Effect of the Moso Bamboo Pyllostachys edulis (Carrière) J.Houz. on Soil Phosphorus Bioavailability in a Broadleaf Forest (Jiangxi Province, China)
Abstract
1. Introduction
2. Materials and Methods
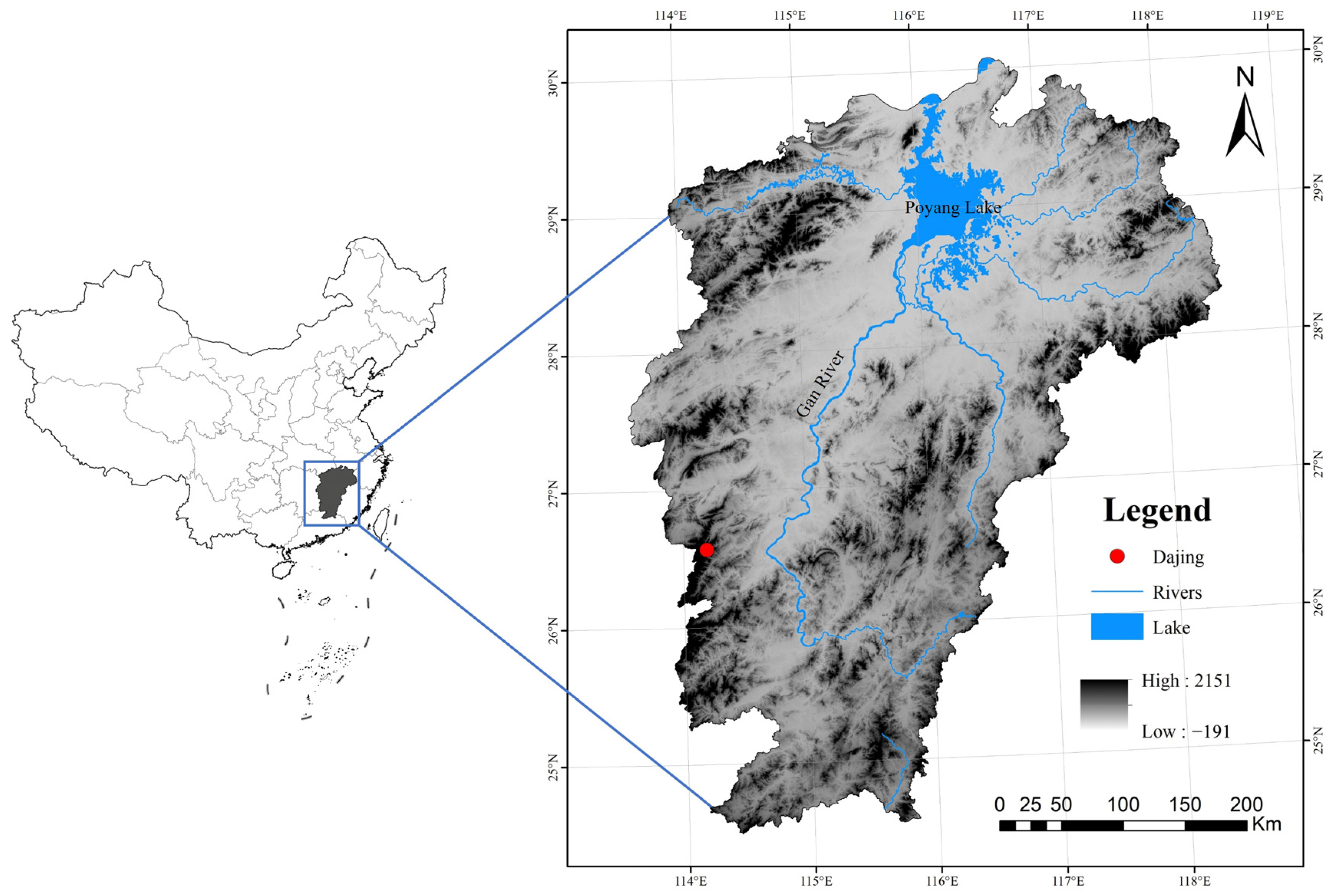
2.1. Study Sites
2.2. Experimental Design and Soil Sampling
2.3. Soil Chemical Properties
2.4. Soil P Fractions
2.5. Soil Acid Phosphatase Activity
2.6. Calculation and Statistical Analyses
3. Results
3.1. Soil Chemical Properties
3.2. Soil P Pool, P Fractions, and Acid Phosphatase Activity
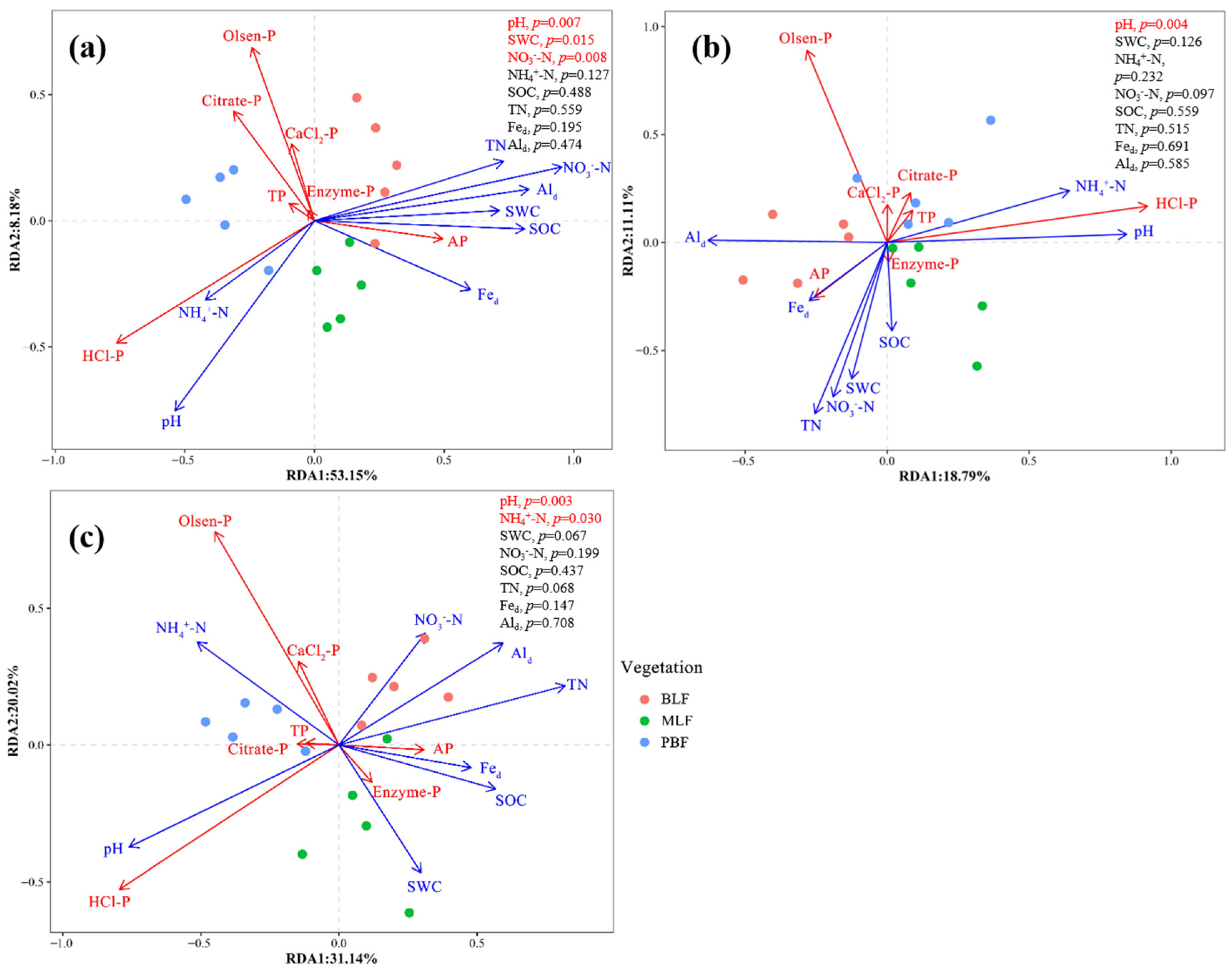
3.3. Linkages between Soil Chemical Properties and P-Related Indicators
4. Discussion
4.1. P. edulis Expansion Significantly Altered Soil P Status
4.2. Soil pH Drives Changes in P Fraction in the Soil Layer during Expansion
5. Conclusions
- (1)
- The P. edulis expansion altered the soil P pool and bioavailability in the subtropical region of China. The Olsen-P content and CaCl2-P were significantly lower in the mixed bamboo–broadleaf forest than in the broadleaf forest and the pure P. edulis forest, whereas the soil total P pool content in the 0–10 cm depths was increased. The HCl-P and Enzyme-P content were higher in the mixed bamboo–broadleaf forest than in the other two forest types.
