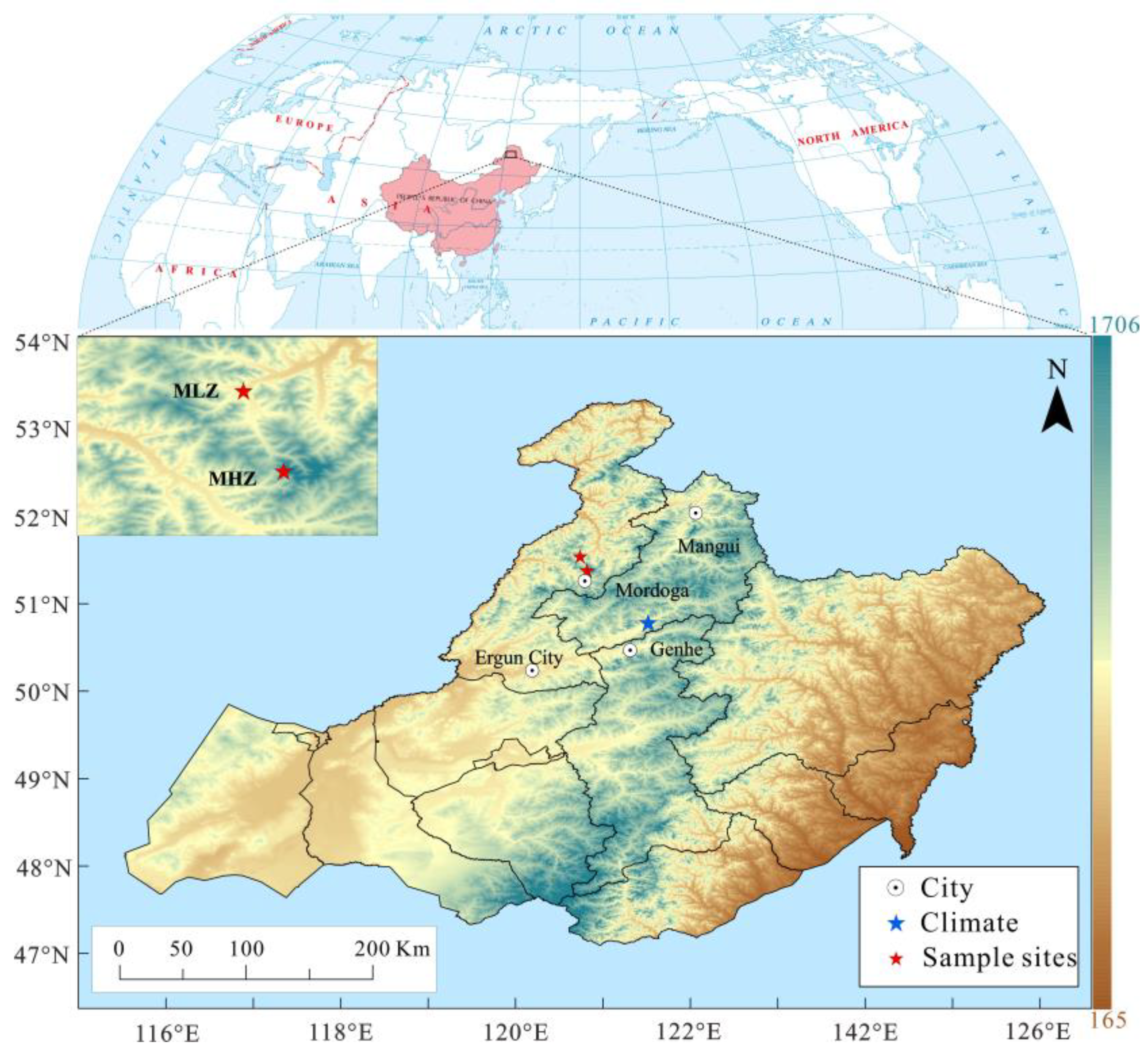
2.1. Study Area
2.2. Tree Ring Sampling and Dendrochronology Development
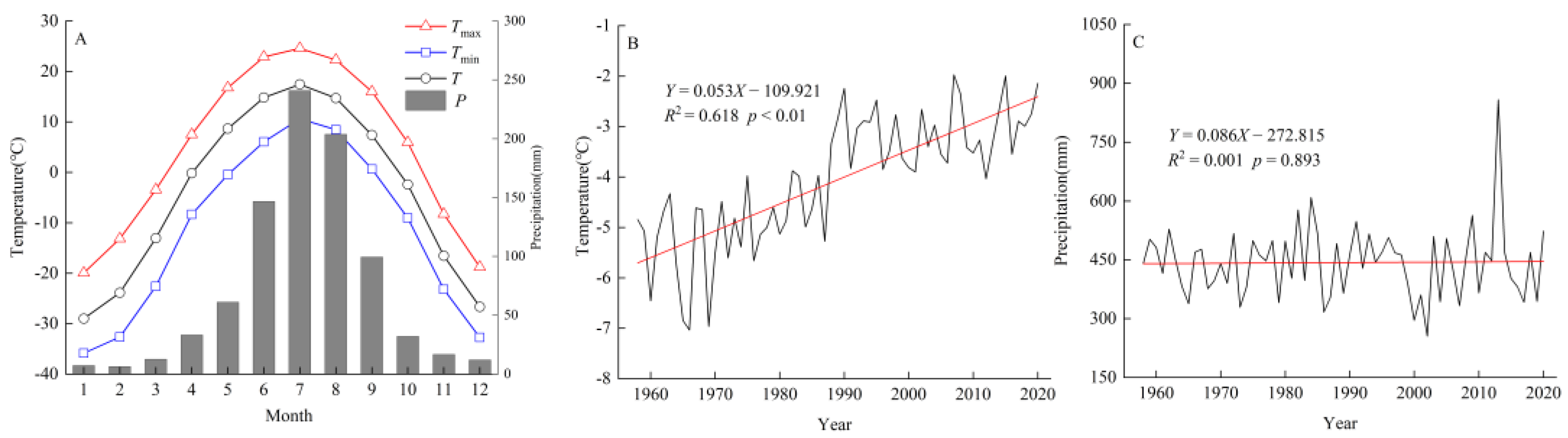
2.3. Meteorological Data
2.4. Research Methods
3. Results
3.1. Chronological Characteristics
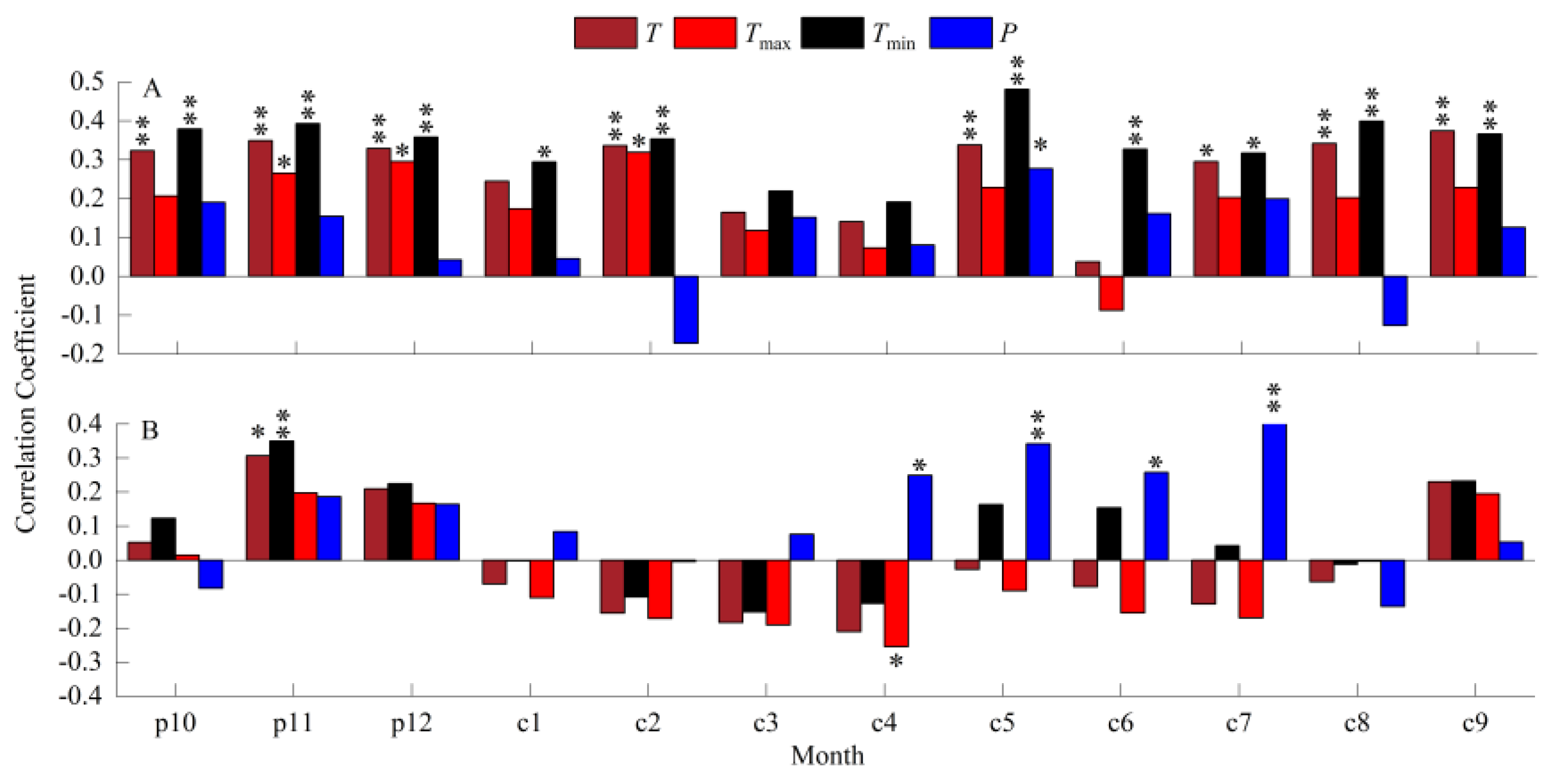
3.2. Correlation between Radial Growth of MP and Climatic Factors
3.3. Correlation between Radial Growth of MP and Climatic Factors
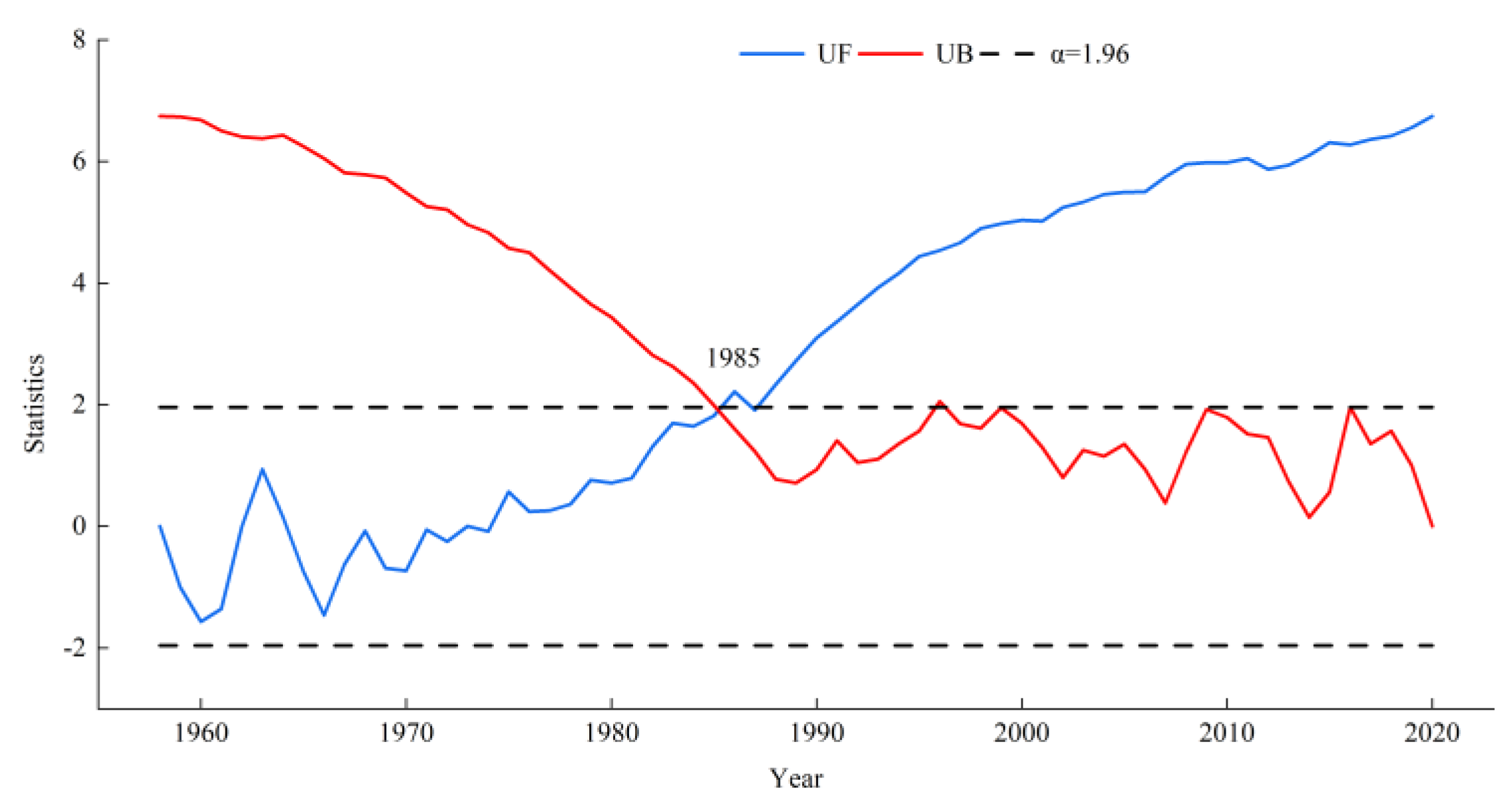
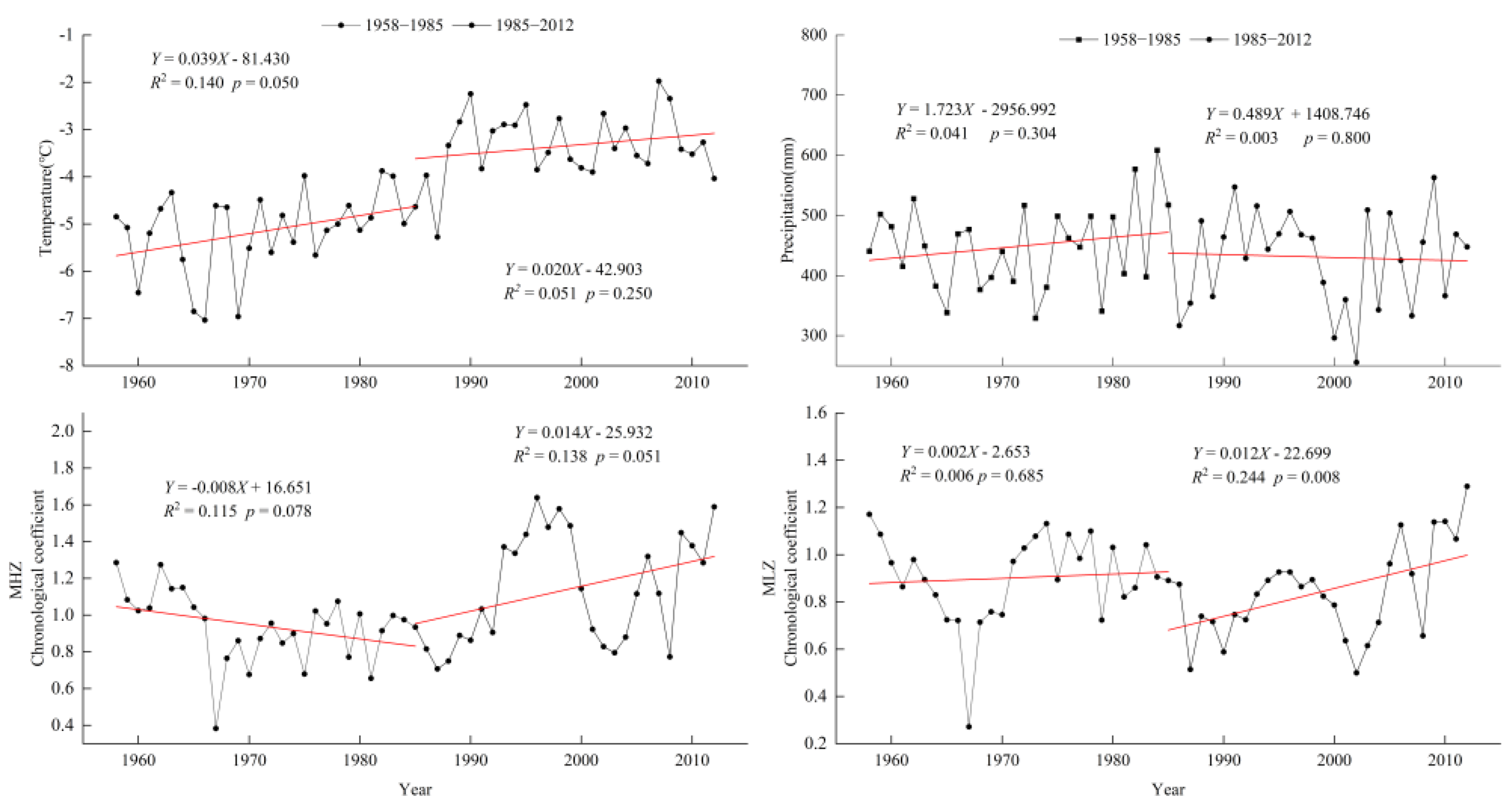
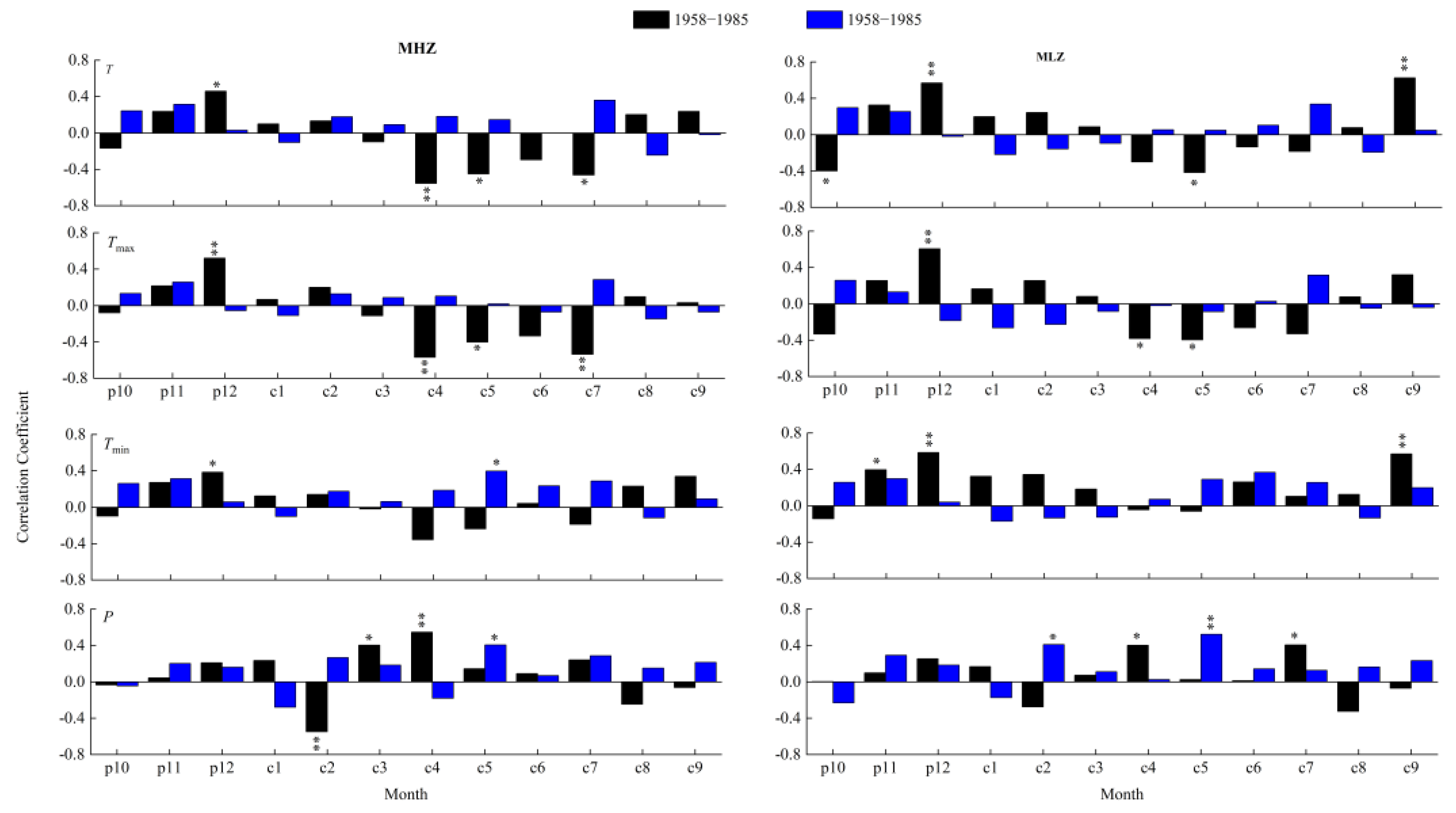
3.4. Response of Radial Growth to Climate before and after 1985
3.5. Temporal Stability of Climate–Growth Relationships
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, G.; Fu, B. Impact of global climate change on forest ecosystems. J. Nat. Resour. 2001, 6, 71–78. [Google Scholar]
- Kikstra, J.S.; Nicholls, Z.R.; Smith, C.J.; Lewis, J.; Lamboll, R.D.; Byers, E.; Sandstad, M.; Meinshausen, M.; Gidden, M.J.; Rogelj, J. The IPCC Sixth Assessment Report WGIII climate assessment of mitigation pathways: From emissions to global temperatures. Geosci. Model Dev. 2022, 15, 9075–9109. [Google Scholar] [CrossRef]
- Song, M.; Yang, B.; Ljungqvist, F.C.; Shi, F.; Qin, C.; Wang, J. Tree-ring-based winter temperature reconstruction for East Asia over the past 700 years. Sci. China Earth Sci. 2021, 64, 872–889. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Corlett, R.T. Impacts of warming on tropical lowland rainforests. Trends Ecol. Evol. 2011, 26, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Dapao, Y.; Zhou, L.; Dai, L.; Wang, Q.; Liu, M. Application of dendrochronology in global change research. Chin. J. Ecol. 2003, 6, 91–96. [Google Scholar]
