Growth Rings in Nine Tree Species on a Neotropical Island with High Precipitation: Coco Island, Costa Rica
Abstract
1. Introduction
2. Materials and Methods
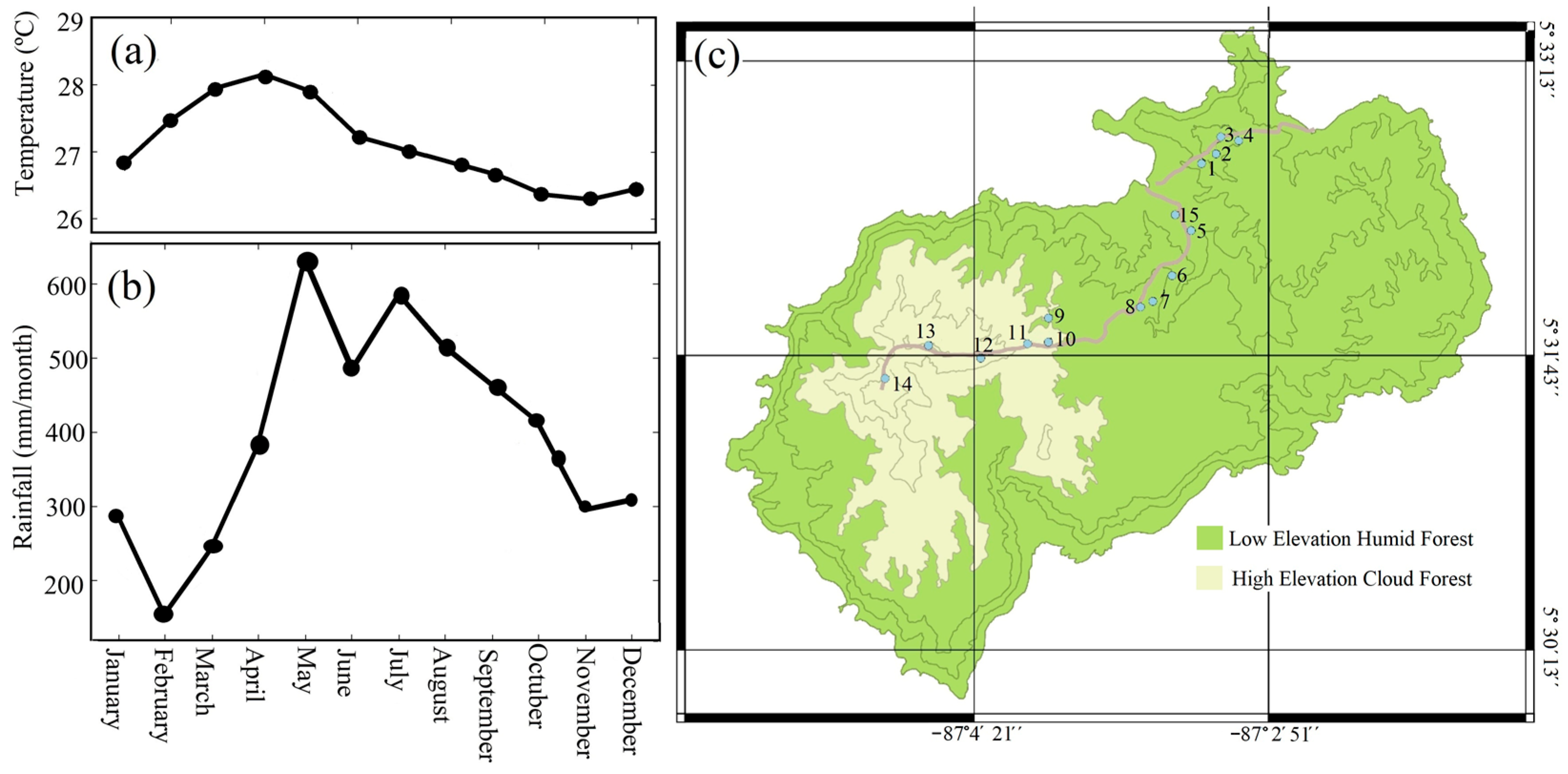
2.1. Geographical and Climatological Characteristics of Coco Island
2.2. Tree Sample
2.3. Wood Sample Preparation for Antomical Observation for Rings Boundary
2.4. Growth Ring Distinctiveness
- Type 1, density variation: the growth ring’s boundary is marked by several rows of fibers with a shortened radial diameter and thickened walls;
- Type 2, marginal parenchyma: the growth ring’s boundary is marked with a uniseriate or multiseriate marginal parenchyma band;
- Type 3, fiber/parenchyma pattern: the growth ring’s boundary is marked by periodically recurring patterns of alternating parenchyma and fiber bands;
- Type 4, vessel distribution: there is a variation in the diameter or frequency of vessel elements;
- Type 5, fiber band: the growth ring’s boundary is marked by a band of fibers.
