The Impact of Canopy on Nutrient Fluxes through Rainfall Partitioning in a Mixed Broadleaf and Coniferous Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Site
2.2. Field Measurement and Sample Collection
2.3. pH and Nutrient Concentration Analysis of Collected Samples
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Rainfall Redistribution
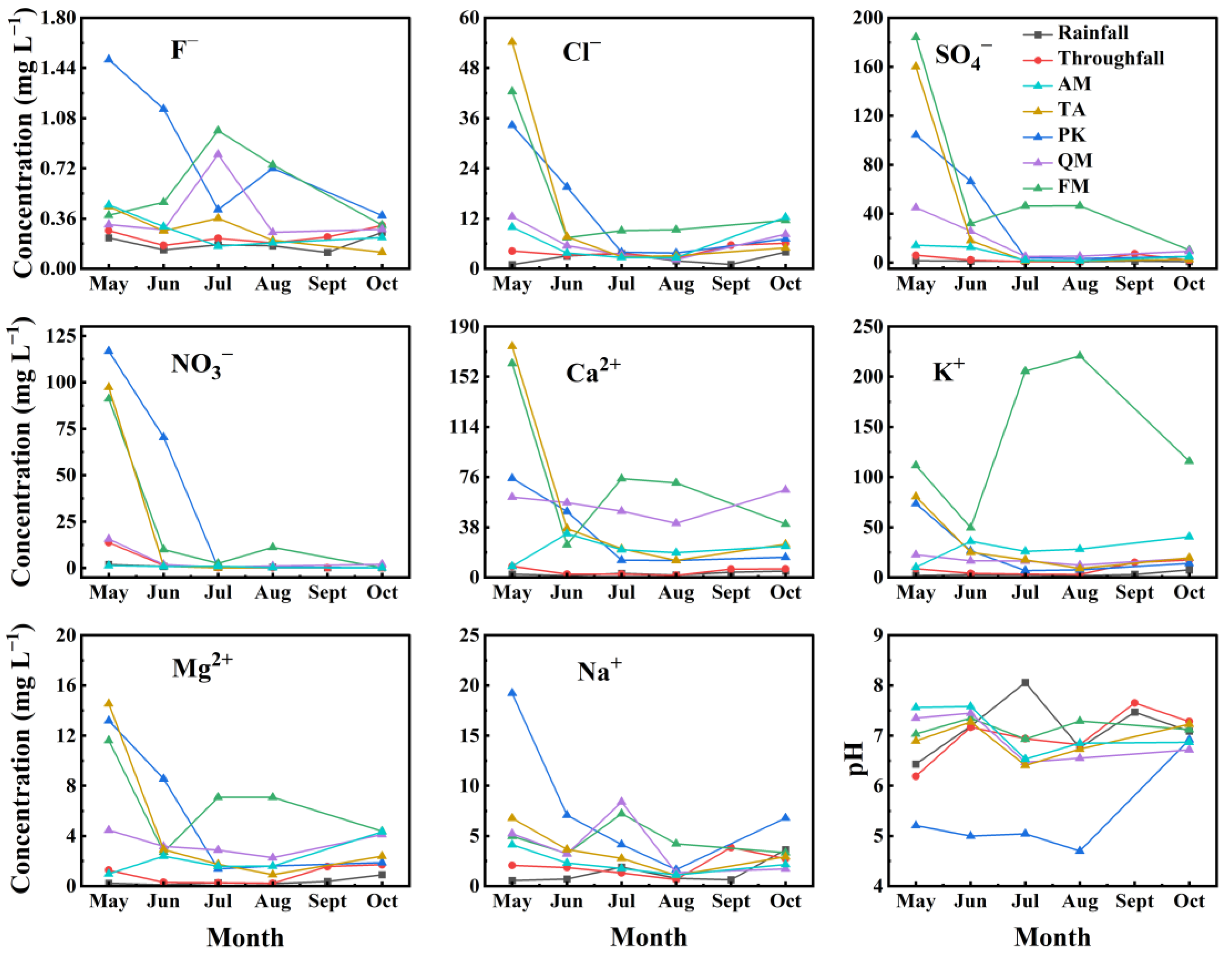
3.2. Variation in Nutrient Concentration across Different Tree Species
3.3. The Impact of Stemflow on Soil pH and Temporal Dynamics of Water Chemistry
3.4. Nutrient Fluxes through Different Water Input Pathways
4. Discussion
4.1. Morphological Characteristics of Trees Affect Nutrient Concentrations in Stemflow
4.2. Throughfall and Stemflow Fluxes Vary between Different Regions and Forest Types
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Van Stan, J.T.; Ponette-Gonzalez, A.G.; Swanson, T.; Weathers, K.C. Throughfall and stemflow are major hydrologic highways for particulate traffic through tree canopies. Front. Ecol. Environ. 2021, 19, 404–410. [Google Scholar] [CrossRef]
- Parker, G.G. Throughfall and Stemflow in the Forest Nutrient Cycle. In Advances in Ecological Research; MacFadyen, A., Ford, E.D., Eds.; Academic Press: Cambridge, MA, USA, 1983; Volume 13, pp. 57–133. [Google Scholar] [CrossRef]
- Sun, X.C.; Zhang, Z.; Cao, Y.H.; Liu, L.; Hu, F.L.; Lu, X.Q. Canopy modification of base cations deposition in a subtropical broadleaved forest: Spatial characteristics, canopy budgets and acid neutralizing capacity. For. Ecol. Manag. 2021, 482, 118863. [Google Scholar] [CrossRef]
- Stubbins, A.; Guillemette, F.; Van Stan, J.T. Throughfall and Stemflow: The Crowning Headwaters of the Aquatic Carbon Cycle. In Precipitation Partitioning by Vegetation: A Global Synthesis; Van Stan, J.T., Gutmann, E., Friesen, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 121–131. [Google Scholar] [CrossRef]
- Levia, D.F.; Keim, R.F.; Carlyle-Moses, D.E.; Frost, E.E. Throughfall and Stemflow in Wooded Ecosystems. In Forest Hydrology and Biogeochemistry: Synthesis of Past Research and Future Directions; Levia, D.F., Carlyle-Moses, D., Tanaka, T., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 425–443. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Yuan, C.; Chen, N.; Levia, D.F. Rainfall partitioning by vegetation in China: A quantitative synthesis. J. Hydrol. 2023, 617, 128946. [Google Scholar] [CrossRef]
- Yan, T.; Wang, Z.H.; Liao, C.G.; Xu, W.Y.; Wan, L. Experimental data on the adsorption of water by branches and leaves as affected by different the morphological characteristics of plants. Data Brief 2021, 34, 106689. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Wang, Z.H.; Liao, C.G.; Xu, W.Y.; Wan, L. Effects of the morphological characteristics of plants on rainfall interception and kinetic energy. J. Hydrol. 2021, 592, 125807. [Google Scholar] [CrossRef]
