Monitoring Intra-Annual Wood Formation of Pinus nigra J.F. Arnold (Black Pine) to Understand the Fire Seasonality in Western Anatolia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites

2.2. Microcore Sampling and Tree-Ring Measurements
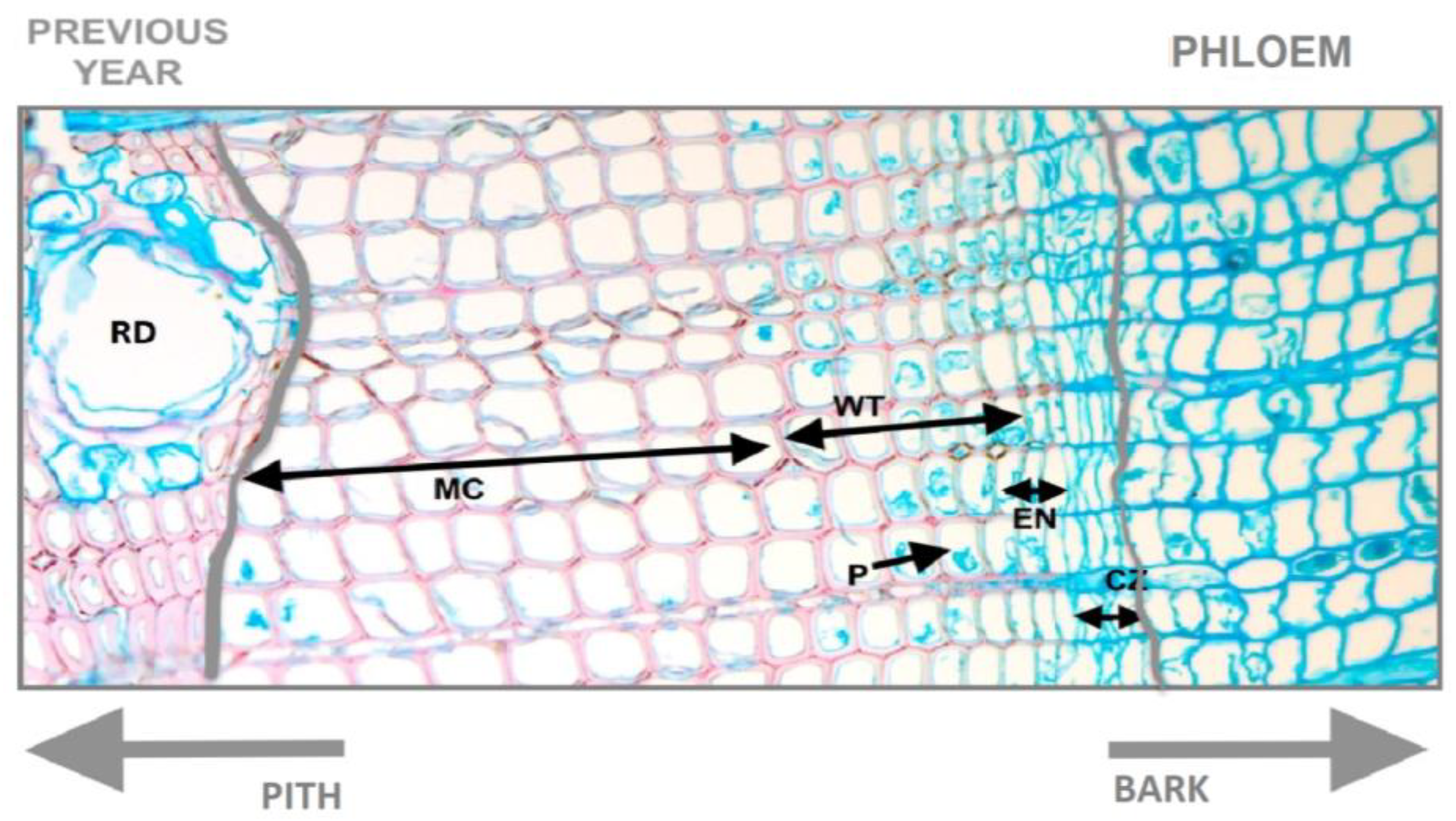
2.3. Laboratory Methods and Microscope Observations
2.4. Data Analysis for Dynamics of Xylem Formation
3. Results
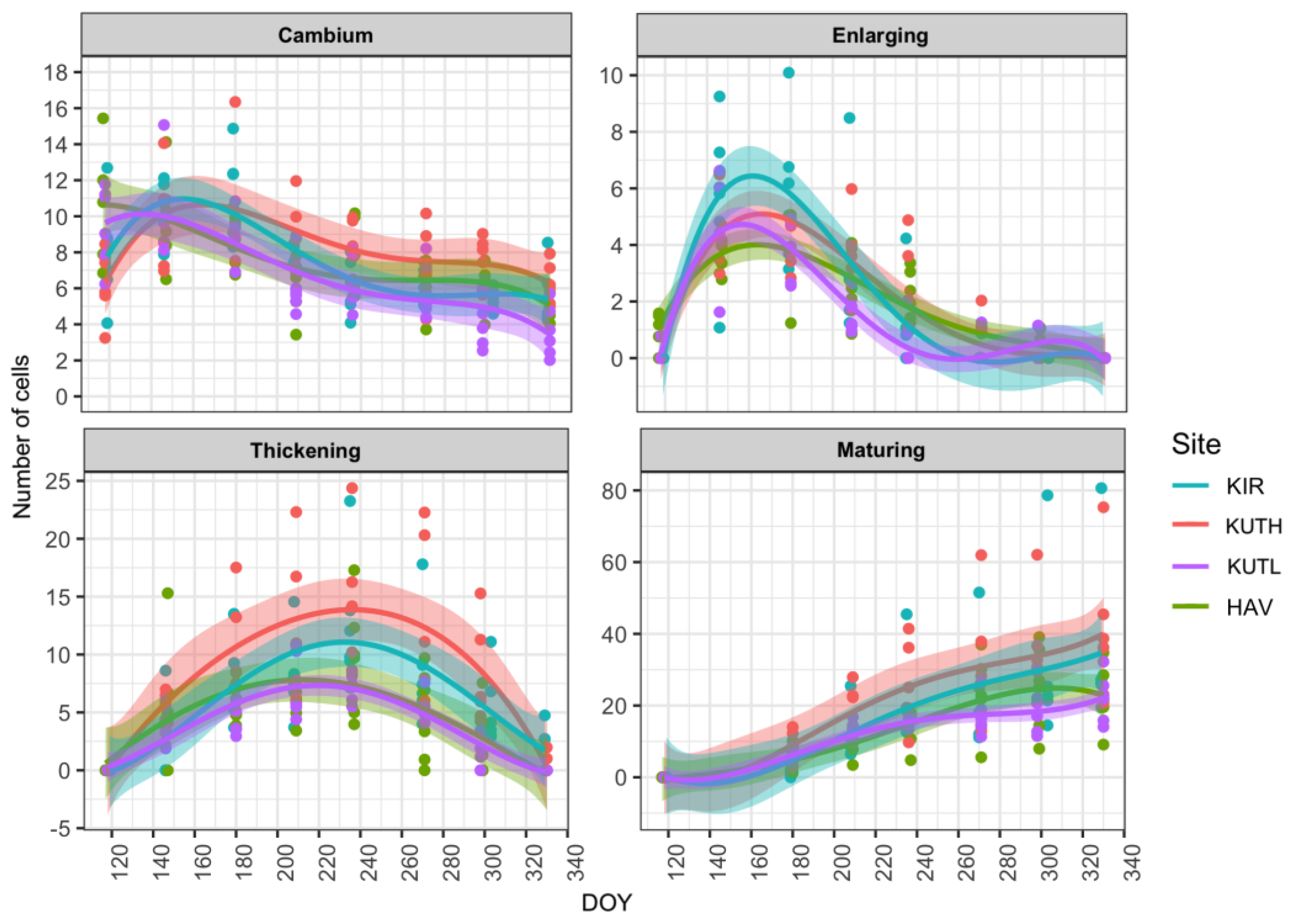
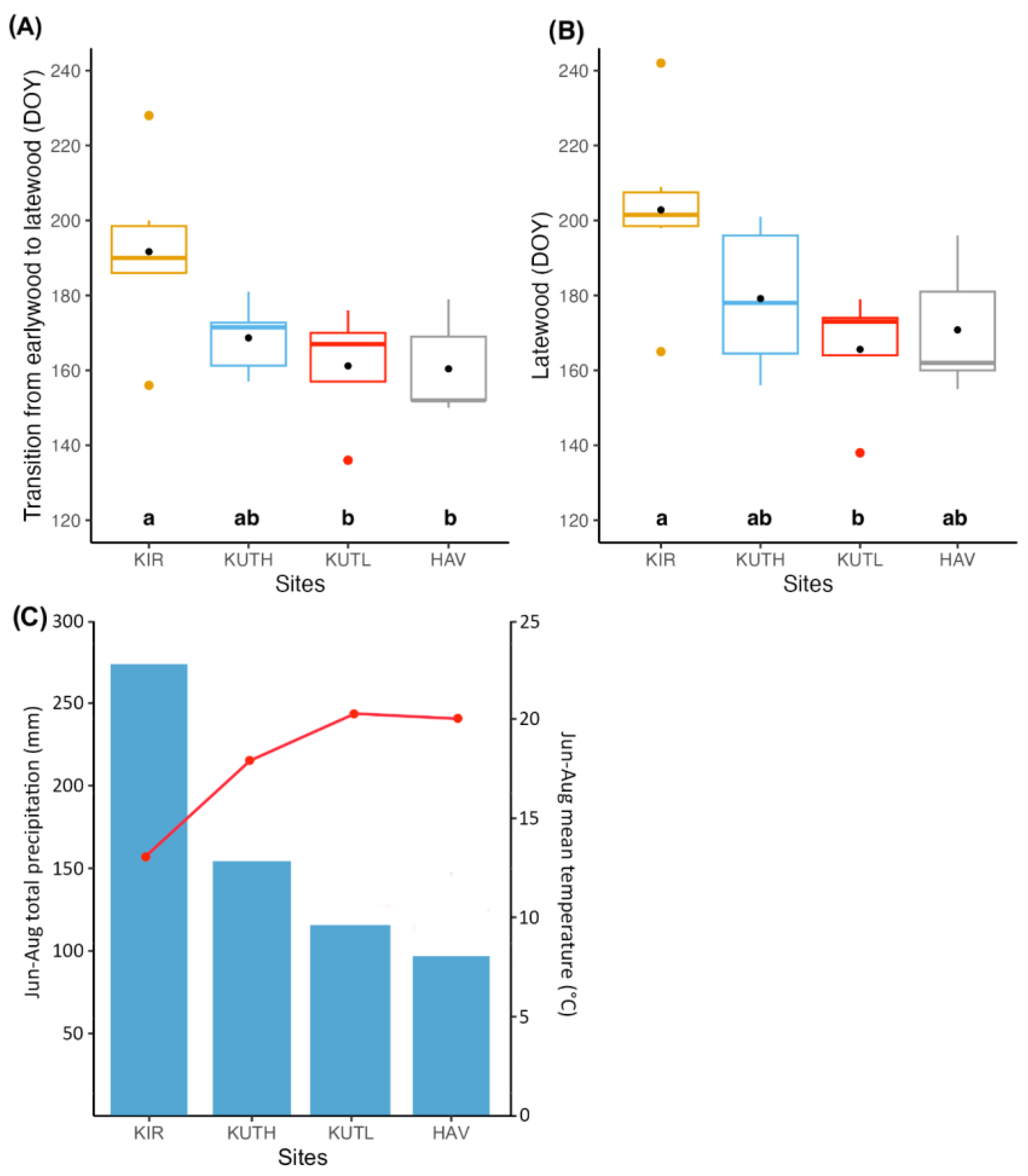
3.1. Cambial Activity across Sites
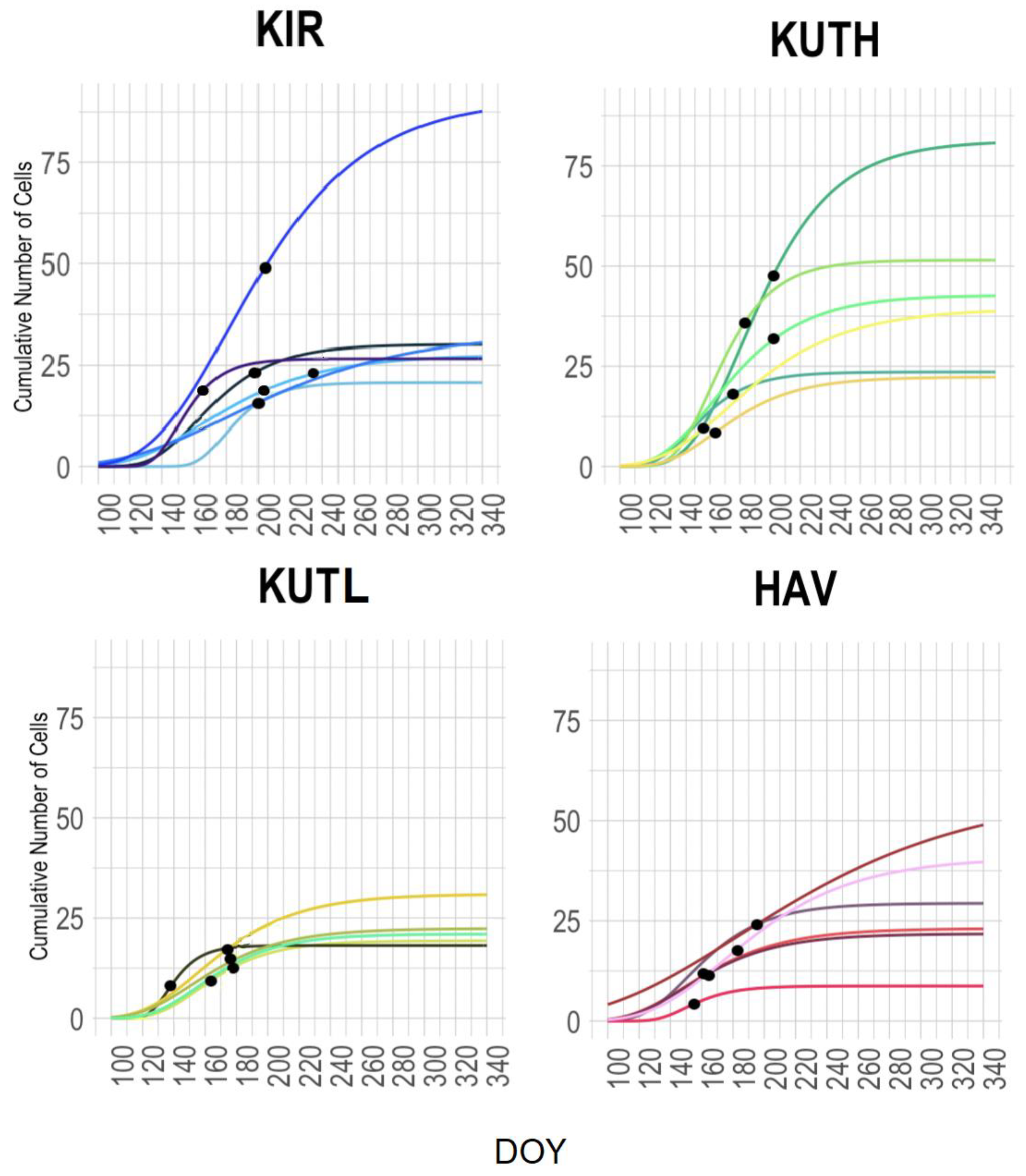
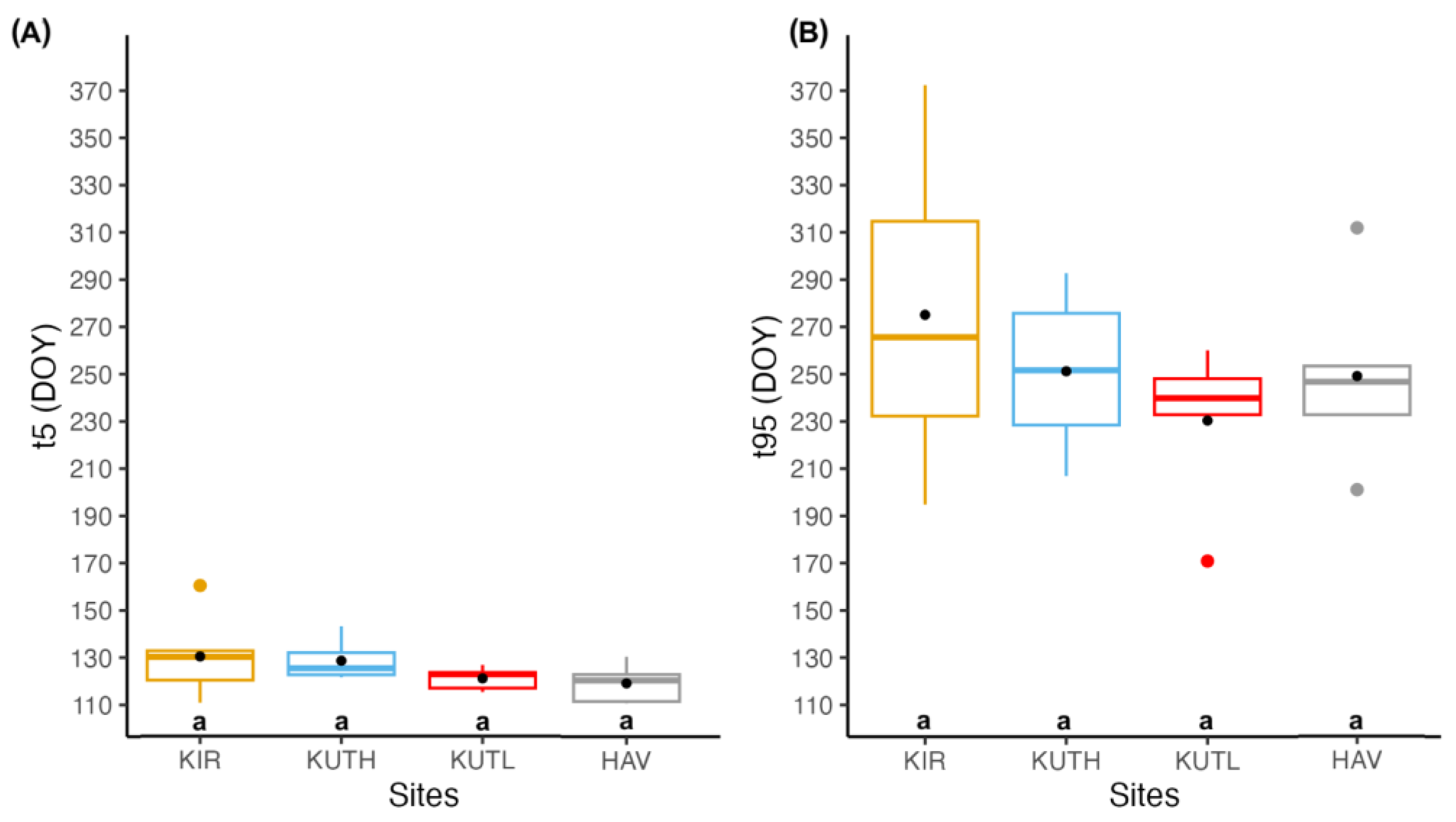
3.2. Determining the Seasonality of Cambial Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Le Page, Y.; Oom, D.; Silva, J.M.N.; Jönsson, P.; Pereira, J.M.C. Seasonality of vegetation fires as modified by human action: Observing the deviation from eco-climatic fire regimes. Glob. Ecol. Biogeogr. 2010, 19, 575–588. [Google Scholar] [CrossRef]
