Differences in Fine Root Foraging Traits of Two Dominant Tree Species (Cunninghamia lanceolata and Quercus acutissima) in Subtropical Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Species Selection
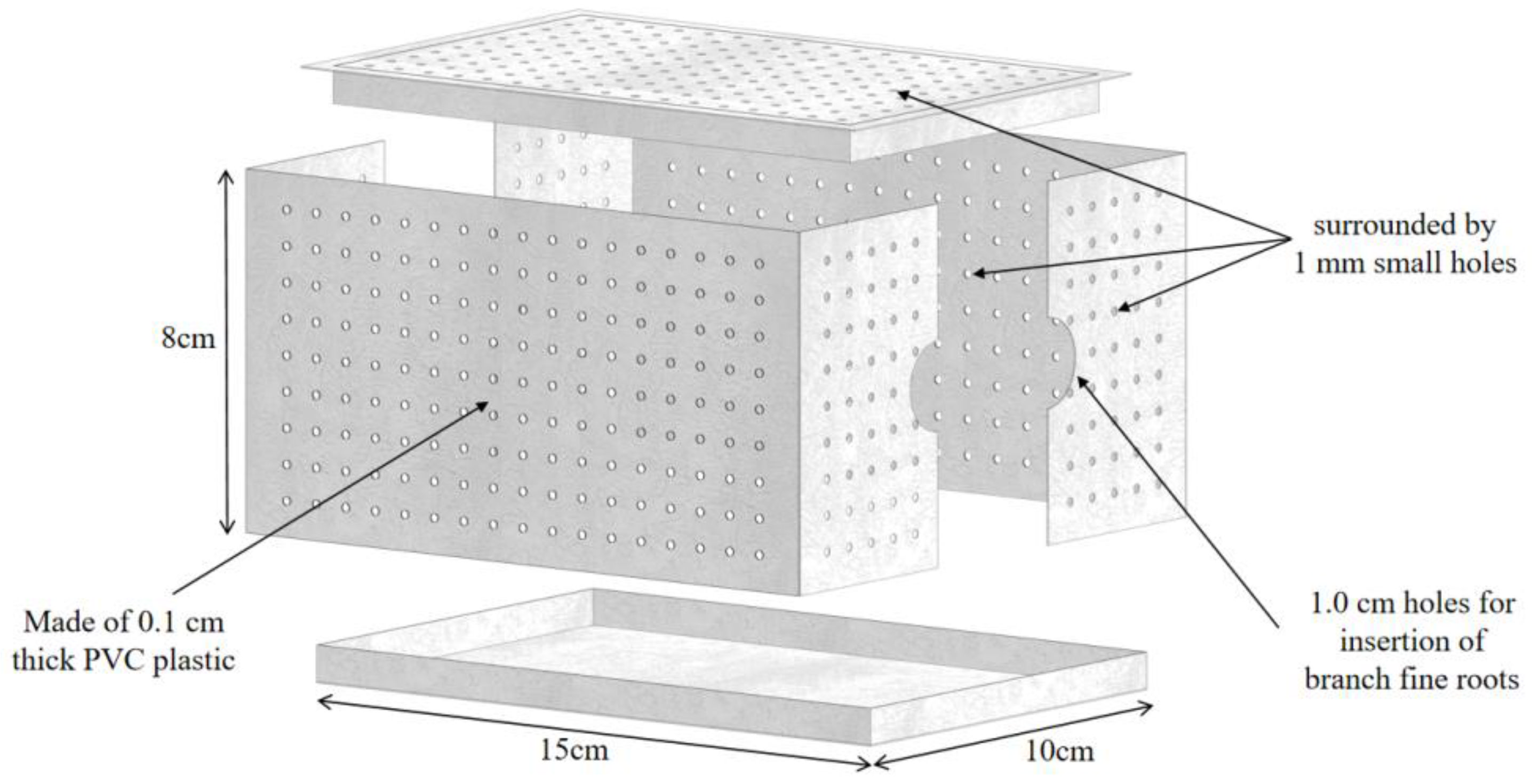
2.2. Nutrient Treatment and Root Chamber Installation
2.3. Root Chamber Harvesting and Processing
2.4. Data Analysis
3. Results
3.1. Response of Fine Root Growth to Nutrient Addition
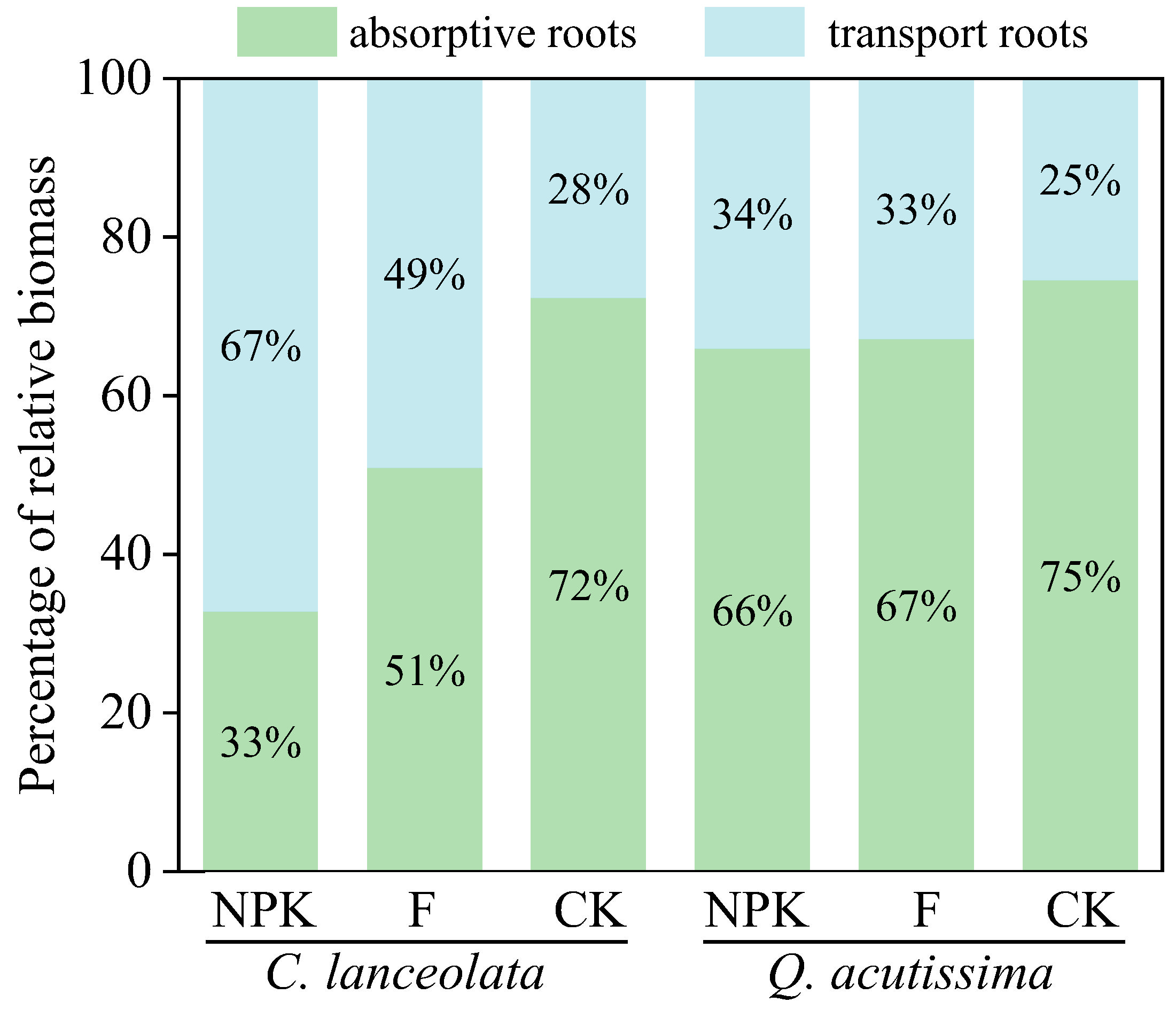
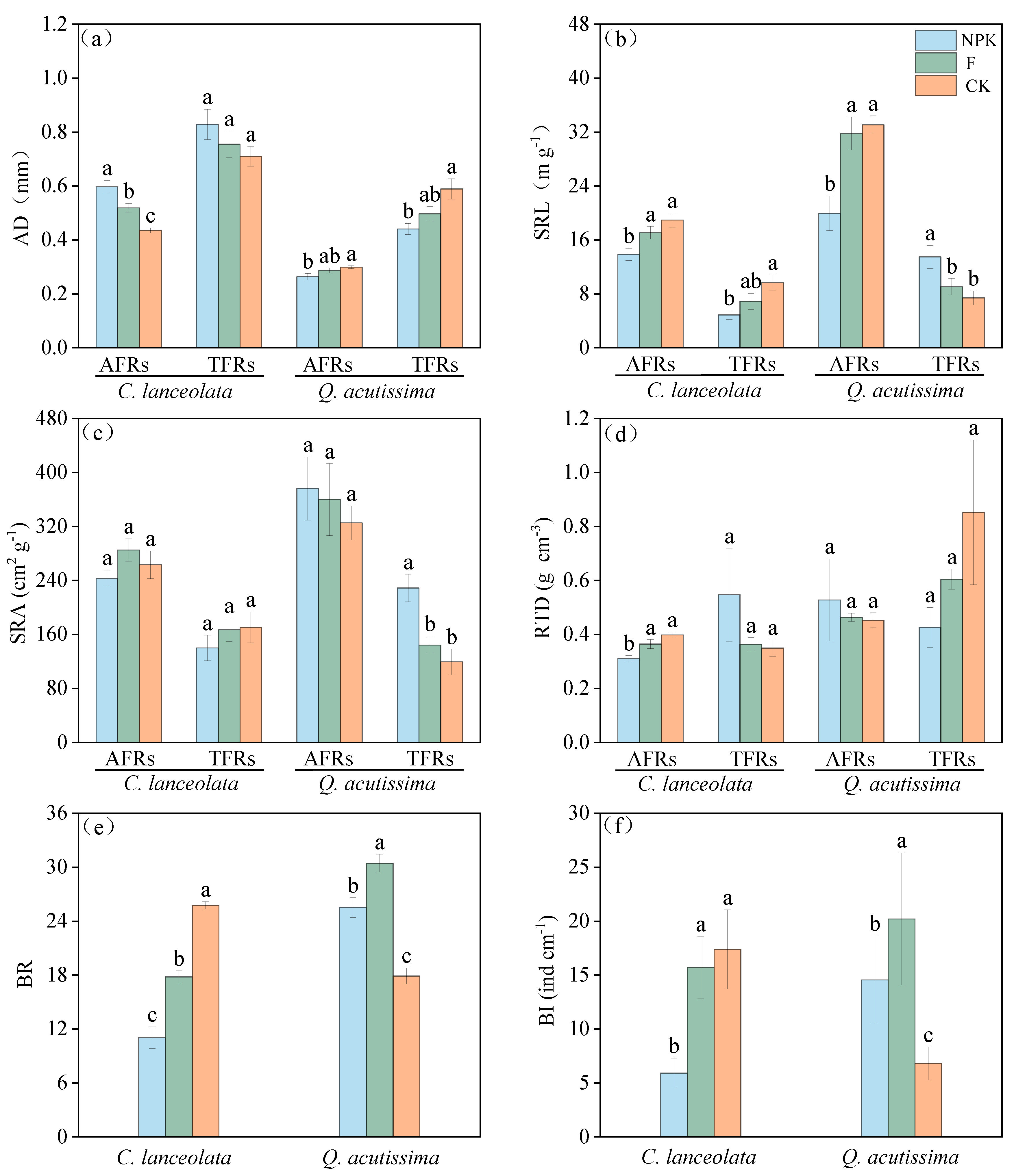
3.2. Response of Root Functional Traits and Mycorrhizal Colonization Rate to Nutrient Addition
3.3. Plasticity of Fine Root Proliferation and Functional Traits to Nutrient Addition
4. Discussion
4.1. Responses of Fine Root Proliferation and Mycorrhizae to Nutrient Addition
4.2. Response of Fine Root Functional Traits to Nutrient Addition
4.3. Comparison of the Magnitude of Plasticity between Root Growth and Morphological Traits
4.4. Responses of Roots and Mycorrhizal Fungi to Different Types of Nutrient-Rich Patches
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bardgett, R.D.; Mommer, L.; De Vries, F.T. Going underground: Root traits as drivers of ecosystem processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef]
- Bergmann, J.; Weigelt, A.; van Der Plas, F.; Laughlin, D.C.; Kuyper, T.W.; Guerrero-Ramirez, N.; Valverde-Barrantes, O.J.; Bruelheide, H.; Freschet, G.T.; Iversen, C.M. The fungal collaboration gradient dominates the root economics space in plants. Sci. Adv. 2020, 6, eaba3756. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Fan, H.; Wei, X.; Wang, H.; Shen, F.; Hu, L.; Li, Y.; Fang, H.; Huang, R. Shifting of the first-order root foraging strategies of Chinese fir (Cunninghamia lanceolata) under varied environmental conditions. Trees 2023, 37, 921–932. [Google Scholar] [CrossRef]
