Valorization of Wood Residues from Vegetation Suppression during Wind Energy Plant Implementation and Its Potential for Renewable Phenolic Compounds through Flash Pyrolysis: A Case Study in Northeast Brazil’s Semi-Arid Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedures for Conducting the Physicochemical Characterization Analyses
2.2. Procedure for Micro-Scale Flash Pyrolysis Experiments
3. Results and Discussion
3.1. Physicochemical Characterization Results for Wood Residues
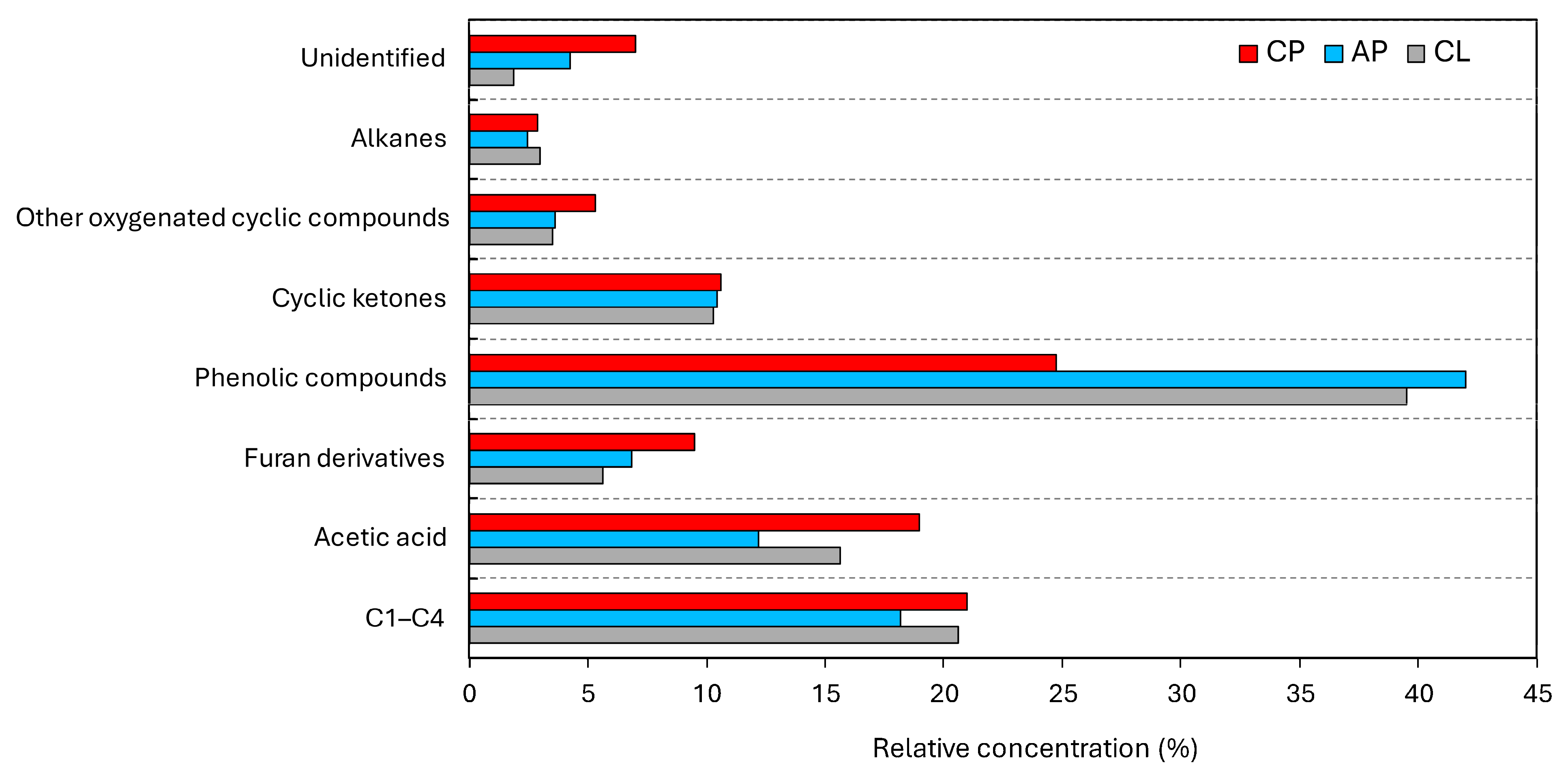
3.2. Examining the Potential for Producing Renewable Chemicals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salvia, A.L.; Brandli, L.L. Energy Sustainability at Universities and Its Contribution to SDG 7: A Systematic Literature Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; ISBN 9783030156046. [Google Scholar]
- Albanito, F.; Roberts, S.; Shepherd, A.; Hastings, A. Quantifying the Land-Based Opportunity Carbon Costs of Onshore Wind Farms. J. Clean. Prod. 2022, 363, 132480. [Google Scholar] [CrossRef]
- Steffen, B.; Patt, A. A Historical Turning Point? Early Evidence on How the Russia-Ukraine War Changes Public Support for Clean Energy Policies. Energy Res. Soc. Sci. 2022, 91, 102758. [Google Scholar] [CrossRef]
- Agaton, C.B. Will a Geopolitical Conflict Accelerate Energy Transition in Oil-Importing Countries? A Case Study of the Philippines from a Real Options Perspective. Resources 2022, 11, 59. [Google Scholar] [CrossRef]
- Schneider, F.; Kallis, G.; Martinez-Alier, J. Crisis or Opportunity? Economic Degrowth for Social Equity and Ecological Sustainability. Introduction to This Special Issue. J. Clean. Prod. 2010, 18, 511–518. [Google Scholar] [CrossRef]
- Khoshnava, S.M.; Rostami, R.; Zin, R.M.; Štreimikiene, D.; Yousefpour, A.; Strielkowski, W.; Mardani, A. Aligning the Criteria of Green Economy (GE) and Sustainable Development Goals (SDGs) to Implement Sustainable Development. Sustainability 2019, 11, 4615. [Google Scholar] [CrossRef]
- Lima, M.A.; Mendes, L.F.R.; Mothé, G.A.; Linhares, F.G.; de Castro, M.P.P.; da Silva, M.G.; Sthel, M.S. Renewable Energy in Reducing Greenhouse Gas Emissions: Reaching the Goals of the Paris Agreement in Brazil. Environ. Dev. 2020, 33, 100504. [Google Scholar] [CrossRef]
- Soares, M.C.; Borba, B.; Szklo, A.; Schaeffer, R. Plug-in Hybrid Electric Vehicles as a Way to Maximize the Integration of Variable Renewable Energy in Power Systems: The Case of Wind Generation in Northeastern Brazil. Energy 2012, 37, 469–481. [Google Scholar] [CrossRef]