- Rother, M.; Huffman, J.; Harley, G.; Platt, W.; Jones, N.; Robertson, K.; Orzell, S. Cambial Phenology Informs Tree-Ring Analysis of Fire Seasonality in Coastal Plain Pine Savannas. Fire Ecol. 2018, 14, 164–185. [Google Scholar] [CrossRef]
- Baisan, C.H.; Swetnam, T.W. Fire history on a desert mountain range: Rincon Mountain Wilderness, Arizona, USA. Can. J. For. Res. 1990, 20, 1559–1569. [Google Scholar] [CrossRef]
- Caprio, A.C.; Swetnam, T.W. Historic fire regimes along an elevational gradient on the west slope of the Sierra Nevada, California. In Symposium on Fire in Wilderness and Park Management: Past Lessons and Future Opportunities; Brown, J.K., Mutch, R.W., Spoon, C.W., Wakimoto, R.H., Technical Coordinators, Eds.; USDA Forest Service General Technical Report INT-GTR-320; DIANE Publishing: Ogden, UT, USA, 1995; pp. 173–179. [Google Scholar]
- Rossi, S.; Deslauriers, A.; Gričar, J.; Seo, J.; Rathgeber, C.; Anfodillo, T.; Morin, H.; Levanic, T.; Oven, P.; Jalkanen, R. Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 2008, 17, 696–707. [Google Scholar] [CrossRef]
- Stephens, S.L.; Maier, L.; Gonen, L.; York, J.D.; Collins, B.M.; Fry, D.L. Variation in fire scar phenology from mixed conifer trees in the Sierra Nevada. Can. J. For. Res. 2018, 48, 101–104. [Google Scholar] [CrossRef]
- Camarero, J.; Olano, J.; Parras, A. Plastic bimodal xylogenesis in conifers from continental Mediterranean climates. New Phytol. 2009, 185, 471–480. [Google Scholar] [CrossRef]
- Pérez-de-Lis, G.; Rathgeber, C.B.; Fernández-de-Uña, L.; Ponton, S. Cutting tree rings into time slices: How intra-annual dynamics of Wood Formation help decipher the space-for-Time Conversion. New Phytol. 2021, 233, 1520–1534. [Google Scholar] [CrossRef]
