Effects of Stand Density, Age, and Drought on the Size–Growth Relationship in Larix principis-rupprechtii Forests
Abstract
1. Introduction
2. Materials and Methods
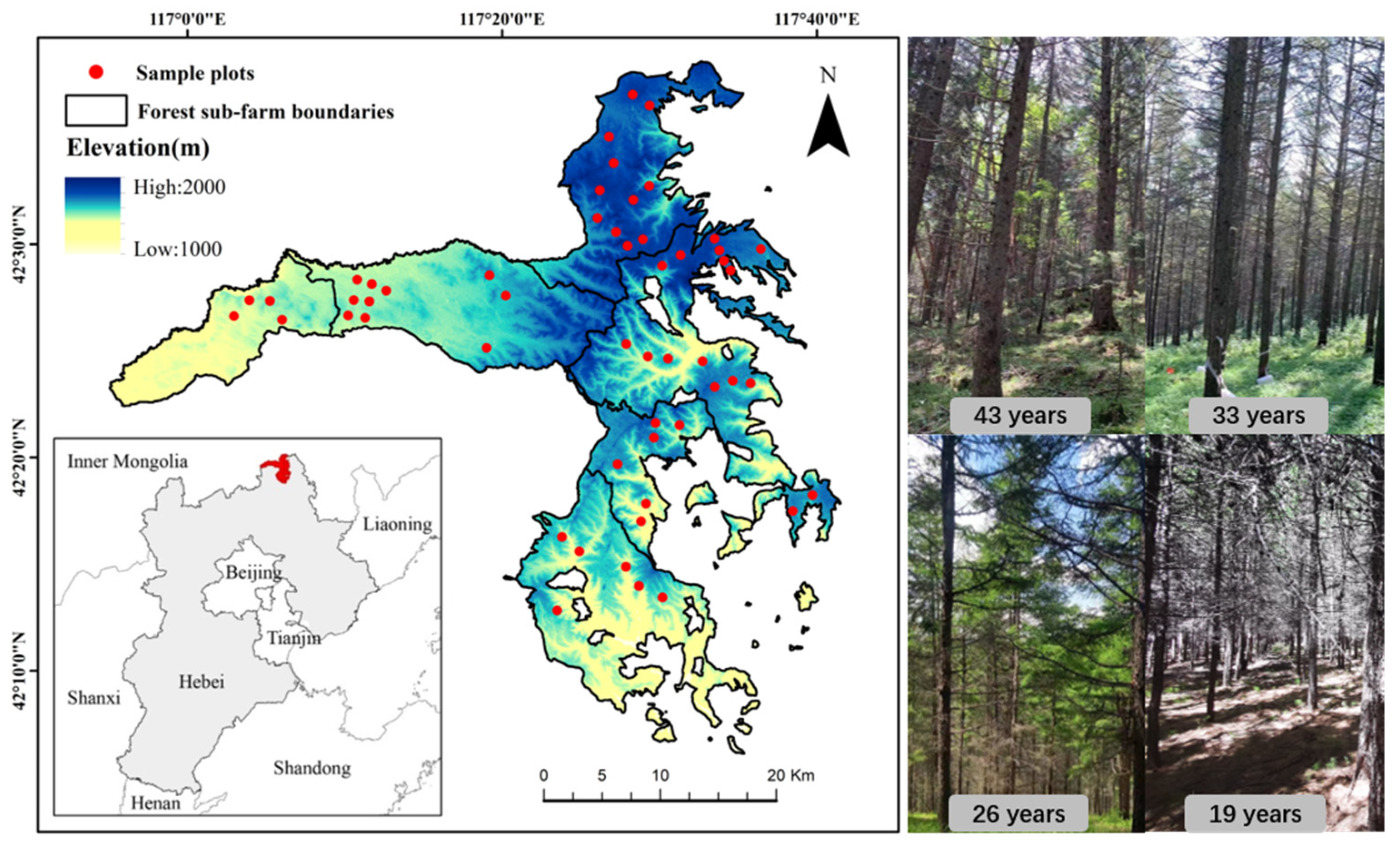
2.1. Study Area
2.2. Data Collection
2.2.1. Study Plots and Sample Trees
2.2.2. Climate
2.3. Statistical Analysis
2.3.1. Calculation of Size Inequality
2.3.2. Calculation of Productivity
2.3.3. Calculation of the Size–Growth Relationship
2.3.4. Calculation of Growth Dominance Coefficient
2.3.5. Construction of the Linear Mixed Model
3. Results
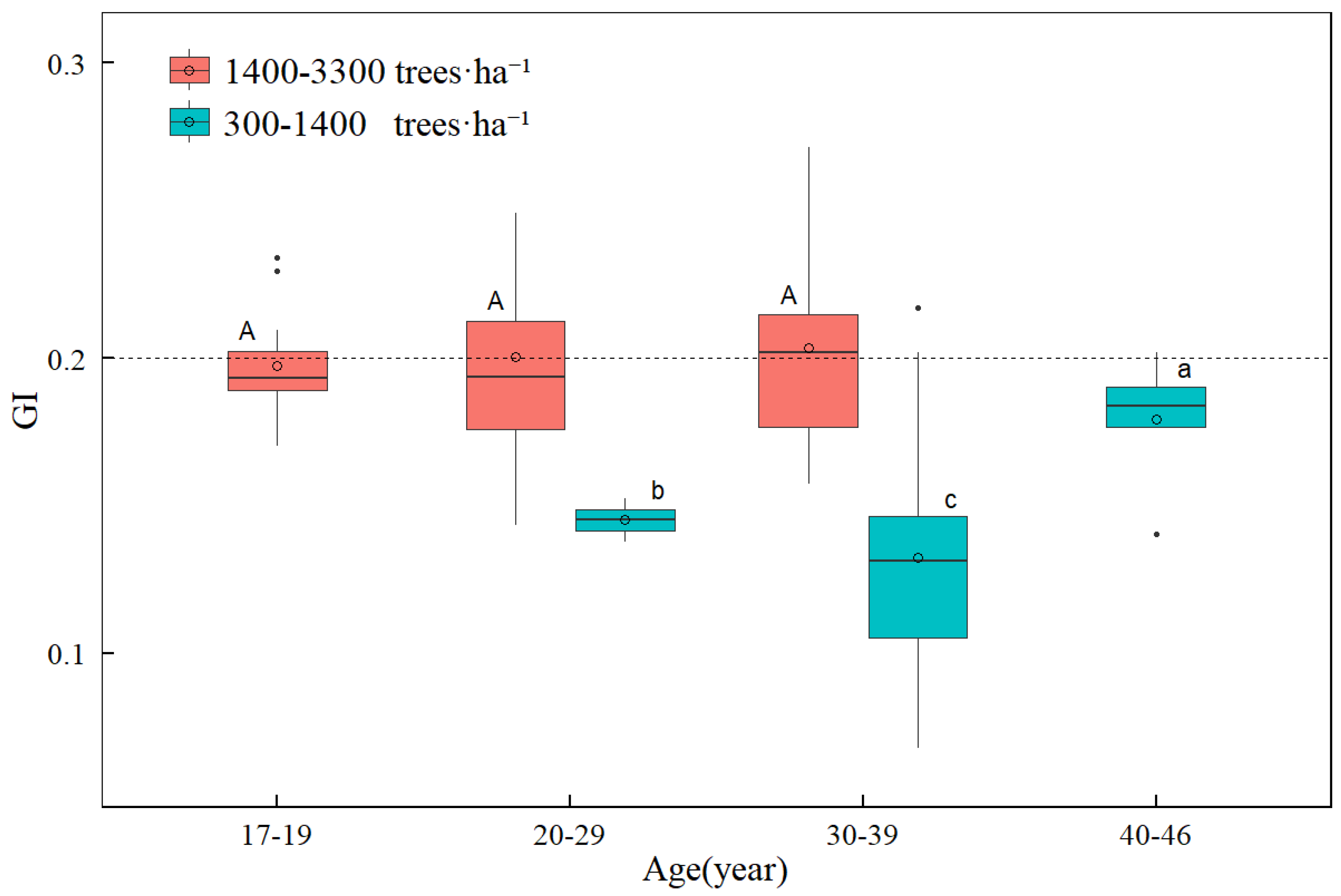
3.1. Change in Size Inequality with Tree Age and Stand Density
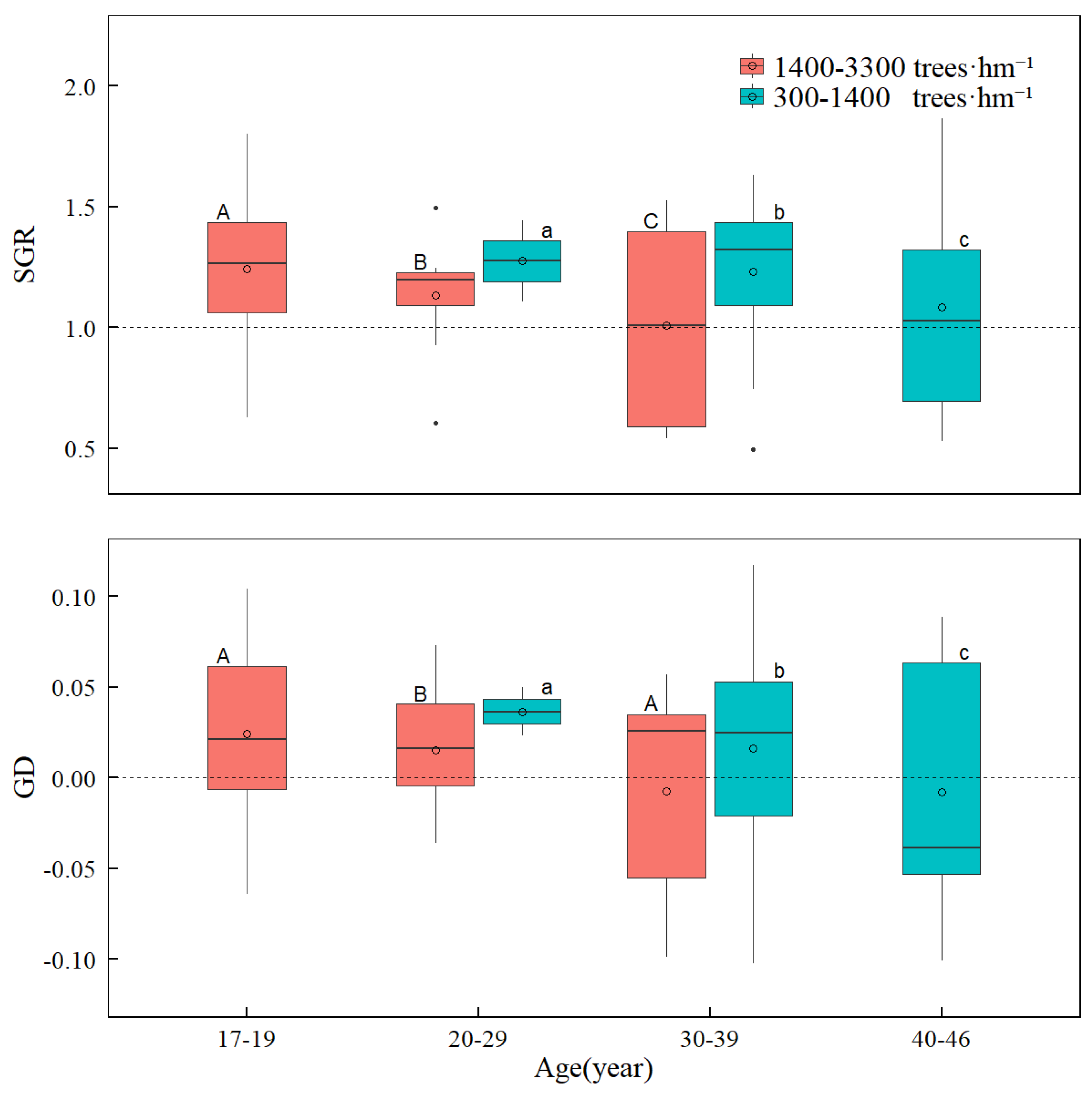
3.2. Change in SGR and GD with Tree Age and Stand Density
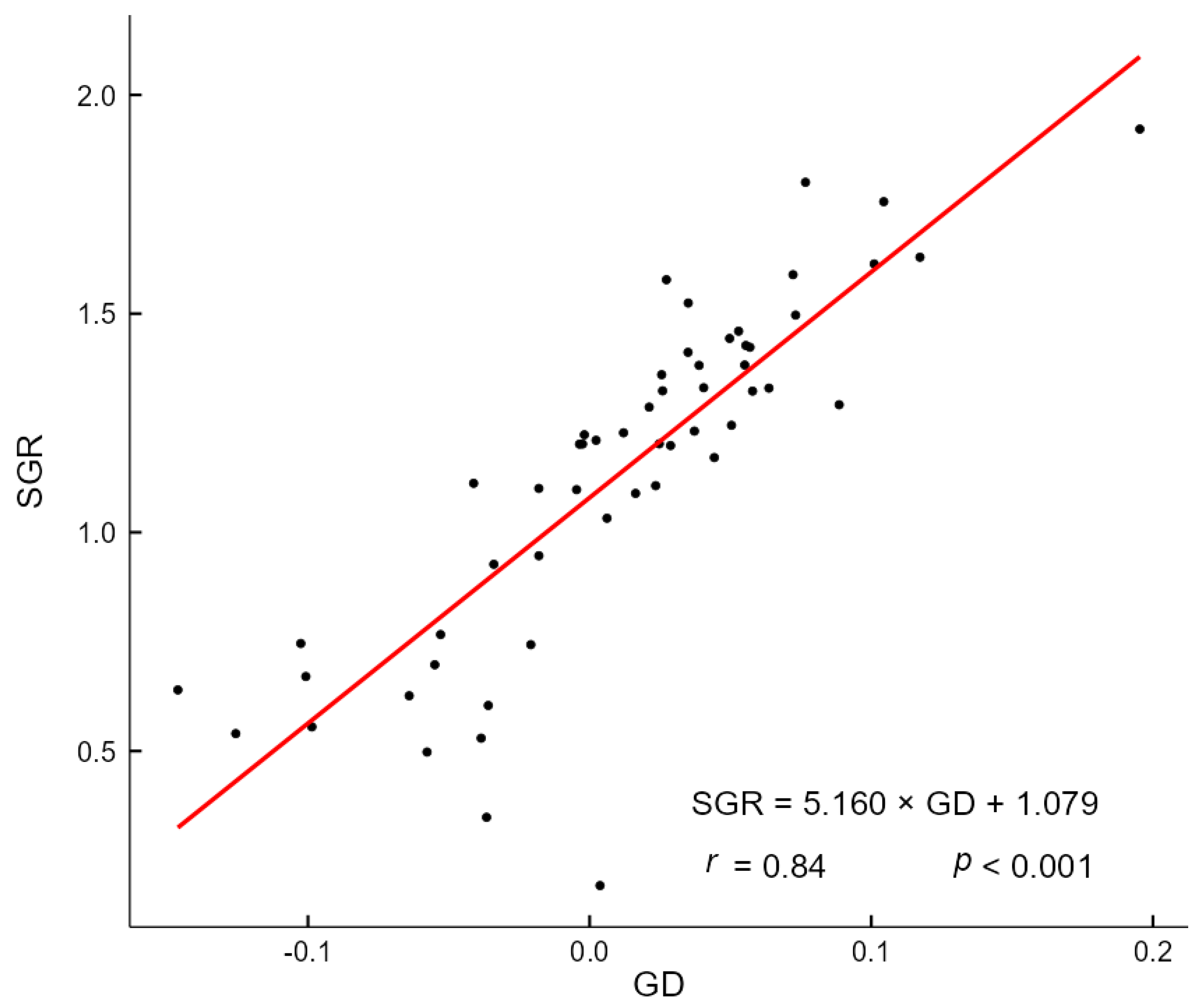
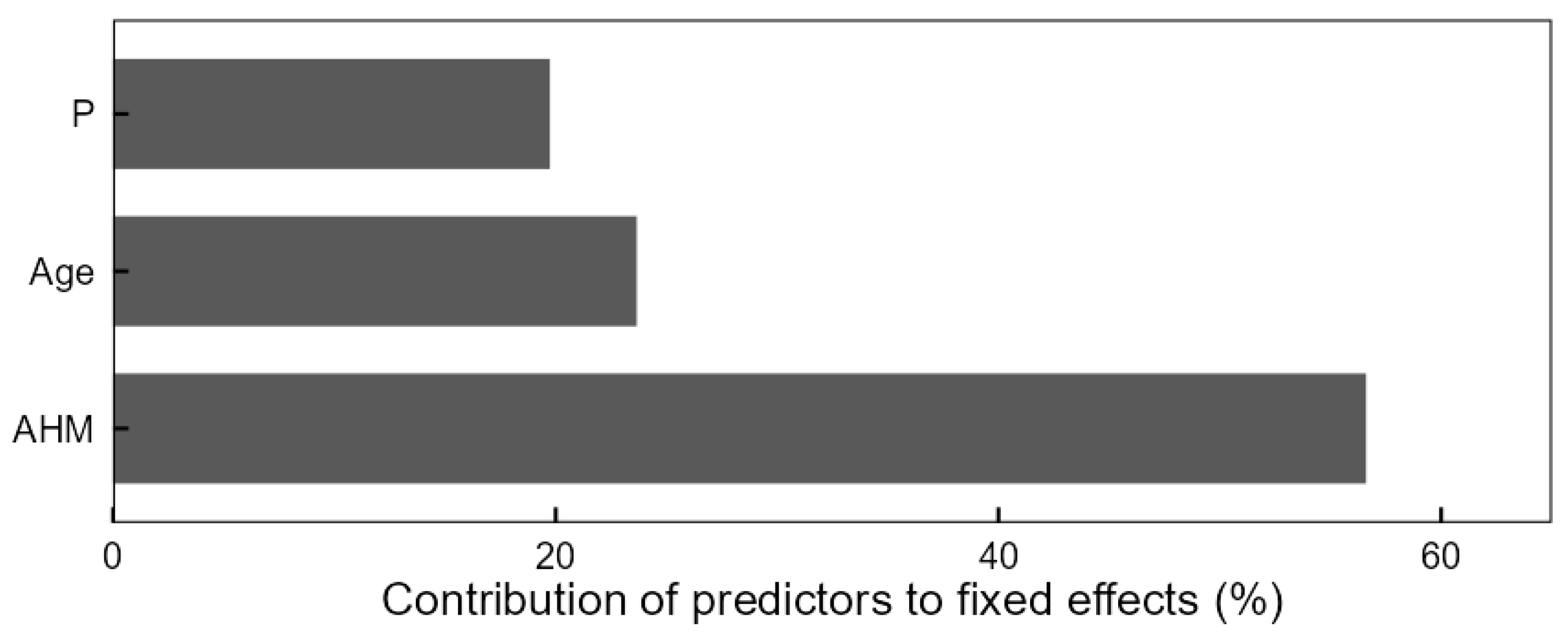
3.3. The Impact of Drought on Size–Growth Relationship
4. Discussion
4.1. Effects of Stand Age and Stand Density on Size Inequality
4.2. Effects of Stand Age and Stand Density on Size–Growth Relationship
4.3. Effects of Drought on Size–Growth Relationship
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Binkley, D.; Stape, J.L.; Ryan, M.G.; Barnard, H.R.; Fownes, J. Age-related decline in forest ecosystem growth: An individual-tree, stand-structure hypothesis. Ecosystems 2002, 5, 58–67. [Google Scholar] [CrossRef]
- Schall, P.; Schulze, E.-D.; Fischer, M.; Ayasse, M.; Ammer, C. Relations between forest management, stand structure and productivity across different types of Central European forests. Basic Appl. Ecol. 2018, 32, 39–52. [Google Scholar] [CrossRef]
- Fernández-Tschieder, E.; Binkley, D. Linking competition with growth dominance and production ecology. For. Ecol. Manag. 2018, 414, 99–107. [Google Scholar] [CrossRef]
