Spatial Pattern of Genetic Diversity and Demographic History Revealed by Population Genomic Analysis: Resilience to Climate Fluctuations of Acer truncatum Bunge
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Collection
2.2. Whole Genome Sequencing
2.3. SNP Calling and Quality Filtering
2.4. Sampling Strategy for One Population
2.5. Genetic Diversity and Linkage Disequilibrium (LD) Decay
2.6. Genetic Structure Analysis
2.7. Inference of Demographic History
3. Results
3.1. Identification and Characterization of SNPs
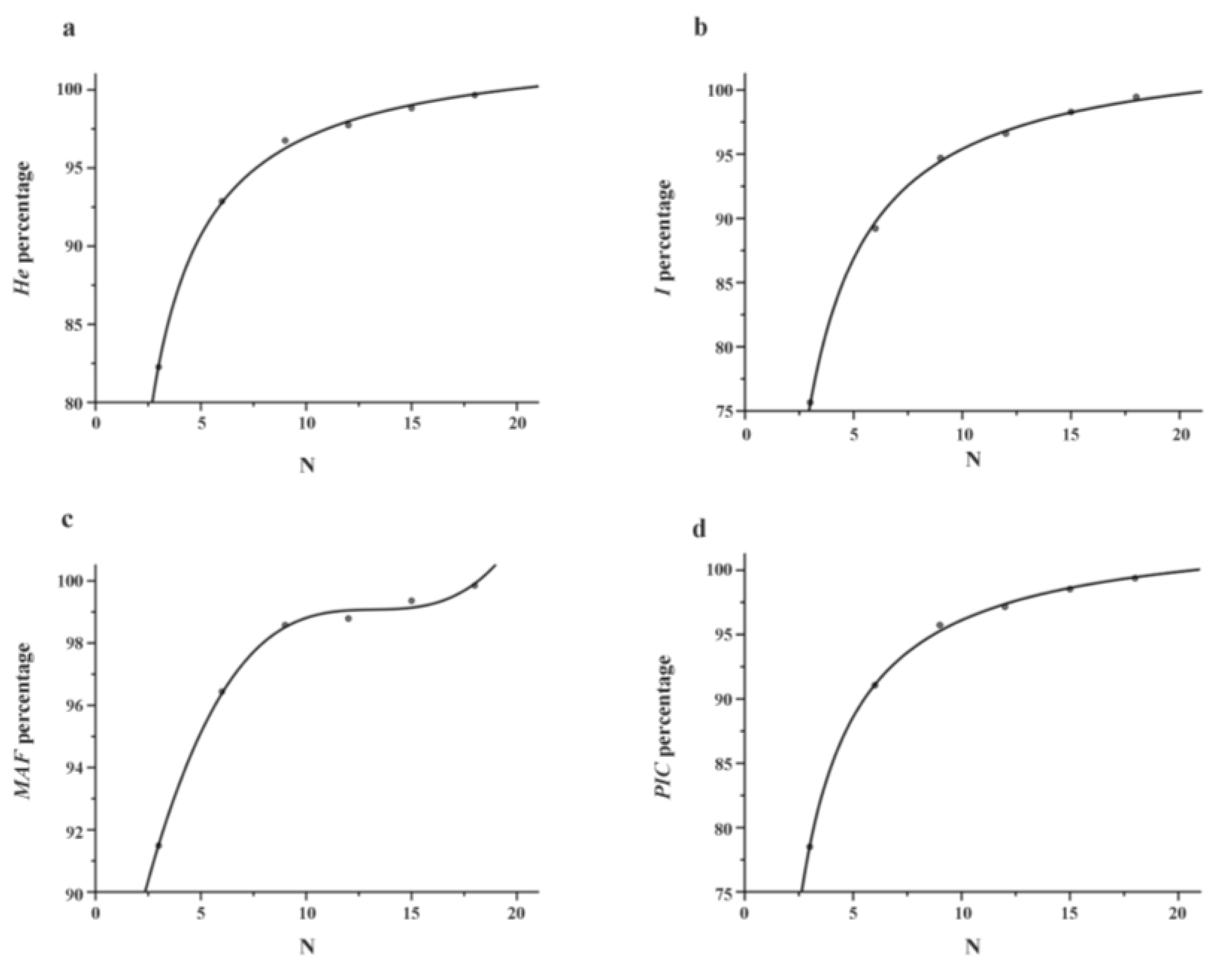
3.2. Sampling Strategy for Analysis of Genetic Diversity of Population
3.3. Genetic Diversity of Populations
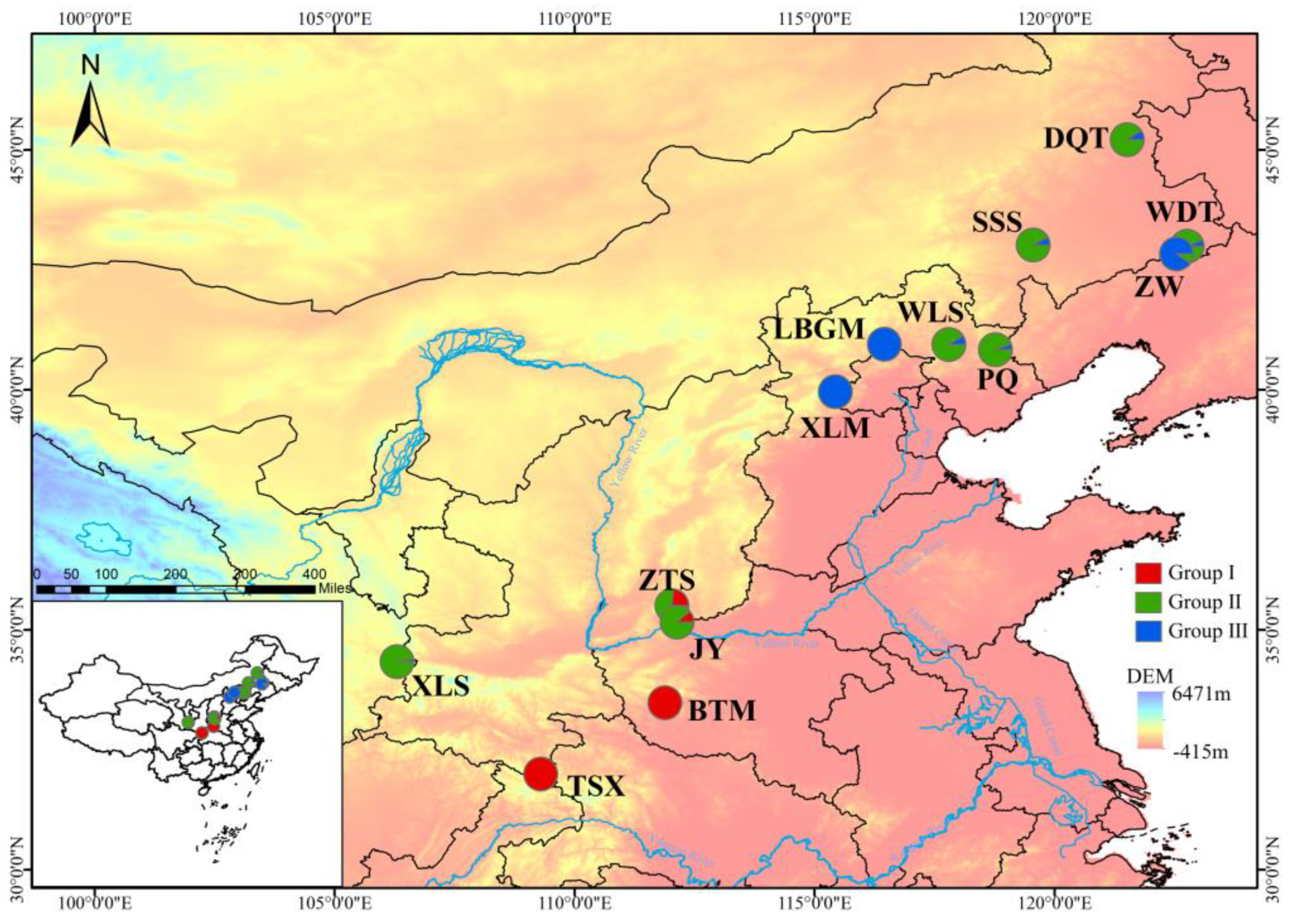
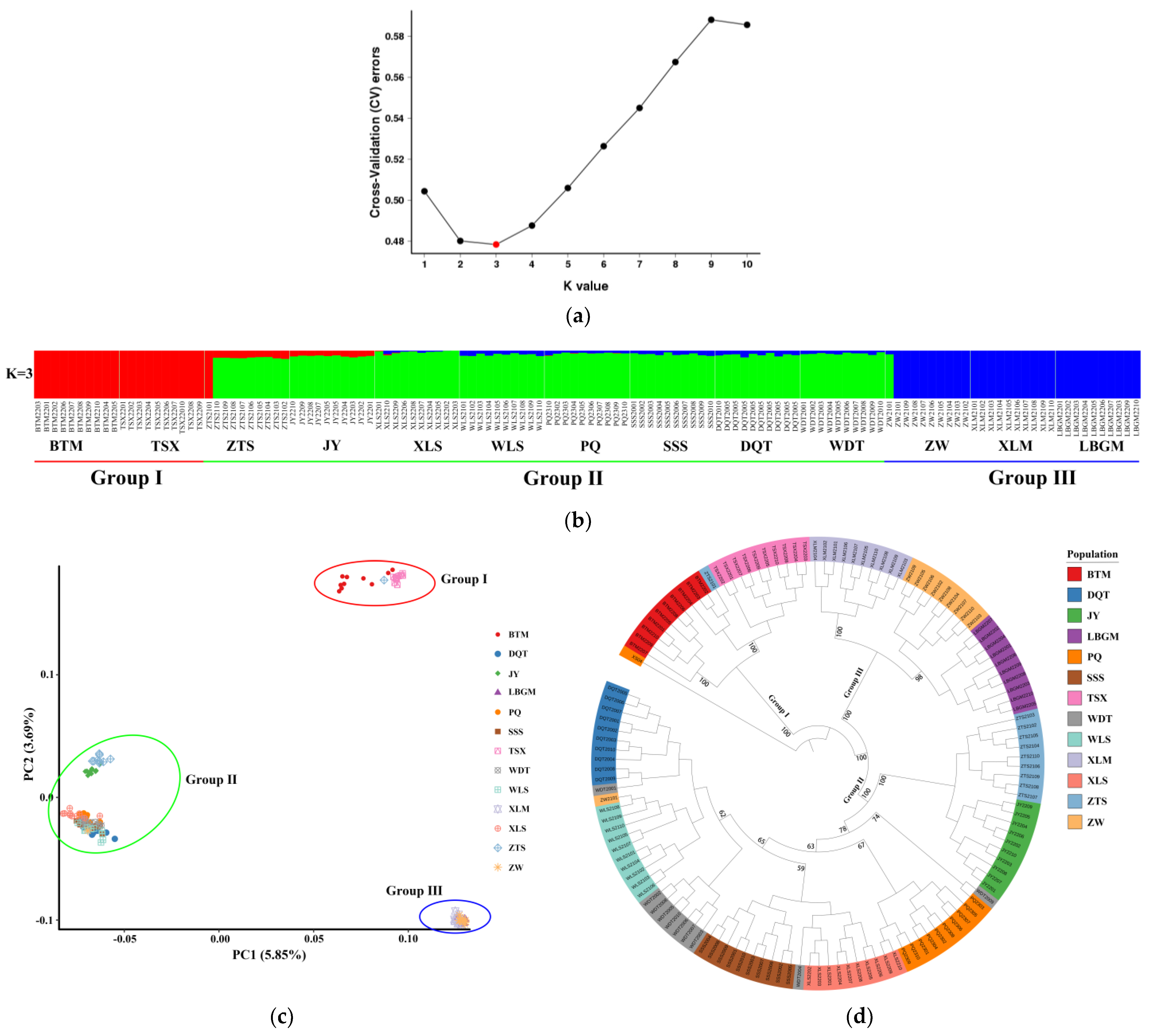
3.4. Genetic Structure and Gene Flow
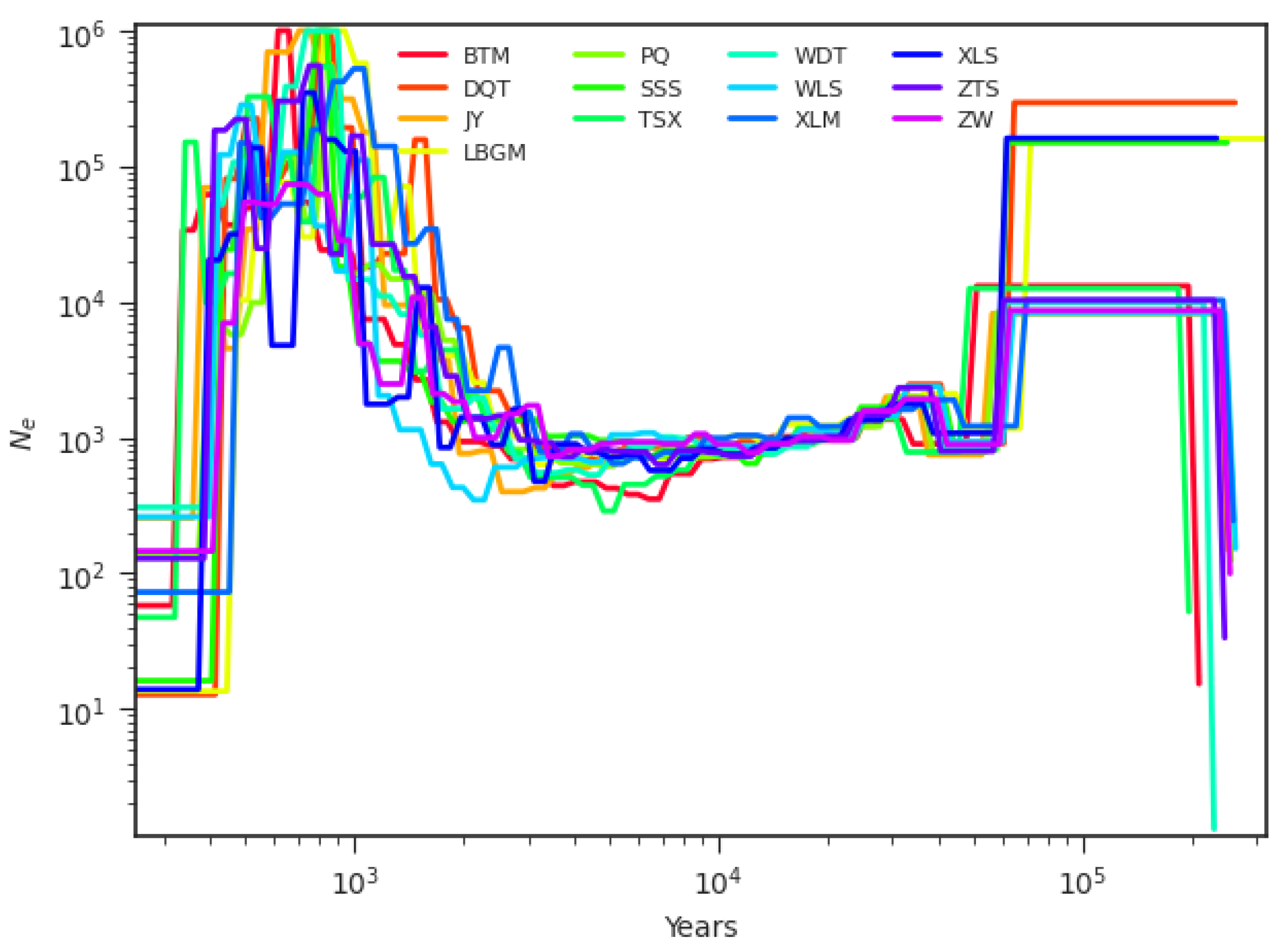
3.5. Inference of Demographic History
4. Discussion
4.1. Sampling Strategy for Genetic Diversity of Populations
4.2. Maintaining Mechanism of Genetic Diversity within Populations
4.3. Genetic Differentiation among Populations
4.4. Demographic History
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawson, C.; Kamalesh, A. Biodiversity, Genetic Resources and Intellectual Property; Routledge: New York, NY, USA, 2018; p. 25. [Google Scholar] [CrossRef]
- Boller, B.; Greene, S.L. Genetic resources. In Fodder Crops and Amenity Grasses; Springer: Berlin/Heidelberg, Germany, 2010; pp. 13–38. [Google Scholar] [CrossRef]
- Abbott, R.J.; Smith, L.C.; Milne, R.I.; Crawforo, R.M.M.; Wolff, K.; Balfour, J. Molecular analysis of plant migration and refugia in the Arctic. Science 2000, 289, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, R.; Wang, L.; Qiu, Q.; Chen, K.; Liu, J. Phylogeographic analyses suggest that a deciduous species (Ostryopsis davidiana decne., betulaceae) survived in northern China during the last glacial maximum. J. Biogeogr. 