Comparative Analysis of Water Isotopic Compositions: Evaluating Isotope Analyzer for Soil and Extraction Method for Stem Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sample Collection
2.2. Soil Water Extraction
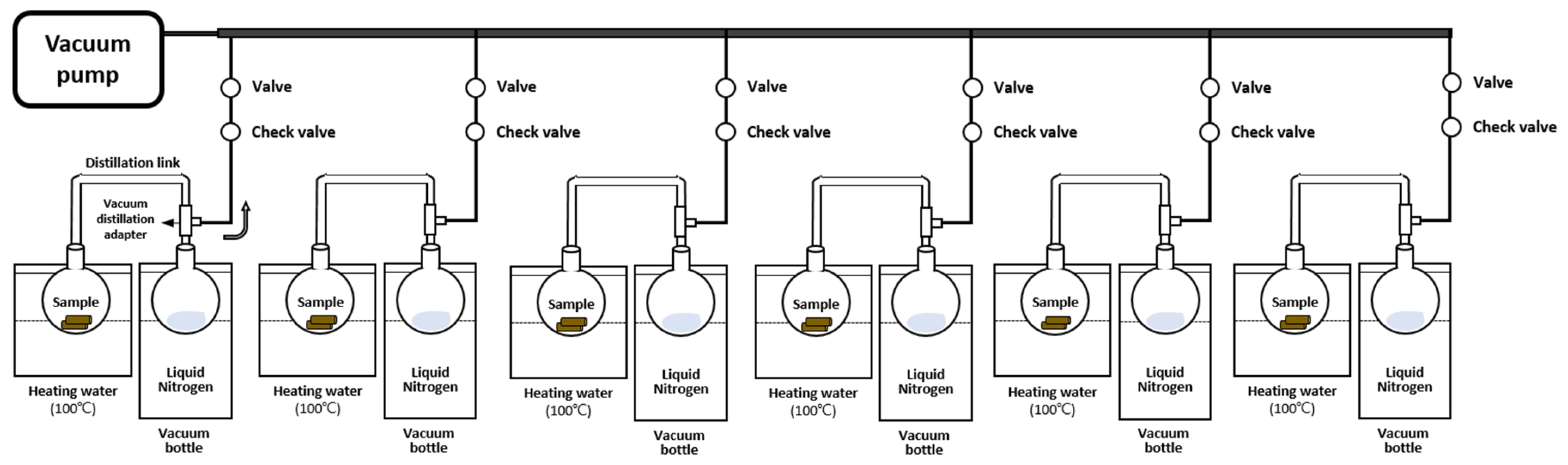
2.3. Xylem Water Extraction
- (1)
- CVD
- (2)
- PC
- (3)
- IM
2.4. Isotope Analyses
- (1)
- IRMS
- (2)
- CRDS
2.5. Data Analyses
3. Results
3.1. Differences in Stable Isotope Ratios between IRMS and CRDS
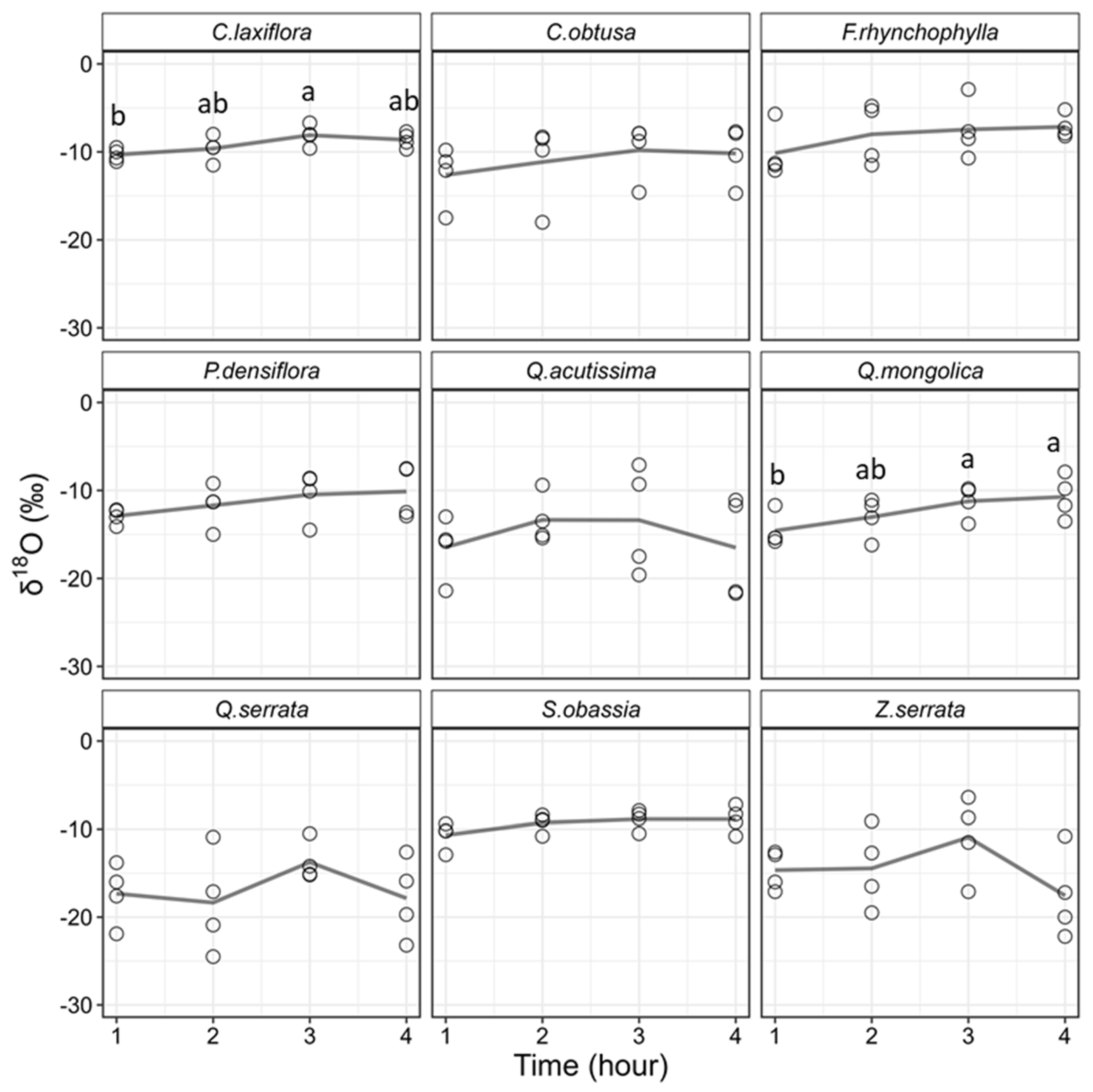
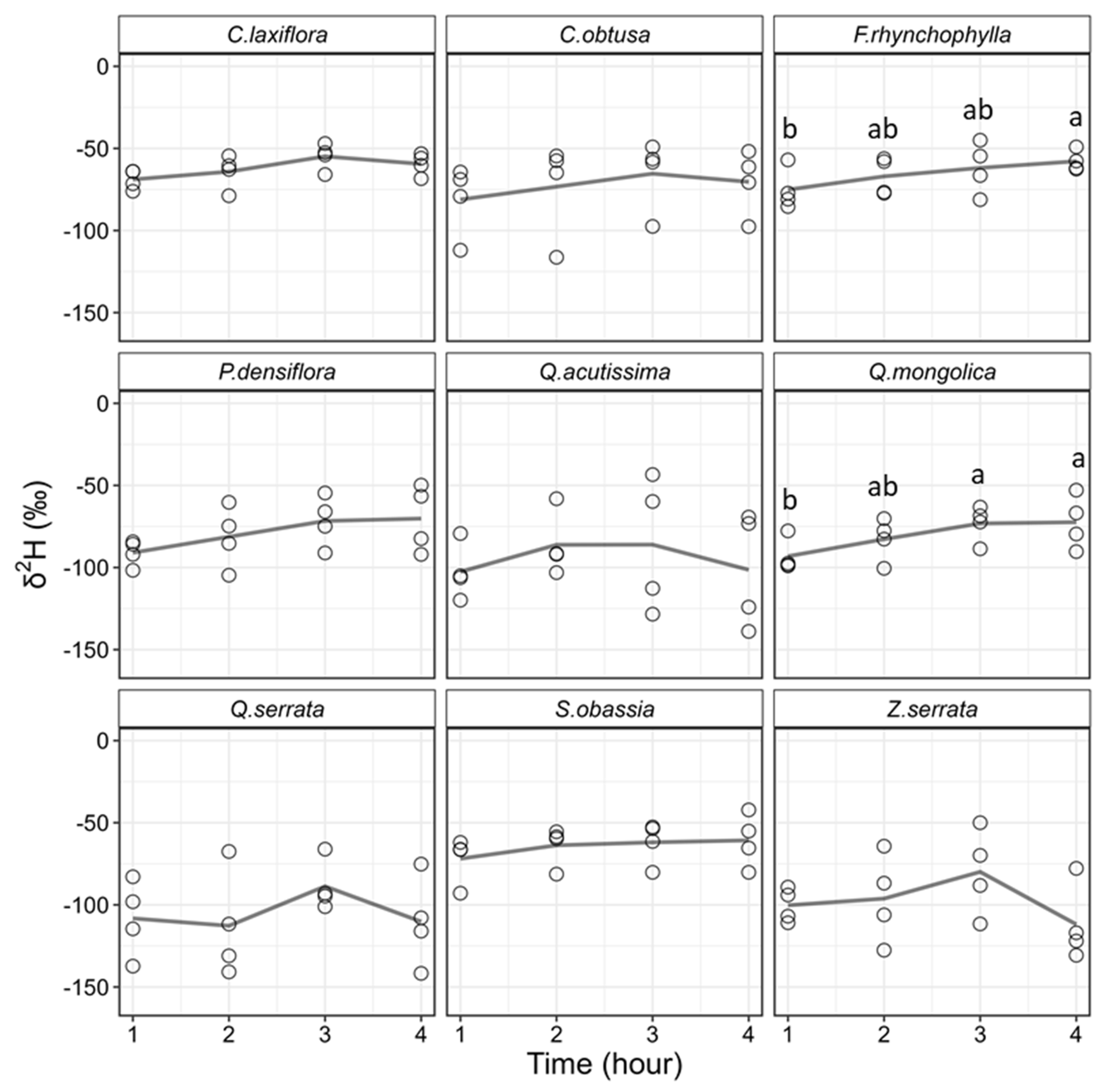
3.2. Differences in Stable Isotope Ratios among Extraction Times
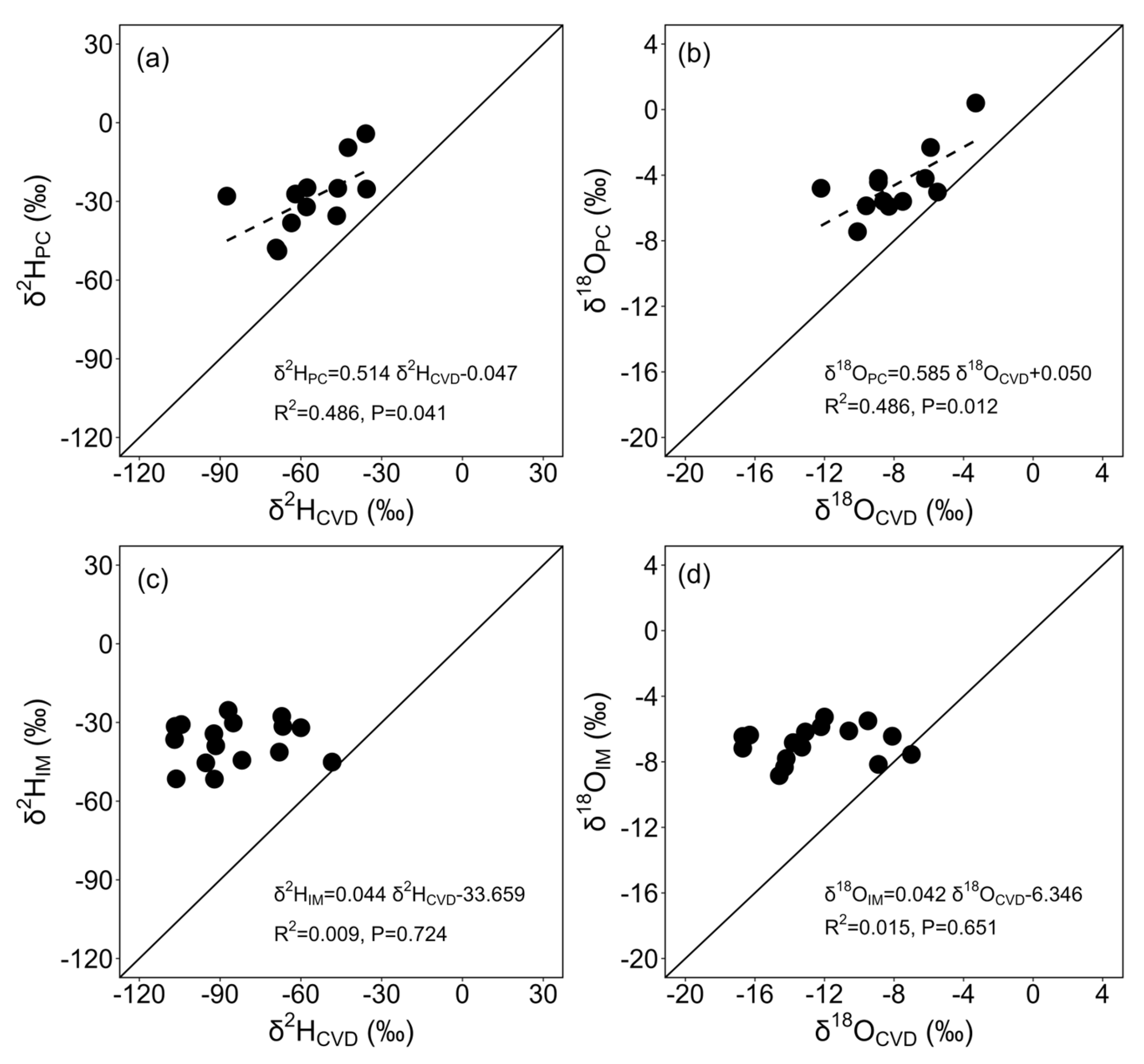
3.3. Differences in Stable Isotope Ratios among Extraction Methods
4. Discussion
4.1. Effects of Soil Properties on the Differences between IRMS and CRDS
4.2. Effects of Extraction Time on Stable Isotope Ratios
4.3. Effects of Stem Water Extraction Method on Stable Isotope Ratios
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tetzlaff, D.; Buttle, J.; Carey, S.K.; Kohn, M.J.; Laudon, H.; McNamara, J.P.; Smith, A.; Sprenger, M.; Soulsby, C. Stable isotopes of water reveal differences in plant–soil water relationships across northern environments. Hydrol. Process. 2021, 35, e14023. [Google Scholar] [CrossRef]
- Miguez-Macho, G.; Fan, Y. Spatiotemporal origin of soil water taken up by vegetation. Nature 2021, 598, 624–628. [Google Scholar] [CrossRef]
