The phoD-Harboring Microorganism Communities and Networks in Karst and Non-Karst Forests in Southwest China
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Field Sampling
2.3. General Soil Parameters
2.4. DNA Extraction and Illumina Sequencing
2.5. Analysis of Illumina Sequencing Data
2.6. Statistical Analysis
3. Results
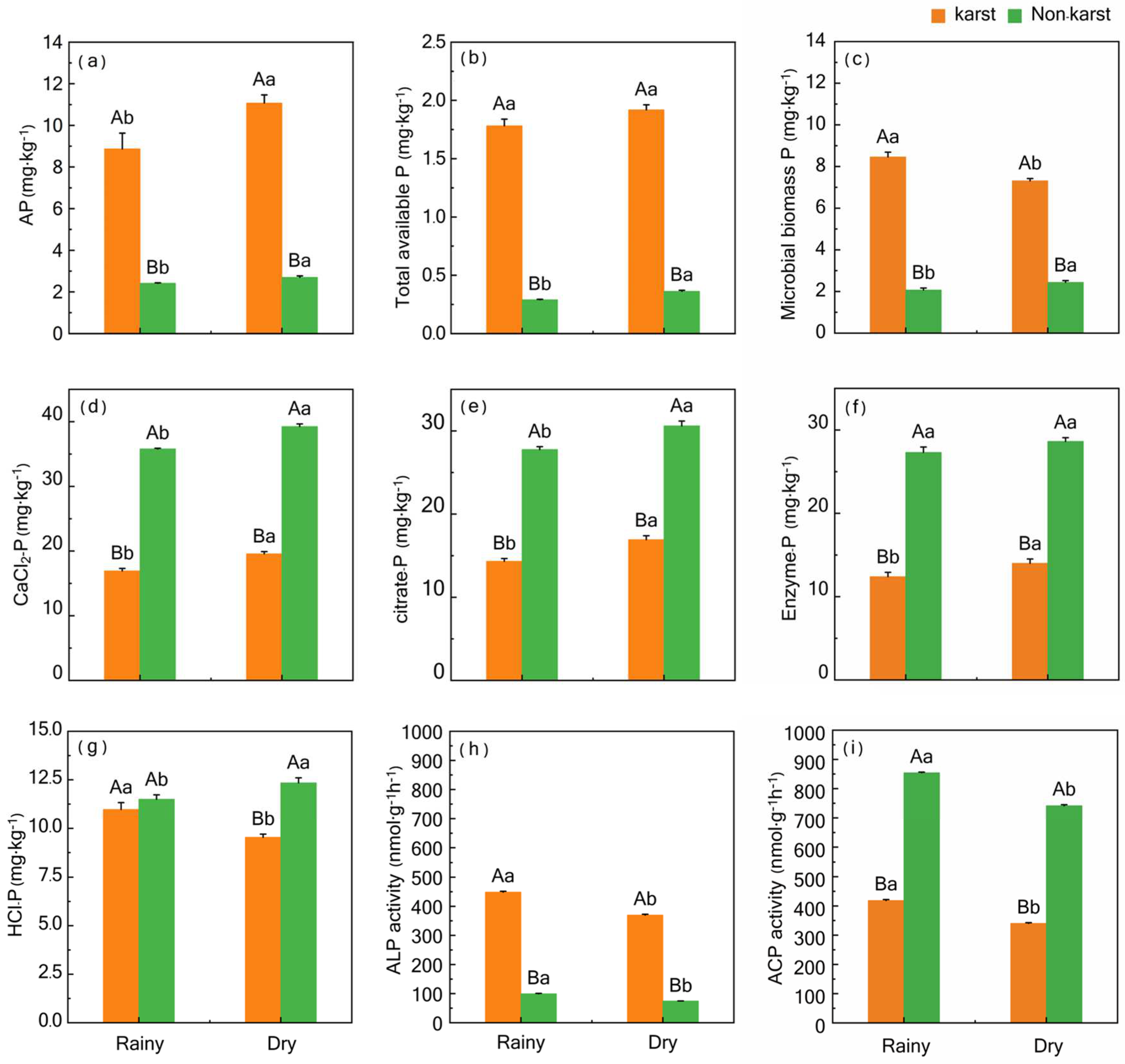
3.1. Soil P Fractions and Phosphatase Activity
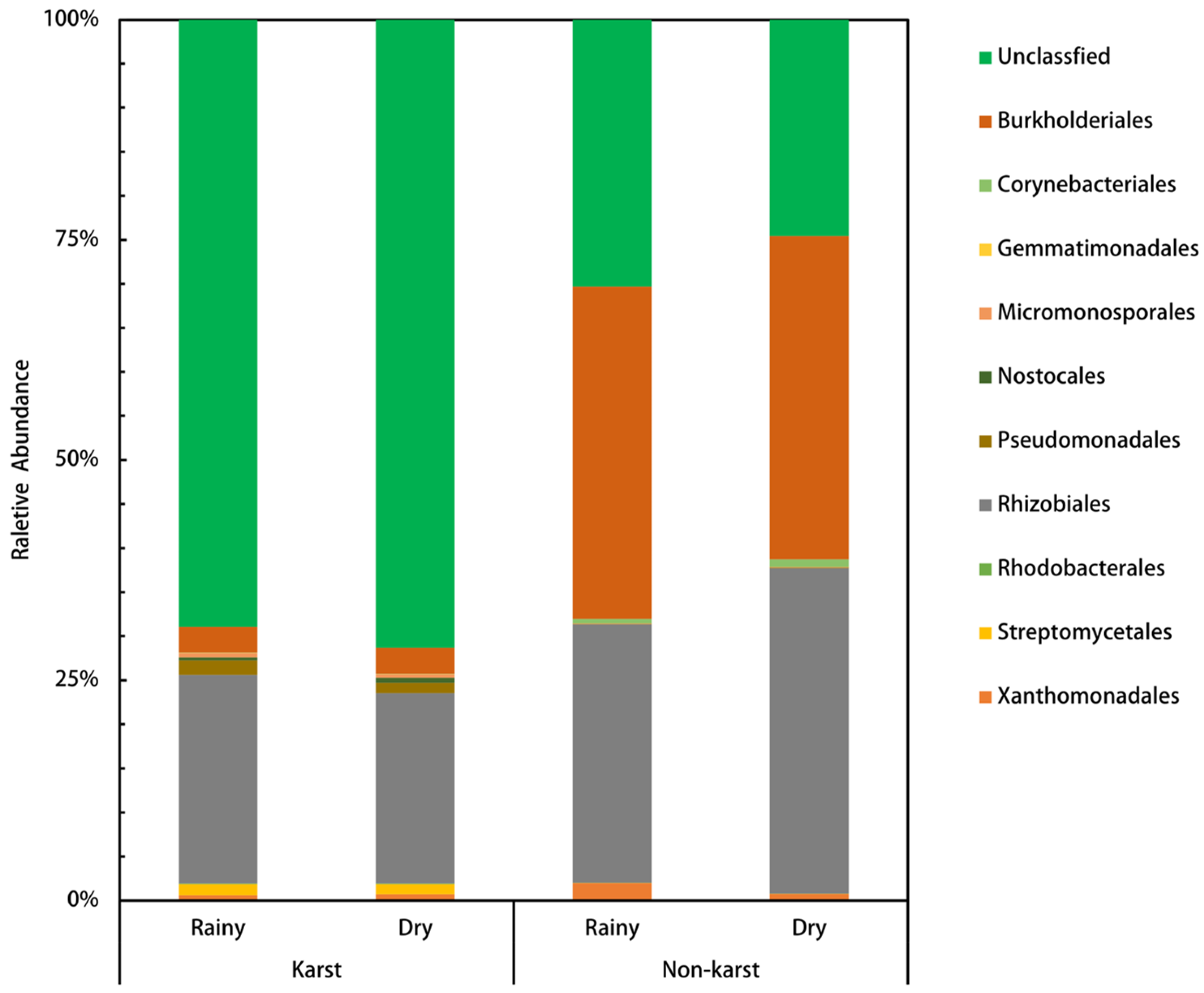
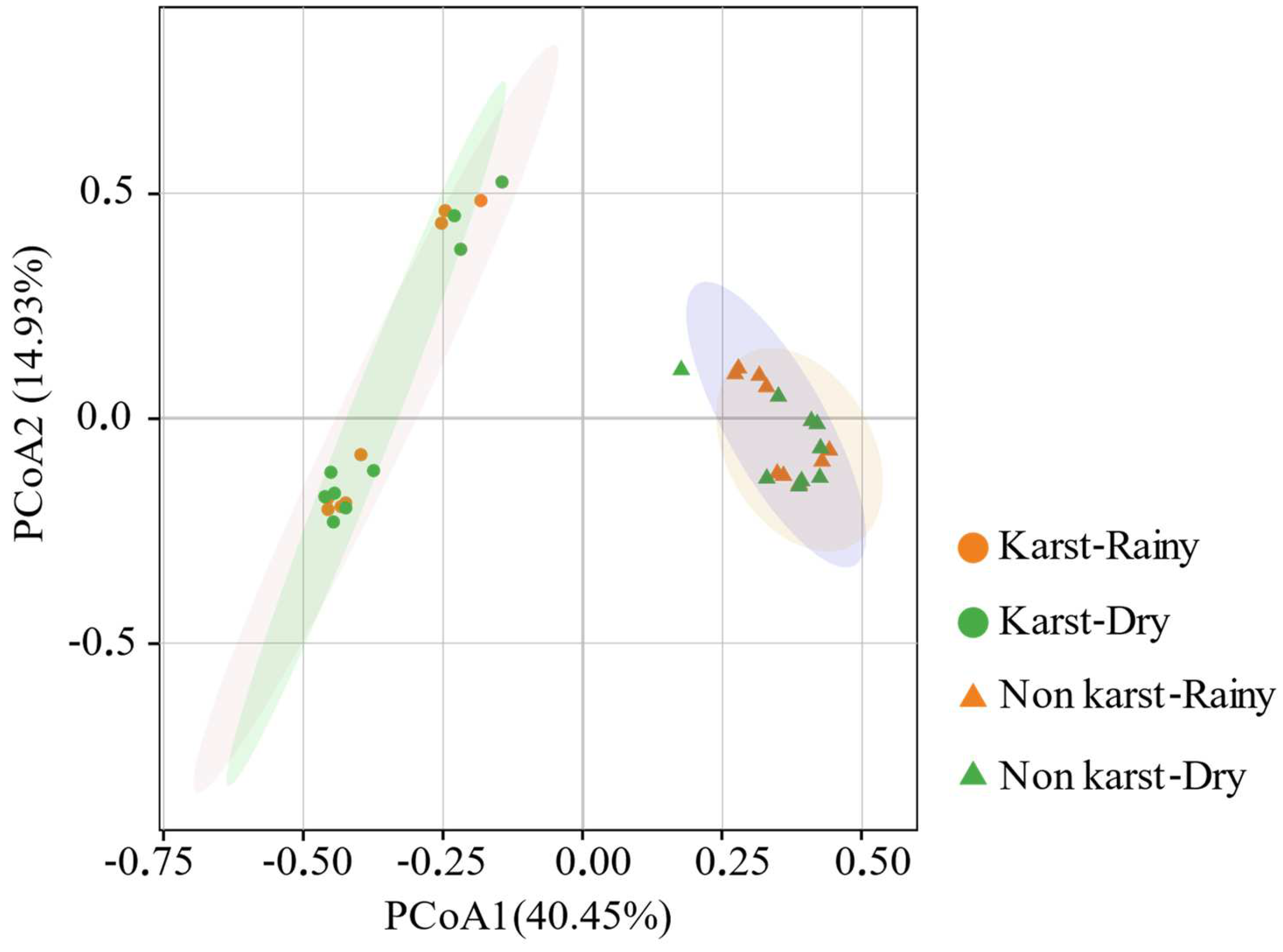
3.2. Diversity and Community Structure of phoD-Harboring Microorganisms
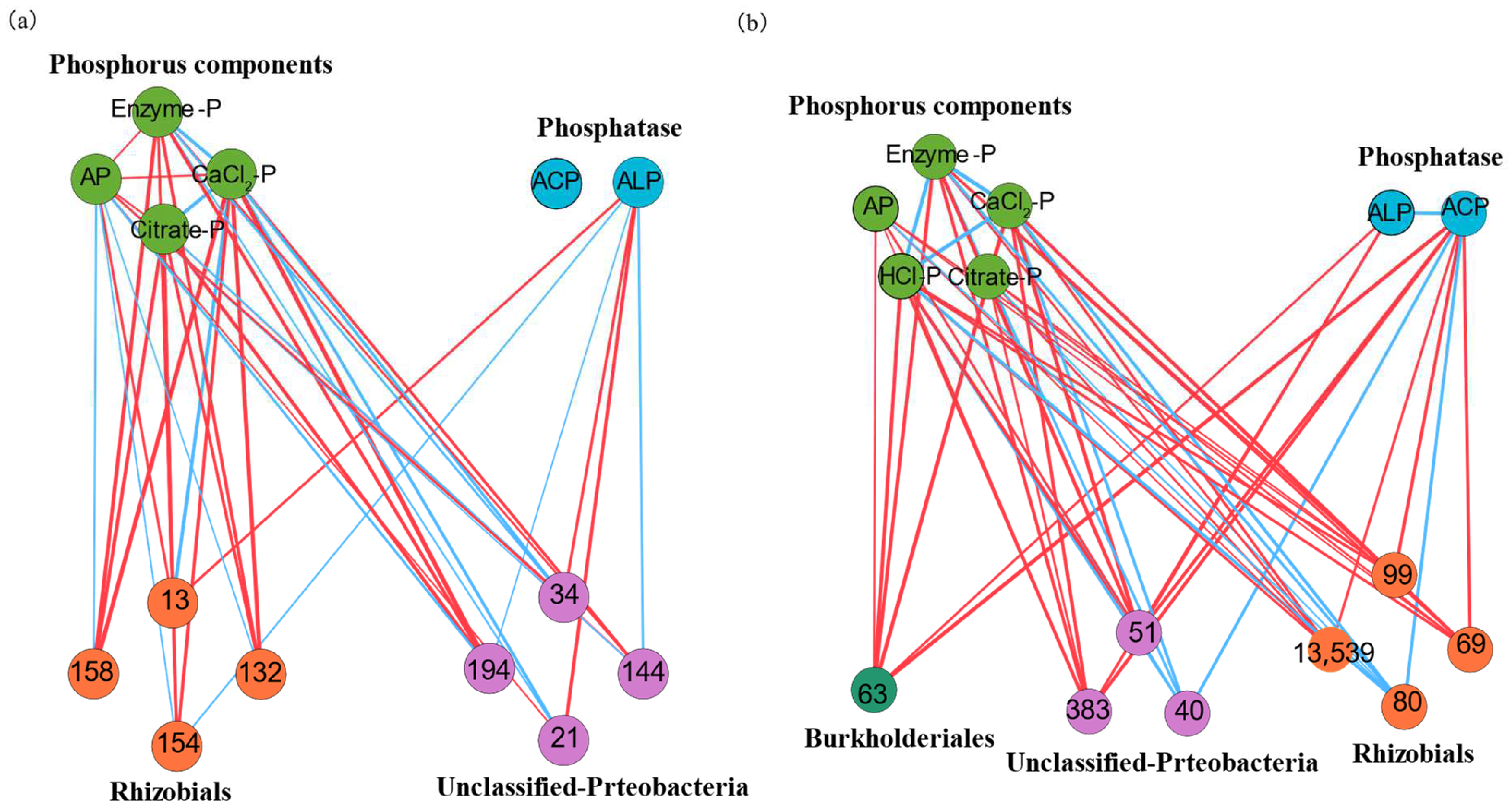
3.3. Network Connectivity of phoD-Harboring Microorganisms
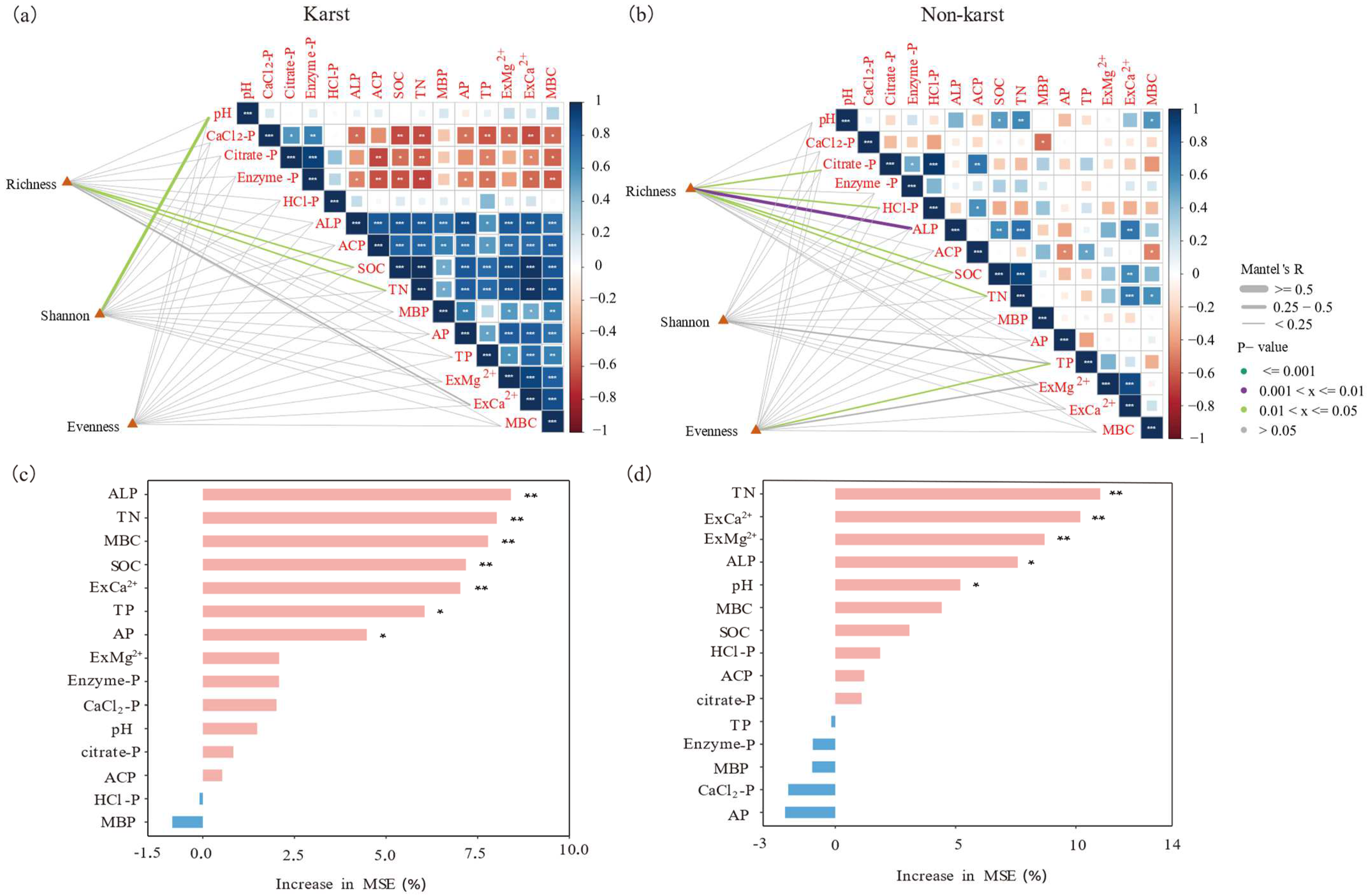
3.4. Correlation between Diversity and Structure of phoD-Harboring Microorganisms and Soil Physicochemical Parameters
4. Discussion
4.1. Difference Patterns of Soil P Fractions between Karst and Non-Karst Forest Soils
4.2. Communities and Networks of phoD-Harboring Microorganisms in Non-Karst and Karst Forest Soils
4.3. Diversities of phoD-Harboring Microorganisms in Non-Karst and Karst Forest Soils
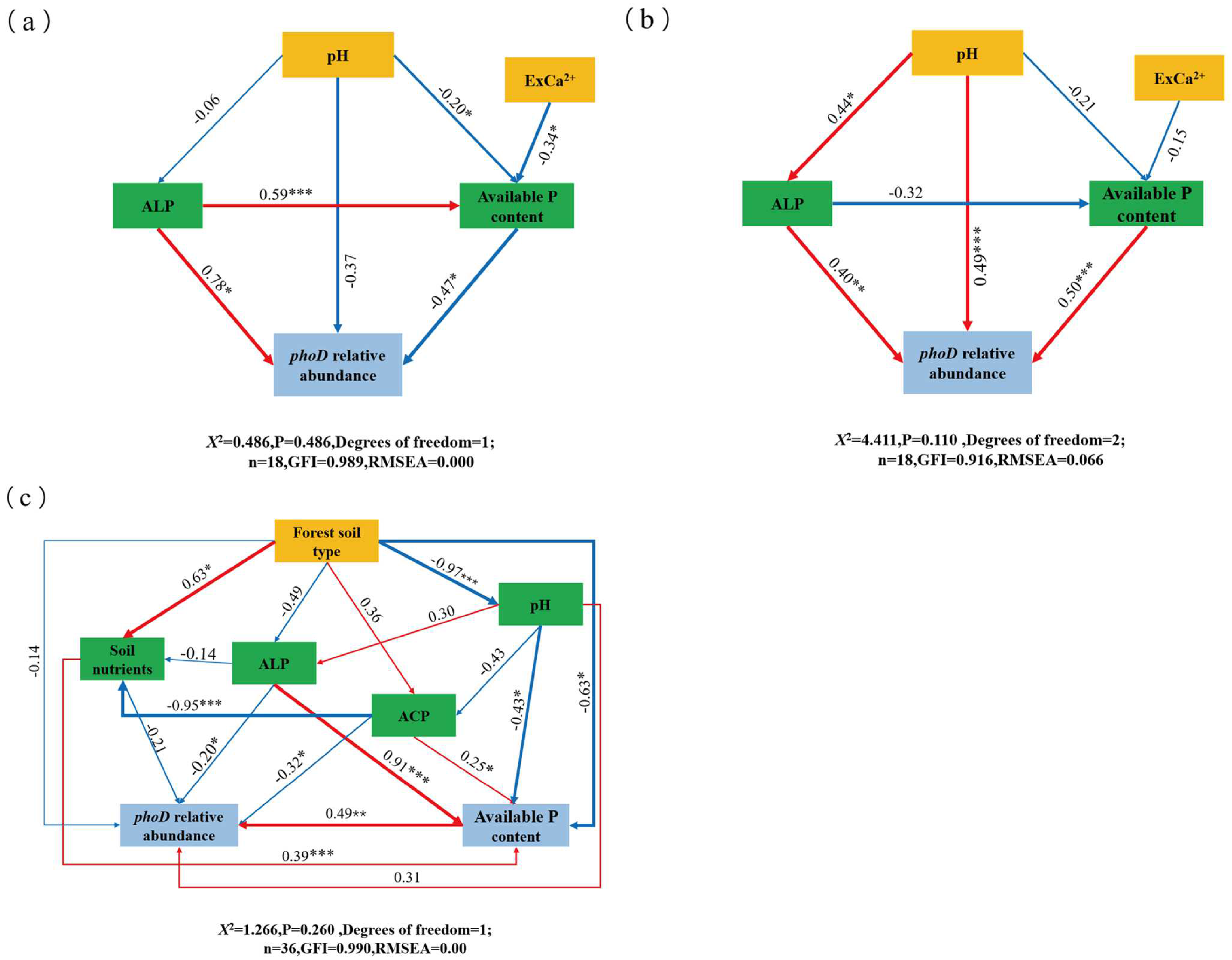
4.4. Factors Affecting the Diversity and Structure of phoD-Harboring Microorganisms and Their Implications for Future Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Souza Kulmann, M.S.; Aguilar, M.V.M.; Tassinari, A.; Schwalbert, R.; Tabaldi, L.A.; Araujo, M.M.; Antoniolli, Z.I.; Nicoloso, F.T.; Brunetto, G.; Schumacher, M.V. Effects of increasing soil phosphorus and association with ectomycorrhizal fungi (Pisolithus microcarpus) on morphological, nutritional, biochemical, and physiological parameters of Pinus taeda L. For. Ecol. Manag. 