- Junior, M.F.S.; Hernandez, M.C.R.; Amora, S.S.A.; de Morais, E.R.C. Perception of Environmental Impacts of Wind Farms in Agricultural Areas of Northeast Brazil. Energies 2022, 15, 101. [Google Scholar] [CrossRef]
- Ma, B.; Yang, J.; Chen, X.; Zhang, L.; Zeng, W. Revealing the Ecological Impact of Low-Speed Mountain Wind Power on Vegetation and Soil Erosion in South China: A Case Study of a Typical Wind Farm in Yunnan. J. Clean. Prod. 2023, 419, 138020. [Google Scholar] [CrossRef]
- Solís-Chaves, J.S.; Rocha-Osorio, C.M.; Murari, A.L.L.; Lira, V.M.; Sguarezi Filho, A.J. Extracting Potable Water from Humid Air plus Electric Wind Generation: A Possible Application for a Brazilian Prototype. Renew. Energy 2018, 121, 102–115. [Google Scholar] [CrossRef]
- Urziceanu, M.; Anastasiu, P.; Rozylowicz, L.; Sesan, T.E. Local-Scale Impact of Wind Energy Farms on Rare, Endemic, and Threatened Plant Species. PeerJ 2021, 9, e11390. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.C.; Mac Nally, R.; Baker, P.J.; Cavagnaro, T.R.; Beringer, J.; Thomson, J.R.; Thompson, R.M. Balancing the Environmental Benefits of Reforestation in Agricultural Regions. Perspect. Plant Ecol. Evol. Syst. 2015, 17, 301–317. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Tran, K.-Q.; Skreiberg, Ø. Combustion Kinetics of Wet-Torrefied Forest Residues Using the Distributed Activation Energy Model (DAEM). Appl. Energy 2017, 185, 1059–1066. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Liu, C.-G.; Nawaz, M.; Tawab, A.; Shen, X.; Shen, B.; Mehmood, M.A. Elucidating the Pyrolysis Reaction Mechanism of Calotropis Procera and Analysis of Pyrolysis Products to Evaluate Its Potential for Bioenergy and Chemicals. Bioresour. Technol. 2021, 322, 124545. [Google Scholar] [CrossRef] [PubMed]
- Alhumade, H.; Alayed, O.S.; Iqbal, M.W.; Shahid, A.; Iqbal, T.; Ahmad, M.S.; Elkamel, A.; Al-Turki, Y.; Mehmood, M.A.; Ashraf, G.A. Exploration of the Bioenergy Potential of Dactyloctenium Aegyptium through Pyrolysis, Kinetics, and Thermodynamic Parameters to Produce Clean Fuels and Biochemicals. Fuel 2023, 341, 127663. [Google Scholar] [CrossRef]
- Tagade, A.; Kirti, N.; Sawarkar, A.N. Pyrolysis of Agricultural Crop Residues: An Overview of Researches by Indian Scientific Community. Bioresour. Technol. Rep. 2021, 15, 100761. [Google Scholar] [CrossRef]
- Yao, Z.; Yu, S.; Su, W.; Wu, W.; Tang, J.; Qi, W. Kinetic Studies on the Pyrolysis of Plastic Waste Using a Combination of Model-Fitting and Model-Free Methods. Waste Manag. Res. 2020, 38, 77–85. [Google Scholar] [CrossRef]