- Landgraf, J.; Tetzlaff, D.; Dubbert, M.; Dubbert, D.; Smith, A.; Soulsby, C. Xylem water in riparian willow trees (Salix alba) reveals shallow sources of root water uptake by in situ monitoring of stable water isotopes. Hydrol. Earth Syst. Sci. 2022, 26, 2073–2092. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, X.; Wang, L.; Wang, R.; Luo, Z.; He, X.; Rao, Z. Water stable isotope characteristics and water use strategies of co-occurring plants in ecological and economic forests in subtropical monsoon regions. J. Hydrol. 2023, 621, 129565. [Google Scholar] [CrossRef]
- Orlowski, N.; Rinderer, M.; Dubbert, M.; Ceperley, N.; Hrachowitz, M.; Gessler, A.; Rothfuss, Y.; Sprenger, M.; Heidbüchel, I.; Kübert, A. Challenges in studying water fluxes within the soil-plant-atmosphere continuum: A tracer-based perspective on pathways to progress. Sci. Total Environ. 2023, 881, 163510. [Google Scholar] [CrossRef]
- Brand, W.A.; Geilmann, H.; Crosson, E.R.; Rella, C.W. Cavity ring-down spectroscopy versus high-temperature conversion isotope ratio mass spectrometry; a case study on δ2H and δ18O of pure water samples and alcohol/water mixtures. Rapid Commun. Mass Spectrom. RCM 2009, 23, 1879–1884. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Maseyk, K.; Lett, C.; Biron, P.; Richard, P.; Bariac, T.; Seibt, U. Reducing and correcting for contamination of ecosystem water stable isotopes measured by isotope ratio infrared spectroscopy. Rapid Commun. Mass Spectrom. 2012, 26, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Martín-Gómez, P.; Barbeta, A.; Voltas, J.; Peñuelas, J.; Dennis, K.; Palacio, S.; Dawson, T.E.; Ferrio, J.P. Isotope-ratio infrared spectroscopy: A reliable tool for the investigation of plant-water sources? New Phytol. 2015, 207, 914–927. [Google Scholar] [CrossRef] [PubMed]