- McCormack, M.L.; Guo, D.; Iversen, C.M.; Chen, W.; Eissenstat, D.M.; Fernandez, C.W.; Li, L.; Ma, C.; Ma, Z.; Poorter, H. Building a better foundation: Improving root-trait measurements to understand and model plant and ecosystem processes. New Phytol. 2017, 215, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Koide, R.T.; Adams, T.S.; Deforest, J.L.; Cheng, L.; Eissenstat, D.M. Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proc. Natl. Acad. Sci. USA 2016, 113, 8741. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, H.; Zhu, B.; Koide, R.T.; Eissenstat, D.M.; Guo, D. Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytol. 2015, 208, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Chen, W.; Adams, T.S.; Wei, X.; Li, L.; McCormack, M.L.; DeForest, J.L.; Koide, R.T.; Eissenstat, D.M. Mycorrhizal fungi and roots are complementary in foraging within nutrient patches. Ecology 2016, 97, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ni, X.; Guo, Z.; Zou, X.; Chen, J.; Shen, W.; Kuzyakov, Y. Nitrogen addition influences fine root growth and mycorrhizal symbiosis formation in trees with contrasting root morphology. Appl. Soil Ecol. 2023, 189, 104987. [Google Scholar] [CrossRef]
- Ostonen, I.; Helmisaari, H.S.; Borken, W.; Tedersoo, L.; Kukumägi, M.; Bahram, M.; Lindroos, A.J.; Nöjd, P.; Uri, V.; Merilä, P. Fine root foraging strategies in Norway spruce forests across a European climate gradient. Glob. Change Biol. 2011, 17, 3620–3632. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J.; Wu, H.; Valverde-Barrantes, O.J.; Wang, R.; Zeng, H.; Kardol, P.; Zhang, H.; Feng, Y. Nonlinearity of root trait relationships and the root economics spectrum. Nat. Commun. 2019, 10, 2203. [Google Scholar] [CrossRef]
- Weemstra, M.; Freschet, G.T.; Stokes, A.; Roumet, C. Patterns in intraspecific variation in root traits are species-specific along an elevation gradient. Funct. Ecol. 2021, 35, 342–356. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root traits are multidimensional: Specific root length is independent from root tissue density and the plant economic spectrum. J. Ecol. 2016, 104, 1299–1310. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef]