- Fritts, H. Tree Rings and Climate. Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Truettner, C.; Anderegg, W.R.L.; Biondi, F.; Koch, G.W.; Ogle, K.; Schwalm, C.; Litvak, M.E.; Shaw, J.D.; Ziaco, E. Conifer radial growth response to recent seasonal warming and drought from the southwestern USA. For. Ecol. Manag. 2018, 418, 55–62. [Google Scholar] [CrossRef]
- Gou, X.; Chen, F.; Jacoby, G.; Cook, E.; Yang, M.; Peng, J.; Zhang, Y. Rapid tree growth with respect to the last 400 years in response to climate warming, northeastern Tibetan Plateau. Int. J. Climatol. A J. R. Meteorol. Soc. 2007, 27, 1497–1503. [Google Scholar] [CrossRef]
- Hollesen, J.; Buchwal, A.; Rachlewicz, G.; Hansen, B.U.; Hansen, M.O.; Stecher, O.; Elberling, B. Winter warming as an important co-driver for Betulanana growth in western Greenland during the past century. Glob. Chang. Biol. 2015, 21, 2410–2423. [Google Scholar] [CrossRef]
- Zhu, L.; Li, S.; Wang, X. Tree-ring reconstruction of February-March mean minimum temperature back to 1790A.D. in Yichun, Northeast China. Quat. Sci. 2015, 35, 1175–1184. [Google Scholar]
- Yu, D.; Wang, Q.; Wang, G.G.; Dai, L. Dendroclimatic response of Picea jezoensis along an altitudinal gradient in Changbai Mountains. Sci. China 2006, 49, 150–159. [Google Scholar] [CrossRef]
- Pellizzari, E.; Pividori, M.; Carrer, M. Winter precipitation effect in a mid-latitude temperature-limited environment: The case of common juniper at high elevation in the Alps. Environ. Res. Lett. 2014, 9, 104021. [Google Scholar] [CrossRef]
- Lamarche, V.C. Frequency-dependent relationships between tree-ring series along an ecological gradient and some dendroclimatic implications. Tree-Ring Bull. 1974, 20, 1–20. [Google Scholar]