- West, A.G.; Goldsmith, G.R.; Brooks, P.D.; Dawson, T.E. Discrepancies between isotope ratio infrared spectroscopy and isotope ratio mass spectrometry for the stable isotope analysis of plant and soil waters. Rapid Commun. Mass Spectrom. 2010, 24, 1948–1954. [Google Scholar] [CrossRef]
- West, A.; Goldsmith, G.; Matimati, I.; Dawson, T. Spectral analysis software improves confidence in plant and soil water stable isotope analyses performed by isotope ratio infrared spectroscopy (IRIS). Rapid Commun. Mass Spectrom. 2011, 25, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Hamann, L.; Dettman, D.; Kim-Hak, D.; Leavitt, S.; Monson, R.; Papuga, S. Performance of induction module cavity ring-down spectroscopy (IM-CRDS) for measuring δ18O and δ2H values of soil, stem, and leaf waters. Rapid Commun. Mass Spectrom. 2017, 31, 547–560. [Google Scholar] [CrossRef]
- Millar, C.; Pratt, D.; Schneider, D.J.; McDonnell, J.J. A comparison of extraction systems for plant water stable isotope analysis. Rapid Commun. Mass Spectrom. 2018, 32, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.; Janzen, K.; Nehemy, M.F.; Koehler, G.; Hervé-Fernández, P.; McDonnell, J.J. Organic contamination detection for isotopic analysis of water by laser spectroscopy. Rapid Commun. Mass Spectrom. 2021, 35, e9118. [Google Scholar] [CrossRef]
- Orlowski, N.; Breuer, L.; McDonnell, J.J. Critical issues with cryogenic extraction of soil water for stable isotope analysis. Ecohydrology 2016, 9, 1–5. [Google Scholar] [CrossRef]