- Binkley, D. A hypothesis about the interaction of tree dominance and stand production through stand development. For. Ecol. Manag. 2004, 190, 265–271. [Google Scholar] [CrossRef]
- Pretzsch, H.; Dieler, J. The dependency of the size-growth relationship of Norway spruce (Picea abies L. Karst.) and European beech (Fagus sylvatica L.) in forest stands on long-term site conditions, drought events, and ozone stress. Trees 2011, 25, 355–369. [Google Scholar] [CrossRef]
- Forrester, D.I. Linking forest growth with stand structure: Tree size inequality, tree growth or resource partitioning and the asymmetry of competition. For. Ecol. Manag. 2019, 447, 139–157. [Google Scholar] [CrossRef]
- Looney, C.E.; D’Amato, A.W.; Jovan, S. Investigating linkages between the size-growth relationship and drought, nitrogen deposition, and structural complexity in western US Forests. For. Ecol. Manag. 2021, 497, 119494. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P. Size-symmetric versus size-asymmetric competition and growth partitioning among trees in forest stands along an ecological gradient in central Europe. Can. J. For. Res. 2010, 40, 370–384. [Google Scholar] [CrossRef]
- Metsaranta, J.M. Influence of past mortality and measurement thresholds on tree-ring inferred trends in tree distribution and size-growth relationships at a Populus tremuloides stand in the Northwest Territories, Canada. For. Ecol. Manag. 2020, 466, 118138. [Google Scholar] [CrossRef]
- Hitsuma, G.; Himmapan, W.; Yagihashi, T.; Miyamoto, K.; Vacharangkura, T. Effects of tree density and size symmetry of competition on diameter growth in the early stages of growth in planted teak (Tectona grandis) trees in northern Thailand. J. For. Res. 2021, 26, 321–327. [Google Scholar] [CrossRef]