- Leal, S.; Melvin, T.M.; Grabner, M.; Wimmer, R.; Briffa, K.R. Erratum: Tree-ring growth variability in the Austrian Alps: The influence of site, altitude, tree species and climate. Boreas 2007, 36, 459. [Google Scholar] [CrossRef]
- Dittmar, C.; Zech, W.; Elling, W. Growth variations of Common beech (Fagus sylvatica L.) under different climatic and environmental conditions in Europe—A dendroecological study. For. Ecol. Manag. 2003, 173, 63–78. [Google Scholar] [CrossRef]
- Huo, Y.; Gou, X.; Liu, W.; Li, J.; Zhang, F.; Fang, K.J.T. Climate–growth relationships of Schrenk spruce (Picea schrenkiana) along an altitudinal gradient in the western Tianshan mountains, northwest China. Trees 2017, 31, 429–439. [Google Scholar] [CrossRef]
- Li, Z.S.; Liu, G.H.; Fu, B.J.; Hu, C.J.; Luo, S.Z.; Liu, X.L.; He, F. Anomalous temperature–growth response of Abies faxoniana to sustained freezing stress along elevational gradients in China’s Western Sichuan Province. Trees 2012, 26, 1373–1388. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, D.; Tian, K.; He, R.; He, M.; Li, Y.; Sun, D.; Zhang, W. Relationship between radial growth of Abies georgei and climate factors at different altitudes on the eastern slope of Yulong Snow Mountain, China. J. Appl. Ecol. 2018, 29, 2355–2361. [Google Scholar]
- Yang, R.; Fan, Z.; Li, Z.; Wen, Q. Radial growth of Pinus yunnanensis at different elevations and their responses to climatic factors in the Yulong Snow Mountain Northwest Yunnan, China. Acta Ecol. Sin. 2018, 38, 8983–8991. [Google Scholar]
- Wang, S.; Ye, J.; Gong, D.; Zhu, J.; Yao, T. Establishment of annual temperature series in China in the last 100 years. J. Appl. Meteorol. 1998, 1, 11–19. [Google Scholar]
- Wang, X.; Song, L.; Zhang, Y. Climate-tree growth relationships of Pinus sylvestris var. mongolica in the northern Daxing’an Mountains, China. Chin. J. Plant Ecol. 2011, 35, 294–302. [Google Scholar] [CrossRef]