- Gupta, G.K.; Mondal, M.K. Kinetics and Thermodynamic Analysis of Maize Cob Pyrolysis for Its Bioenergy Potential Using Thermogravimetric Analyzer. J. Therm. Anal. Calorim. 2019, 137, 1431–1441. [Google Scholar] [CrossRef]
- Azizi, S.; Mowla, D. CFD Modeling of Algae Flash Pyrolysis in the Batch Fluidized Bed Reactor Including Heat Carrier Particles. Int. J. Chem. React. Eng. 2016, 14, 463–480. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A. Bio-Oil Production and Upgrading Research: A Review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]
- Iha, O.K.; Alves, F.C.S.C.; Suarez, P.A.Z.; de Oliveira, M.B.F.; Meneghetti, S.M.P.; Santos, B.P.T.; Soletti, J.I. Physicochemical Properties of Syagrus Coronata and Acrocomia Aculeata Oils for Biofuel Production. Ind. Crops Prod. 2014, 62, 318–322. [Google Scholar] [CrossRef]
- Tahir, M.H.; Irfan, R.M.; Cheng, X.; Ahmad, M.S.; Jamil, M.; Shah, T.U.H.; Karim, A.; Ashraf, R.; Haroon, M. Mango Peel as Source of Bioenergy, Bio-Based Chemicals via Pyrolysis, Thermodynamics and Evolved Gas Analyses. J. Anal. Appl. Pyrolysis 2021, 155, 105066. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K.; Wang, X. Pyrolysis Kinetic Behavior and Py-GC–MS Analysis of Waste Dahlia Flowers into Renewable Fuel and Value-Added Chemicals. Fuel 2020, 260, 116338. [Google Scholar] [CrossRef]
- Pal, S.K.; Garcés-Sánchez, G.; Kranert, M.; Vinu, R. Characterization and Evaluation of Resource Recovery Potential of Beach Plastic Wastes Using Analytical Py-GC/MS. J. Anal. Appl. Pyrolysis 2023, 172, 105996. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, B.; Zong, P.; Tian, Y.; Xu, F.; Li, D.; Li, F.; Tian, Y. Thermal Behavior, Kinetics and Fast Pyrolysis Characteristics of Palm Oil: Analytical TG-FTIR and Py-GC/MS Study. Energy Convers. Manag. 2019, 199, 111964. [Google Scholar] [CrossRef]
- ASTM E871-82; Standard Test Method for Moisture Analysis of Particulate Wood Fuels. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM E872-82; Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM E1755-01; Standard Test Method for Ash in Biomass. ASTM International: West Conshohocken, PA, USA, 2010.
- Correia, L.A.d.S.; da Silva, J.E.; Calixto, G.Q.; Melo, D.M.d.A.; Braga, R.M. Pachira Aquatica Fruits Shells Valorization: Renewables Phenolics through Analytical Pyrolysis Study (Py-GC/MS). Cienc. Rural 2022, 52, 1–11. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- ASTM E873-82; Standard Test Method for Bulk Density of Densified Particulate Biomass Fuels. ASTM International: West Conshohocken, PA, USA, 2013.