- Weemstra, M.; Mommer, L.; Visser, E.J.; van Ruijven, J.; Kuyper, T.W.; Mohren, G.M.; Sterck, F.J. Towards a multidimensional root trait framework: A tree root review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Mo, Q.; Han, X.; Hui, D.; Shen, W. Fine root dynamics responses to nitrogen addition depend on root order, soil layer, and experimental duration in a subtropical forest. Biol. Fertil. Soils 2019, 55, 723–736. [Google Scholar] [CrossRef]
- Wang, G.; Fahey, T.J.; Xue, S.; Liu, F. Root morphology and architecture respond to N addition in Pinus tabuliformis, west China. Oecologia 2013, 171, 583–590. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, B.; Nie, Y.; Liu, Y.; Kuzyakov, Y. Root and mycorrhizal strategies for nutrient acquisition in forests under nitrogen deposition: A meta-analysis. Soil Biol. Biochem. 2021, 163, 108418. [Google Scholar] [CrossRef]
- Li, W.; Jin, C.; Guan, D.; Wang, Q.; Wang, A.; Yuan, F.; Wu, J. The effects of simulated nitrogen deposition on plant root traits: A meta-analysis. Soil Biol. Biochem. 2015, 82, 112–118. [Google Scholar] [CrossRef]
- Zadworny, M.; McCormack, M.L.; Żytkowiak, R.; Karolewski, P.; Mucha, J.; Oleksyn, J. Patterns of structural and defense investments in fine roots of Scots pine (Pinus sylvestris L.) across a strong temperature and latitudinal gradient in Europe. Glob. Change Biol. 2017, 23, 1218–1231. [Google Scholar] [CrossRef]
- Ostonen, I.; Truu, M.; Helmisaari, H.S.; Lukac, M.; Borken, W.; Vanguelova, E.; Godbold, D.L.; Lõhmus, K.; Zang, U.; Tedersoo, L. Adaptive root foraging strategies along a boreal–temperate forest gradient. New Phytol. 2017, 215, 977–991. [Google Scholar] [CrossRef]
- Freschet, G.T.; Swart, E.M.; Cornelissen, J.H. Integrated plant phenotypic responses to contrasting above-and below-ground resources: Key roles of specific leaf area and root mass fraction. New Phytol. 2015, 206, 1247–1260. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Laughlin, D.C. Root nutrient concentration and biomass allocation are more plastic than morphological traits in response to nutrient limitation. Plant Soil 2017, 416, 539–550. [Google Scholar] [CrossRef]