2.5. Dendrochronological Potential
- (1)
- Null: growth rings are non-defined;
- (2)
- Low: growth rings are slightly defined; it is difficult to observe growth ring boundaries, there are false growth rings, growth rings are too close together, or they have frequent discontinuity;
- (3)
- Medium: there are false growth rings or few frequent, more or less distinctive, or poorly defined growth rings;
- (4)
- High: growth rings are clearly defined in tangential orientation, and there is no presence of false growth rings.
3. Results
4. Discussion
4.1. Growth Ring Presence
4.2. The Dendrochronological Potential for Climate and Ecology Studies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caludino-Sales, V. Cocos Island National Park, Costa Rica. In Coastal World Heritage Sites. Coastal Research Library; Springer: Dordrecht, The Netherlands, 2019; Volume 28. [Google Scholar] [CrossRef]
- Cortes, J. Isla del Coco: Coastal and Marine Ecosystems. In Costa Rica Ecosystem; Kappelle, M., Ed.; The University of Chicago Press: Chicago, IL, USA, 2016; pp. 97–191, Chapter 7. [Google Scholar]
- Estrada-Chavarría, A.; Sánchez-González, J.; Rodríguez-González, A. Catálogo actualizado de las plantas vasculares del Parque Nacional Isla del Coco, Costa Rica. Rev. Biol. Trop. 2020, 68, 73–88. [Google Scholar] [CrossRef]
- Bonilla-Mata, R.; Acosta-Vargas, L.G. Dynamic and growth of the forests of the Isla del Coco National Park, Costa Rica. Rev. Biol. Trop. 2020, 68, 89–102. [Google Scholar] [CrossRef]
- Bonilla-Mata, R. Evaluación de los bosques del Parque Nacional Isla del Coco, Costa Rica. Bachelor’s Thesis, Escuela de Ingeniería Forestal, Instituto Tecnológico de Costa Rica, Cartago, Costa Rica, 2017; 145p. [Google Scholar]
- Pearl, J.K.; Keck, J.R.; Tintor, W.; Siekacz, L.; Herrick, H.M.; Meko, M.D.; Pearson, C.L. New frontiers in tree-ring research. Holocene 2020, 30, 923–941. [Google Scholar] [CrossRef]
- Porras-Jiménez, M.A.; Acosta-Vargas, L.G.; Castillo-Ugalde, M.; Quesada-Monge, R. Estructura y composición florística del bosque nuboso de la Isla del Coco. Rev. Tecnol. Marcha 2014, 27, 22–36. [Google Scholar] [CrossRef]
- Alfaro, E.J. Ciclo diario y anual de variables troposfericas y oceanicas en la Isla del Coco, Costa Rica (Pacifico Oriental). Rev. Biologia Trop. 2008, 56 (Suppl. 2), 19–29. [Google Scholar]