- Bradford, J.B.; D’Amato, A.W.; Palik, B.J.; Fraver, S. A new method for evaluating forest thinning: Growth dominance in managed Pinus resinosa stands. Can. J. For. Res. 2010, 40, 843–849. [Google Scholar] [CrossRef]
- Pothier, D. Relationships between patterns of stand growth dominance and tree competition mode for species of various shade tolerances. For. Ecol. Manag. 2017, 406, 155–162. [Google Scholar] [CrossRef]
- Binkley, D.; Kashian, D.M.; Boyden, S.; Kaye, M.W.; Bradford, J.B.; Arthur, M.A.; Fornwalt, P.J.; Ryan, M.G. Patterns of growth dominance in forests of the Rocky Mountains, USA. For. Ecol. Manag. 2006, 236, 193–201. [Google Scholar] [CrossRef]
- Castagneri, D.; Nola, P.; Cherubini, P.; Motta, R. Temporal variability of size-growth relationships in a Norway spruce forest: The influences of stand structure, logging, and climate. Can. J. For. Res. 2012, 42, 550–560. [Google Scholar] [CrossRef]
- Pérez-de-Lis, G.; García-González, I.; Rozas, V.; Arévalo, J.R. Effects of thinning intensity on radial growth patterns and temperature sensitivity in Pinus canariensis afforestations on Tenerife Island, Spain. Ann. For. Sci. 2011, 68, 1093. [Google Scholar] [CrossRef]
- Turski, M.; Kwasna, H.; Beker, C.; Jaszczak, R.; Kazmierczak, K.; Najgrakowski, T.; Borzyszkowski, W. The influence of age and crown position on growth efficiency along a Scots pine chronosequence. iForest—Biogeosc. For. 2019, 12, 474–479. [Google Scholar] [CrossRef]
- Trouvé, R.; Bontemps, J.-D.; Collet, C.; Seynave, I.; Lebourgeois, F. Growth partitioning in forest stands is affected by stand density and summer drought in sessile oak and Douglas-fir. For. Ecol. Manag. 2014, 334, 358–368. [Google Scholar] [CrossRef]