2010, 36, 2148–2155. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Y.; Wang, Y.; Chen, J.J.; Li, Y.H.; Huang, H.W.; Qiu, L.J.; Wang, Y. Population structure of the wild soybean (Glycine soja) in China: Implications from microsatellite analyses. Ann. Bot. 2012, 110, 777–785. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giarla, T.C.; Jansa, S.A. The impact of Quaternary climate oscillations on divergence times and historical population sizes in Thylamys opossums from the Andes. Mol. Ecol. 2015, 24, 2495–2506. [Google Scholar] [CrossRef] [PubMed]
- Gelderen, D.V.; Jong, P.C.; Oterdoom, H. Maples of the World; Timber Press, Inc.: Portland, OR, USA, 2005; p. 65. [Google Scholar]
- Taberlet, P.; Fumagalli, L.; Wust-Saucy, A.G.; Cosson, J.F. Comparative phylogeography and postglacial colonization routes in Europe. Mol. Ecol. 1998, 7, 453–464. [Google Scholar] [CrossRef]
- Hewitt, G. The genetic legacy of the Quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-P.; Fan, G.; Yin, P.-P.; Sun, S.; Li, N.; Hong, X.; Hu, G.; Zhang, H.; Zhang, F.-M.; Han, J.-D.; et al. Resequencing 545 ginkgo genomes across the world reveals the evolutionary history of the living fossil. Nat. Commun. 2019, 10, 4201. [Google Scholar] [CrossRef]
- Gong, W.; Liu, W.; Gu, L.; Kaneko, S.; Koch, M.A.; Zhang, D. From glacial refugia to wide distribution range: Demographic expansion of Loropetalum chinense (Hamamelidaceae) in Chinese subtropical evergreen broadleaved forest. Org. Divers. Evol. 2016, 16, 23–38. [Google Scholar] [CrossRef]
- Hamrick, J.L.; Godt, M.J.W.; Sherman-Broyles, S.L. Factors influencing levels of genetic diversity in woody plant species. In New Forests; Springer: Berlin/Heidelberg, Germany, 1992; pp. 95–124. [Google Scholar] [CrossRef]