- Tabakova, M.A.; Arzac, A.; Martínez, E.; Kirdyanov, A.V. Climatic factors controlling Pinus sylvestris radial growth along a transect of increasing continentality in southern Siberia. Dendrochronologia 2020, 62, 125709. [Google Scholar] [CrossRef]
- Bozkurt, A.E.; Sahan, E.A.; Kose, N. Growth responses of Pinus sylvestris L. to climate from the southeastern limit of its natural distribution area, Turkey. Dendrochronologia 2021, 70, 125897. [Google Scholar] [CrossRef]
- Liu, N.; Liu, Y.; Bao, g.; Bao, M.; Wang, Y.; Zhang, L.; Bao, W.; Tian, H. Drought reconstruction in eastern Hulun Buir steppe, China and its linkages to the sea surface temperatures in the Pacific Ocean. Asian Earth 2016, 115, 298–307. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, M.; Semyung, K.; Pan, L.; Han, H.; Yang, X.; Liu, Y.; Shi, Z. Radial growth responses of Mongolian pine (pinus sylvestris var. mongolica) plantations at different ages to climate and groundwater level changes. Acta Ecol. Sin. 2022, 42, 6827–6837. [Google Scholar]
- Bao, G.; Liu, Z.; Liu, N.; Wu, M. Simulation analysis of the radial growth characteristics of Pinus sylvestris var. mongolica in Hulunbuir Sandy Land by Vaganov-Shashkin Model. J. Appl. Ecol. 2021, 32, 3448–3458. [Google Scholar]
- Lv, S.; Wang, X. Growth-climate response and winter precipitation reconstruction of Pinus sylvestris var. mongolicain A’li River of Greater Khingan Range. J. Northeast. Norm. Univ. (Nat. Sci. Ed.) 2014, 46, 110–116. [Google Scholar]
- Li, L.; Li, L.G.; Chen, Z.J.; Zhou, Y.B.; Xiao, J.Q. Responses of Pinus sylvestris var. mongolica to gradient change of hydrothermal in plantations in Liaoning Province. Acta Ecol. Sin. 2015, 35, 4508–4517. [Google Scholar]