- Newberry, S.; Nelson, D.; Kahmen, A. Cryogenic vacuum artifacts do not affect plant water-uptake studies using stable isotope analysis. Ecohydrology 2017, 10, e1892. [Google Scholar] [CrossRef]
- Lazarus, B.E.; Germino, M.J. Methodological considerations regarding online extraction of water from soils for stable isotope determination. Rapid Commun. Mass Spectrom. 2017, 31, 1677–1680. [Google Scholar] [CrossRef]
- Millar, C.; Janzen, K.; Nehemy, M.F.; Koehler, G.; Hervé-Fernández, P.; Wang, H.; Orlowski, N.; Barbeta, A.; McDonnell, J.J. On the urgent need for standardization in isotope-based ecohydrological investigations. Hydrol. Process. 2022, 36, e14698. [Google Scholar] [CrossRef]
- Goebel, T.S.; Lascano, R.J. System for high throughput water extraction from soil material for stable isotope analysis of water. J. Anal. Sci. Methods Instrum. 2012, 2, 203–207. [Google Scholar] [CrossRef][Green Version]
- Orlowski, N.; Frede, H.-G.; Brüggemann, N.; Breuer, L. Validation and application of a cryogenic vacuum extraction system for soil and plant water extraction for isotope analysis. J. Sens. Sens. Syst. 2013, 2, 179–193. [Google Scholar] [CrossRef]
- Munksgaard, N.C.; Cheesman, A.W.; Wurster, C.M.; Cernusak, L.A.; Bird, M.I. Microwave extraction–isotope ratio infrared spectroscopy (ME-IRIS): A novel technique for rapid extraction and in-line analysis of δ18O and δ2H values of water in plants, soils and insects. Rapid Commun. Mass Spectrom. 2014, 28, 2151–2161. [Google Scholar] [CrossRef]