- Bourdier, T.; Cordonnier, T.; Kunstler, G.; Piedallu, C.; Lagarrigues, G.; Courbaud, B. Tree size inequality reduces forest productivity: An analysis combining inventory data for ten european species and a light competition model. PLoS ONE 2016, 11, e0151852. [Google Scholar] [CrossRef]
- Del Río, M.; Condés, S.; Pretzsch, H. Analyzing size-symmetric vs. size-asymmetric and intra- vs. inter-specific competition in beech (Fagus sylvatica L.) mixed stands. For. Ecol. Manag. 2014, 325, 90–98. [Google Scholar] [CrossRef]
- Giuggiola, A.; Ogee, J.; Rigling, A.; Gessler, A.; Bugmann, H.; Treydte, K. Improvement of water and light availability after thinning at a xeric site: Which matters more? A dual isotope approach. New Phytol. 2016, 210, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, T.; Moon, M.; Cho, S.; Ryu, D.; Seok Kim, H. Effects of thinning intensities on tree water use, growth, and resultant water use efficiency of 50-year-old Pinus koraiensis forest over four years. For. Ecol. Manag. 2018, 408, 121–128. [Google Scholar] [CrossRef]
- Keyser, T.L. Patterns of growth dominance in thinned yellow-poplar stands in the southern Appalachian Mountains, USA. Can. J. For. Res. 2012, 42, 406–412. [Google Scholar] [CrossRef]
- Liang, R.T.; Xie, Y.H.; Sun, Y.Y.; Wang, B.Y.; Ding, Z.D. Temporal changes in size inequality and stand growth partitioning between tree sizes under various thinning intensities in subtropical Cunninghamia lanceolata plantations. For. Ecol. Manag. 2023, 547, 121363. [Google Scholar] [CrossRef]