- Scavotto, N.; Siegert, C.M.; Alexander, H.D.; Varner, J.M. Bark and crown morphology drive differences in rainwater distribution in an upland oak forest. For. Ecol. Manag. 2024, 553, 121642. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Lv, X.Z.; Yu, X.X.; Ni, Y.X.; Ma, L.; Liu, Z.Q. Species and spatial differences in vegetation rainfall interception capacity: A synthesis and meta-analysis in China. Catena 2022, 213, 106223. [Google Scholar] [CrossRef]
- Grunicke, S.; Queck, R.; Bernhofer, C. Long-term investigation of forest canopy rainfall interception for a spruce stand. Agric. For. Meteorol. 2020, 292, 108125. [Google Scholar] [CrossRef]
- Zabret, K.; Sraj, M. How Characteristics of a Rainfall Event and the Meteorological Conditions Determine the Development of Stemflow: A Case Study of a Birch Tree. Front. For. Glob. Chang. 2021, 4, 663100. [Google Scholar] [CrossRef]
- Tonello, K.C.; Van Stan, J.T.; Rosa, A.G.; Balbinot, L.; Pereira, L.C.; Bramorski, J. Stemflow variability across tree stem and canopy traits in the Brazilian Cerrado. Agric. For. Meteorol. 2021, 308, 108551. [Google Scholar] [CrossRef]
- Yue, K.; De Frenne, P.; Fornara, D.A.; Van Meerbeek, K.; Li, W.; Peng, X.; Ni, X.; Peng, Y.; Wu, F.; Yang, Y.; et al. Global patterns and drivers of rainfall partitioning by trees and shrubs. Glob. Chang. Biol. 2021, 27, 3350–3357. [Google Scholar] [CrossRef] [PubMed]
- Staelens, J.; Houle, D.; De Schrijver, A.; Neirynck, J.; Verheyen, K. Calculating dry deposition and canopy exchange with the canopy budget model: Review of assumptions and application to two deciduous forests. Water Air Soil Pollut. 2008, 191, 149–169. [Google Scholar] [CrossRef]
- Hyder, P.W.; Fredrickson, E.L.; Estell, R.E.; Lucero, M.E. Transport of phenolic compounds from leaf surface of creosotebush and tarbush to soil surface by precipitation. J. Chem. Ecol. 2002, 28, 2475–2482. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.B.; Hong, W. Estimation of dry deposition and canopy exchange in Chinese fir plantations. For. Ecol. Manag. 2001, 147, 99–107. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ogawa, T.; Ishikawa, M.; Hirai, A.; Watanabe, Y.; Nakano, T. Organic and inorganic nitrogen deposition on the red pine forests at the northern foot of Mt. Fuji, Japan. Atmospheric Environ. 2020, 237, 117676. [Google Scholar] [CrossRef]