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Biomass Sources for Thermal Conversion. Techno-Economical Overview. Fuel 2017, 195, 182–189. [Google Scholar] [CrossRef]
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Spanish Biofuels Heating Value Estimation. Part I: Ultimate Analysis Data. Fuel 2014, 117, 1130–1138. [Google Scholar] [CrossRef]
- Yang, Y.B.; Ryu, C.; Khor, A.; Yates, N.E.; Sharifi, V.N.; Swithenbank, J. Effect of Fuel Properties on Biomass Combustion. Part II. Modelling Approach—Identification of the Controlling Factors. Fuel 2005, 84, 2116–2130. [Google Scholar] [CrossRef]
- Díez, D.; Urueña, A.; Piñero, R.; Barrio, A.; Tamminen, T. Determination of Hemicellulose, Cellulose, and Lignin Content in Different Types of Biomasses by Thermogravimetric Analysis and Pseudocomponent Kinetic Model (TGA-PKM Method). Processes 2020, 8, 1048. [Google Scholar] [CrossRef]
- Geng, W.; Narron, R.; Jiang, X.; Pawlak, J.J.; Chang, H.m.; Park, S.; Jameel, H.; Venditti, R.A. The Influence of Lignin Content and Structure on Hemicellulose Alkaline Extraction for Non-Wood and Hardwood Lignocellulosic Biomass. Cellulose 2019, 26, 3219–3230. [Google Scholar] [CrossRef]
- Bertero, M.; de la Puente, G.; Sedran, U. Fuels from Bio-Oils: Bio-Oil Production from Different Residual Sources, Characterization and Thermal Conditioning. Fuel 2012, 95, 263–271. [Google Scholar] [CrossRef]
- Subagyono, R.D.J.N.; Miten, P.D.; Sinaga, R.J.; Wijayanti, A.; Qi, Y.; Marshall, M.; Sanjaya, A.S.; Chaffee, A.L. Pyrolysis of Fast Growing Wood Macaranga Gigantea: Product Characterisation and Kinetic Study. Fuel 2022, 315, 123182. [Google Scholar] [CrossRef]
- Braga, R.M.; Melo, D.M.A.; Melo, M.A.F.; Freitas, J.C.O.; Boateng, A.A. Effect of Pretreatment on Pyrolysis Products of Pennisetum Purpureum Schum. by Py-GC/MS. J. Therm. Anal. Calorim. 2022, 147, 6655–6663. [Google Scholar] [CrossRef]
- Dias Júnior, A.F.; Andrade, C.R.; Protásio, T.D.P.; Brito, J.O.; Trugilho, P.F.; Oliveira, M.P.; Dambroz, G.B.V. Thermal Profile of Wood Species from the Brazilian Semi-Arid Region Submitted to Pyrolysis. Cerne 2019, 25, 44–53. [Google Scholar] [CrossRef]
- Aboyade, A.O.; Hugo, T.J.; Carrier, M.; Meyer, E.L.; Stahl, R.; Knoetze, J.H.; Görgens, J.F. Non-Isothermal Kinetic Analysis of the Devolatilization of Corn Cobs and Sugar Cane Bagasse in an Inert Atmosphere. Thermochim. Acta 2011, 517, 81–89. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, D.; Gu, J.; Bao, B.; Zhang, Q. Determination of Pyrolysis Characteristics and Kinetics of Palm Kernel Shell Using TGA-FTIR and Model-Free Integral Methods. Energy Convers. Manag. 2015, 89, 251–259. [Google Scholar] [CrossRef]
- Pinzi, S.; Buratti, C.; Bartocci, P.; Marseglia, G.; Fantozzi, F.; Barbanera, M. A Simplified Method for Kinetic Modeling of Coffee Silver Skin Pyrolysis by Coupling Pseudo-Components Peaks Deconvolution Analysis and Model Free-Isoconversional Methods. Fuel 2020, 278, 118260. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, G.K.; Mondal, M.K. Thermal Degradation Characteristics, Kinetics, Thermodynamic, and Reaction Mechanism Analysis of Pistachio Shell Pyrolysis for Its Bioenergy Potential. Biomass Convers. Biorefinery 2020, 12, 4847–4861. [Google Scholar] [CrossRef]