- Orlowski, N.; Pratt, D.L.; McDonnell, J.J. Intercomparison of soil pore water extraction methods for stable isotope analysis. Hydrol. Process. 2016, 30, 3434–3449. [Google Scholar] [CrossRef]
- Orlowski, N.; Breuer, L.; Angeli, N.; Boeckx, P.; Brumbt, C.; Cook, C.S.; Dubbert, M.; Dyckmans, J.; Gallagher, B.; Gralher, B. Inter-laboratory comparison of cryogenic water extraction systems for stable isotope analysis of soil water. Hydrol. Earth Syst. Sci. 2018, 22, 3619–3637. [Google Scholar] [CrossRef]
- Yang, B.; Dossa, G.G.; Hu, Y.H.; Liu, L.L.; Meng, X.J.; Du, Y.Y.; Li, J.Y.; Zhu, X.A.; Zhang, Y.J.; Singh, A.K. Uncorrected soil water isotopes through cryogenic vacuum distillation may lead to a false estimation on plant water sources. Methods Ecol. Evol. 2023, 14, 1443–1456. [Google Scholar] [CrossRef]
- Allen, S.T.; Kirchner, J.W. Potential effects of cryogenic extraction biases on plant water source partitioning inferred from xylem-water isotope ratios. Hydrol. Process 2022, 36, e14483. [Google Scholar] [CrossRef]
- Bowers, W.H.; Williams, D.G. Isotopic heterogeneity of stem water in conifers is correlated to xylem hydraulic traits and supports multiple residence times. Front. Water 2022, 46, 861590. [Google Scholar] [CrossRef]
- He, D.; Wen, M.; Wang, Y.; Du, G.; Zhang, C.; He, H.; Jin, J.; Li, M.; Si, B. Xylem water cryogenic vacuum extraction: Testing correction methods with CaviTron-based apple twig sampling. J. Hydrol. 2023, 621, 129572. [Google Scholar] [CrossRef]