- (2)
- The acid phosphatase activity was significantly higher in the mixed bamboo–broadleaf forest compared to the P. edulis forest.
- (3)
- The positive relationship between the soil pH and HCl-P fraction indicated that the soil pH was an important factor in altering the P bioavailability during the P. edulis expansion.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paz-Ares, J.; Puga, M.I.; Rojas-Triana, M.; Martinez-Hevia, I.; Diaz, S.; Poza-Carrión, C.; Miñambres, M.; Leyva, A. Plant adaptation to low phosphorus availability: Core signaling, crosstalks, and applied implications. Mol. Plant 2022, 15, 104–124. [Google Scholar] [CrossRef]
- Zhu, M.K.; Huang, B.C.; Liu, Z.Y.; Wang, Y.; Teng, J.; Tian, X.S.; Ai, X.Y.; Sheng, M.H.; Ai, Y.W. The distribution, effectiveness and environmental threshold of soil aggregate phosphorus fractions in the sub-alpine region of Southwest China. Land Degrad. Dev. 2023, 34, 3–15. [Google Scholar] [CrossRef]
- Han, Y.; White, P.J.; Cheng, L.Y. Mechanisms for improving phosphorus utilization efficiency in plants. Ann. Bot. 2022, 129, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Darcy, J.L.; Schmidt, S.K.; Knelman, J.E.; Cleveland, C.C.; Castle, S.C.; Nemergut, D.R. Phosphorus, not nitrogen, limits plants and microbial primary producers following glacial retreat. Sci. Adv. 2018, 4, eaaq0942. [Google Scholar] [CrossRef] [PubMed]
- Du, E.Z.; Terrer, C.; Pellegrini, A.F.A.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.H.; Robert, B.J. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Qi, X.X.; Chen, L.; Zhu, J.A.; Li, Z.; Lei, H.M.; Shen, Q.; Wu, H.L.; Ouyang, S.; Zeng, Y.L.; Hu, Y.T.; et al. Increase of soil phosphorus bioavailability with ectomycorrhizal tree dominance in subtropical secondary forests. For. Ecol. Manag. 2022, 521, 120435. [Google Scholar] [CrossRef]
- Hou, E.Q.; Tan, X.; Heenan, M.; Wen, D.Z. A global dataset of plant available and unavailable phosphorus in natural soils derived by Hedley method. Sci. Data 2018, 5, 180166. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.K.; Chen, R.; Men, X.X.; Cheng, X.L. Divergent linkages of soil phosphorus fractions to edaphic properties following afforestation in the riparian zone of the upper Yangtze river, China. Chemosphere 2023, 313, 137452. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Yan, X.J.; Wang, M.K.; Cai, Y.Y.; Weng, X.F.; Su, D.; Guo, J.X.; Wang, W.Q.; Hou, Y.; Ye, D.L.; et al. Long-term excessive phosphorus fertilization alters soil phosphorus fractions in the acidic soil of pomelo orchards. Soil Tillage Res. 2022, 215, 105214. [Google Scholar] [CrossRef]
- Wu, H.L.; Xiang, W.H.; Chen, L.; Ouyang, S.; Xiao, W.F.; Li, S.G.; Forrester, D.I.; Lei, P.F.; Zeng, Y.L.; Deng, X.W.; et al. Soil phosphorus bioavailability and recycling increased with stand age in Chinese fir plantations. Ecosystems 2019, 23, 973–988. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Shi, L.L.; Lu, H.B.; Shao, Y.H.; Liu, S.R.; Fu, S.L. Drought promotes soil phosphorus transformation and reduces phosphorus bioavailability in a temperate forest. Sci. Total Environ. 2020, 732, 139295. [Google Scholar] [CrossRef]