- Looney, C.E.; Zhang, J.W. Site quality and intensive early stand management practices affect growth dominance, structural complexity, and tree growth in ponderosa pine plantations. For. Ecol. Manag. 2022, 519, 120318. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q.; Wang, D.Z.; Zhang, Z.D. Soil microbial community, soil quality, and productivity along a chronosequence of Larix principis-rupprechtii forests. Plants 2023, 12, 2913. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; del Río, M.; Grote, R.; Klemmt, H.-J.; Ordóñez, C.; Oviedo, F.B. Tracing drought effects from the tree to the stand growth in temperate and Mediterranean forests: Insights and consequences for forest ecology and management. Eur. J. For. Res. 2022, 141, 727–751. [Google Scholar] [CrossRef]
- Au, T.F.; Maxwell, J.T.; Robeson, S.M.; Li, J.B.; Siani, S.M.O.; Novick, K.A.; Dannenberg, M.P.; Phillips, R.P.; Li, T.; Chen, Z.J.; et al. Younger trees in the upper canopy are more sensitive but also more resilient to drought. Nat. Clim. Chang. 2022, 12, 1168–1174. [Google Scholar] [CrossRef]
- Li, W.B.; Lv, Z.G.; Zhang, Z.D.; Huang, X.R. Predicting productivity and spatial distribution of Larix principis-rupprechtii plantation. J. Nat. Resour. 2019, 34, 1365–1375. [Google Scholar] [CrossRef]
- He, H. Measurement of Tree-Ring Width with WinDENDRO and Crossdating Methods. J. Chongqing Norm. Univ. Nat. Sci. Ed. 2005, 22, 6. [Google Scholar] [CrossRef]
- Grissino-Mayer, H.D. Evaluating Crossdating Accuracy: A Manual and Tutorial for the Computer Program COFECHA. Tree-Ring Res. 2001, 57, 205–221. [Google Scholar]
- Wang, T.L.; Wang, G.Y.; Innes, J.L.; Seely, B.; Chen, B.Z. ClimateAP: An application for dynamic local downscaling of historical and future climate data in Asia Pacific. Front. Agric. Sci. Eng. 2017, 4, 448–458. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Manzanedo, R.D.; D’Orangeville, L.; Lv, P.; Wang, C.; Xu, C.; Hou, M.; Huang, X.; Rademacher, T. High risk of growth cessation of planted larch under extreme drought. Environ. Res. Lett. 2021, 16, 014040. [Google Scholar] [CrossRef]