- Vargas, J.L.; Alfaro, E.J. Radiación ultravioleta (UV) en el Parque Nacional Isla del Coco, Costa Rica. Rev. Biol. Trop. 2016, 64, 75–86. [Google Scholar] [CrossRef]
- Giraldo, J.A.; Valle, J.I.; Sierra, C.A.; Melo, O. Dendrochronological potential of trees from America’s rainiest region. In Latin American Dendroecology: Combining Tree-Ring Sciences and Ecology in a Megadiverse Territory; Pompa-García, M., Camarero, J.J., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 79–119. [Google Scholar]
- Esper, J.; Krusic, P.J.; Ljungqvist, F.C.; Luterbacher, J.; Carrer, M.; Cook, E.; Davi, N.K.; Hartl-Meier, C.; Kirdyanov, A.; Konter, O.; et al. Ranking of tree-ring based temperature reconstructions of the past millennium. Quat. Sci. Rev. 2016, 145, 134–151. [Google Scholar] [CrossRef]
- Silva, M.; Funch, L.S.; da Silva, L.B. The growth ring concept: Seeking a broader and unambiguous approach covering tropical species. Biol. Rev. 2019, 94, 1161–1178. [Google Scholar] [CrossRef]
- Locosselli, G.M.; Brienen, R.J.; Leite, M.D.S.; Gloor, M.; Krottenthaler, S.; Oliveira, A.A.D.; Barichivich, J.; Anhuf, D.; Ceccantini, G.; Schöngart, H.; et al. Global tree-ring analysis reveals rapid decrease in tropical tree longevity with temperature. Proc. Natl. Acad. Sci. USA 2020, 117, 33358–33364. [Google Scholar] [CrossRef]
- Quesada-Román, A.; Ballesteros-Cánovas, J.A.; George, S.S.; Stoffel, M. Tropical and subtropical dendrochronology: Approaches, applications, and prospects. Ecol. Indic. 2022, 144, 109506. [Google Scholar] [CrossRef]
- Quesada-Román, A.; Ballesteros-Cánovas, J.A.; Granados-Bolaños, S.; Birkel, C.; Stoffel, M. Improving regional flood risk assessment using flood frequency and dendrogeomorphic analyses in mountain catchments impacted by tropical cyclones. Geomorphology 2022, 396, 108000. [Google Scholar] [CrossRef]
- Alves, E.S.; Angyalossy-Alfonso, V. Ecological trends in the wood anatomy of some Brazilian species. 1. Growth rings and vessels. IAWA J. 2000, 21, 3–30. [Google Scholar] [CrossRef]
- Sánchez-Calderón, O.D.; Carlón-Allende, T.; Mendoza, M.E.; Villanueva-Díaz, J. Dendroclimatology in Latin America: A Review of the State of the Art. Atmosphere 2022, 13, 748. [Google Scholar] [CrossRef]
- Pacheco-Solana, A.; Oelkers, R.; D’Arrigo, R.; Santos, G.M.; Rodriguez-Caton, M.; Tejedor, E.; Ferrero, E.; Fuentes, A.F.; Maldonado, C.; Andreu-Hayles, L. Radiocarbon and wood anatomy as complementary tools for generating tree-ring records in Bolivia. Front. Plant Sci. 2023, 14, 1135480. [Google Scholar] [CrossRef]