- Song, Q.N.; Lu, H.; Liu, J.; Yang, J.; Yang, G.Y.; Yang, Q.P. Accessing the impacts of bamboo expansion on NPP and N cycling in evergreen broadleaved forest in subtropical China. Sci. Rep. 2017, 7, 40383. [Google Scholar] [CrossRef]
- Xu, Q.F.; Liang, C.F.; Chen, J.H.; Li, Y.C.; Qin, H.; Fuhrmann, J.J. Rapid bamboo invasion (expansion) and its effects on biodiversity and soil processes. Glob. Ecol. Conserv. 2020, 21, e00787. [Google Scholar] [CrossRef]
- Qi, S.H.; Song, B.; Liu, C.; Gong, P.; Luo, J.; Zhang, M.A.; Xiong, T.W. Bamboo forest mapping in China using the dense landsat 8 image archive and google earth engine. Remote Sens. 2022, 14, 762. [Google Scholar] [CrossRef]
- Ouyang, M.; Tian, D.; Pan, J.M.; Chen, G.P.; Su, H.J.; Yan, Z.B.; Yang, Q.P.; Ji, C.J.; Tang, Z.Y.; Fang, J.Y. Moso bamboo (Phyllostachys edulis) invasion increases forest soil pH in subtropical China. Catena 2022, 215, 106339. [Google Scholar] [CrossRef]
- Liu, C.X.; Zhou, Y.; Qin, H.; Liang, C.F.; Shao, S.; Fuhrmann, J.J.; Chen, J.H.; Xu, Q.F. Moso bamboo invasion has contrasting effects on soil bacterial and fungal abundances, co-occurrence networks and their associations with enzyme activities in three broadleaved forests across subtropical China. For. Ecol. Manag. 2021, 498, 119549. [Google Scholar] [CrossRef]
- Chen, Z.H.; Li, Y.C.; Chang, S.X.; Xu, Q.F.; Li, Y.F.; Ma, Z.L.; Qin, H.; Cai, Y.J. Linking enhanced soil nitrogen mineralization to increased fungal decomposition capacity with Moso bamboo invasion of broadleaf forests. Sci. Total Environ. 2021, 771, 144779. [Google Scholar] [CrossRef]
- Fu, D.G.; Wu, X.N.; Duan, C.Q.; Chadwick, D.R.; Jones, D.L. Response of soil phosphorus fractions and fluxes to different vegetation restoration types in a subtropical mountain ecosystem. Catena 2020, 193, 104663. [Google Scholar] [CrossRef]
- Wan, S.Z.; Lin, G.G.; Liu, B.; Ding, Y.D.; Li, S.L.; Mao, R. Contrasting responses of soil phosphorus pool and bioavailability to alder expansion in a boreal peatland, Northeast China. Catena 2022, 212, 106128. [Google Scholar] [CrossRef]
- Weihrauch, C.; Opp, C. Ecologically relevant phosphorus pools in soils and their dynamics: The story so far. Geoderma 2018, 325, 183–194. [Google Scholar] [CrossRef]
- Hou, E.Q.; Wen, D.Z.; Kuang, Y.W.; Cong, J.; Chen, C.R.; He, X.J.; Heenan, M.; Lu, H.; Zhang, Y.G. Soil pH predominantly controls the forms of organic phosphorus in topsoils under natural broadleaved forests along a 2500 km latitudinal gradient. Geoderma 2018, 315, 65–74. [Google Scholar] [CrossRef]
- Giesler, R.; Satoh, F.; Ilstedt, U.; Nordgren, A. Microbially available phosphorus in boreal forests: Effects of aluminum and iron accumulation in the humus layer. Ecosystems 2004, 7, 208–217. [Google Scholar] [CrossRef]
- Fan, Y.X.; Zhong, X.J.; Lin, F.; Liu, C.C.; Yang, L.M.; Wang, M.H.; Chen, G.S.; Chen, Y.; Yang, Y.S. Responses of soil phosphorus fractions after nitrogen addition in a subtropical forest ecosystem: Insights from decreased Fe and Al oxides and increased plant roots. Geoderma 2019, 337, 246–255. [Google Scholar] [CrossRef]