- Diao, H.; Schuler, P.; Goldsmith, G.R.; Siegwolf, R.T.; Saurer, M.; Lehmann, M.M. On uncertainties in plant water isotopic composition following extraction by cryogenic vacuum distillation. Hydrol. Earth Syst. Sci. 2022, 26, 5835–5847. [Google Scholar] [CrossRef]
- Wen, M.; He, D.; Li, M.; Ren, R.; Jin, J.; Si, B. Causes and factors of cryogenic extraction biases on isotopes of xylem water. Water Resour. Res. 2022, 58, e2022WR032182. [Google Scholar] [CrossRef]
- Sobota, M.; Li, K.; Hren, M.; Knighton, J. Evidence for variations in cryogenic extraction deuterium biases of plant xylem water across foundational northeastern US trees. Hydrol. Process. 2024, 38, e15079. [Google Scholar] [CrossRef]
- Zuecco, G.; Amin, A.; Frentress, J.; Engel, M.; Marchina, C.; Anfodillo, T.; Borga, M.; Carraro, V.; Scandellari, F.; Tagliavini, M. A comparative study of plant water extraction methods for isotopic analyses: Scholander-type pressure chamber vs. cryogenic vacuum distillation. Hydrol. Earth Syst. Sci. 2022, 26, 3673–3689. [Google Scholar] [CrossRef]
- Gee, G.; Or, D. Particle size analysis. In ‘Methods of soil analysis. Part 4. Physical methods’. Soil Sci. Soc. Am. Book Ser. 2002, 5, 255–293. [Google Scholar]
- Thomas, G.W. Soil pH and soil acidity. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 475–490. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 1201–1229. [Google Scholar]
- Soil Survey Staff. Web Soil Survey. United States Department of Agriculture: Retrieved from Natural Resources Conservation Service. 2019. Available online: https://websoilsurvey.sc.egov.usda.gov/ (accessed on 12 December 2017).