- da Rocha Mendes, K.; Portela, J.C.; Gondim, J.E.F.; Ribeiro, M.A.; de Medeiros, J.F.; de Queiroz, G.C.M. Physical, Chemical and Structural Attributes of Soil in Agroecosystems in the Brazilian Semiarid Region. Rev. Cienc. Agron. 2022, 53, 1–10. [Google Scholar] [CrossRef]
- de Paiva, K.F.; Severo, P.J.d.S.; de Oliveira, F.S.; Soares, L.A.D.A.; Araujo, J.L. Potassium Availability in Brazilian Semiarid Soils Cultivated with Melon. Pesqui. Agropecu. Trop. 2020, 50, 1–8. [Google Scholar] [CrossRef]
- Mahadevan, R.; Adhikari, S.; Shakya, R.; Wang, K.; Dayton, D.; Lehrich, M.; Taylor, S.E. Effect of Alkali and Alkaline Earth Metals on In-Situ Catalytic Fast Pyrolysis of Lignocellulosic Biomass: A Microreactor Study. Energy Fuels 2016, 30, 3045–3056. [Google Scholar] [CrossRef]
- Nik-Azar, M.; Hajaligol, M.R.; Sohrabi, M.; Dabir, B. Mineral Matter Effects in Rapid Pyrolysis of Beech Wood. Fuel Process. Technol. 1997, 51, 7–17. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Satrio, J.A.; Brown, R.C.; Shanks, B.H. Influence of Inorganic Salts on the Primary Pyrolysis Products of Cellulose. Bioresour. Technol. 2010, 101, 4646–4655. [Google Scholar] [CrossRef]
- Collard, F.X.; Blin, J.; Bensakhria, A.; Valette, J. Influence of Impregnated Metal on the Pyrolysis Conversion of Biomass Constituents. J. Anal. Appl. Pyrolysis 2012, 95, 213–226. [Google Scholar] [CrossRef]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of Physicochemical Properties and Analytical Characterization of Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef]
- Kumar, M.; Upadhyay, S.N.; Mishra, P.K. A Comparative Study of Thermochemical Characteristics of Lignocellulosic Biomasses. Bioresour. Technol. Rep. 2019, 8, 100186. [Google Scholar] [CrossRef]
- Abdullah, M.Z.; Husain, Z.; Yin Pong, S.L. Analysis of Cold Flow Fluidization Test Results for Various Biomass Fuels. Biomass Bioenergy 2003, 24, 487–494. [Google Scholar] [CrossRef]
- Lenis, Y.A.; Osorio, L.F.; Pérez, J.F. Fixed Bed Gasification of Wood Species with Potential as Energy Crops in Colombia: The Effect of the Physicochemical Properties. Energy Sources Part A Recovery Util. Environ. Eff. 2013, 35, 1608–1617. [Google Scholar] [CrossRef]
- Protásio, T.d.P.; Bufalino, L.; Tonoli, G.H.D.; Guimarães Junior, M.; Trugilho, P.F.; Mendes, L.M. Brazilian Lignocellulosic Wastes for Bioenergy Production: Characterization and Comparison with Fossil Fuels. BioResources 2013, 8, 1166–1185. [Google Scholar] [CrossRef]
- Tumuluru, J.S. Effect of Pellet Die Diameter on Density and Durability of Pellets Made from High Moisture Woody and Herbaceous Biomass. Carbon Resour. Convers. 2018, 1, 44–54. [Google Scholar] [CrossRef]