- Bozkurt, Y. Belgrad Ormanında Önemli Bazı Ağaç Türlerinde Yıllık Halka Gelişimi Üzerine Araştırmalar. Istanb. Univ. Orman Fak. Derg. 1960, 10, 29–56. (In Turkish) [Google Scholar]
- Guada, G.; Camarero, J.; Sánchez-Salguero, R.; Cerrillo, R. Limited Growth Recovery after Drought-Induced Forest Dieback in Very Defoliated Trees of Two Pine Species. Front. Plant Sci. 2016, 7, 418. [Google Scholar] [CrossRef] [PubMed]
- Akkemik, Ü.; Çınar Yılmaz, H.; Sevgi, O. Cambial Activity of the Sessile Oak (Quercus petraea) in Belgrade Forest, Istanbul. Turk. J. Agric. For. 2006, 30, 429–438. [Google Scholar]
- İnan, M. Belgrad Ormanında Doğal Yetişen Bazı Ağaçların Kambiyum Faaliyeti. Ph.D. Thesis, İstanbul Üniversitesi, İstanbul, Turkey, 2015. (In Turkish). [Google Scholar]
- Yılmaz, H. Weather Response of Sweet Chestnus (Castanea sativa Mill.) Radial Growth Increment in Belgrad Forest. Fresenius Environ. Bull. 2015, 24, 2491–2495. [Google Scholar]