2023, 544, 121207. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Q.; Wei, L.M.; Sun, Q.Y.; Zeng, D.H. Tree roots exert greater impacts on phosphorus fractions than aboveground litter in mineral soils under a Pinus sylvestris var. mongolica plantation. For. Ecol. Manag. 2023, 545, 121242. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Yan, Y.P.; Wang, X.M.; Liu, F.; Feng, X.H. Progress in Researches on Interactions between Organic Phosphates and Soil Minerals and Their Environmental Impacts. Acta Pedol. Sin. 2019, 56, 1290–1299. [Google Scholar]
- Wu, H.L.; Xiang, W.H.; Chen, L.; Ouyang, S.; Xiao, W.F.; Li, S.G.; Forrester, D.I.; Lei, P.F.; Zeng, Y.L.; Deng, X.W.; et al. Soil Phosphorus Bioavailability and Recycling Increased with Stand Age in Chinese Fir Plantations. Ecosystems 2020, 23, 973–988. [Google Scholar] [CrossRef]
- Wang, L.J.; Lu, J.W.; Xu, F.S.; Zhang, F.S. Dynamics of crystallization and dissolution of calcium orthophosphates at the near-molecular level. Chin. Sci. Bull. 2010, 55, 2794–2802. [Google Scholar] [CrossRef]
- Ruttenberg, K.C. The global phosphorus cycle. Treatise Geochem. 2003, 8, 585–643. [Google Scholar]
- Fang, X.M.; Zhang, X.L.; Zong, Y.Y.; Zhang, Y.; Wan, S.Z.; Bu, W.S.; Chen, F.S. Soil phosphorus functional fractions and tree tissue nutrient concentrations influenced by stand density in subtropical Chinese fir plantation forests. PLoS ONE 2017, 12, e0186905. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.G.; Wu, X.N.; Duan, C.Q.; Zhao, L.Q.; Li, B. Different life-form plants exert different rhizosphere effects on phosphorus biogeochemistry in subtropical mountainous soils with low and high phosphorus content. Soil Tillage Res. 2020, 199, 104516. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action; Bünemann, E., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 215–243. [Google Scholar]