- Daco, L.; Matthies, D.; Hermant, S.; Colling, G. Genetic diversity and differentiation of populations of Anthyllis vulneraria along elevational and latitudinal gradients. Ecol. Evol. 2022, 12, e9167. [Google Scholar] [CrossRef]
- Guo, X.; Wang, R.; Chang, R.; Liang, X.; Wang, C.; Luo, Y.; Yuan, Y.; Guo, W. Effects of nitrogen addition on growth and photosynthetic characteristics of Acer truncatum seedlings. Dendrobiology 2014, 72, 151–161. [Google Scholar] [CrossRef]
- Li, L.; Manning, W.; Tong, L.; Wang, X. Chronic drought stress reduced but not protected Shantung maple (Acer truncatum Bunge) from adverse effects of ozone (O3) on growth and physiology in the suburb of Beijing, China. Environ. Pollut. 2015, 201, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Su, D.L. Acer truncatum Bunge is full of treasures. Land Green. 2018, 11, 55–56. [Google Scholar]
- Wang, X.Y.; Xie, S.J.; Wang, G.H. Application research status and prospects of Acer truncatum Bunge. seed oil rich in nervonic acid in China. China Oils Fats 2018, 43, 93–95. [Google Scholar]
- Yang, L.; Yin, P.; Fan, H.; Xue, Q.; Li, K.; Li, X.; Sun, L.; Liu, Y. Response surface methodology optimization of ultrasonic-assisted extraction of Acer truncatum leaves for maximal phenolic yield and antioxidant activity. Molecules 2017, 22, 232. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, B.; Bussmann, R.W. Acer tataricum L. Acer truncatum Bunge Sapindaceae. In Ethnobotany of the Mountain Regions of Central Asia and Altai; Springer: Berlin/Heidelberg, Germany, 2020; pp. 21–31. [Google Scholar] [CrossRef]
- Liu, X.Y.; Fu, H.; Qiu, Z.H. Analysis of Acer truncatum tea of yunnan. Yunnan Chem. Technol. 2004, 2, 17–19. [Google Scholar]
- Gu, R.H. Chemical Constituents and Metabolomics of Acer truncatum an Arbor Species Native to China. Ph.D. Thesis, Minzu University of China, Beijing, China, 2019. [Google Scholar]