- Recep, E.; Süleyman, S.; Isa, C.; Abdullah, S. Subalpine Ecosystem and Possible Impact of Climate Change on Vegetation of Kaz Mountain (Mount Ida—NW Turkey); Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 645–663. [Google Scholar]
- Pepin, N.; Bradley, R.S.; Diaz, H.F.; Baraer, M.; Caceres, E.B.; Forsythe, N.; Fowler, H.; Greenwood, G.; Hashmi, M.Z.; Liu, X.D. Elevation-dependent warming in mountain regions of the world. Nature Climate Chang. 2015, 5, 424–430. [Google Scholar]
- Vicente-Serrano, S.M.; Gouveia, C.; Camarero, J.J.; Beguería, S.; Sanchez-Lorenzo, A. Response of vegetation to drought time-scales across global land biomes. Proc. Natl. Acad. Sci. USA 2012, 110, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Stokes, M.A.; Smiley, T.L. An Introduction to Tree-Ring Dating; University of Arizona Press: Tucson, AZ, USA, 1968. [Google Scholar]
- Hart, S.J. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, AZ, USA, 2011. [Google Scholar]
- Holmes, R.L. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree-Ring Bull. 1983, 43, 51–67. [Google Scholar]
- Chacón-de la Cruz, J.E.; Pompa-García, M. Response of tree radial growth to evaporation, as indicated by earlywood and latewood. Rev. Chapingo Ser. Cienc. For. Y Del Ambiente 2015, 21, 57–65. [Google Scholar] [CrossRef]
- Feng, K.; Lan, X.; Shao, S.; Pin, W. Prediction of Regional Differences in the Influence of Geoengineering on Temperature in China under 1.5 °C Temperature Control Target. Sci. Technol. Eng. 2019, 6, 230–251. [Google Scholar]
- Gou, X.; Chen, F.; Yang, M.; Gordon, J.; Fang, K.; Tian, Q.; Zhang, Y. Asymmetric variability between maximum and minimum temperatures in Northeastern Tibetan Plateau: Evidence from tree rings. Int. J. Climatol. A J. R. Meteorol. Soc. 2008, 51, 41–55. [Google Scholar] [CrossRef]