- Kong, D.; Ma, C.; Zhang, Q.; Li, L.; Chen, X.; Zeng, H.; Guo, D. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol. 2014, 203, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Bolte, A.; Villanueva, I. Interspecific competition impacts on the morphology and distribution of fine roots in European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst.). Eur. J. For. Res. 2006, 125, 15–26. [Google Scholar] [CrossRef]
- Cahill, J.F., Jr.; McNickle, G.G.; Haag, J.J.; Lamb, E.G.; Nyanumba, S.M.; St. Clair, C.C. Plants integrate information about nutrients and neighbors. Science 2010, 328, 1657. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Jiang, L.; Fu, X.; Dai, X.; Wang, H.; Li, S. Nitrogen deposition increases root production and turnover but slows root decomposition in Pinus elliottii plantations. New Phytol. 2018, 218, 1450–1461. [Google Scholar] [CrossRef] [PubMed]
- Fitter, A.; Caldwell, M.; Pearcy, R. Architecture and biomass allocation as components of the plastic response of root systems to soil heterogeneity. In Exploitation of Environmental Heterogeneity by Plants: Ecophysiological Processes Above-and Belowground; Academic Press: New York, NY, USA, 1994; Volume 7, pp. 305–323. [Google Scholar]
- Grime, J.P.; Mackey, J. The role of plasticity in resource capture by plants. Evol. Ecol. 2002, 16, 299–307. [Google Scholar] [CrossRef]
- Farley, R.; Fitter, A. The responses of seven co-occurring woodland herbaceous perennials to localized nutrient-rich patches. J. Ecol. 1999, 87, 849–859. [Google Scholar] [CrossRef]
- Johnson, H.A.; Biondini, M.E. Root morphological plasticity and nitrogen uptake of 59 plant species from the Great Plains grasslands, USA. Basic Appl. Ecol. 2001, 2, 127–143. [Google Scholar] [CrossRef][Green Version]
- Peng, Y.; Guo, D.; Yang, Y. Global patterns of root dynamics under nitrogen enrichment. Glob. Ecol. Biogeogr. 2017, 26, 102–114. [Google Scholar] [CrossRef]
- Hodge, A.; Fitter, A.H. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. USA 2010, 107, 13754–13759. [Google Scholar] [CrossRef]