- Olugbade, T.; Ojo, O.; Mohammed, T. Influence of Binders on Combustion Properties of Biomass Briquettes: A Recent Review. BioEnergy Res. 2019, 12, 241–259. [Google Scholar] [CrossRef]
- de Souza, H.J.P.L.; Arantes, M.D.C.; Vidaurre, G.B.; Andrade, C.R.; de Cássia Oliveira Carneiro, A.; de Souza, D.P.L.; de Paula Protásio, T. Pelletization of Eucalyptus Wood and Coffee Growing Wastes: Strategies for Biomass Valorization and Sustainable Bioenergy Production. Renew. Energy 2020, 149, 128–140. [Google Scholar] [CrossRef]
- Sarchami, T.; Batta, N.; Berruti, F. Production and Separation of Acetic Acid from Pyrolysis Oil of Lignocellulosic Biomass: A Review. Biofuels Bioprod. Biorefining 2021, 15, 1912–1937. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Liu, X.; Shi, X.; Wang, C.; Zhong, H.; Jin, F. Sustainable Production of Formic Acid and Acetic Acid from Biomass. Mol. Catal. 2023, 545, 113199. [Google Scholar] [CrossRef]
- Veses, A.; Aznar, M.; López, J.M.; Callén, M.S.; Murillo, R.; García, T. Production of Upgraded Bio-Oils by Biomass Catalytic Pyrolysis in an Auger Reactor Using Low Cost Materials. Fuel 2015, 141, 17–22. [Google Scholar] [CrossRef]
- Chagas, B.M.E.; Dorado, C.; Serapiglia, M.J.; Mullen, C.A.; Boateng, A.A.; Melo, M.A.F.; Ataíde, C.H. Catalytic Pyrolysis-GC/MS of Spirulina: Evaluation of a Highly Proteinaceous Biomass Source for Production of Fuels and Chemicals. Fuel 2016, 179, 124–134. [Google Scholar] [CrossRef]
- Urrutia, R.I.; Yeguerman, C.; Jesser, E.; Gutierrez, V.S.; Volpe, M.A.; Werdin González, J.O. Sunflower Seed Hulls Waste as a Novel Source of Insecticidal Product: Pyrolysis Bio-Oil Bioactivity on Insect Pests of Stored Grains and Products. J. Clean. Prod. 2021, 287, 125000. [Google Scholar] [CrossRef]
- Ma, Z.; Sun, Q.; Ye, J.; Yao, Q.; Zhao, C. Study on the Thermal Degradation Behaviors and Kinetics of Alkali Lignin for Production of Phenolic-Rich Bio-Oil Using TGA–FTIR and Py–GC/MS. J. Anal. Appl. Pyrolysis 2016, 117, 116–124. [Google Scholar] [CrossRef]
- Kaur, R.; Tarun Kumar, V.; Krishna, B.B.; Bhaskar, T. Characterization of Slow Pyrolysis Products from Three Different Cashew Wastes. Bioresour. Technol. 2023, 376, 128859. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.C.G.; Pereira, J.L.C.; Andersen, S.L.F.; Moreira, R.d.F.P.M.; José, H.J. Torrefaction of Ponkan Peel Waste in Tubular Fixed-Bed Reactor: In-Depth Bioenergetic Evaluation of Torrefaction Products. Energy 2020, 210, 118569. [Google Scholar] [CrossRef]
- Menezes, J.C.; Neto, O.C.C.; Azevedo, I.F.P.; Machado, A.O.; Nunes, Y.R.F. Soil Seed Bank at Different Depths and Light Conditions in a Dry Forest in Northern Minas Gerais. Floresta Ambient. 2019, 26, 1–9. [Google Scholar] [CrossRef]