- Güney, A.; Kerr, D.; Sökücü, A.; Zimmermann, R.; Küppers, M. Cambial activity and xylogenesis in stems of Cedrus libani A. Rich at different altitudes. Bot. Stud. 2015, 56, 20. [Google Scholar] [CrossRef] [PubMed]
- Şahan, E.; Köse, N.; Akkemik, Ü.; Güner, H.; Tavşanoğlu, Ç.; Bahar, A.; Dalfes, H.N. Fire history of Pinus nigra in Western Anatolia: A first dendrochronological study. Dendrochronologia 2021, 69, 125874. [Google Scholar] [CrossRef]
- Şahan, E.; Köse, N.; Güner, H.; Trouet, V.; Tavşanoğlu, Ç.; Akkemik, Ü.; Dalfes, H.N. Multi-century spatiotemporal patterns of fire history in black pine forests, Türkiye. For. Ecol. Manag. 2022, 518, 120296. [Google Scholar] [CrossRef]
- Şahan, E.A.; Gürçay, B.; Tuncay Güner, H. The history of fire, human and climate in black pine forests of Western Anatolia: The Taurus Mountains. Dendrochronologia 2023, 82, 126149. [Google Scholar] [CrossRef]
- Şensoy, S.; Demircan, M.; Ulupınar, Y.; Balta, İ.; Climate of Turkey. Devlet Meteoroloji İşleri Genel Müdürlüğü. 2016. Available online: https://www.mgm.gov.tr/FILES/genel/makale/13_turkiye_iklimi.pdf (accessed on 9 September 2023).
- Akça, E.; Kapur, S. Toprak. In Resimli Türkiye Florası; Güner, A., ve Ekim, T., Eds.; Ali Nihat Gökyiğit Vakfı, Flora Araştırmaları Derneği ve Türkiye İş Bankası Kültür Yayınları yayını: İstanbul, Turkey, 2014; Volume 1. [Google Scholar]
- EUFORGEN. Technical Guidelines for Genetic Conservation and Use for European Black Pine (Pinus nigra); International Plant Genetic Resources Institute: Rome, Italy, 2004. [Google Scholar]
- Rossi, S.; Anfodillo, T.; Menardi, R. Trephor: A new tool for sampling microcores from tree stems. IAWA J. 2006, 27, 89–97. [Google Scholar] [CrossRef]
- Larsson, L. Coorecorder and Cdendro Programs of the Coorecorder/Cdendropackage Version 7.7. 2014. Available online: https://www.cybis.se/forfun/dendro.htm (accessed on 10 November 2023).