- Moya, R.; Quesada-Román, A.; Quesada-Monge, R. Potential use of Costa Rican wood data for neotropical dendrochronology. New For. 2024; submitted. [Google Scholar]
- Aragão, J.R.V.; Groenendijk, P.; Lisi, C.S. Dendrochronological potential of four neotropical dry-forest tree species: Climate-growth correlations in northeast Brazil. Dendrochronologia 2019, 53, 5–16. [Google Scholar] [CrossRef]
- Fontana, C.; Pérez-de-Lis, G.; Santini Junior, L.; Botosso, P.C.; Nabais, C.; Tomazello-Filho, M.; Lousada, J.L.P.C. Wood anatomy and growth ring boundaries of Copaifera lucens (Fabaceae). IAWA J. 2018, 39, 395–405. [Google Scholar] [CrossRef]
- Quesada-Román, A.; Ballesteros-Cánovas, J.A.; Guillet, S.; Madrigal-González, J.; Stoffel, M. Neotropical Hypericum irazuense shrury reveal recent ENSO variability in Costa Rican páramo. Dendrochronologia 2020, 61, 125704. [Google Scholar] [CrossRef]
- López, R.; Cano, F.J.; Rodríguez-Calcerrada, J.; Sangüesa-Barreda, G.; Gazol, A.; Camarero, J.J.; Rozenberg, P.; Gil, L. Tree-ring density and carbon isotope composition are early-warning signals of drought-induced mortality in the drought tolerant Canary Island pine. Agricul. For. Meteorol. 2021, 310, 108634. [Google Scholar] [CrossRef]
- Brienen, R.J.; Lebrija-Trejos, E.; Van Breugel, M.; Pérez-García, E.A.; Bongers, F.; Meave, J.A.; Martínez-Ramos, M. The potential of tree rings for the study of forest succession in southern Mexico. Biotropica 2009, 41, 186–195. [Google Scholar] [CrossRef]
- Marques, J.B.; Callado, C.H.; Rabelo, G.R.; Silva Neto, S.J.D.; Cunha, M.D. Comparative wood anatomy of species of Psychotria L. (Rubiaceae) in Atlantic Rainforest remnants of Rio de Janeiro State, Brazil. Acta Bot. Brasilica 2015, 29, 433–444. [Google Scholar] [CrossRef]
- Campbell, G.; Mielke, M.S.; Rabelo, G.R.; Da Cunha, M. Key anatomical attributes for occurrence of Psychotria schlechtendaliana (Müll. Arg.) Müll. Arg. (Rubiaceae) in different successional stages of a tropical moist forest. Flora 2018, 246, 33–41. [Google Scholar] [CrossRef]
- Reis-Avila, G.; Oliveira, J.M. Lauraceae: A promising family for the advance of neotropical dendrochronology. Dendrochronologia 2017, 44, 103–116. [Google Scholar] [CrossRef]