- Vamosi, S.M. A Reconsideration of the Reproductive Biology of the Atlantic Forest in the Volta Velha Reserve. Biodivers. Conserv. 2006, 15, 1417–1424. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-Oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Kim, J.S. Production, Separation and Applications of Phenolic-Rich Bio-Oil—A Review. Bioresour. Technol. 2015, 178, 90–98. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Luo, K.H.; Wang, S.R.; Fang, M.X. The Pyrolytic Degradation of Wood-Derived Lignin from Pulping Process. Bioresour. Technol. 2010, 101, 6136–6146. [Google Scholar] [CrossRef]
- Asmadi, M.; Kawamoto, H.; Saka, S. Characteristics of Softwood and Hardwood Pyrolysis in an Ampoule Reactor. J. Anal. Appl. Pyrolysis 2017, 124, 523–535. [Google Scholar] [CrossRef]
- Oliveira, G.P.D.; Araújo, L.; Siqueira, D.M.; Quintela, G.; Maria, D.; Melo, D.A.; Antonio, M.; Melo, D.F.; Pimenta, A.S.; Braga, R.M. Kinetic Study and Effect of Flash Pyrolysis Temperature of Kraft Lignin on the Yield of Aromatic Compounds. J. Therm. Anal. Calorim. 2023, 148, 12725–12737. [Google Scholar] [CrossRef]
- Sobek, S.; Zeng, K.; Werle, S.; Junga, R.; Sajdak, M. Brewer’s Spent Grain Pyrolysis Kinetics and Evolved Gas Analysis for the Sustainable Phenolic Compounds and Fatty Acids Recovery Potential. Renew. Energy 2022, 199, 157–168. [Google Scholar] [CrossRef]
- Awasthi, A.; Dhyani, V.; Biswas, B.; Kumar, J.; Bhaskar, T. Production of Phenolic Compounds Using Waste Coir Pith: Estimation of Kinetic and Thermodynamic Parameters. Bioresour. Technol. 2019, 274, 173–179. [Google Scholar] [CrossRef]
- Norouzi, O.; Jafarian, S.; Safari, F.; Tavasoli, A.; Nejati, B. Promotion of Hydrogen-Rich Gas and Phenolic-Rich Bio-Oil Production from Green Macroalgae Cladophora Glomerata via Pyrolysis over Its Bio-Char. Bioresour. Technol. 2016, 219, 643–651. [Google Scholar] [CrossRef]
- Lazzari, E.; Schena, T.; Primaz, C.T.; da Silva Maciel, G.P.; Machado, M.E.; Cardoso, C.A.L.; Jacques, R.A.; Caramão, E.B. Production and Chromatographic Characterization of Bio-Oil from the Pyrolysis of Mango Seed Waste. Ind. Crops Prod. 2016, 83, 529–536. [Google Scholar] [CrossRef]
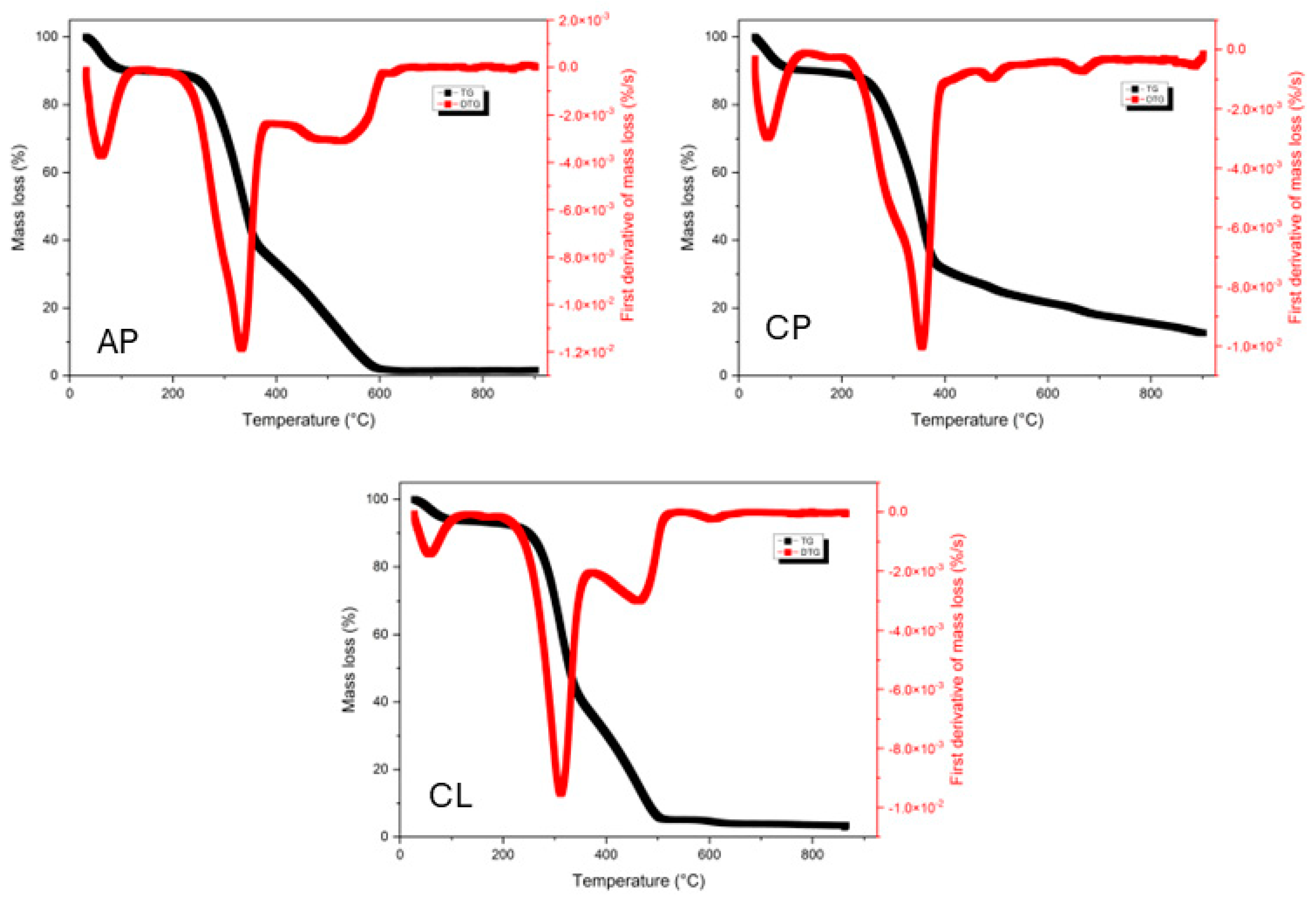

| Aspidosperma pyrifolium | Cenostigma pyramidale | Commiphora leptophloeos | |
|---|---|---|---|
| Proximate composition (wt.%) | |||
| Moisture | 7.11 ± 0.13 | 10.04 ± 0.06 | 10.85 ± 0.06 |
| Volatile matter | 89.57 ± 1.37 | 79.40 ± 0.26 | 78.50 ± 1.81 |
| Fixed carbon | 2.55 a | 9.45 a | 7.81 a |
| Ash | 0.77 ± 0.15 | 1.20 ± 0.10 | 2.84 ± 0.11 |
| Lignocellulosic composition (wt.%) | |||
| Hemicellulose | 11.34 | 19.79 | 13.48 |
| Cellulose | 42.64 | 39.03 | 46.26 |
| Lignin | 29.76 | 19.11 | 30.61 |
| Heating values (MJ kg−1) | |||
| HHV | 18.39 ± 0.64 | 17.47 ± 0.20 | 17.99 ± 0.01 |
| LHV | 17.58 | 16.66 | 17.18 |
| Bulk density (kg m−3) | 268.70 ± 0.61 | 237.10 ± 0.10 | 126.50 ± 0.17 |
| Chemical composition of ash (wt.%) | |||
| CaO | 65.33 | 80.12 | 37.61 |
| K2O | 32.45 | 18.78 | 57.59 |
| Fe2O3 | 2.03 | -- | 2.34 |
| SiO2 | -- | -- | 1.66 |
| Others | 0.36 | 1.11 | 0.80 |
| Compound | Molecular Formula | Molecular Weight | Relative Concentration (%) | ||
|---|---|---|---|---|---|
| AP | CP | CL | |||
| C1–C4 | – | – | 18.19 | 20.98 | 20.59 |