- Liang, Y.M.; Li, M.J.; Pan, F.J.; Ma, J.; Yang, Z.Q.; Ling, T.W.; Qin, J.S.; Lu, S.H.; Zhong, F.Y.; Song, Z.R. Alkaline Phosphomonoesterase-Harboring Microorganisms Mediate Soil Phosphorus Transformation with Stand Age in Chinese Pinus massoniana Plantations. Front. Microbiol. 2020, 11, 571209. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Li, H.M.; Lakshmanan, P.; Chen, Y.X.; Chen, X.P. phoD-harboring bacterial community composition dominates organic P mineralization under long-term P fertilization in acid purple soil. Front. Microbiol. 2022, 13, 1045919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Gu, Y.F.; Chen, Y.L.; Wang, Y.; Luo, L.; Liang, J.P.; Chen, Q. Effects of Crop Rotation Systems on Soil phoD Gene Diversity and Community Structure in Panxi Plateau, China. Chin. J. Soil Sci. 2023, 54, 913–919. [Google Scholar]
- Ikpyi, I.; Fowler, A.; Schmalenberger, A. One-time phosphate fertilizer application to grassland columns modifies the soil microbiota and limits its role in ecosystem services. Sci. Total Environ. 2018, 630, 849–858. [Google Scholar]
- Ragot, S.A.; Kertesz, M.A.; Buenemann, E.K. phoD Alkaline Phosphatase Gene Diversity in Soil. Appl. Environ. Microbiol. 2015, 81, 7281–7289. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.Z.; Li, C.N.; Kou, Y.P.; Li, J.B.; Yao, M.J.; Zhang, B.C.; Wang, L.X.; Xu, H.W.; You, C.M.; et al. Disentangling the relative importance of precipitation, biocrust succession, and shrub cover in mediating soil phoD-harbouring communities and organic phosphorus mineralisation. Soil Biol. Biochem. 2023, 186, 109165. [Google Scholar] [CrossRef]