- Wang, X.Y. Chinese Acer truncatum; Sicuan National Press: Chengdu, China, 2003; pp. 232–238. [Google Scholar]
- Chang, P.; Ma, J.; Xin, H.; Wang, S.; Chen, Z.; Hong, X.; Zhang, B.; Li, L. Comparative Study of the Fatty Acid Composition of the Acer truncatum Bunge from Different Producing Areas. Forests 2022, 13, 1409. [Google Scholar] [CrossRef]
- Tanaka, K.; Shimizu, T.; Ohtsuka, Y.; Yamashiro, Y.; Oshida, K. Early dietary treatments with Lorenzo’s oil and docosahexaenoic acid for neurological development in a case with Zellweger syndrome. Brain Dev. Jpn. 2007, 29, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Terluk, M.R.; Tieu, J.; Sahasrabudhe, S.A.; Moser, A.; Watkins, P.A.; Raymond, G.V.; Kartha, R.V. Nervonic acid attenuates accumulation of very long-chain fatty acids and is a potential therapy for adrenoleukodystrophy. Neurotherapeutics 2022, 19, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Lewkowicz, N.; Piątek, P.; Namiecińska, M.; Domowicz, M.; Bonikowski, R.; Szemraj, J.; Przygodzka, P.; Stasiołek, M.; Lewkowicz, P. Naturally occurring nervonic acid ester improves myelin synthesis by human oligodendrocytes. Cells 2019, 8, 786. [Google Scholar] [CrossRef]
- Feng, J.P. Let Acer truncatum benefit China. China For. Ind. 2021, 5, 2. [Google Scholar]
- Wang, S.; Xie, Y. China Species Red List. Vol.Ⅰ; Higher Education Press: Beijing, China, 2004; p. 356. [Google Scholar]
- Qiao, Q.; Ye, M.; Wu, C.; Wang, J.; Liu, Q.; Tao, J.; Zhang, L.; Feng, Z. Analysis of leaf morphology variation and genetic diversity via SRAP markers for near-threatened plant Acer truncatum. Glob. Ecol. Conserv. 2022, 33, e01980. [Google Scholar] [CrossRef]