- Chen, W.; Koide, R.T.; Eissenstat, D.M. Nutrient foraging by mycorrhizas: From species functional traits to ecosystem processes. Funct. Ecol. 2018, 32, 858–869. [Google Scholar] [CrossRef]
- Li, L.; Zhou, G.; Liu, J.; Li, H. The resource investigation and community structure characteristics of mycorrhizal fungi associated with Chinese fir. Afr. J. Biotechnol. 2011, 10, 5719–5724. [Google Scholar]
- McCormack, M.L.; Kaproth, M.A.; Carlson, E.; Hipp, A.L.; Han, Y.; Kennedy, P.G. Climate and phylogenetic history structure morphological and architectural trait variation among fine-root orders. New Phytol. 2020, 228, 1824–1834. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Xia, M.; Guo, D.; Pregitzer, K.S. Ephemeral root modules in Fraxinus mandshurica. New Phytol. 2010, 188, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Huang, Z.; He, Z.; Hu, Z.; Yang, J.; Yu, Z.; Wang, M. Effects of broadleaf plantation and Chinese fir (Cunninghamia lanceolata) plantation on soil carbon and nitrogen pools. Chin. J. Appl. Ecol. 2013, 24, 345–350. [Google Scholar]
- Sharma, A.; Chetani, R. A review on the effect of organic and chemical fertilizers on plants. Int. J. Res. Appl. Sci. Eng. Technol. 2017, 5, 677–680. [Google Scholar] [CrossRef]
- Adams, T.S.; McCormack, M.L.; Eissenstat, D.M. Foraging strategies in trees of different root morphology: The role of root lifespan. Tree Physiol. 2013, 33, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Hertel, D.; Leuschner, C. The in situ root chamber: A novel tool for the experimental analysis of root competition in forest soils. Pedobiologia 2006, 50, 217–224. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; DeForest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine root architecture of nine North American trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Yang, N.; Wang, B.; Liu, D.; Wang, X.; Li, X.; Zhang, Y.; Xu, Y.; Peng, S.; Ge, Z.; Mao, L. Long-term nitrogen deposition alters ectomycorrhizal community composition and function in a poplar plantation. J. Fungi 2021, 7, 791. [Google Scholar] [CrossRef] [PubMed]