- Sheng, H.C.; Guo, N.; Ren, S.Y.; Zhang, J.W.; Ju, C.Y.; Cai, T.J. Hydrochemical fluxes in rainfall, throughfall, and stemflow in Pinus sylvestris var. mongolica plantation, northeast China. Glob. Nest J. 2021, 23, 333–339. [Google Scholar] [CrossRef]
- Dowtin, A.L.; Siegert, C.M.; Levia, D.F. Comparisons of flux-based stemflow enrichment ratios for two Quercus spp. within the megalopolis of the eastern USA. Urban Ecosyst. 2021, 24, 675–690. [Google Scholar] [CrossRef]
- Lima, M.T.; Urso-Guimarães, M.V.; Van Stan, J.T.; Tonello, K.C. Stemflow metazoan transport from common urban tree species (São Paulo, Brazil). Ecohydrology 2022, 16, 2517. [Google Scholar] [CrossRef]
- Guidone, M.; Gordon, D.A.; Van Stan, J.T. Living particulate fluxes in throughfall and stemflow during a pollen event. Biogeochemistry 2021, 153, 323–330. [Google Scholar] [CrossRef]
- Teachey, M.E.; Ottesen, E.A.; Pound, P.; Van Stan, J.T., II. Under the canopy: Disentangling the role of stemflow in shaping spatial patterns of soil microbial community structure underneath trees. Environ. Microbiol. 2022, 24, 4001–4012. [Google Scholar] [CrossRef]
- Metzger, J.C.; Filipzik, J.; Michalzik, B.; Hildebrandt, A. Stemflow Infiltration Hotspots Create Soil Microsites Near Tree Stems in an Unmanaged Mixed Beech Forest. Front. For. Glob. Chang. 2021, 4, 701293. [Google Scholar] [CrossRef]
- Yang, B.; Wang, R.; Xiao, H.; Cao, Q.Q.; Liu, T. Spatio-temporal variations of soil water content and salinity around individual Tamarix ramosissima in a semi-arid saline region of the upper Yellow River, Northwest China. J. Arid Environ. 2018, 10, 101–114. [Google Scholar] [CrossRef]
- Li, C.J.; Li, Y.; Ma, J. Spatial heterogeneity of soil chemical properties at fine scales induced by Haloxylon ammodendron (Chenopodiaceae) plants in a sandy desert. Ecol. Res. 2011, 26, 385–394. [Google Scholar] [CrossRef]
- Wang, H.F.; Cai, Y.; Yang, Q.; Gong, Y.M.; Lv, G.H. Factors that alter the relative importance of abiotic and biotic drivers on the fertile island in a desert-oasis ecotone. Sci. Total Environ. 2019, 697, 134096. [Google Scholar] [CrossRef]
- Whitford, W.G.; Anderson, J.; Rice, P.M. Stemflow contribution to the ‘fertile island’ effect in creosotebush, Larrea tridentata. J. Arid Environ. 1997, 35, 451–457. [Google Scholar] [CrossRef]
- Sheng, H.C.; Guo, N.; Ju, C.Y.; Cai, T.J. Variation of nutrient fluxes by rainfall redistribution processes in the forest canopy of an urban larch plantation in northeast China. J. For. Res. 2022, 33, 1259–1269. [Google Scholar] [CrossRef]
- Jana, P.; Dasgupta, S.; Todaria, N.P. Throughfall and stemflow nutrient flux in deodar and oak forests, Garhwal Himalaya, India. Water Supply 2021, 21, 1649–1656. [Google Scholar] [CrossRef]