- Aslam, M.M.; Pueyo, J.J.; Pang, J.Y.; Yang, J.Y.; Chen, W.G.; Chen, H.; Waseem, M.; Li, Y.; Zhang, J.H.; Xu, W.F. Root acid phosphatases and rhizobacteria synergistically enhance white lupin and rice phosphorus acquisition. Plant Physiol. 2022, 190, 2449–2465. [Google Scholar] [CrossRef]
- Jindo, K.; Audette, Y.; Olivares, F.L.; Canellas, L.P.; Smith, D.S.; Paul Voroney, R. Biotic and abiotic effects of soil organic matter on the phytoavailable phosphorus in soils: A review. Chem. Biol. Technol. Agric. 2023, 10, 29. [Google Scholar] [CrossRef]
- Li, Y.F.; Li, G.H. Mechanisms of straw biochar’s improvement of phosphorus bioavailability in soda saline-alkali soil. Environ. Sci. Pollut. Res. 2022, 29, 47867–47872. [Google Scholar] [CrossRef]
- Wu, C.S.; Mo, Q.F.; Wang, H.K.; Zhang, Z.J.; Huang, G.X.; Ye, Q.; Zou, Q.; Kong, F.Q.; Liu, Y.Q.; Wang, G.G. Moso bamboo (Phyllostachys edulis (Carriere) J. Houzeau) invasion affects soil phosphorus dynamics in adjacent coniferous forests in subtropical China. Ann. For. Sci. 2018, 75, 24. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Liang, C.F.; Shao, S.; Chen, J.H.; Qin, H.; Xu, Q.F. Linkages of litter and soil C: N: P stoichiometry with soil microbial resource limitation and community structure in a subtropical broadleaf forest invaded by Moso bamboo. Plant Soil 2021, 465, 473–490. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B. Method to measure microbial phosphate in soils. Soil Biol. Biochem. 1982, 14, 377–385. [Google Scholar] [CrossRef]
- Tiessen, H.; Moir, J.O. Characterization of available P by sequential extraction. Soil Sampl. Methods Anal. 1993, 824, 75–86. [Google Scholar]
- DeLuca, T.H.; Glanville, H.C.; Harris, M.; Emmett, B.A.; Pingree, M.R.A.; de Sosa, L.L.; Cerdá-Moreno, C.; Jones, D.L. A novel biologically-based approach to evaluating soil phosphorus availability across complex landscapes. Soil Biol. Biochem. 2015, 88, 110–119. [Google Scholar] [CrossRef]
- Wu, H.L.; Xiang, W.H.; Ouyang, S.; Forrester, D.I.; Zhou, B.; Chen, L.X.; Ge, T.D.; Lei, P.F.; Chen, L.X.; Zeng, Y.L.; et al. Linkage between tree species richness and soil microbial diversity improves phosphorus bioavailability. Funct. Ecol. 2019, 33, 1549–1560. [Google Scholar] [CrossRef]
- Lu, R.K. Soil and Agro-Chemistry Analytical Methods; China Agricultural Sciences Press: Beijing, China, 2000. [Google Scholar]
- Mehra, O.P.; Jackson, M.L. Iron oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonate. In Clays and Clay Minerals; Pergamon: Oxford, UK, 2013; pp. 317–327. [Google Scholar]
- Ohno, T.; Zibilske, L.M. Determination of low concentrations of phosphorus in soil extracts using malachite green. Soil Sci. Soc. Am. J. 1991, 55, 892–895. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Lai, J.S.; Peres-Neto, P.; Nimon, K. R Package; Version 0.2.0; rdacca.hp: Hierarchical Partitioning for Canonical Analysis; R Foundation for Statistical Computation: Vienna, Austria, 2021. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.; Simpson, G.; Solymos, P. R Package; Version 1.17-3; vegan: Community Ecology Package; R Foundation for Statistical Computation: Vienna, Austria, 2010; Available online: http://CRAN.R-project.org/package=vegan (accessed on 24 December 2023).