- Bao, S. Agricultural Chemical Analysis of Soil, 3rd ed.; China Agriculture Press: Beijing, China, 2016. [Google Scholar]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 489–500. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.; Fairchild, G.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Kou, L.; Guo, D.; Yang, H.; Gao, W.; Li, S. Growth, morphological traits and mycorrhizal colonization of fine roots respond differently to nitrogen addition in a slash pine plantation in subtropical China. Plant Soil 2015, 391, 207–218. [Google Scholar] [CrossRef]
- Schlichting, C.D.; Levin, D.A. Phenotypic plasticity: An evolving plant character. Biol. J. Linn. Soc. 1986, 29, 37–47. [Google Scholar] [CrossRef]
- Eissenstat, D.; Yanai, R. The ecology of root lifespan. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 1997; Volume 27, pp. 1–60. [Google Scholar]
- Li, L.; McCormack, M.L.; Chen, F.; Wang, H.; Ma, Z.; Guo, D. Different responses of absorptive roots and arbuscular mycorrhizal fungi to fertilization provide diverse nutrient acquisition strategies in Chinese fir. For. Ecol. Manag. 2019, 433, 64–72. [Google Scholar] [CrossRef]
- Lee, M.-H.; Comas, L.H.; Callahan, H.S. Experimentally reduced root–microbe interactions reveal limited plasticity in functional root traits in Acer and Quercus. Ann. Bot. 2014, 113, 513–521. [Google Scholar] [CrossRef]
- Noguchi, K.; Nagakura, J.; Konôpka, B.; Sakata, T.; Kaneko, S.; Takahashi, M. Fine-root dynamics in sugi (Cryptomeria japonica) under manipulated soil nitrogen conditions. Plant Soil 2013, 364, 159–169. [Google Scholar] [CrossRef]
- Ding, J.; Kong, D.; Zhang, Z.; Cai, Q.; Xiao, J.; Liu, Q.; Yin, H. Climate and soil nutrients differentially drive multidimensional fine root traits in ectomycorrhizal-dominated alpine coniferous forests. J. Ecol. 2020, 108, 2544–2556. [Google Scholar] [CrossRef]
- Li, H.; Liu, B.; McCormack, M.L.; Ma, Z.; Guo, D. Diverse belowground resource strategies underlie plant species coexistence and spatial distribution in three grasslands along a precipitation gradient. New Phytol. 2017, 216, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S.; Matson, P.A.; Vitousek, P.M. Principles of Terrestrial Ecosystem Ecology; Springer: New York, NY, USA, 2011. [Google Scholar]