| Acetic acid | 12.19 | 18.96 | 15.63 | ||
| 1,3–Dioxane | C4H8O2 | 88 | 3.62 | 2.94 | 2.55 |
| Unknown | – | – | 4.25 | 7.00 | 1.89 |
| 3–Furaldehyde | C5H4O2 | 96 | 3.29 | 4.30 | 3.07 |
| 2–Furanmethanol | C5H6O2 | 98 | 3.57 | 5.18 | 2.54 |
| Cyclopentanone | C5H8O | 84 | 2.07 | 2.33 | 2.20 |
| 2–Hydroxy–2–cyclopenten–1–one | C5H6O2 | 98 | 4.84 | 5.27 | 4.25 |
| Phenol | C6H6O | 94 | – | – | 3.68 |
| 2–Hydroxy–3–methyl–2–cyclopenten–1–one | C6H8O2 | 112 | 2.91 | 2.10 | 2.94 |
| 4–Methylphenol | C7H8O | 108 | – | – | 2.48 |
| Mequinol | C7H8O2 | 124 | 8.36 | 3.19 | 2.48 |
| 2,7–Dimethyl–octane | C10H22 | 142 | 2.44 | 2.88 | 2.98 |
| 2–Hydroxy–3,5–dimethylcyclopent–2–en–1–one | C7H10O2 | 126 | 0.62 | 0.93 | 0.92 |
| 2–Methoxy–5–methylphenol | C8H10O2 | 138 | 7.48 | 3.08 | 3.29 |
| 4–Ethyl–2–methoxyphenol | C9H12O2 | 152 | 2.44 | 0.82 | 1.81 |
| 2–Methoxy–4–vinylphenol | C9H10O2 | 150 | 10.48 | 6.07 | 11.59 |
| 2,6–Dimethoxyphenol | C8H10O3 | 154 | 2.30 | 5.60 | 6.99 |
| Eugenol | C10H10O2 | 164 | 2.34 | 1.12 | 1.09 |
| 2–Methoxy–4–(1–propenyl)–phenol | C10H12O2 | 164 | 1.33 | 0.65 | 0.85 |
| 1,2,4–Trimethoxybenzene | C9H12O3 | 168 | – | 2.39 | 0.96 |
| 2–Methoxy–6–(2–propenyl)–phenol | C10H12O2 | 164 | 7.28 | 4.23 | 5.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, M.C.P.d.S.; Silva, J.E.d.; Batista, W.G.d.S.; Alves, J.L.F.; Melo, D.M.d.A.; Pimenta, A.S.; Braga, R.M. Valorization of Wood Residues from Vegetation Suppression during Wind Energy Plant Implementation and Its Potential for Renewable Phenolic Compounds through Flash Pyrolysis: A Case Study in Northeast Brazil’s Semi-Arid Region. Forests 2024, 15, 621. https://doi.org/10.3390/f15040621
Almeida MCPdS, Silva JEd, Batista WGdS, Alves JLF, Melo DMdA, Pimenta AS, Braga RM. Valorization of Wood Residues from Vegetation Suppression during Wind Energy Plant Implementation and Its Potential for Renewable Phenolic Compounds through Flash Pyrolysis: A Case Study in Northeast Brazil’s Semi-Arid Region. Forests. 2024; 15(4):621. https://doi.org/10.3390/f15040621
Chicago/Turabian StyleAlmeida, Marcela Cristina Pereira dos Santos, Janduir Egito da Silva, Willame Gomes da Silva Batista, José Luiz Francisco Alves, Dulce Maria de Araújo Melo, Alexandre Santos Pimenta, and Renata Martins Braga. 2024. "Valorization of Wood Residues from Vegetation Suppression during Wind Energy Plant Implementation and Its Potential for Renewable Phenolic Compounds through Flash Pyrolysis: A Case Study in Northeast Brazil’s Semi-Arid Region" Forests 15, no. 4: 621. https://doi.org/10.3390/f15040621
APA StyleAlmeida, M. C. P. d. S., Silva, J. E. d., Batista, W. G. d. S., Alves, J. L. F., Melo, D. M. d. A., Pimenta, A. S., & Braga, R. M. (2024). Valorization of Wood Residues from Vegetation Suppression during Wind Energy Plant Implementation and Its Potential for Renewable Phenolic Compounds through Flash Pyrolysis: A Case Study in Northeast Brazil’s Semi-Arid Region. Forests, 15(4), 621. https://doi.org/10.3390/f15040621