- Brubaker, L.B. Spatial Patterns of Tree Growth Anomalies in the Pacific Northwest. Ecology 1980, 61, 798–807. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Li, J.; Davi, N.; Chen, Z.; Cui, M.; Chen, W.; Li, N. Temperature reconstruction (1750–2008) from Dahurian larch tree-rings in an area subject to permafrost in Inner Mongolia, Northeast China. Clim. Res. 2011, 47, 151–159. [Google Scholar] [CrossRef]
- He, M.; Wei, J.-S.; Shi, L.; Zhou, M.; Zhao, P.-W. The response of radial growth and death of Populus davidiana to regional climate change in southern Greater Xing’an Mountains. Chin. J. Ecol. 2018, 37, 3237–3244. [Google Scholar]
- Ren, P.; Rossi, S.; Gricar, J.; Liang, E.; Cufar, K. Is precipitation a trigger for the onset of xylogenesis in Juniper przewalskii on the north-eastern Tibetan Plateau? Ann. Bot. 2015, 115, 629–639. [Google Scholar] [CrossRef]
- Cui, J.; Peng, J.; Li, J.; Li, X.; Peng, M.; Yang, L. Responses of tree-ring width of Pinus tabuliformis plantation to climatic factors in Songshan Mountains, central China. Chin. J. Appl. Ecol. 2021, 32, 3497–3504. [Google Scholar]
- Sidor, C.G.; Popa, I.; Vlad, R.; Cherubini, P. Different tree-ring responses of Norway spruce to air temperature across an altitudinal gradient in the Eastern Carpathians (Romania). Trees 2015, 29, 985–997. [Google Scholar] [CrossRef]
- Kai, T.U.; Yan, Z.; Dong, W. Climatic Jumps in Precipitation and Extremes in Drying North China during 1954–2006. J. Meteorol. Soc. Jpn. Ser. II 2010, 88, 29–42. [Google Scholar]
- You, Q.; Kang, S.; Enric, A.; Nick, P.; Wolfgang, A.; Flügel. Changes in daily climate extremes in China and their connection to the large scale atmospheric circulation during 1961–2003. Clim. Dyn. Obs. Theor. Comput. Res. Clim. Syst. 2011, 36, 2399–2417. [Google Scholar] [CrossRef]