- Holmes, R.L. Quality control of cross-dating and measuring. A users manual for computer program COFECHA. In Tree-Rings Chronologies of Western North America: California, Eastern Oregon and Northern Great Basin; Holmes, R.L., Adams, R.K., Fritts, H.C., Eds.; Chronology Ser. 6; University of Arizona: Tucson, AZ, USA, 1986; p. 41249. [Google Scholar]
- Wilson, J.W. The Growing Tree; The University of Massachusetts Press: Amherst, MA, USA, 1970. [Google Scholar]
- Denne, M.P. Definition of latewood according to Mork (1928). IAWA J. 1989, 10, 59–62. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Morin, H. Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia 2003, 21, 33–39. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2013. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 28 February 2024).
- Cuny, H.E.; Fonti, P.; Rathgeber, C.B.K.; Arx, G.; Peters, R.L.; Frank, D.C. Couplings in cell differentiation kinetics mitigate air temperature influence on conifer wood anatomy. Plant Cell Environ. 2018, 42, 1222–1232. [Google Scholar] [CrossRef]
- Gričar, J.; Čufar, K.; Eler, K.; Gryc, V.; Vavrčík, H.; de Luis, M.; Prislan, P. Transition Dates from Earlywood to Latewood and Early Phloem to Late Phloem in Norway Spruce. Forests 2021, 12, 331. [Google Scholar] [CrossRef]
- Camarero, J.J.; Collado, E.; Martínez-de-Aragón, J.; de-Miguel, S.; Büntgen, U.; Martinez-Peña, F.; Martín-Pinto, P.; Ohenoja, E.; Romppanen, T.; Salo, K.; et al. Associations between climate and Earlywood and latewood width in boreal and Mediterranean Scots pine forests. Trees 2020, 35, 155–169. [Google Scholar] [CrossRef]
- Vieira, J.; Rossi, S.; Campelo, F.; Freitas, H.; Nabais, C. Xylogenesis of Pinus pinaster under a Mediterranean climate. Ann. For. Sci. 2014, 71, 71–80. [Google Scholar] [CrossRef]
- Cabon, A.; Fernández-de-Uña, L.; Gea-Izquierdo, G.; Meinzer, F.C.; Woodruff, D.R.; Martínez-Vilalta, J.; De Cáceres, M. Water potential control of turgor-driven tracheid enlargement in Scots pine at its xeric distribution edge. New Phytol. 2019, 225, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Buttò, V.; Khare, S.; Drolet, G.; Sylvain, J.D.; Gennaretti, F.; Deslauriers, A.; Morin, H.; Rossi, S. Regionwide temporal gradients of carbon allocation allow for shoot growth and latewood formation in boreal black spruce. Glob. Ecol. Biogeogr. 2021, 30, 1657–1670. [Google Scholar] [CrossRef]
- Körner, C. A re-assessment of high elevation treeline positions and their explanation. Oecologia 1998, 115, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Deslauriers, A.; Morin, H. Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees 2005, 19, 402–408. [Google Scholar] [CrossRef]
- Vieira, J.; Carvalho, A.; Campelo, F. Tree Growth Under Climate Change: Evidence from Xylogenesis Timings and Kinetics. Front. Plant Sci. 2020, 11, 90. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Carraro, V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 2006, 152, 1–12. [Google Scholar] [CrossRef]
- Deslauriers, A.; Rossi, S.; Anfodillo, T.; Saracino, A. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiol. 2008, 28, 863–871. [Google Scholar] [CrossRef]
- Köse, N.; Akkemik, Ü.; Dalfes, H.; Özeren, M.; Tolunay, D. Tree-ring growth of Pinus nigra Arn. subsp. pallasiana under different climate conditions throughout western Anatolia. Dendrochronologia 2012, 30, 295–301. [Google Scholar] [CrossRef]