- Lv, Z.G.; Li, W.B.; Huang, X.R.; Zhang, Z.D. Predicting suitable distribution area of three dominant tree species under climate change scenarios in Hebei Province. Sci. Silvae. Sin. 2019, 55, 13–21. [Google Scholar] [CrossRef]
- Lan, J.; Lei, X.D.; He, X.; Gao, W.Q.; Li, Y.T. Multi-functionality of natural mixed broad-leaved forests and driving forces in Jilin Province. Acta Ecol. Sin. 2021, 41, 5128–5141. [Google Scholar] [CrossRef]
- Weiner, J.; Solbrig, O.T. The meaning and measurement of size hierarchies in plant populations. Oecologia 1984, 61, 334–336. [Google Scholar] [CrossRef]
- Cordonnier, T.; Kunstler, G. The Gini index brings asymmetric competition to light. Perspect. Plant Ecol. Evol. Syst. 2015, 17, 107–115. [Google Scholar] [CrossRef]
- Dong, X.T.; Zhang, J.; Zhang, Z.D.; Huang, X.R. Effects of tree species interaction, stand density, and tree size on the productivity of Larix principis-rupprechtii. Chin. J. Appl. Ecol. 2022, 32, 2722–2728. [Google Scholar] [CrossRef]
- Metsaranta, J.M.; Lieffers, V.J. Patterns of inter-annual variation in the size asymmetry of growth in Pinus banksiana. Oecologia 2010, 163, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, J.; Ishii, H.; Vetrova, V.; Sumida, A.; Hara, T. Tree growth and competition in a Betula platyphylla-Larix cajanderi Post-fire Forest in Central Kamchatka. Ann. Bot. 2004, 94, 333–343. [Google Scholar] [CrossRef] [PubMed]
- West, P.W. Calculation of a growth dominance statistic for forest stands. For. Sci. 2014, 60, 1021–1023. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org (accessed on 1 October 2022).