- Worbes, M. One hundred years of tree-ring research in the tropics—A brief history and an outlook to future challenges. Dendrochronologia 2002, 20, 217–231. [Google Scholar] [CrossRef]
- Godoy-Veiga, M.; Slotta, F.; Alecio, P.C.; Ceccantini, G.; Buckeridge, M.S.; Locosselli, G.M. Improved tree-ring visualization using autofluorescence. Dendrochronologia 2019, 55, 33–42. [Google Scholar] [CrossRef]
- Worbes, M.; Raschke, N. Carbon allocation in a Costa Rican dry forest derived from tree ring analysis. Dendrochronologia 2012, 30, 231–238. [Google Scholar] [CrossRef]
- Marcelo-Peña, J.L.; Roig, F.A.; Goodwin, Z.A.; Tomazello-Filho, M. Characterizing growth rings in the trees of Perú: A wood anatomical overview for potential applications in dendroecological-related fields. Dendrochronologia 2020, 62, 125728. [Google Scholar] [CrossRef]
- Quintilhan, M.T.; Santini, L., Jr.; Rodriguez, D.R.O.; Guillemot, J.; Cesilio, G.H.M.; Chambi-Legoas, R.; Nouvellon, Y.; Tomazello-Filho, M. Growth-ring boundaries of tropical tree species: Aiding delimitation by long histological sections and wood density profiles. Dendrochronologia 2021, 69, 125878. [Google Scholar] [CrossRef]
- Giraldo, J.A.; del Valle, J.I.; González-Caro, S.; David, D.A.; Taylor, T.; Tobón, C.; Sierra, C.A. Tree growth periodicity in the ever-wet tropical forest of the Americas. J. Ecol. 2023, 111, 889–902. [Google Scholar] [CrossRef]
- Giraldo, J.A.; del Valle, J.I.; González-Caro, S.; Sierrra, C.A. Intra-annual isotope variations in tree rings reveal growth rhythms within the least rainy season of an ever-wet tropical forest. Trees 2022, 36, 1039–1052. [Google Scholar] [CrossRef]
- Xu, G.; Liu, X.; Hu, J.; Dorado-Liñán, I.; Gagen, M.; Szejner, P.; Chen, T.; Trouet, V. Intra-annual tree-ring δ18O and δ13C reveal a trade-off between isotopic source and humidity in moist environments. Tree Physiol. 2022, 42, 2203–2223. [Google Scholar] [CrossRef]
- Vetter, R.E.; Botosso, P.C. Remarks on age and growth rate determination of Amazonian trees. IAWA J. 1989, 10, 133–145. [Google Scholar] [CrossRef]
- Groenendijk, P.; Sass-Klaassen, U.; Bongers, F.; Zuidema, P.A. Potential of tree-ring analysis in a wet tropical forest: A case study on 22 commercial tree species in Central Africa. For. Ecol. Manag. 2014, 323, 65–78. [Google Scholar] [CrossRef]
- Azim, A.A.; Okada, N. Occurrence and anatomical features of growth rings in tropical rainforest trees in Peninsular Malaysia: A preliminary study. Tropics 2014, 23, 15–31. [Google Scholar] [CrossRef]