- Luna-Robles, E.O.; Cantu-Silva, I.; Gonzalez-Rodriguez, H.; Marmolejo-Monsivais, J.G.; Yanez-Diaz, M.I.; Bejar-Pulido, S.J. Nutrient input via gross rainfall, throughfall and stemflow in scrubland species in northeastern Mexico. Rev. Chapingo Ser. Cienc. For. Ambient. 2019, 25, 235–251. [Google Scholar] [CrossRef]
- Duval, T.P. Rainfall partitioning through a mixed cedar swamp and associated C and N fluxes in Southern Ontario, Canada. Hydrol. Process. 2019, 33, 1510–1524. [Google Scholar] [CrossRef]
- Turtscher, S.; Grabner, M.; Berger, T.W. Reconstructing Soil Recovery from Acid Rain in Beech (Fagus sylvatica) Stands of the Vienna Woods as Indicated by Removal of Stemflow and Dendrochemistry. Water Air Soil Pollut. 2019, 230, 30. [Google Scholar] [CrossRef]
- Lombardo, L.; Trujillo, C.; Vanwalleghem, T.; Gomez, J.A. Organic carbon fluxes by precipitation, throughfall and stemflow in an olive orchard in Southern Spain. Plant Biosyst. 2018, 152, 1039–1047. [Google Scholar] [CrossRef]
- Pinos, J.; Llorens, P.; Latron, J. High-resolution temporal dynamics of intra-storm isotopic composition of stemflow and throughfall in a Mediterranean Scots pine forest. Hydrol. Process. 2022, 36, 14641. [Google Scholar] [CrossRef]
- Fan, J.L.; Oestergaard, K.T.; Guyot, A.; Lockington, D.A. Measuring and modeling rainfall interception losses by a native Banksia woodland and an exotic pine plantation in subtropical coastal Australia. J. Hydrol. 2014, 515, 156–165. [Google Scholar] [CrossRef]
- Tonello, K.C.; Rosa, A.G.; Pereira, L.C.; Matus, G.N.; Guandique, M.E.G.; Navarrete, A.A. Rainfall partitioning in the Cerrado and its influence on net rainfall nutrient fluxes. Agric. For. Meteorol. 2021, 303, 108372. [Google Scholar] [CrossRef]
- Tan, S.Y.; Zhao, H.R.; Yang, W.Q.; Tan, B.; Ni, X.Y.; Yue, K.; Zhang, Y.; Wu, F.Z. The effect of canopy exchange on input of base cations in a subalpine spruce plantation during the growth season. Sci. Rep. 2018, 8, 9373. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, S.X.; Fang, J.P.; Yan, H.A.; Li, J.R. Nutrient Fluxes in Rainfall, Throughfall, and Stemflow in Pinus densata Natural Forest of Tibetan Plateau. Clean 2017, 45, 1600008. [Google Scholar] [CrossRef]
- Germer, S.; Zimmermann, A.; Neill, C.; Krusche, A.V.; Elsenbeer, H. Disproportionate single-species contribution to canopy-soil nutrient flux in an Amazonian rainforest. For. Ecol. Manag. 2012, 267, 40–49. [Google Scholar] [CrossRef]
- Cayuela, C.; Levia, D.F.; Latron, J.; Llorens, P. Particulate Matter Fluxes in a Mediterranean Mountain Forest: Interspecific Differences Between Throughfall and Stemflow in Oak and Pine Stands. J. Geophys. Res. Atmos. 2019, 124, 5106–5116. [Google Scholar] [CrossRef]