| Root Traits | Abbreviation | Units | Description |
|---|---|---|---|
| Growth traits | |||
| Fine root biomass | FRB | mg | Dry mass of fine roots in root chamber |
| Total root length | TRL | mm | Total length of fine roots in root chamber |
| Morphological traits | |||
| Average diameter | AD | mm | Average root diameter |
| Specific root length | SRL | m g−1 | Length per unit root dry mass |
| Specific root area | SRA | cm2 g−1 | Area per unit root dry mass |
| Root tissue density | RTD | g cm−3 | Mass per unit root volume |
| Architectural traits | |||
| Branching ratio | BR | The number of AFRs/the number of TFRs | |
| Branching intensity | BI | ind cm−1 | The number of AFRs/total root length of TFRs |
| Chemical traits | |||
| Root N concentration | RN | g kg−1 | Total nitrogen content per unit root dry mass |
| Root P concentration | RP | g kg−1 | Total phosphorus content per unit root dry mass |
| S | R | T | S × R | S × T | R × T | S × R × T | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | F | p | |
| TRL | 18.44 | <0.001 ** | 86.61 | <0.001 ** | 40.72 | <0.001 ** | 10.89 | 0.001 ** | 4.96 | 0.009 ** | 41.17 | <0.001 ** | 8.05 | 0.001 ** |
| AD | 109.62 | <0.001 ** | 140.40 | <0.001 ** | 0.02 | 0.977 | 0.20 | 0.653 | 8.02 | 0.001 ** | 1.97 | 0.146 | 0.52 | 0.598 |
| FRB | 9.51 | 0.003 ** | 7.97 | 0.006 ** | 10.82 | <0.001 ** | 4.56 | 0.036 * | 5.40 | 0.006 ** | 5.09 | 0.008 ** | 1.48 | 0.233 |
| SRL | 41.90 | <0.001 ** | 200.72 | <0.001 ** | 7.81 | <0.001 ** | 15.39 | <0.001 ** | 0.49 | 0.614 | 7.62 | 0.001 ** | 6.42 | 0.003 ** |
| SRA | 5.82 | 0.018 * | 48.85 | <0.001 ** | 0.90 | 0.412 | 5.40 | 0.022 * | 2.53 | 0.086 | 1.40 | 0.253 | 0.67 | 0.514 |
| RTD | 6.95 | 0.010 * | 1.66 | 0.202 | 0.51 | 0.604 | 0.17 | 0.684 | 0.31 | 0.738 | 0.04 | 0.962 | 4.04 | 0.021 * |
| BR | 23.89 | <0.001 ** | —— | 2.91 | 0.003 ** | —— | 22.23 | <0.001 ** | —— | —— | ||||
| BI | 1.32 | 0.273 ** | —— | 7.14 | 0.009 ** | —— | 10.50 | 0.002 ** | —— | —— | ||||