- Li, Z.; Yan, Z. Homogenized daily mean/maximum/minimum temperature series for China from 1960–2008. Atmos. Ocean. Sci. Lett. 2009, 2, 237–243. [Google Scholar]
- Fritts, H. Tree Rings and Climate; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Oberhuber, W.; Stumböck, M.; Kofler, W. Climate-tree-growth relationships of Scots pine stands (Pinus sylvestris L.) exposed to soil dryness. Trees 1998, 13, 19–27. [Google Scholar] [CrossRef]
- Xu, J.R.; Wang, H.; Zhao, M.S.; Shi, S.Y.; Zhang, Y.P.; Shi, J.F. Reconstructing mean temperature of April-July in 1809-2018 based on tree-ring of Pinus taiwanensis in the Tianmu Mountain. East China. Chin. J. Appl. Ecol. 2022, 33, 2347–2355. [Google Scholar]
- Qin, J.; Bai, H.; Zhou, Q.; Wang, J.; Li, S.; Gan, Z.; Bao, G. Radial growth response of Abies fargesii to climate change from different elevations at timberline of Niubeiliang Natural Reserve. Arid. Land Geogr. 2017, 40, 147–155. [Google Scholar]
- Yiru, Y.; Mingshan, Z.; Lingnan, Z.; Qiangqiang, L.; Yixue, H.; Xiaohong, L. Different responses of radial growth of Pinus tabuliformis to climate in the middle and western Qinling Mountains. Acta Ecol. Sin. 2022, 42, 1474–1486. [Google Scholar]
- Bao, A.; Yang, L.; Liu, B. Radial Growth of Pinus koraiensis and Juglans mandshurica in Response to Climate Change in Laoyeling Mountains. J. Northeast. For. Univ. 2019, 47, 16–21. [Google Scholar]


| Site Code | High Altitude (MHZ) | Low Altitude (MLZ) |
|---|---|---|
| Latitude (N) | 51.35 | 51.53 |
| Longitude (E) | 120.86 | 120.74 |
| Average altitude (m) | 1100 (±20) | 650 (±20) |
| Slope direction | SE | N |
| Slope (°) | 25 | 25 |
| Canopy density | 0.6 | 0.6 |
| Tree/cores | 24/53 | 23/48 |
| Parameter | MHZ | MLZ |