- Zang, Z.R.; Yan, L.P.; Wang, Y.H.; Wang, K.F.; Liang, Y.; Zhong, W.G.; Wu, D.J.; Quan, Z.X. Analysis of Genetic Diversity of Acer truncatum Germplasm Resources by Fluorescent SSR Markers. Mol. Plant Breed. 2023, 21, 8163–8171. [Google Scholar] [CrossRef]
- Idrees, M.; Irshad, M. Molecular markers in plants for analysis of genetic diversity: A review. Eur. Acad. Res. 2014, 2, 1513–1540. [Google Scholar]
- Xia, W.; Luo, T.; Zhang, W.; Mason, A.S.; Huang, D.; Huang, X.; Tang, W.; Dou, Y.; Zhang, C.; Xiao, Y. Development of high-density SNP markers and their application in evaluating genetic diversity and population structure in Elaeis guineensis. Front. Plant Sci. 2019, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Guichoux, E.; Lagache, L.; Wagner, S.; Chaumeil, P.; Leger, P.; Lepsis, O.; Lepoittevin, C.; Malausa, T.; Revardel, E.; Salin, F.; et al. Current trends in microsatellite genotyping. Mol. Ecol. Resour. 2011, 11, 591–611. [Google Scholar] [CrossRef] [PubMed]
- Mammadov, J.; Aggarwal, R.; Buyyarapu, R.; Kumpatla, S. SNP markers and their impact on plant breeding. Int. J. Plant Genom. 2012, 2012, 728398. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Y.; Sun, T.L.; Li, S.S.; Wen, J.; Zhu, L.; Yin, T.M.; Yan, K.Y.; Xu, X.; Li, S.X.; Mao, J.F.; et al. The Acer truncatum genome provides insights into nervonic acid biosynthesis. Plant J. 2020, 104, 662–678. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Bian, L.; Zhang, X.; Zhao, B.; Zheng, R.; Su, S.; Ye, D.; Zheng, X.; El-Kassaby, Y.A.; Shi, J. Genetic diversity and structure of the 4th cycle breeding population of Chinese fir (Cunninghamia lanceolata (lamb.) hook). Front. Plant. Sci. 2023, 14, 1106615. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabrie, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Analysis: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 18 March 2024).