- Yang, G.J.; Hu, H.F.; Sun, H.G.; Zhang, J.G.; Duan, A.G. The influences of stand age, planting density and self-thinning on relationship between size inequality and periodic annual increment in chinese Fir (Cunninghamia lanceolata) plantations. Sci. Silvae. Sin. 2019, 55, 126–136. [Google Scholar] [CrossRef]
- Vorster, A.G.; Evangelista, P.H.; Stovall, A.E.L.; Ex, S. Variability and uncertainty in forest biomass estimates from the tree to landscape scale: The role of allometric equations. Carbon Balance Manag. 2020, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Boyden, S.; Binkley, D.; Stape, J.L. Competition among Eucalyptus trees depends on genetic variation and resource supply. Ecology 2008, 89, 2850–2859. [Google Scholar] [CrossRef]
- Looney, C.E.; D’Amato, A.W.; Palik, B.J.; Fraver, S.; Kastendick, D.N. Size-growth relationship, tree spatial patterns, and tree-tree competition influence tree growth and stand complexity in a 160-year red pine chronosequence. For. Ecol. Manag. 2018, 424, 85–94. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.Y.H. Individual size inequality links forest diversity and above-ground biomass. J. Ecol. 2015, 103, 1245–1252. [Google Scholar] [CrossRef]
- Zhao, D.H.; Bullock, B.P.; Montes, C.R.; Wang, M.L. Production, tree size inequality and growth dominance in loblolly pine plantations under different silvicultural management regimes. For. Ecol. Manag. 2022, 526, 120594. [Google Scholar] [CrossRef]
- Soares, A.A.V.; Leite, H.G.; Souza, A.L.; Silva, S.R.; Lourenço, H.M.; Forrester, D.I. Increasing stand structural heterogeneity reduces productivity in Brazilian Eucalyptus monoclonal stands. For. Ecol. Manag. 2016, 373, 26–32. [Google Scholar] [CrossRef]
- Fronhofer, E.A.; Govaert, L.; O’Connor, M.I.; Schreiber, S.J.; Altermatt, F. The shape of density dependence and the relationship between population growth, intraspecific competition and equilibrium population density. Oikos 2024, 2024, e09824. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.M.; Cheng, R.M.; Ge, Z.X.; Zhang, Z.D. Effects of neighborhood competition and stand structure on the productivity of pure and mixed Larix principis-rupprechtii forests. Forests 2022, 13, 1318. [Google Scholar] [CrossRef]
- Del Río, M.; Bravo-Oviedo, A.; Ruiz-Peinado, R.; Condés, S. Tree allometry variation in response to intra- and inter-specific competitions. Trees 2019, 33, 121–138. [Google Scholar] [CrossRef]
- Geng, Y.; Yue, Q.; Zhang, C.; Zhao, X.; von Gadow, K. Dynamics and drivers of aboveground biomass accumulation during recovery from selective harvesting in an uneven-aged forest. Eur. J. For. Res. 2021, 140, 1163–1178. [Google Scholar] [CrossRef]
- Baret, M.; Pepin, S.; Ward, C.; Pothier, D. Long-term changes in stand growth dominance as related to resource acquisition and utilization in the boreal forest. For. Ecol. Manag. 2017, 400, 408–416. [Google Scholar] [CrossRef]
- Binkley, D.; Kashian, D.M. Tree-Level patterns of lodgepole pine growth and leaf area in yellowstone national park: Explaining anomalous patterns of growth dominance within stands. Ecosystems 2015, 18, 251–259. [Google Scholar] [CrossRef]
- Pretzsch, H.; Bravo-Oviedo, A.; Hilmers, T.; Ruiz-Peinado, R.; Coll, L.; Löf, M.; Ahmed, S.; Aldea, J.; Ammer, C.; Avdagić, A.; et al. With increasing site quality asymmetric competition and mortality reduces Scots pine (Pinus sylvestris L.) stand structuring across Europe. For. Ecol. Manag. 2022, 520, 120365. [Google Scholar] [CrossRef]
- Britwum Acquah, S.; Marshall, P.L.; Eskelson, B.N.I.; Moss, I.; Barbeito, I. Changes in size inequality and growth dominance in thinned and unthinned uneven-aged interior Douglas-fir dominated stands. For. Ecol. Manag. 2022, 524, 120517. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, H.; Dean, T.J.; Zhang, J.; Zhang, X. Growth dominance and growth efficiency in response to thinning treatments in Chinese fir plantations with long-term spacing trials. For. Ecol. Manag. 2022, 521, 120438. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G.; Biber, P. Drought can favour the growth of small in relation to tall trees in mature stands of Norway spruce and European beech. For. Ecosyst. 2018, 5, 20. [Google Scholar] [CrossRef]