- Zheng, C.H.; Wan, L.; Wang, R.S.; Wang, G.; Dong, L.; Yang, T.; Yang, Q.L.; Zhou, J.X. Effects of rock lithology and soil nutrients on nitrogen and phosphorus mobility in trees in non-karst and karst forests of southwest China. For. Ecol. Manag. 2023, 548, 121392. [Google Scholar] [CrossRef]
- Ahmed, Y.A.R.; Pichler, V.; Homolak, M.; Goemoeryova, E.; Nagy, D.; Pichlerova, M.; Gregor, J. High organic carbon stock in a karstic soil of the Middle-European Forest Province persists after centuries-long agroforestry management. Eur. J. For. Res. 2012, 131, 1669–1680. [Google Scholar] [CrossRef]
- He, X.J.; Augusto, L.; Goll, D.S.; Ringeval, B.; Wang, Y.P.; Helfenstein, J.; Huang, Y.Y.; Yu, K.L.; Wang, Z.Q.; Yang, Y.C.; et al. Global patterns and drivers of soil total phosphorus concentration. Earth Syst. Sci. Data 2021, 13, 5831–5846. [Google Scholar] [CrossRef]
- Li, M.; You, Y.M.; Tan, X.M.; Wen, Y.G.; Yu, S.Z.; Xiao, N.; Shen, W.J.; Huang, X.M. Mixture of N2-fixing tree species promotes organic phosphorus accumulation and transformation in topsoil aggregates in a degraded karst region of subtropical China. Geoderma 2022, 413, 115752. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.J.; Xiao, K.C.; Wang, K.L. Soil microbial processes and resource limitation in karst and non karst forests. Funct. Ecol. 2018, 32, 1400–1409. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Pan, F.J.; Li, D.J.; Chen, H.S.; Wang, K.L. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil. 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community composition at the continental scale. Appl. Environ. Microb. 2009, 75, 5111–5120. [Google Scholar] [CrossRef]