- Welle, B.J.H.; Jifke Koek-Noorman, J. Wood anatomy of the Neotropical Melastomataceae. Blumea 1981, 27, 335–394. [Google Scholar]
- León-Hernández, W.J. Anatomía xilemática caulinar de 14 especies de la familia Lauraceae. Rev. For. Venez. 2002, 46, 15–25. [Google Scholar]
- León, H.W. Anatomía de la madera de 31 especies de la subfamilia mimosaceae (leguminaceae) en Venezuela. Rev. Colombia For. 2008, 11, 113–135. [Google Scholar]
- Marqués, P.A.; Callado, C.H.; Barros, C.F.; Costa, C.G. Variação intraespecífica do lenho de Eugenia uniflora L. em duas diferentes fitofisionomias do complexo vegetacional atlântico. Floresta Ambiente 2012, 19, 483–496. [Google Scholar] [CrossRef]
- Beltrán, L.A.; Valencia, G.M. Anatomía de anillos de crecimiento de 80 especies arbóreas potenciales para estudios dendrocronológicos en la Selva Central, Perú. Rev. Biología Trop. 2013, 61, 1025–1037. [Google Scholar]
- Quirós-Badilla, E.; Alfaro, E. Algunos aspectos relacionados con la variabilidad climática en la Isla del Coco, Costa Rica. Rev. Climatol. 2009, 9, 33–44. [Google Scholar]
- Bahtiar, E.T.; Iswanto, A.H. Annual tree-ring curve-fitting for graphing the growth curve and determining the increment and cutting cycle period of sungkai (Peronema canescens). Forests 2023, 14, 1643. [Google Scholar] [CrossRef]
- Andreu-Hayles, L.; Tejedor, E.; D’arrigo, R.; Locosselli, G.M.; Rodríguez-Catón, M.; Daux, V.; Oelkers, R.; Pacheco-Solana, A.; Paredes-Villanueva, K.; Rodríguez-Morata, C. Dendrochronological advances in the tropical and subtropical Americas: Research priorities and future directions. Dendrochronologia 2023, 81, 126124. [Google Scholar] [CrossRef]
- Moreno, M.L.; Jiménez, K.; Villalobos, C. Approximation of the benefits of socioeconomic activities in Cocos Island National Park and the effects of climate change. Rev. Interamer. Amb. Tur. 2021, 17, 14–26. [Google Scholar] [CrossRef]
- Cabon, A.; Kannenberg, S.A.; Arain, A.; Babst, F.; Baldocchi, D.; Belmecheri, S.; Delpierre, N.; Guerrieri, R.; Maxwell, J.T.; McKenzie, S.; et al. Cross-biome synthesis of source versus sink limits to tree growth. Science 2022, 376, 758–761. [Google Scholar] [CrossRef]
- Gruber, N.; Bakker, D.C.; DeVries, T.; Gregor, L.; Hauck, J.; Landschützer, P.; McKinley, G.A.; Müller, J.D. Trends and variability in the ocean carbon sink. Nat. Rev. Earth Environ. 2023, 4, 119–134. [Google Scholar] [CrossRef]