|---|---|---|
| Mean sensitivity | 0.144 | 0.182 |
| Standard deviation | 0.216 | 0.282 |
| First-order autocorrelation | 0.683 | 0.719 |
| Mean within tree correlation | 0.608 | 0.676 |
| Signal-to-noise ratio | 14.574 | 20.174 |
| Expressed population signal | 0.936 | 0.953 |
| The first year of subsample signal strength > 0.85 | 1797 | 1826 |
| Chronological length | 1754–2022 | 1774–2022 |
| Time Interval | Mean Annual Temperature (°C) | Annual Precipitation (mm) | Tree Ring Index | |
|---|---|---|---|---|
| MHZ | MLZ | |||
| Before abrupt change (1958–1985) | −5.15 | 449 | 0.94 | 0.90 |
| After abrupt change (1985–2012) | −3.35 | 431 | 1.14 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, Z.; Zhang, D.; Luo, T.; Li, X.; Du, B.; Zhong, S. Climate–Growth Relationships of Mongolian Pine (Pinussylvestris var. mongholica) along an Altitudinal Gradient of Northeast China. Forests 2024, 15, 922. https://doi.org/10.3390/f15060922
Wang X, Wang Z, Zhang D, Luo T, Li X, Du B, Zhong S. Climate–Growth Relationships of Mongolian Pine (Pinussylvestris var. mongholica) along an Altitudinal Gradient of Northeast China. Forests. 2024; 15(6):922. https://doi.org/10.3390/f15060922
Chicago/Turabian StyleWang, Xinrui, Zhaopeng Wang, Dongyou Zhang, Taoran Luo, Xiangyou Li, Bingyun Du, and Shubing Zhong. 2024. "Climate–Growth Relationships of Mongolian Pine (Pinussylvestris var. mongholica) along an Altitudinal Gradient of Northeast China" Forests 15, no. 6: 922. https://doi.org/10.3390/f15060922
APA StyleWang, X., Wang, Z., Zhang, D., Luo, T., Li, X., Du, B., & Zhong, S. (2024). Climate–Growth Relationships of Mongolian Pine (Pinussylvestris var. mongholica) along an Altitudinal Gradient of Northeast China. Forests, 15(6), 922. https://doi.org/10.3390/f15060922