- Wan, W.J.; Tan, T.; Wang, J.D.; Qin, Y.; He, H.G.; Wu, H.Q.; Zuo, E.L.; He, D.G. Responses of the rhizosphere bacterial community in acidic crop soil to pH: Changes in diversity, composition, interaction, and function. Sci. Total Environ. 2020, 700, 134418. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The ecology of the microbiome: Networks, competition, and stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Karimi, B.; Maron, P.A.; Boure, N.C.P.; Bernard, N.; Gilbert, D.; Ranjard, L. Microbial diversity and ecological networks as indicators of environmental quality. Environ. Chem. Lett. 2017, 15, 265–281. [Google Scholar] [CrossRef]
- Wei, X.M.; Hu, Y.J.; Cai, G.; Yao, H.Y.; Ye, J.; Sun, Q.; Veresoglou, S.D.; Li, Y.Y.; Zhu, Z.K.; Guggenberger, G.; et al. Organic phosphorus availability shapes the diversity of phoD-harboring bacteria in agricultural soil. Soil Biol. Biochem. 2021, 161, 108364. [Google Scholar] [CrossRef]
- Hu, M.J.; Yan, R.B.; Wu, H.; Ni, R.X.; Zhang, D.Q.; Zou, S.Q. Linking soil phosphorus availability and phosphatase functional genes to coastal marsh erosion: Implications for nutrient cycling and wetland restoration. Sci. Total Environ. 2023, 898, 165559. [Google Scholar] [CrossRef] [PubMed]
- Morrien, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; De Hollander, M.; Soto, R.L.; Bouffaud, M.L.; Buee, M.; Dimmers, W. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef]