| Species | Botanical Family | Distribution | Forest | Diameter (cm) | Altitudinal Zone (msl) | TECw Code |
|---|---|---|---|---|---|---|
| Ardisia compressa Kunth | Primulaceae | Native | LEHF | 7.27 | 50–450 | 895 |
| Cecropia pittieri B.L. Rob | Urticaceae | Endemic | LEHF | 15.53 | 10–300 (550) | 889 |
| Eugenia pacifica Benth | Myrtaceae | Endemic | LEHF | 8.04 | 50–450 | 897 |
| Henriettea succosa (Aubl.) DC. | Melastomataceae | Native | LEHF | 9.99 | 10–450 | 893 |
| Henriettella fascicularis (Sw.) C. Wright | Melastomataceae | Native | LEHF | 8.73 | 10–450 | 894 |
| Henriettella odorata Markgr. | Melastomataceae | Native | LEHF | 9.57 | 50–450 | 890 |
| Ocotea insularis (Meisn.) Mez | Lauraceae | Native | LEHF, HECF | 14.08 | 0–550 | 892 |
| Psychotria cocosensis C.W. Ham. | Rubiaceae | Endemic | LEHF, HECF | 7.28 | 150–600 | 896 |
| Sacoglottis holdridgei Cuatrec. | Humiriaceae | Endemic | LEHF, HECF | 30.41 | 0–630 | 891 |
| Species | Core Sample | Distinctiveness | Intensity of Expression | Growth Ring Type | Dendrocronological Potential | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||||
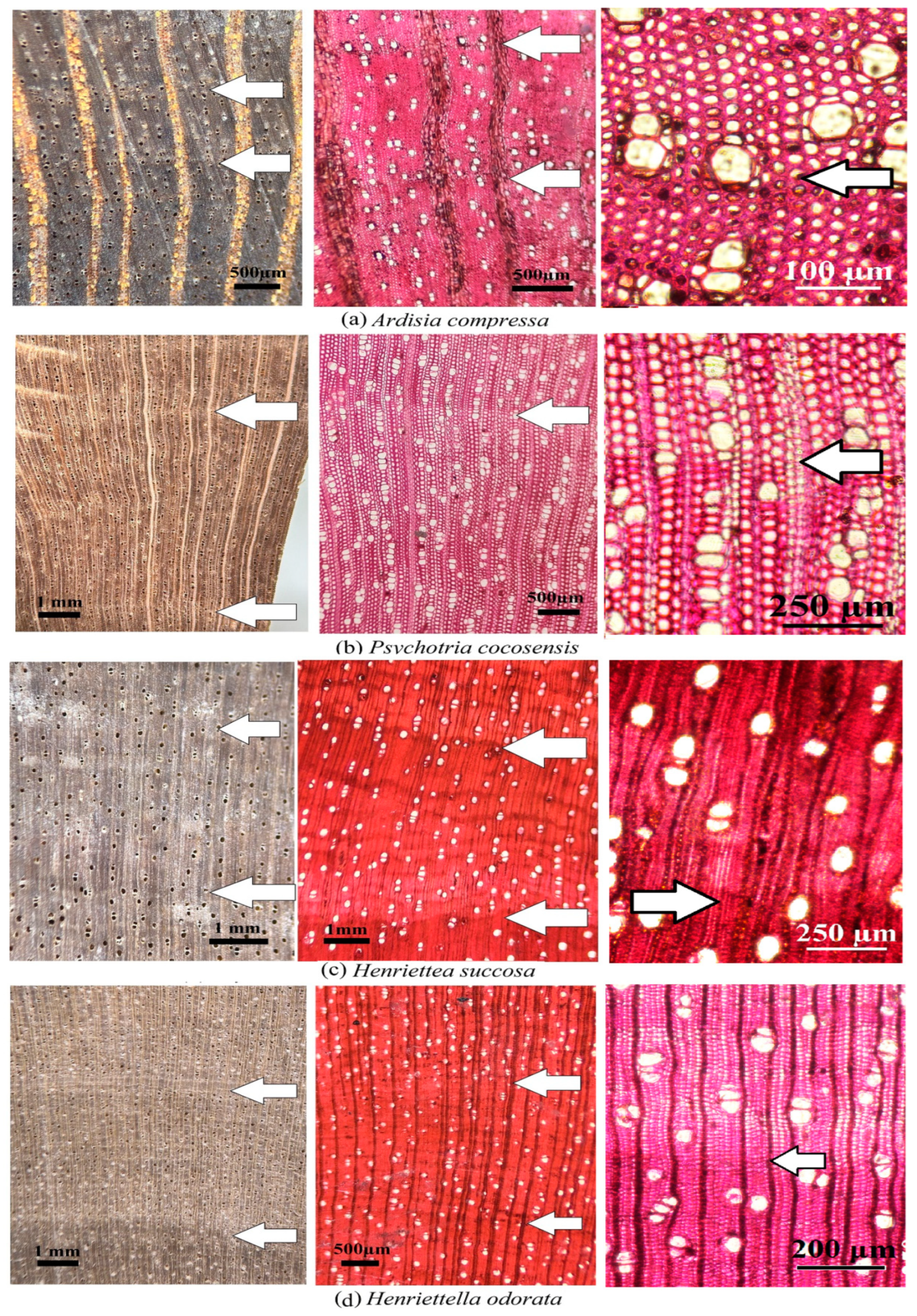
| Ardisia compressa | 1 | +/− | Low | X | Low | ||||
| 2 | +/− | Low | X | Low | |||||
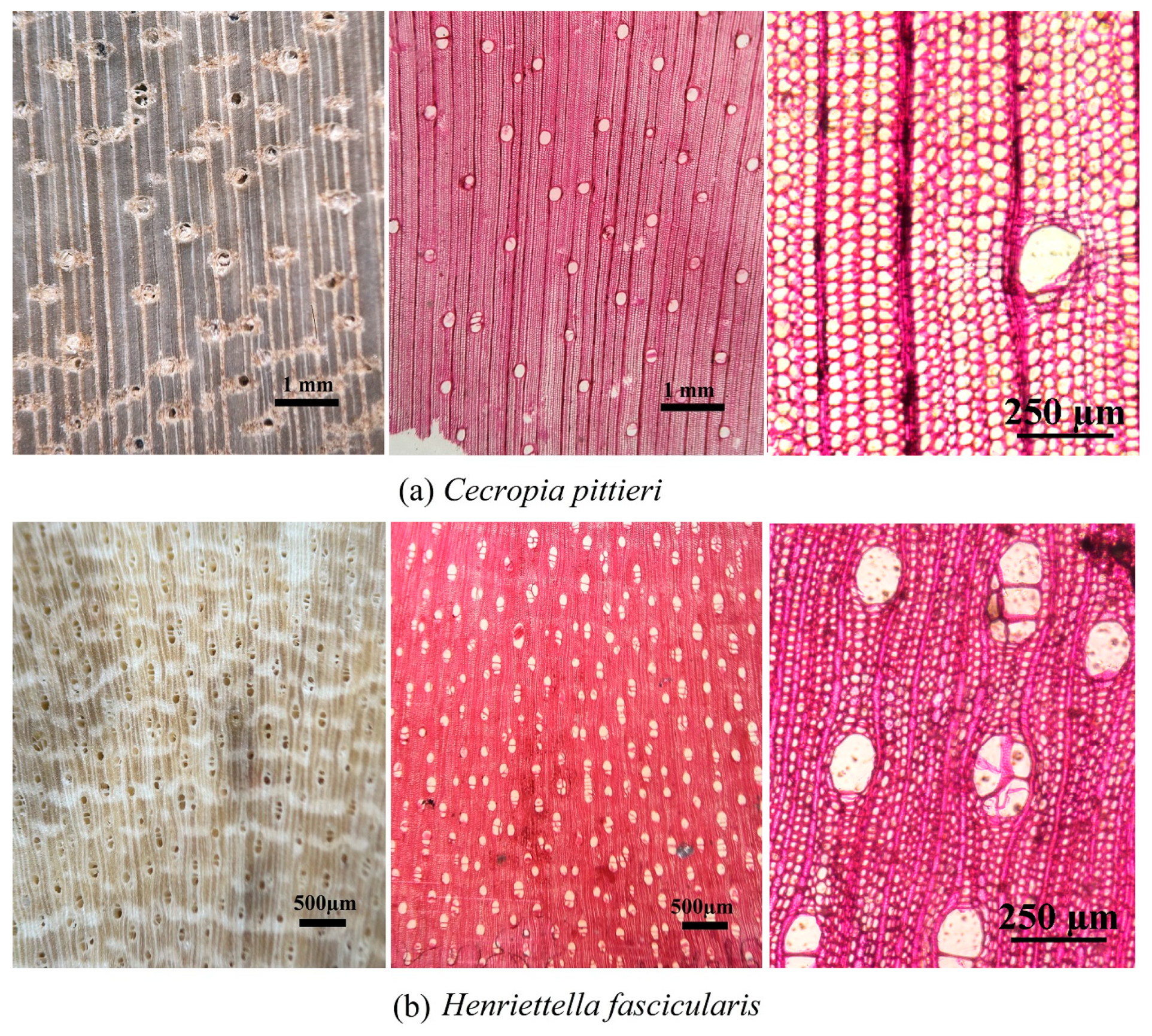
| Cecropia pittieri | 1 | − | Null | Null | |||||
| 2 | − | Null | Null | ||||||
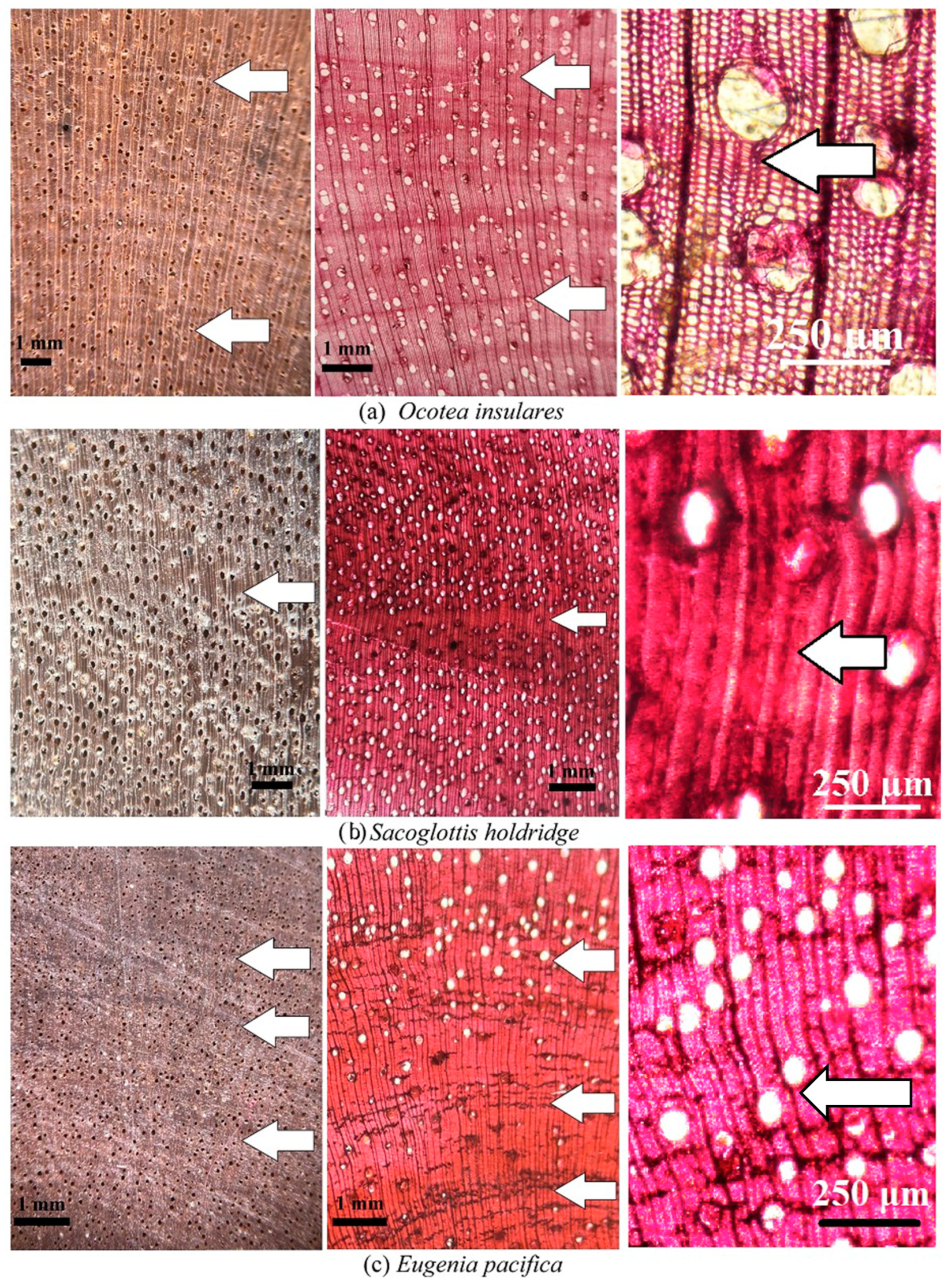
| Eugenia pacifica | 1 | +/− | Low | X | X | Low | |||
| 2 | +/− | Low | X | X | Low | ||||
| Henriettea succosa | 1 | +/− | Medium | X | Medium | ||||
| 2 | +/− | Medium | X | Medium | |||||
| Henriettella fascicularis | 1 | − | Null | Null | |||||
| 2 | − | Null | Null | ||||||
| Henriettella odorata | 1 | +/− | Medium | X | Medium | ||||
| 2 | +/− | Medium | X | Medium | |||||
| Ocotea insularis | 1 | +/− | Medium | X | Medium | ||||
| 2 | +/− | Medium | X | Medium | |||||
| Psychotria cocosensis | 1 | +/− | Low | X | Low | ||||
| 2 | +/− | Low | X | Low | |||||
| Sacoglottis holdridgei | 1 | +/− | Medium | X | Medium | ||||
| 2 | +/− | Medium | X | Medium | |||||
| Country and Reference | Precipitation (mm year−1) | Quantity Species and Results |
|---|---|---|
| Brazil [36] | 2500–3000 | A total of 29 species, 100% of species presented annual rings and ten different types of anatomical features were observed in annual ring boundaries. |