- Pypker, T.G.; Levia, D.F.; Staelens, J.; Van Stan, J.T. Canopy Structure in Relation to Hydrological and Biogeochemical Fluxes. In Forest Hydrology and Biogeochemistry: Synthesis of Past Research and Future Directions; Levia, D.F., Carlyle-Moses, D., Tanaka, T., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 371–388. [Google Scholar] [CrossRef]
- Schooling, J.T.; Levia, D.F.; Carlyle-Moses, D.E.; Dowtin, A.L.; Brewer, S.E.; Donkor, K.K.; Borden, S.A.; Grzybowski, A.A. Stemflow chemistry in relation to tree size: A preliminary investigation of eleven urban park trees in British Columbia, Canada. Urban For. Urban Green. 2017, 21, 129–133. [Google Scholar] [CrossRef]
- De Schrijver, A.; Geudens, G.; Augusto, L.; Staelens, J.; Mertens, J.; Wuyts, K.; Gielis, L.; Verheyen, K. The effect of forest type on throughfall deposition and seepage flux: A review. Oecologia 2007, 153, 663–674. [Google Scholar] [CrossRef]
- Hamdan, K.; Schmidt, M. The influence of bigleaf maple on chemical properties of throughfall, stemflow, and forest floor in coniferous forest in the Pacific Northwest. Can. J. For. Res. 2012, 42, 868–878. [Google Scholar] [CrossRef]
- Hofhansl, F.; Wanek, W.; Drage, S.; Huber, W.; Weissenhofer, A.; Richter, A. Controls of hydrochemical fluxes via stemflow in tropical lowland rainforests: Effects of meteorology and vegetation characteristics. J. Hydrol. 2012, 452, 247–258. [Google Scholar] [CrossRef]
- Levia, D.F.; Herwitz, S.R. Physical properties of water in relation to stemflow leachate dynamics: Implications for nutrient cycling. Can. J. For. Res. 2000, 30, 662–666. [Google Scholar] [CrossRef]
- You, Y.Y.; Xiang, W.H.; Ouyang, S.; Zhao, Z.H.; Chen, L.; Zeng, Y.L.; Lei, P.F.; Deng, X.W.; Wang, J.R.; Wang, K.L. Hydrological fluxes of dissolved organic carbon and total dissolved nitrogen in subtropical forests at three restoration stages in southern China. J. Hydrol. 2020, 583, 124656. [Google Scholar] [CrossRef]
- Hofhansl, F.; Wanek, W.; Drage, S.; Huber, W.; Weissenhofer, A.; Richter, A. Topography strongly affects atmospheric deposition and canopy exchange processes in different types of wet lowland rainforest, Southwest Costa Rica. Biogeochemistry 2010, 106, 371–396. [Google Scholar] [CrossRef]
- Berger, T.W.; Untersteiner, H.; Schume, H.; Jost, G. Throughfall fluxes in a secondary spruce (Picea abies), a beech (Fagus sylvatica) and a mixed spruce–beech stand. For. Ecol. Manag. 2008, 255, 605–618. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Zhi, Q.Y.; Van Stan, J.T.; Zhang, S.Y.; Xiao, Y.H.; Chen, X.Y.; Yang, X.; Zhou, H.Y.; Hu, Z.M.; Wu, H.W. Rainfall partitioning and associated chemical alteration in three subtropical urban tree species. J. Hydrol. 2021, 603, 127109. [Google Scholar] [CrossRef]