- Pan, Y.Q.; Kang, P.; Hu, J.P.; Song, N.P. Bacterial community demonstrates stronger network connectivity than fungal community in desert-grassland salt marsh. Sci. Total Environ. 2021, 798, 149118. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.B.; Li, S.F.; Tang, R.; Su, J.R. Microbial network complexity and diversity together drive the soil ecosystem multifunctionality of forests during different woodland use intensity in dry and wet season. For. Ecol. Manag. 2023, 542, 121086. [Google Scholar] [CrossRef]
- Wang, S.; Fu, Z.Y.; Chen, H.S.; Nie, Y.P.; Xu, Q.X. Mechanisms of surface and subsurface runoff generation in subtropical soil-epikarst systems: Implications of rainfall simulation experiments on karst slope. J. Hydrol. 2020, 580, 124370. [Google Scholar] [CrossRef]
- Sun, M.M.; Yang, R.; Tang, Y.X.; Xiao, D.; Zhang, W.; Xu, Z.H.; Shi, Z.H.; Hu, P.L.; Wu, H.Q.; Wang, K.L. Lithologic control of soil C:N:P stoichiometry across a climatic gradient in southwest China. J. Soils Sediments 2023, 23, 1662–1673. [Google Scholar] [CrossRef]
- Pan, F.J.; Liang, Y.M.; Zhang, W.; Zhao, J.; Wang, K.L. Enhanced Nitrogen Availability in Karst Ecosystems by Oxalic Acid Release in the Rhizosphere. Front. Plant Sci. 2016, 7, 687. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.J.; Zhang, W.; Liang, Y.M.; Wang, K.L.; Jin, Z.J. Seasonal changes of soil organic acid concentrations in relation to available N and P at different stages of vegetation restoration in a karst ecosystem. Chin. J. Ecol. 2020, 39, 1112–1120. [Google Scholar]
- Qin, J.S.; Wang, Y.Q.; Ma, J.M.; Ou, J.; Lan, C.Z.; Yang, Z.Q. Composition and Diversity of Woody Plants of Pinus massoniana Plantations under Different Climatic Conditions in Guangxi. Guangxi Sci. 2020, 27, 154–164. [Google Scholar]
- Olsen, S.R. (Ed.) Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Circular/United States Department of Agriculture: Washington, DC, USA, 1954.