- Harvey, J.E.; Smiljanić, M.; Scharnweber, T.; Buras, A.; Cedro, A.; Cruz-García, R.; Drobyshev, I.; Janecka, K.; Jansons, Ā.; Kaczka, R.; et al. Tree growth influenced by warming winter climate and summer moisture availability in northern temperate forests. Glob. Chang. Biol. 2020, 26, 2505–2518. [Google Scholar] [CrossRef] [PubMed]
- McGregor, I.R.; Helcoski, R.; Kunert, N.; Tepley, A.J.; Gonzalez-Akre, E.B.; Herrmann, V.; Zailaa, J.; Stovall, A.E.L.; Bourg, N.A.; McShea, W.J.; et al. Tree height and leaf drought tolerance traits shape growth responses across droughts in a temperate broadleaf forest. New Phytol. 2021, 231, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Gleason, K.E.; Bradford, J.B.; D’Amato, A.W.; Fraver, S.; Palik, B.J.; Battaglia, M.A. Forest density intensifies the importance of snowpack to growth in water-limited pine forests. Ecol. Appl. 2021, 31, e02211. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, T.; Häberle, K.-H.; Matyssek, R.; Schulz, C.; Ammer, C. The more, the better? Water relations of Norway spruce stands after progressive thinning. Agric. For. Meteorol. 2014, 197, 235–243. [Google Scholar] [CrossRef]
- Schmied, G.; Pretzsch, H.; Ambs, D.; Uhl, E.; Schmucker, J.; Fäth, J.; Biber, P.; Hoffmann, Y.-D.; Šeho, M.; Mellert, K.H.; et al. Rapid beech decline under recurrent drought stress: Individual neighborhood structure and soil properties matter. For. Ecol. Manag. 2023, 545, 121305. [Google Scholar] [CrossRef]
- Bose, A.K.; Rohner, B.; Bottero, A.; Ferretti, M.; Forrester, D.I. Did the 2018 megadrought change the partitioning of growth between tree sizes and species? A Swiss case-study. Plant Biol. 2022, 24, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Zang, C.; Pretzsch, H.; Rothe, A. Size-dependent responses to summer drought in Scots pine, Norway spruce and common oak. Trees 2012, 26, 557–569. [Google Scholar] [CrossRef]
| Stand Age (Year) | Density (Trees·ha−1) | Number of Plots | Mean DBH (Min–Max) (cm) | Mean Height (Min–Max) (m) | Mean BAI (cm2·year−1) | Crown Width (m) |
|---|---|---|---|---|---|---|
| 17–19 | 2270 | 13 | 11.82 (9.21–14.54) | 10.86 (6.47–16.08) | 7.63 | 0.7–1.9 |
| 20–29 | 2049 | 13 | 12.58 (9.07–18.66) | 10.66 (7.38–19.85) | 8.48 | 0.9–2.6 |
| 30–39 | 1034 | 23 | 20.51 (15.88–26.38) | 15.49 (7.89–23.06) | 10.32 | 0.9–3.0 |
| 40–46 | 785 | 6 | 23.93 (21.77–26.49) | 20.47 (16.67–24.20) | 8.99 | 1.0–3.3 |
| Response | Hypothesis | Response Varies as a Function of: |
|---|---|---|
| SGR | SGR0 | Null model (P) |
| SGR | SGR1 | Null model, Age |
| SGR | SGR2 | Null model, Age, P × Age |
| SGR | SGR3 | Null model, AHM |
| SGR | SGR4 | Null model, Age, AHM |
| SGR | SGR5 | Null model, Age, P × Age, AHM |
| Model | P | Age | AHM | R2 | AIC | BIC | MAE |
|---|---|---|---|---|---|---|---|
| SGR0 | −0.003 | 0.40 | 70.83 | 82.87 | 0.23 | ||
| SGR4 | 0.300 | 0.328 | 0.558 * | 0.47 | 69.52 | 79.56 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chen, D.; Yang, X.; Fan, N.; Wang, Y.; Zhang, Z. Effects of Stand Density, Age, and Drought on the Size–Growth Relationship in Larix principis-rupprechtii Forests. Forests 2024, 15, 413. https://doi.org/10.3390/f15030413
Li J, Chen D, Yang X, Fan N, Wang Y, Zhang Z. Effects of Stand Density, Age, and Drought on the Size–Growth Relationship in Larix principis-rupprechtii Forests. Forests. 2024; 15(3):413. https://doi.org/10.3390/f15030413
Chicago/Turabian StyleLi, Jiajing, Dongsheng Chen, Xin Yang, Niqiao Fan, Yiwen Wang, and Zhidong Zhang. 2024. "Effects of Stand Density, Age, and Drought on the Size–Growth Relationship in Larix principis-rupprechtii Forests" Forests 15, no. 3: 413. https://doi.org/10.3390/f15030413
APA StyleLi, J., Chen, D., Yang, X., Fan, N., Wang, Y., & Zhang, Z. (2024). Effects of Stand Density, Age, and Drought on the Size–Growth Relationship in Larix principis-rupprechtii Forests. Forests, 15(3), 413. https://doi.org/10.3390/f15030413