- West, A.G.; Patrickson, S.J.; Ehleringer, J.R. Water extraction times for plant and soil materials used in stable isotope analysis. Rapid Commun. Mass Spectrom. 2006, 20, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R–project.org/ (accessed on 31 December 2023).
- McDonnell, J.J. The two water worlds hypothesis: Ecohydrological separation of water between streams and trees? Wiley Interdiscip. Rev. Water 2014, 1, 323–329. [Google Scholar] [CrossRef]
- Sánchez-Murillo, R.; Todini-Zicavo, D.; Poca, M.; Birkel, C.; Esquivel-Hernández, G.; Chavarría, M.M.; Zuecco, G.; Penna, D. Dry season plant water sourcing in contrasting tropical ecosystems of Costa Rica. Ecohydrology 2023, 16, e2541. [Google Scholar] [CrossRef]
- Berry, S.L.; Roderick, M.L. Plant–water relations and the fibre saturation point. New Phytol. 2005, 168, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Jupa, R.; Plavcová, L.; Gloser, V.; Jansen, S. Linking xylem water storage with anatomical parameters in five temperate tree species. Tree Physiol. 2016, 36, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Barbeta, A.; Burlett, R.; Martín-Gómez, P.; Fréjaville, B.; Devert, N.; Wingate, L.; Domec, J.-C.; Ogée, J. Evidence for distinct isotopic compositions of sap and tissue water in tree stems: Consequences for plant water source identification. New Phytol. 2022, 233, 1121–1132. [Google Scholar] [CrossRef]
- Ellsworth, P.Z.; Williams, D.G. Hydrogen isotope fractionation during water uptake by woody xerophytes. Plant Soil 2007, 291, 93–107. [Google Scholar] [CrossRef]
- Evaristo, J.; McDonnell, J.J.; Clemens, J. Plant source water apportionment using stable isotopes: A comparison of simple linear, two-compartment mixing model approaches. Hydrol. Process. 2017, 31, 3750–3758. [Google Scholar] [CrossRef]
- Chen, Y.; Helliker, B.R.; Tang, X.; Li, F.; Zhou, Y.; Song, X. Stem water cryogenic extraction biases estimation in deuterium isotope composition of plant source water. Proc. Natl. Acad. Sci. USA 2020, 117, 33345–33350. [Google Scholar] [CrossRef]
- Zhao, Y. An incorrect wetness-based correction method for deuterium offset. Proc. Natl. Acad. Sci. USA 2021, 118, e2026641118. [Google Scholar] [CrossRef] [PubMed]
- Barbeta, A.; Gimeno, T.E.; Clavé, L.; Fréjaville, B.; Jones, S.P.; Delvigne, C.; Wingate, L.; Ogée, J. An explanation for the isotopic offset between soil and stem water in a temperate tree species. New Phytol. 2020, 227, 766–779. [Google Scholar] [CrossRef] [PubMed]






| Method | Species | Number of Samples |
|---|---|---|
| PC and CVD | P. densiflora | 3 |
| C. laxiflora | 3 | |
| C. obtusa | 4 | |
| S. obassia | 2 | |
| IM and CVD | P. densiflora | 6 |
| C. obtusa | 8 | |
| S. obassia | 2 |