- Wu, J.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of soil microbial biomass C by fumigation-extraction—An automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Glanville, H.C.; Harris, M.; Emmett, B.A.; Pingree, M.R.A.; de Sosa, L.L.; Cerda-Moreno, C.; Jones, D.L. A novel biologically-based approach to evaluating soil phosphorus availability across complex landscapes. Soil Biol. Biochem. 2015, 88, 110–119. [Google Scholar] [CrossRef]
- Finzi, A.C.; Sinsabaugh, R.L.; Long, T.M.; Osgood, M.P. Microbial Community Responses to Atmospheric Carbon Dioxide Enrichment in a Warm-Temperate Forest. Ecosystems 2006, 9, 215–226. [Google Scholar] [CrossRef]
- Sakurai, M.; Wasaki, J.; Shinano, T.; Osaki, M. Analysis of bacterial communities on alkaline phosphatase genes in soil supplied with organic matter. Soil Sci. Plant Nutr. 2008, 54, 62–71. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Wei, X.M.; Hu, Y.J.; Razavi, B.S.; Zhou, J.; Shen, J.L.; Nannipieri, P.; Wu, J.S.; Ge, T. Rare taxa of alkaline phosphomonoesterase-harboring microorganisms mediate soil phosphorus mineralization. Soil Biol. Biochem. 2019, 131, 62–70. [Google Scholar] [CrossRef]
- Fish, J.A.; Chai, B.L.; Wang, Q.; Sun, Y.N.; Brown, C.T.; Tiedje, J.M.; Cole, J.R. FunGene: The functional gene pipeline and repository. Front. Microbiol. 2013, 4, 291. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.C.; Zhang, Z.H.; Liu, G.H.; Lv, M.; Feng, Y.J. Enhancing methane production of synthetic brewery water with granular activated carbon modified with nanoscale zero-valent iron (NZVI) in anaerobic system. Sci. Total Environ. 2021, 760, 143933. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.; Coughlan, J.; Mullen, M. Structural Equation Modelling: Guidelines for Determining Model Fit; University Press of America: New York, NY, USA, 2008; Volume 6, pp. 53–60. [Google Scholar]
- Wu, Y.J.; Tian, X.; Zhang, M.Y.; Wang, R.Z.; Wang, S. A Case Study of Initial Vegetation Restoration Affecting the Occurrence Characteristics of Phosphorus in Karst Geomorphology in Southwest China. Sustainability 2022, 14, 12277. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Ladd, J.N. Microbial Biomass in Soil: Measurement and Turnover. Soil Biochem. 1981, 5, 415–471. [Google Scholar]
- Janes-Bassett, V.; Blackwell, M.S.A.; Blair, G.; Davies, J.; Haygarth, P.M.; Mezeli, M.M.; Stewart, G. A meta-analysis of phosphatase activity in agricultural settings in response to phosphorus deficiency. Soil Biol. Biochem. 2022, 165, 108537. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A Critical Review on Soil Chemical Processes that Control How Soil pH Affects Phosphorus Availability to Plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Pardo, M.T.; Guadalix, M.E.; Garcia-Gonzalez, M.T. Effect of pH and background electrolyte on P sorption by variable charge soils. Geoderma 1992, 54, 275–284. [Google Scholar] [CrossRef]
- Lopez-Gutierrez, J.C. Seasonality of organic phosphorus mineralization in the rhizosphere of the native savanna grass, Trachypogon plumosus. Soil Biol. Biochem. 2004, 36, 1675–1684. [Google Scholar] [CrossRef]
- Babur, E. Effects of parent material on soil microbial biomass carbon and basal respiration within young afforested areas. Scand. J. For. Res. 2019, 34, 94–101. [Google Scholar] [CrossRef]
- Shi, J.Y.; Gong, J.R.; Li, X.B.; Zhang, Z.H.; Zhang, W.Y.; Li, Y.; Song, L.Y.; Zhang, S.Q.; Dong, J.J.; Baoyin, T. Phosphorus application promoted the sequestration of orthophosphate within soil microorganisms and regulated the soil solution P supply in a temperate grassland in northern China: A 31P NMR study. Soil Tillage Res. 2023, 227, 105612. [Google Scholar] [CrossRef]
- Valdespino, P.; Romualdo, R.; Cadenazzi, L.; Campo, J. Phosphorus cycling in primary and secondary seasonally dry tropical forests in Mexico. Ann. For. Sci. 2009, 66, 107. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Lu, L.; Chang, X.L.; Jiang, F.; Gao, X.D.; Yao, Y.F.; Li, C.S.; Cao, S.N.; Zhou, Q.M.; Peng, F. Small-Scale Soil Microbial Community Heterogeneity Linked to Landform Historical Events on King George Island, Maritime Antarctica. Front. Microbiol. 2018, 9, 03065. [Google Scholar] [CrossRef] [PubMed]
- Berkelmann, D.; Schneider, D.; Meryandini, A.; Daniel, R. Unravelling the effects of tropical land use conversion on the soil microbiome. Environ. Microbiome 2020, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.J.; Liang, Y.M.; Ma, J.M.; Yang, Z.Q.; Ling, T.W.; Li, M.J.; Lu, S.H.; Zhong, F.Y. Stand age and density affected litter nutrient changes in planted Pinus massoniana forests. Guihaia 2020, 40, 237–246. [Google Scholar]
- Jiao, S.; Liu, Z.S.; Lin, Y.B.; Yang, J.; Chen, W.M.; Wei, G.H. Bacterial communities in oil contaminated soils: Biogeography and co-occurrence patterns. Soil Biol. Biochem. 2016, 98, 64–73. [Google Scholar] [CrossRef]
- Banerjee, S.; Zhao, C.; Kirkby, C.A.; Coggins, S.; Zhao, S.; Bissett, A.; van der Heijden, M.G.A.; Kirkegaard, J.A.; Richardson, A.E. Microbial interkingdom associations across soil depths reveal network connectivity and keystone taxa linked to soil fine-fraction carbon content. Agric. Ecosyst. Environ. 2021, 320, 107559. [Google Scholar] [CrossRef]