- Dawoe, E.K.; Barnes, V.R.; Oppong, S.K. Spatio-temporal dynamics of gross rainfall partitioning and nutrient fluxes in shaded-cocoa (Theobroma cocoa) systems in a tropical semi-deciduous forest. Agrofor. Syst. 2017, 92, 397–413. [Google Scholar] [CrossRef]
- Coble, A.A.; Hart, S.C. The significance of atmospheric nutrient inputs and canopy interception of precipitation during ecosystem development in piñon–juniper woodlands of the southwestern USA. J. Arid Environ. 2013, 98, 79–87. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.H.; Chen, R.S.; Ni, X.; Cao, J. Effects of Forest Type on Nutrient Fluxes in Throughfall, Stemflow, and Litter Leachate within Acid-Polluted Locations in Southwest China. Int. J. Environ. Res. Public Health 2022, 19, 2810. [Google Scholar] [CrossRef]
- Arisci, S.; Rogora, M.; Marchetto, A.; Dichiaro, F. The role of forest type in the variability of DOC in atmospheric deposition at forest plots in Italy. Environ. Monit. Assess. 2012, 184, 3415–3425. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.C.; Wu, F.Z.; Peng, Y.; Hedenec, P.; Ni, X.Y.; Tan, S.Y.; Huang, Y.B.; Yue, K. Effects of Forest Transformation on the Fluxes of Potassium, Calcium, Sodium, and Magnesium Along with Rainfall Partitioning. Pol. J. Environ. Stud. 2023, 32, 4341–4351. [Google Scholar] [CrossRef] [PubMed]
- Arfanuzzaman, M.; Betts, R.A.; Gelfan, A.; Hirabayashi, Y.; Lissner, T.K.; Gunn, E.L.; Liu, J.; Morgan, R.; Mwanga, S.; Supratid, S. Water. In Climate Change 2022—Impacts, Adaptation and Vulnerability: Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022; pp. 551–712. [Google Scholar] [CrossRef]
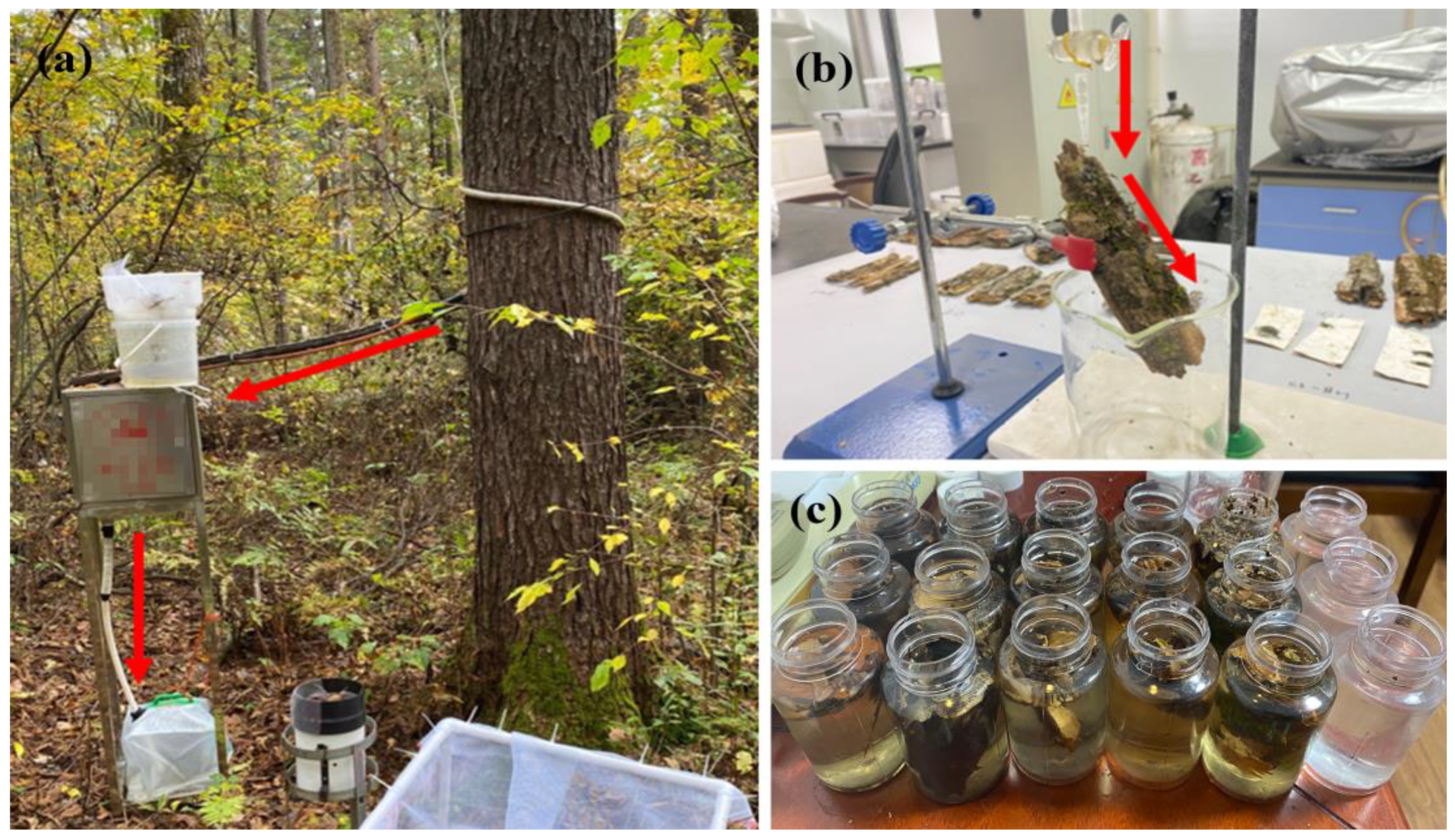

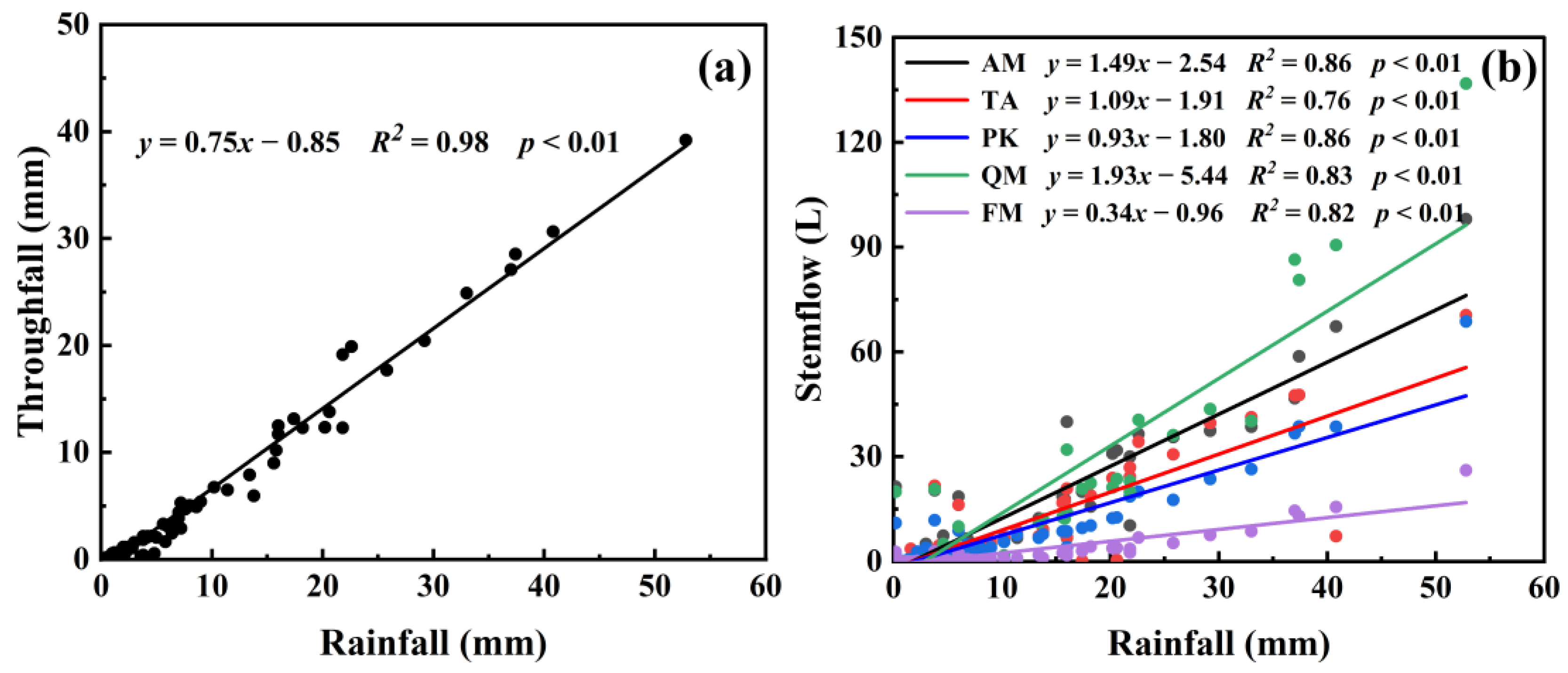
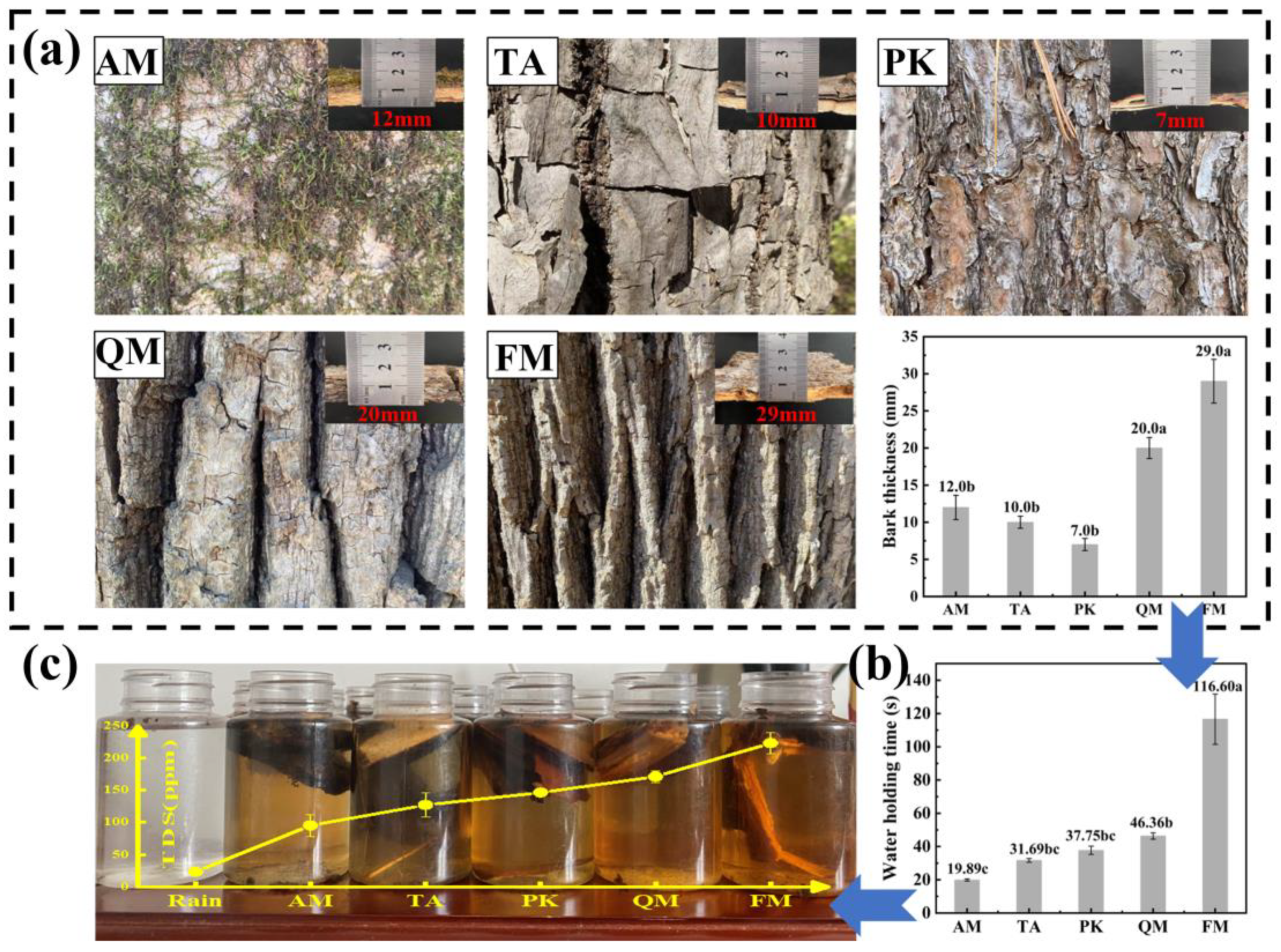
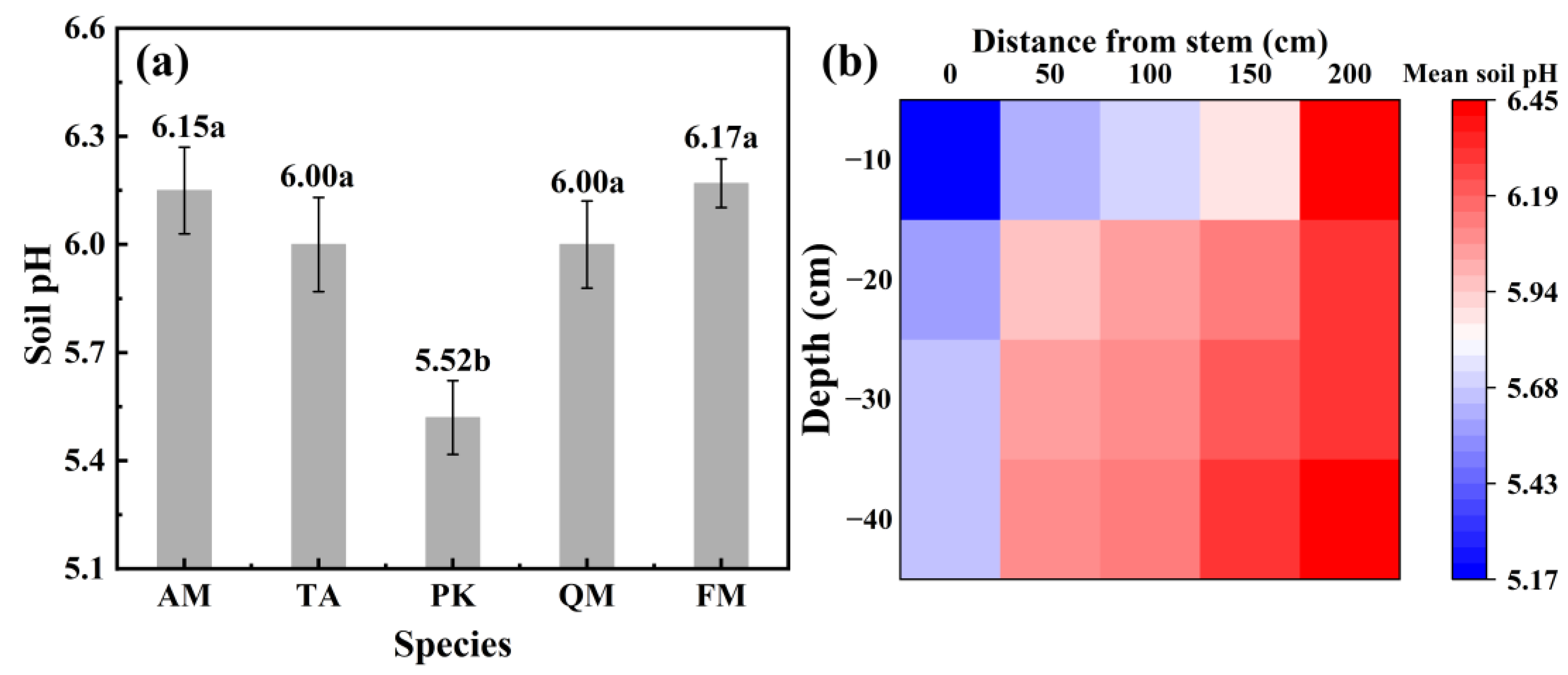
| Item | DBH (cm) | Height (m) | Crown Area (m2) | Tree Density (Trees ha−1) | Leaf Area Index | Canopy Density |
|---|---|---|---|---|---|---|
| Stand | 50.49 ± 8.93 | 25.67 ± 2.09 | — | 545 | 4.92 ± 0.32 | 0.80 |
| AM | 29.30 ± 2.43 | 19.33 ± 0.72 | 4.91 ± 0.94 | 140 | — | — |
| TA | 44.59 ± 5.95 | 26.00 ± 1.25 | 9.93 ± 1.30 | 100 | — | — |
| PK | 39.81 ± 2.36 | 26.00 ± 0.47 | 8.43 ± 0.75 | 166 | — | — |
| QM | 60.51 ± 0.99 | 28.33 ± 0.72 | 17.27 ± 1.93 | 14 | — | — |
| FM | 65.29 ± 0.77 | 28.67 ± 0.27 | 14.79 ± 0.97 | 82 | — | — |
| Chemical Variable | Rainfall (n = 30) | Throughfall (n = 40) | Stemflow (n = 150) | ||||
|---|---|---|---|---|---|---|---|
| AM | TA | PK | QM | FM | |||
| F− | 0.17 ± 0.02 c | 0.20 ± 0.03 bc | 0.22 ± 0.02 bc | 0.28 ± 0.07 bc | 0.76 ± 0.08 a | 0.41 ± 0.20 ab | 0.69 ± 0.16 a |