| TN | 807.88 | <0.001 ** | —— | 462.76 | <0.001 ** | —— | 418.86 | <0.001 ** | —— | —— | ||||
| TP | 13.50 | 0.003 ** | —— | 131.59 | <0.001 ** | —— | 4.98 | 0.027 * | —— | —— | ||||
| Traits | C. lanceolata | Q. acutissima | Average CV | ||||
|---|---|---|---|---|---|---|---|
| AFRs | TFRs | AFRs | TFRs | Total | Total | Total | |
| IN-OR-CK | IN-OR-CK | IN-CK | OR-CK | ||||
| TRL | 91.2 | 44.7 | 106.1 | 45.8 | 71.9 | 79.1 | 52.3 |
| TP | 71.4 | 62.5 | 66.9 | 71.9 | 48.1 | ||
| FRB | 73.4 | 10.8 | 80.1 | 62.0 | 56.6 | 73.4 | 35.4 |
| BI | 46.2 | 51.3 | 48.7 | 59.9 | 30.1 | ||
| TN | 27.5 | 48.7 | 38.1 | 32.6 | 44.3 | ||
| BR | 39.1 | 22.3 | 30.7 | 37.9 | 22.9 | ||
| SRL | 15.5 | 33.5 | 25.6 | 31.4 | 26.5 | 36.1 | 12.0 |
| RTD | 12.4 | 26.3 | 8.5 | 34.2 | 20.3 | 26.7 | 8.7 |
| SRA | 8.0 | 10.5 | 7.3 | 35.0 | 15.2 | 18.6 | 6.9 |
| AD | 15.6 | 7.8 | 6.4 | 14.7 | 11.1 | 15.6 | 7.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Tan, R.; Shao, H.; Gu, J.; Wang, W.; Wang, G.; Yu, S. Differences in Fine Root Foraging Traits of Two Dominant Tree Species (Cunninghamia lanceolata and Quercus acutissima) in Subtropical Forests. Forests 2024, 15, 336. https://doi.org/10.3390/f15020336
Xu X, Tan R, Shao H, Gu J, Wang W, Wang G, Yu S. Differences in Fine Root Foraging Traits of Two Dominant Tree Species (Cunninghamia lanceolata and Quercus acutissima) in Subtropical Forests. Forests. 2024; 15(2):336. https://doi.org/10.3390/f15020336
Chicago/Turabian StyleXu, Xinying, Rui Tan, Huimei Shao, Jiacun Gu, Weifeng Wang, Guobing Wang, and Shuiqiang Yu. 2024. "Differences in Fine Root Foraging Traits of Two Dominant Tree Species (Cunninghamia lanceolata and Quercus acutissima) in Subtropical Forests" Forests 15, no. 2: 336. https://doi.org/10.3390/f15020336
APA StyleXu, X., Tan, R., Shao, H., Gu, J., Wang, W., Wang, G., & Yu, S. (2024). Differences in Fine Root Foraging Traits of Two Dominant Tree Species (Cunninghamia lanceolata and Quercus acutissima) in Subtropical Forests. Forests, 15(2), 336. https://doi.org/10.3390/f15020336