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Pallant, J. SPSS Survival Manual: A Step by Step Guide to Data Analysis Using IBM SPSS; McGraw-Hill Education: London, UK, 2020. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dong, S.S.; Xu, J.; He, W.M.; Yang, T.L. PopLDdecay: A fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics 2019, 35, 1786–1788. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Pickrell, J.; Pritchard, J. Inference of population splits and mixtures from genome-wide allele frequency data. Nat. Prec. 2012, 8, 11. [Google Scholar] [CrossRef]
- Wright, S. The genetical structure of populations. Ann. Eugen. 1949, 15, 323–354. [Google Scholar] [CrossRef]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef]
- Terhorst, J.; Kamm, J.A.; Song, Y.S. Robust and scalable inference of population history from hundreds of unphased whole genomes. Nat. Genet. 2017, 49, 303–309. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, D.; Wariss, H.M.; Zhang, R.; Tao, L.; Milne, R.I.; Sun, W. Demographic history and identification of threats revealed by population genomic analysis provide insights into conservation for an endangered maple. Mol. Ecol. 2022, 31, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Tsumura, Y.; Kimura, M.; Nakao, K.; Uchiyama, K.; Ujino-Ihara, T.; Wen, Y.; Tong, Z.; Han, W. Effects of the last glacial period on genetic diversity and genetic differentiation in Cryptomeria japonica in East Asia. Tree Genet. Genomes 2020, 16, 19. [Google Scholar] [CrossRef]
- Sjögren, P.; Wyöni, P.I. Conservation genetics and detection of rare alleles in finite populations. Conserv. Biol. 1994, 8, 267–270. Available online: https://www.jstor.org/stable/2386741 (accessed on 18 March 2023). [CrossRef]
- Nazareno, A.G.; Bemmels, J.B.; Dick, C.W.; Lohmann, L.G. Minimum sample sizes for population genomics: An empirical study from an Amazonian plant species. Mol. Ecol. Resour. 2017, 17, 1136–1147. [Google Scholar] [CrossRef]
- Sun, R.X. Genetic Diversity and Phylogeography of Liquidambar formosana Hance in China. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2017. [Google Scholar]
- Liu, J.; Qiao, W.Y.; Qiu, Y.B.; Liu, X.S.; Chen, R.H.; Zhong, Z.Z.; Yu, F.; Jiang, J.M. Discussion on Genetic Diversity and Sampling Strategy of Natural Toona ciliata var. pubescens. Popul. For. Res. 2019, 32, 175–184. [Google Scholar] [CrossRef]
- Zhu, X.; Zou, R.; Tang, J.; Deng, L.; Wei, X. Genetic diversity variation during the natural regeneration of Vatica guangxiensis, an endangered tree species with extremely small populations. Glob. Ecol. Conserv. 2023, 42, e02400. [Google Scholar] [CrossRef]
- Jia, Z.; Yu, T.; Jiang, B.; Song, X.; Li, J. Genetic Structure and Historical Dynamics of Pinus densiflora Siebold & Zucc. Populations. Forests 2022, 13, 2078. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.L.; Ye, H.L.; Liu, Y.; Su, S.C. Flower bud differentiation and flowering process of Acer truncatum. Acta Bot. Boreali-Occident. Sin. 2021, 41, 1662–1672. [Google Scholar] [CrossRef]
- Endress, P.K. Structural and temporal modes of heterodichogamy and similar patterns across angiosperms. Bot. J. Linn. Soc. 2020, 193, 5–18. [Google Scholar] [CrossRef]
- Zhang, Q.D.; Jia, R.Z.; Meng, C.; Ti, C.W.; Wang, Y.L. Diversity and population structure of a dominant deciduous tree based on morphological and genetic data. AoB Plants 2015, 7, plv103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Motahari, B.; Shabanian, N.; Rahmani, M.S.; Mohammad-Hasanit, F. Genetic diversity and genetic structure of Acer monspessulanum L. across Zagros forests of Iran using molecular markers. Gene 2021, 769, 145245. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, J.L.; Godt, M.J.W. Allozyme diversity in plant species. In Plant Population Genetics, Breeding and Genetic Resource; Sinauer Associates, Inc.: Sunderland, UK, 1989; pp. 43–63. Available online: https://www.researchgate.net/publication/245264868 (accessed on 18 March 2024).