| Soil Depth (cm) | Sand (%) | Silt (%) | Clay (%) | Total C (%) | Total N (%) | pH (H2O) | CEC (cmolc kg−1) |
|---|---|---|---|---|---|---|---|
| 10 | 44.8 ± 4.1 | 41.9 ± 3.9 | 13.2 ± 0.6 | 4.8 ± 0.5 | 0.36 ± 0.03 | 4.54 ± 0.09 | 4.28 ± 0.27 |
| 30 | 44.7 ± 4.9 | 40 ± 4.4 | 15.3 ± 1.5 | 3.26 ± 0.57 | 0.25 ± 0.04 | 4.7 ± 0.05 | 3.23 ± 0.3 |
| 50 | 45.5 ± 4.3 | 37.6 ± 6.4 | 16.9 ± 3.3 | 2.19 ± 0.93 | 0.16 ± 0.04 | 4.74 ± 0.07 | 2.75 ± 0.23 |
| 100 | 54.4 ± 15.3 | 25.6 ± 9.5 | 20 ± 6.3 | 0.73 ± 0.47 | 0.06 ± 0.03 | 5.2 ± 0.34 | 2.27 ± 0.51 |
| 120 | 51.1 ± 17.2 | 26.6 ± 9.4 | 22.4 ± 8.5 | 0.6 ± 0.3 | 0.07 ± 0.02 | 5.39 ± 0.04 | 2.45 ± 0.5 |
| Isotope | Soil Depth (cm) | Paired t-Test | Pearson’s Correlation Coefficient (r) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| p | Sand | Silt | Clay | Total C | Total N | pH | CEC | ||
| δ2H | 10 | 0.060 | −0.177 | 0.141 | 0.288 | −0.083 | 0.024 | −0.328 | 0.731 |
| 30 | <0.001 | 0.577 | −0.378 | −0.793 | 0.762 | 0.529 | −0.196 | −0.419 | |
| 50 | <0.001 | 0.169 | 0.185 | −0.578 | 0.409 | 0.411 | −0.245 | 0.011 | |
| 100 | 0.062 | 0.566 | −0.431 | −0.722 | 0.103 | −0.059 | 0.329 | −0.696 | |
| 120 | <0.05 | 0.295 | −0.032 | −0.516 | 0.470 | 0.386 | 0.059 | −0.264 | |
| δ18O | 10 | 0.129 | −0.376 | 0.403 | −0.025 | −0.312 | −0.156 | −0.016 | 0.609 |
| 30 | 0.625 | 0.388 | −0.115 | −0.949 * | 0.987 ** | 0.949 * | −0.574 | 0.275 | |
| 50 | <0.001 | −0.779 | 0.914 * | −0.752 | 0.867 | 0.823 | −0.681 | 0.498 | |
| 100 | <0.05 | 0.455 | −0.322 | −0.617 | 0.262 | 0.016 | −0.082 | −0.501 | |
| 120 | 0.053 | 0.740 | −0.574 | −0.833 | −0.025 | −0.162 | −0.044 | −0.539 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, J.; Lee, H.; Lee, M.; Hong, J.; Kim, S.; Park, C.; Kim, H.S. Comparative Analysis of Water Isotopic Compositions: Evaluating Isotope Analyzer for Soil and Extraction Method for Stem Water. Forests 2024, 15, 420. https://doi.org/10.3390/f15030420
Jeon J, Lee H, Lee M, Hong J, Kim S, Park C, Kim HS. Comparative Analysis of Water Isotopic Compositions: Evaluating Isotope Analyzer for Soil and Extraction Method for Stem Water. Forests. 2024; 15(3):420. https://doi.org/10.3390/f15030420
Chicago/Turabian StyleJeon, Jihyeon, Hojin Lee, Minsu Lee, Jeonghyun Hong, Seohyun Kim, Chanoh Park, and Hyun Seok Kim. 2024. "Comparative Analysis of Water Isotopic Compositions: Evaluating Isotope Analyzer for Soil and Extraction Method for Stem Water" Forests 15, no. 3: 420. https://doi.org/10.3390/f15030420
APA StyleJeon, J., Lee, H., Lee, M., Hong, J., Kim, S., Park, C., & Kim, H. S. (2024). Comparative Analysis of Water Isotopic Compositions: Evaluating Isotope Analyzer for Soil and Extraction Method for Stem Water. Forests, 15(3), 420. https://doi.org/10.3390/f15030420