| Cl− | 2.44 ± 0.82 b | 3.45 ± 0.43 b | 3.46 ± 1.04 b | 6.09 ± 0.98 b | 10.05 ± 0.45 ab | 4.02 ± 0.80 b | 8.66 ± 1.30 a |
| SO42− | 0.90 ± 0.08 e | 1.50 ± 0.51 de | 5.84 ± 1.70 bc | 11.35 ± 2.84 cd | 27.18 ± 2.40 b | 13.15 ± 3.36 b | 39.39 ± 10.49 a |
| NO3− | 0.64 ± 0.10 b | 0.80 ± 0.80 b | 0.72 ± 0.25 b | 2.72 ± 0.39 b | 26.56 ± 1.60 ab | 1.34 ± 0.30 ab | 7.90 ± 1.95 a |
| Ca2+ | 2.29 ± 0.33 c | 2.75 ± 0.58 c | 24.41 ± 2.32 b | 29.79 ± 3.52 b | 26.93 ± 1.47 b | 50.48 ± 4.90 a | 53.65 ± 11.00 a |
| K+ | 2.74 ± 0.52 d | 5.08 ± 1.09 d | 30.15 ± 2.45 b | 20.43 ± 2.16 bc | 15.42 ± 1.54 c | 15.38 ± 1.11 c | 147.85 ± 32.77 a |
| Mg2+ | 0.24 ± 0.05 c | 0.44 ± 0.12 c | 1.92 ± 0.31 b | 2.40 ± 0.28 b | 4.15 ± 0.37 b | 2.85 ± 0.28 ab | 5.33 ± 1.01 a |
| Na+ | 1.22 ± 0.23 c | 1.51 ± 0.23 c | 1.81 ± 0.19 bc | 2.91 ± 0.40 bc | 4.97 ± 0.65 b | 3.87 ± 1.39 abc | 4.64 ± 0.99 a |
| pH | 7.32 ± 0.23 a | 6.88 ± 0.18 a | 7.05 ± 0.24 a | 6.86 ± 0.19 a | 5.17 ± 0.25 b | 6.89 ± 0.19 a | 7.16 ± 0.19 a |
| Type of Water Sample | Nutrient Input (kg ha−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| F− | Cl− | SO42− | NO3− | Ca2+ | K+ | Mg2+ | Na+ | |
| Rainfall (IP) | 1.13 | 16.43 | 6.02 | 4.29 | 15.39 | 18.43 | 1.60 | 8.23 |
| Throughfall (IT) | 0.86 | 14.76 | 6.40 | 3.41 | 11.76 | 21.73 | 1.88 | 6.45 |
| Stemflow (IS) | 0.11 | 1.67 | 4.05 | 2.48 | 7.61 | 7.49 | 0.77 | 0.85 |
| Total wet input (IT + IS) | 0.98 | 16.42 | 10.45 | 5.89 | 19.38 | 29.22 | 2.65 | 7.31 |
| Exchange effect (IT + IS − IP) | −0.15 | 0.01 | 4.43 | 1.60 | 3.98 | 10.79 | 1.05 | −0.92 |
| Species | Nutrient Inputs through Stemflow (g tree−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| F− | Cl− | SO42− | NO3− | Ca2+ | K+ | Mg2+ | Na+ | |
| AM | 0.17 ± 0.04 a | 2.59 ± 0.29 ab | 4.38 ± 0.18 c | 0.54 ± 0.04 b | 18.32 ± 0.68 b | 22.63 ± 1.16 a | 1.44 ± 0.22 a | 1.36 ± 0.15 a |
| TA | 0.15 ± 0.02 a | 3.41 ± 0.74 ab | 6.36 ± 0.88 c | 1.52 ± 0.05 b | 16.68 ± 0.78 b | 11.44 ± 0.42 b | 1.34 ± 0.09 a | 1.63 ± 0.32 a |
| PK | 0.37 ± 0.04 a | 4.87 ± 1.38 a | 13.16 ± 0.25 a | 12.86 ± 1.16 a | 13.04 ± 0.87 b | 7.47 ± 0.41 c | 2.01 ± 0.46 a | 2.41 ± 0.69 a |
| QM | 0.33 ± 0.14 a | 3.17 ± 0.72 ab | 10.35 ± 0.11 b | 1.05 ± 0.10 b | 39.75 ± 1.72 a | 12.11 ± 0.45 b | 2.25 ± 0.59 a | 3.05 ± 0.97 a |
| FM | 0.10 ± 0.01 a | 1.22 ± 0.06 b | 5.55 ± 0.72 c | 1.11 ± 0.17 b | 7.55 ± 0.63 c | 20.82 ± 0.86 a | 0.75 ± 0.06 a | 0.65 ± 0.09 a |
| Climate Zone (Location) | Forest Type | Precipitation (mm) | Nutrient Inputs (Throughfall + Stemflow, kg ha−1) | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cl− | SO42− | NO3− | Ca2+ | K+ | Mg2+ | Na+ | ||||