| Colombia [10] | Over 7000 | A total of 81 species, 18% of the species were lacking growth ring boundaries, 46% were well defined, and 36% were poorly defined. The thickening of the fiber cell walls presented in 51% of species, 36% of species presented marginal parenchyma bands. |
| Our study, Costa Rica. | 7000 | Nine species, 22% (two species) presented indistinct growth rings, two (22%) species had well defined growth rings, and five species (56%) had more or less distinctive growth rings. Four species presented density variation in growth ring boundary, two species had fiber bands, and one species presented a fiber/parenchyma pattern with vessel distribution. |
| Southwest Region of Camerron [37] | >4000 mm | A total of 22 species, 14 species showed distinct tree-ring boundaries. All four types of tree-ring structures were observed in the screened species, as well as combinations of structures. The most common tree-ring structure was marginal parenchyma bands, sometimes combined with repeated patterns of fibres and parenchyma bands. |
| Peninsular Malaysia [38] | 3000 | A total of 27 species, 17 species showed indefinable growth rings and ten species had no growth rings. In the species that were indefinable, some irregularities were included, such as intermittent growth rings, ambiguous growth rings, discontinuous growth rings, resin, and/or traumatic canals in the parenchyma bands. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moya, R.; Tenorio, C.; Acosta-Vargas, L. Growth Rings in Nine Tree Species on a Neotropical Island with High Precipitation: Coco Island, Costa Rica. Forests 2024, 15, 885. https://doi.org/10.3390/f15050885
Moya R, Tenorio C, Acosta-Vargas L. Growth Rings in Nine Tree Species on a Neotropical Island with High Precipitation: Coco Island, Costa Rica. Forests. 2024; 15(5):885. https://doi.org/10.3390/f15050885
Chicago/Turabian StyleMoya, Róger, Carolina Tenorio, and Luis Acosta-Vargas. 2024. "Growth Rings in Nine Tree Species on a Neotropical Island with High Precipitation: Coco Island, Costa Rica" Forests 15, no. 5: 885. https://doi.org/10.3390/f15050885
APA StyleMoya, R., Tenorio, C., & Acosta-Vargas, L. (2024). Growth Rings in Nine Tree Species on a Neotropical Island with High Precipitation: Coco Island, Costa Rica. Forests, 15(5), 885. https://doi.org/10.3390/f15050885