- Martin-Benito, D.; Pederson, N.; Köse, N.; Doğan, M.; Bugmann, H.; Mosulishvili, M.; Bigler, C. Pervasive effects of drought on tree growth across a wide climatic gradient in the temperate forests of the Caucasus. Glob. Ecol. Biogeogr. 2018, 27, 1314–1325. [Google Scholar] [CrossRef]
- Pompa-García, M.; Camarero, J.J.; Colangelo, M.; Gallardo-Salazar, J.L. Xylogenesis is uncoupled from forest productivity. Trees 2021, 35, 1123–1134. [Google Scholar] [CrossRef]
- Castagneri, D.; Fonti, P.; von Arx, G.; Carrer, M. How does climate influence xylem morphogenesis over the growing season? Insights from long-term intra-ring anatomy in Picea abies. Ann. Bot. 2017, 119, 1011–1020. [Google Scholar] [CrossRef]
- de Micco, V.; Carrer, M.; Rathgeber, C.; Julio Camarero, J.; Voltas, J.; Cherubini, P.; Battipaglia, G. From xylogenesis to tree rings: Wood traits to investigate tree response to environmental changes. IAWA J. 2019, 40, 155–182. [Google Scholar] [CrossRef]
| Site Names | Latitude (N) | Longitude (E) | Elevation (m a.s.l) | DBH Range | Age Range | Climate |
|---|---|---|---|---|---|---|
| KIR (Bolu) | 40°29′ | 31°42′ | 1400 | 30–34 | 59–74 | Black Sea climate |
| KUTH (Kütahya) | 39°24′ | 29°04′ | 1430 | 27–33 | 46–75 | High-altitude site in Kütahya with, Marmara transition climate |
| KUTL (Kütahya) | 39°24′ | 29°02′ | 1160 | 29–37 | 76–95 | Low-altitude site in Kütahya with, Marmara transition climate |
| HAV (Isparta) | 37°47′ | 30°57′ | 1238 | 36–43 | 62–115 | Mediterranean climate |
| Parameters | KIR | KUTH | KUTL | HAV |
|---|---|---|---|---|
| R2 | 0.73–0.98 | 0.87–0.99 | 0.82–0.95 | 0.77–0.98 |
| A | 20.84–90.94 | 22.34–81.31 | 18.14–30.97 | 8.74–57.41 |
| ß | 2.81–9.82 | 4.03–7.15 | 4.42–11.46 | 2.14–8.53 |
| 0.02–0.07 | 0.02–0.05 | 0.03–0.08 | 0.01–0.06 | |
| t5 (DOY) | 110–140 | 121–143 | 115–126 | 88–130 |
| tx (DOY) | 149–181 | 144–180 | 135–155 | 148–183 |
| t95 (DOY) | 194–323 | 206–292 | 170–260 | 201–311 |
| gx (cells/day) | 0.19–0.70 | 0.35–0.87 | 0.25–0.56 | 0.18–0.39 |
| gr (cells/day) | 0.11–0.42 | 0.17–0.53 | 0.15–0.34 | 0.11–0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şahan, E.A.; Köse, N.; Güner, H.T.; Martin-Benito, D.; Gea-Izquierdo, G.; Conde, M.; Almagro, D.; Kızılaslan, I.S.; Akkemik, Ü.; Dalfes, H.N. Monitoring Intra-Annual Wood Formation of Pinus nigra J.F. Arnold (Black Pine) to Understand the Fire Seasonality in Western Anatolia. Forests 2024, 15, 494. https://doi.org/10.3390/f15030494
Şahan EA, Köse N, Güner HT, Martin-Benito D, Gea-Izquierdo G, Conde M, Almagro D, Kızılaslan IS, Akkemik Ü, Dalfes HN. Monitoring Intra-Annual Wood Formation of Pinus nigra J.F. Arnold (Black Pine) to Understand the Fire Seasonality in Western Anatolia. Forests. 2024; 15(3):494. https://doi.org/10.3390/f15030494
Chicago/Turabian StyleŞahan, Evrim A., Nesibe Köse, H. Tuncay Güner, Dario Martin-Benito, Guillermo Gea-Izquierdo, María Conde, David Almagro, Irem Sena Kızılaslan, Ünal Akkemik, and H. Nüzhet Dalfes. 2024. "Monitoring Intra-Annual Wood Formation of Pinus nigra J.F. Arnold (Black Pine) to Understand the Fire Seasonality in Western Anatolia" Forests 15, no. 3: 494. https://doi.org/10.3390/f15030494
APA StyleŞahan, E. A., Köse, N., Güner, H. T., Martin-Benito, D., Gea-Izquierdo, G., Conde, M., Almagro, D., Kızılaslan, I. S., Akkemik, Ü., & Dalfes, H. N. (2024). Monitoring Intra-Annual Wood Formation of Pinus nigra J.F. Arnold (Black Pine) to Understand the Fire Seasonality in Western Anatolia. Forests, 15(3), 494. https://doi.org/10.3390/f15030494