- Fjeldsa, J.; Bowie, R.C.; Rahbek, C. The role of mountain ranges in the diversification of birds. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 249–265. [Google Scholar] [CrossRef]
- Bockelmann, A.C.; Reusch, T.B.; Bijlsma, R.; Bakker, J.P. Habitat differentiation vs. isolation-by-distance: The genetic population structure of Elymus athericus in European salt marshes. Mol. Ecol. 2003, 12, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Deng, Y.W.; Liu, Y.; Zhao, J.; Bao, L.; Ge, J.P.; Wang, H.F. Genetic structure and trait variation within a maple hybrid zone underscore North China as an overlooked diversity hotspot. Sci. Rep. 2022, 12, 13949. [Google Scholar] [CrossRef] [PubMed]
- Uhrinová, V.; Zozomová-Lihová, J.; Bernátová, D.; Paule, J.; Paule, L.; Gömöry, D. Origin and genetic differentiation of pink-flowered Sorbus hybrids in the Western Carpathians. Ann. Bot. 2017, 120, 271–284. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, G.B. Intraspecific phylogeography and its application in conservation strategies of plant genetic diversity. J. Cent. South Univ. For. Technol. 2011, 31, 1–6. [Google Scholar] [CrossRef]
- Sun, X.; Jiao, C.; Schwaninger, H.; Chao, C.T.; Fei, Z. Phased diploid genome assemblies and pan-genomes provide insights into the genetic history of apple domestication. Nat. Genet. 2020, 52, 1423–1432. [Google Scholar] [CrossRef]
- Tao, T.; Milne, R.I.; Li, J.; Yang, H.; Wang, S.; Chen, S.; Mao, K. Conservation Genomic investigation of an endangered conifer, Thuja sutchuenensis, reveals low genetic diversity but also low genetic load. Plant Divers. 2023, 46, 78–90. [Google Scholar] [CrossRef]
- Zhao, Z.L. Research on afforestation in the arid area of Acer truncatum. For. Sci. Technol. 1999, 1, 28–29. [Google Scholar]
- Wang, H.J.; Duan, A.A.; Wu, Y. A study on flowering and fruiting characteristics of Acer truncatumn introducing site of Kunming. J. West China For. Sci. 2006, 4, 68–72. [Google Scholar] [CrossRef]
- Mu, Y.J.; Yang, Y.M.; Wang, L.L.; Han, B.; Xian, Y.; Xie, X.M.; Li, W.Q.; Lu, L.; Lu, Y.Z. Seed germination and response to different disinfection treatments of Acer truncatum. For. Sci. Technol. 2021, 9, 17–22. [Google Scholar] [CrossRef]
- Shen, J.; Jones, R.T.; Yang, X.; Dearing, J.A.; Wang, S. The Holocene vegetation history of Lake Erhai, Yunnan province southwestern China: The role of climate and human forcings. Holocene 2006, 16, 265–276. [Google Scholar] [CrossRef]



| Climatic Zone | Province | Location | Code | Longitude (E) | Latitude (N) | Altitude (m) | N |
|---|---|---|---|---|---|---|---|
| Middle temperate zone | Inner Mongolia | Dai Qin Ta La | DQT | 121°30′41″ | 45°12′54″ | 332 | 10 |
| Song Shu Shan | SSS | 119°33′23″ | 43°1′27″ | 773 | 10 | ||
| Wu Dan Ta La | WDT | 122°45′44″ | 42°59′45″ | 220 | 20 | ||
| Liaoning | Zhang Wu | ZW | 122°32′38″ | 42°50′24″ | 259 | 10 | |
| Warm temperate zone | Beijing | La Ba Gou Men | LBGM | 116°27′81″ | 40°57′18″ | 1313 | 10 |
| Xiao Long Men | XLM | 115°25′57″ | 39°57′59″ | 1188 | 10 | ||
| Gansu | Xiao Long Shan | XLS | 106°17′34″ | 34°20′17″ | 1322 | 10 | |
| Henan | Ping Quan | PQ | 118°46′1″ | 40°50′33″ | 1150 | 10 | |
| Ji Yuan | JY | 112°8′0.6″ | 35°9′39″ | 700 | 10 | ||
| Hebei | Wu Ling Shan | WLS | 117°47′41″ | 40°57′1″ | 1334 | 10 | |
| Shannxi | Zhong Tiao Shan | ZTS | 112°1′34″ | 35°30′12″ | 1379 | 10 | |
| Subtropic zone | Henan | Bao Tian Man | BTM | 111°52′46″ | 33°28′57″ | 1017 | 10 |
| Shaanxi | Tian Shu Xia | TSX | 109°17′18″ | 31°59′51″ | 1804 | 10 |
| Location Code | Total Reads | SNP Num. | Alignment Rate (%) | Average Q30 (%) | Average CG (%) | Ti/Tv |
|---|---|---|---|---|---|---|
| WDT * | 1,054,174,488 | 15,686,473 | 95.20 | 90.33 | 36.63 | 2.02 |