| Temperate (Northeast China) | Mixed broadleaf and coniferous forest | 672.2 | 16.42 | 10.45 | 5.89 | 19.38 | 29.22 | 2.65 | 7.31 | This study |
| Temperate (southwestern USA) | Pinus edulis and Juniperus monosperma | 340 | 1.68 | 0.98 | — | 3.12 | 0.90 | 0.41 | 0.69 | Coble et al. [53] |
| Temperate (Lower Austria) | Secondary spruce | 929 | 4.60 | 18.40 | 36.31 | 9.50 | 13.00 | 2.20 | 1.60 | Berger et al. [50] |
| Temperate (Lower Austria) | Mixed spruce–beech | 929 | 3.30 | 12.20 | 29.23 | 8.90 | 8.70 | 1.00 | 1.60 | Berger et al. [50] |
| Temperate (Lower Austria) | Beech | 929 | 2.50 | 11.00 | 21.70 | 8.80 | 8.90 | 1.00 | 1.30 | Berger et al. [50] |
| Subtropical (Brazil) | Secondary vegetation | 970 | 10.79 | 26.00 | 28.94 | 263.92 | 410.25 | 75.40 | 13.65 | Tonello et al. [37] |
| Subtropical (Southern China) | Roystonea regia | 1630 | 25.63 | 27.91 | 29.80 | 11.78 | 28.49 | 1.53 | 3.09 | Jiang et al. [51] |
| Subtropical (Southern China) | Ficus microcarpa | 1630 | 27.54 | 31.80 | 51.88 | 13.69 | 21.84 | 2.26 | 3.11 | Jiang et al. [51] |
| Subtropical (Southern China) | Lagerstroemia speciosa | 1630 | 12.27 | 32.67 | 34.82 | 13.38 | 20.98 | 2.76 | 3.08 | Jiang et al. [51] |
| Tropical (Southwest Costa Rica) | Primary rainforest | 5850 | 12.30 | 12.00 | 0.90 | 19.40 | 51.10 | 7.10 | 5.20 | Hofhansl et al. [46,49] |
| Tropical (Southwest Costa Rica) | Secondary rainforest | 5850 | 9.30 | 9.00 | 0.50 | 17.30 | 37.20 | 5.20 | 4.90 | Hofhansl et al. [46,49] |
| Tropical (Ghana) | Tropical semi-deciduous forest | 1376 | — | — | 3.14 | 11.70 | 56.90 | 9.50 | — | Dawoe et al. [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Wang, A.; Shen, L.; Dai, G.; Liu, Y.; Zhang, Y.; Fei, W.; Wu, J. The Impact of Canopy on Nutrient Fluxes through Rainfall Partitioning in a Mixed Broadleaf and Coniferous Forest. Forests 2024, 15, 623. https://doi.org/10.3390/f15040623
Yang J, Wang A, Shen L, Dai G, Liu Y, Zhang Y, Fei W, Wu J. The Impact of Canopy on Nutrient Fluxes through Rainfall Partitioning in a Mixed Broadleaf and Coniferous Forest. Forests. 2024; 15(4):623. https://doi.org/10.3390/f15040623
Chicago/Turabian StyleYang, Junhui, Anzhi Wang, Lidu Shen, Guanhua Dai, Yage Liu, Yuan Zhang, Wenli Fei, and Jiabing Wu. 2024. "The Impact of Canopy on Nutrient Fluxes through Rainfall Partitioning in a Mixed Broadleaf and Coniferous Forest" Forests 15, no. 4: 623. https://doi.org/10.3390/f15040623
APA StyleYang, J., Wang, A., Shen, L., Dai, G., Liu, Y., Zhang, Y., Fei, W., & Wu, J. (2024). The Impact of Canopy on Nutrient Fluxes through Rainfall Partitioning in a Mixed Broadleaf and Coniferous Forest. Forests, 15(4), 623. https://doi.org/10.3390/f15040623