- Gao, J.X.; Barzel, B.; Barabási, A.L. Universal resilience patterns in complex networks. Nature 2016, 530, 307–312. [Google Scholar] [CrossRef]
- Xu, L.; Cao, H.L.; Li, C.N.; Wang, C.H.; He, N.P.; Hu, S.Y.; Yao, M.J.; Wang, C.T.; Wang, J.M.; Zhou, S.G.; et al. The importance of rare versus abundant phoD-harboring subcommunities in driving soil alkaline phosphatase activity and available P content in Chinese steppe ecosystems. Soil Biol. Biochem. 2021, 164, 108491. [Google Scholar] [CrossRef]
- Sasaki, T.; Lauenroth, W.K. Dominant species, rather than diversity, regulates temporal stability of plant communities. Oecologia 2011, 166, 761–768. [Google Scholar] [CrossRef]
- Hou, G.; Shi, P.L.; Zhou, T.C.; Sun, J.; Zong, N.; Song, M.H.; Zhang, X.Z. Dominant species play a leading role in shaping community stability in the northern Tibetan grasslands. J. Plant Ecol. 2023, 16, rtac110. [Google Scholar] [CrossRef]
- Xiao, D.; He, X.Y.; Zhang, W.; Hu, P.L.; Sun, M.M.; Wang, K.L. Comparison of bacterial and fungal diversity and network connectivity in karst and non-karst forests in southwest China. Sci. Total Environ. 2022, 822, 153179. [Google Scholar] [CrossRef]
- Liu, H.; Huang, X.; Tan, W.; Di, H.; Xu, J.; Li, Y. High manure load reduces bacterial diversity and network complexity in a paddy soil under crop rotations. Soil Ecol. Lett. 2020, 2, 104–119. [Google Scholar] [CrossRef]
- Wan, W.J.; Hao, X.L.; Xing, Y.H.; Liu, S.; Zhang, X.Y.; Li, X.; Chen, W.L.; Huang, Q.Y. Spatial differences in soil microbial diversity caused by pH-driven organic phosphorus mineralization. Land Degrad. Dev. 2021, 32, 766–776. [Google Scholar] [CrossRef]
- Luo, G.W.; Ling, N.; Nannipieri, P.; Chen, H.; Raza, W.; Wang, M.; Guo, S.W.; Shen, Q.R. Long-term fertilisation regimes affect the composition of the alkaline phosphomonoesterase encoding microbial community of a vertisol and its derivative soil fractions. Biol. Fertil. Soils 2017, 53, 375–388. [Google Scholar] [CrossRef]
- Chen, X.D.; Jiang, N.; Condron, L.M.; Dunfield, K.E.; Chen, Z.H.; Wang, J.K.; Chen, L.J. Impact of long-term phosphorus fertilizer inputs on bacterial phoD gene community in a maize field, Northeast China. Sci. Total Environ. 2019, 669, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Jarosch, K.A.; Kandeler, E.; Frossard, E.; Bünemann, E.K. Is the enzymatic hydrolysis of soil organic phosphorus compounds limited by enzyme or substrate availability. Soil Biol. Biochem. 2019, 139, 107628. [Google Scholar] [CrossRef]
- Ragot, S.A.; Huguenin-Elie, O.; Kertesz, M.A.; Frossard, E.; Bunemann, E.K. Total and active microbial communities and phoD as affected by phosphate depletion and pH in soil. Plant Soil 2016, 408, 15–30. [Google Scholar] [CrossRef]
- Della Monica, I.F.; Godoy, M.S.; Godeas, A.M.; Scervino, J.M. Fungal extracellular phosphatases: Their role in P cycling under different pH and P sources availability. J. Appl. Microbiol. 2018, 124, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Tang, X.X.; Qin, Y.M.; He, Q.S.; Yi, Y.; Ji, Z.L. Karst rocky desertification progress: Soil calcium as a possible driving force. Sci. Total Environ. 2019, 649, 1250–1259. [Google Scholar] [CrossRef] [PubMed]

| Forest Soil Type | Season | Shannon-Wiener | SIMPSON | Evenness | Richness | Chao 1 |
|---|---|---|---|---|---|---|
| Karst forest soil | Rainy | 5.776 ± 0.077 Aa | 0.015 ± 0.001 Ba | 0.087 ± 0.005 Aa | 3776 ± 143 Aa | 3778 ± 142 Aa |
| Dry | 5.890 ± 0.095 Aa | 0.013 ± 0.000 Ba | 0.101 ± 0.010 Aa | 3749 ± 112 Aa | 3751 ± 112 Aa | |
| Non-karst forest soil | Rainy | 4.829 ± 0.096 Ba | 0.045 ± 0.002 Ab | 0.059 ± 0.008 Ba | 2329 ± 113 Ba | 2332 ± 113 Ba |
| Dry | 4.510 ± 0.106 Bb | 0.061 ± 0.002 Aa | 0.043 ± 0.005 Ba | 2307 ± 141 Ba | 2310 ± 141 Ba |
| Network | Nodes/Edges | Avg. Degree | Avg. Path Length | Density | Diameter | Clust. Coeff. |
|---|---|---|---|---|---|---|
| Karst forest soil | 63/670 | 21.270 | 1.73 | 0.343 | 4 | 0.686 |
| Non-karst forest soil | 94/1597 | 35.489 | 1.843 | 0.399 | 4 | 0.744 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Qin, H.; Liang, Y.; Xiao, D.; Yan, P.; Yin, M.; Pan, F. The phoD-Harboring Microorganism Communities and Networks in Karst and Non-Karst Forests in Southwest China. Forests 2024, 15, 341. https://doi.org/10.3390/f15020341
Chen M, Qin H, Liang Y, Xiao D, Yan P, Yin M, Pan F. The phoD-Harboring Microorganism Communities and Networks in Karst and Non-Karst Forests in Southwest China. Forests. 2024; 15(2):341. https://doi.org/10.3390/f15020341
Chicago/Turabian StyleChen, Min, Hanlian Qin, Yueming Liang, Dan Xiao, Peidong Yan, Mingshan Yin, and Fujing Pan. 2024. "The phoD-Harboring Microorganism Communities and Networks in Karst and Non-Karst Forests in Southwest China" Forests 15, no. 2: 341. https://doi.org/10.3390/f15020341
APA StyleChen, M., Qin, H., Liang, Y., Xiao, D., Yan, P., Yin, M., & Pan, F. (2024). The phoD-Harboring Microorganism Communities and Networks in Karst and Non-Karst Forests in Southwest China. Forests, 15(2), 341. https://doi.org/10.3390/f15020341