| BTM | 511,807,662 | 10,313,738 | 97.61 | 90.03 | 35.95 | 2.03 |
| DQT | 511,057,652 | 5,989,638 | 97.70 | 88.88 | 36.17 | 1.93 |
| JY | 495,467,730 | 5,320,385 | 95.38 | 93.60 | 36.04 | 1.92 |
| LBGM | 502,797,720 | 11,099,540 | 96.85 | 89.28 | 35.94 | 1.94 |
| PQ | 474,844,234 | 5,503,809 | 94.51 | 93.49 | 36.50 | 1.92 |
| SSS | 503,270,792 | 5,866,083 | 97.97 | 88.74 | 36.45 | 1.94 |
| TSX | 511,075,076 | 10,485,169 | 96.09 | 89.22 | 36.70 | 2.01 |
| WDT | 519,997,908 | 5,454,954 | 95.16 | 89.74 | 36.50 | 1.91 |
| WLS | 513,698,880 | 6,143,759 | 97.39 | 89.09 | 36.64 | 1.95 |
| XLM | 500,572,490 | 11,008,006 | 95.90 | 89.09 | 36.63 | 1.94 |
| XLS | 541,715,686 | 5,388,349 | 95.79 | 93.33 | 36.21 | 1.91 |
| ZTS | 509,314,274 | 6,476,086 | 97.60 | 89.01 | 36.37 | 1.96 |
| ZW | 510,144,564 | 10,685,342 | 97.14 | 89.27 | 36.19 | 1.94 |
| XS04 | 47,801,750 | 905,848 | 74.70 | 96.09 | 40.28 | 1.52 |
| Location Code | Na | Ne | Ho | He | PIC | I | Nei’s | Tajima’D | Fis |
|---|---|---|---|---|---|---|---|---|---|
| BTM | 1.439 | 1.246 | 0.266 | 0.332 | 0.267 | 0.501 | 0.351 | 0.9490 | 0.058 |
| DQT | 1.511 | 1.215 | 0.267 | 0.267 | 0.221 | 0.423 | 0.281 | 0.2386 | 0.007 |
| JY | 1.423 | 1.191 | 0.262 | 0.280 | 0.231 | 0.439 | 0.296 | −1.0122 | 0.074 |
| LBGM | 1.429 | 1.244 | 0.329 | 0.336 | 0.270 | 0.506 | 0.356 | 1.0783 | −0.068 |
| PQ | 1.513 | 1.205 | 0.243 | 0.255 | 0.212 | 0.407 | 0.269 | 0.4044 | 0.034 |
| SSS | 1.509 | 1.204 | 0.254 | 0.255 | 0.212 | 0.407 | 0.269 | 0.1223 | 0.013 |
| TSX | 1.348 | 1.179 | 0.299 | 0.310 | 0.251 | 0.475 | 0.328 | 1.2503 | −0.058 |
| WDT | 1.459 | 1.192 | 0.250 | 0.266 | 0.220 | 0.421 | 0.280 | 1.0854 | 0.073 |
| WLS | 1.490 | 1.222 | 0.284 | 0.286 | 0.236 | 0.447 | 0.301 | 0.6191 | 0.008 |
| XLM | 1.434 | 1.244 | 0.322 | 0.331 | 0.266 | 0.500 | 0.351 | 0.8866 | −0.054 |
| XLS | 1.439 | 1.188 | 0.256 | 0.269 | 0.223 | 0.425 | 0.284 | −0.7118 | 0.057 |
| ZTS | 1.572 | 1.255 | 0.268 | 0.284 | 0.235 | 0.446 | 0.299 | 1.4664 | 0.026 |
| ZW | 1.717 | 1.321 | 0.280 | 0.282 | 0.233 | 0.443 | 0.299 | −0.5777 | 0.001 |
| Mean | 1.483 | 1.224 | 0.275 | 0.289 | 0.237 | 0.449 | 0.305 | 0.4460 | 0.013 |
| Source of Variation | Df | SS | VC | PV (%) |
|---|---|---|---|---|
| Among Pops | 12 | 1,587,760.90 | 5346.23544 | 17.53% |
| Within Pops | 247 | 6,210,342.35 | 25,155.3671 | 82.47% |
| Total | 259 | 7,798,103.25 | 30,501.60254 | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Yu, X.; Wu, Y.; Pei, S.; Xin, X.; Xia, X.; Mao, S.; Pan, X.; Zheng, Y.; Zhang, C. Spatial Pattern of Genetic Diversity and Demographic History Revealed by Population Genomic Analysis: Resilience to Climate Fluctuations of Acer truncatum Bunge. Forests 2024, 15, 639. https://doi.org/10.3390/f15040639
Liao J, Yu X, Wu Y, Pei S, Xin X, Xia X, Mao S, Pan X, Zheng Y, Zhang C. Spatial Pattern of Genetic Diversity and Demographic History Revealed by Population Genomic Analysis: Resilience to Climate Fluctuations of Acer truncatum Bunge. Forests. 2024; 15(4):639. https://doi.org/10.3390/f15040639
Chicago/Turabian StyleLiao, Jia, Xuedan Yu, Yuxia Wu, Shunxiang Pei, Xuebing Xin, Xinhe Xia, Shan Mao, Xinyue Pan, Yongqi Zheng, and Chuanhong Zhang. 2024. "Spatial Pattern of Genetic Diversity and Demographic History Revealed by Population Genomic Analysis: Resilience to Climate Fluctuations of Acer truncatum Bunge" Forests 15, no. 4: 639. https://doi.org/10.3390/f15040639
APA StyleLiao, J., Yu, X., Wu, Y., Pei, S., Xin, X., Xia, X., Mao, S., Pan, X., Zheng, Y., & Zhang, C. (2024). Spatial Pattern of Genetic Diversity and Demographic History Revealed by Population Genomic Analysis: Resilience to Climate Fluctuations of Acer truncatum Bunge. Forests, 15(4), 639. https://doi.org/10.3390/f15040639