- Sun, H.P.; Hu, W.Y.; Dai, Y.X.; Ai, L.; Wu, M.; Hu, J.; Zuo, Z.; Li, M.Y.; Yang, H.; Ma, J.M. Moso bamboo (Phyllostachys edulis (Carriere) J. Houzeau) invasion affects soil microbial communities in adjacent planted forests in the Lijiang River basin, China. Front. Microbiol. 2023, 14, 1111498. [Google Scholar] [CrossRef]
- Liu, C.X.; Zheng, C.Y.; Wang, L.; Zhang, J.; Wang, Q.Z.; Shao, S.; Qin, H.; Xu, Q.F.; Liang, C.F.; Chen, J.H. Moso bamboo invasion changes the assembly process and interactive relationship of soil microbial communities in a subtropical broadleaf forest. For. Ecol. Manag. 2023, 536, 120901. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Y.F.; Chang, S.X.; Xu, Q.F.; Guo, Z.Y.; Gao, Q.; Qin, Z.Y.; Yang, Y.F.; Chen, J.H.; Liang, X. Bamboo invasion of broadleaf forests altered soil fungal community closely linked to changes in soil organic C chemical composition and mineral N production. Plant Soil 2017, 418, 507–521. [Google Scholar] [CrossRef]
- Bai, S.B.; Conant, R.T.; Zhou, G.M.; Wang, Y.X.; Wang, N.; Li, Y.H.; Zhang, K.Q. Effects of moso bamboo encroachment into native, broad-leaved forests on soil carbon and nitrogen pools. Sci. Rep. 2016, 6, 31480. [Google Scholar] [CrossRef]
- Liu, X.S.; Siemann, E.; Cui, C.; Liu, Y.Q.; Guo, X.M.; Zhang, L. Moso bamboo (Phyllostachys edulis) invasion effects on litter, soil and microbial PLFA characteristics depend on sites and invaded forests. Plant Soil 2019, 438, 85–99. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Q.P.; Song, Q.N.; Yu, D.K.; Yang, G.Y.; Qi, H.Y.; Shi, J.M. Strategy of fine root invasion of Phyllostachys pubescens population into evergreen broad-leaves forest. Chin. J. Plant Ecol. 2013, 37, 230–238. [Google Scholar] [CrossRef]
- Ding, W.L.; Cong, W.F.; Lambers, H. Plant phosphorus-acquisition and -use strategies affect soil carbon cycling. Trends Ecol. Evol. 2021, 36, 899–906. [Google Scholar] [CrossRef]
- Qin, H.; Niu, L.M.; Wu, Q.F.; Chen, J.H.; Li, Y.C.; Liang, C.F.; Xu, Q.F.; Fuhrmann, J.J.; Shen, Y. Bamboo forest expansion increases soil organic carbon through its effect on soil arbuscular mycorrhizal fungal community and abundance. Plant Soil 2017, 420, 407–421. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Azene, B.; Qiu, P.; Zhu, R.H.; Pan, K.W.; Sun, X.M.; Nigussie, Y.; Yigez, B.; Gruba, P.; Wu, X.G.; Zhang, L. Response of soil phosphorus fractions to land use change in the subalpine ecosystems of Southeast margin of Qinghai-Tibet Plateau, Southwest China. Ecol. Indic. 2022, 144, 109432. [Google Scholar] [CrossRef]
- Garau, G.; Morillas, L.; Roales, J.; Castaldi, P.; Mangia, N.P.; Spano, D.; Mereu, S. Effect of monospecific and mixed Mediterranean tree plantations on soil microbial community and biochemical functioning. Appl. Soil Ecol. 2019, 140, 78–88. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Pressler, Y.; Zhou, J.Z.; He, Z.L.; Van Nostrand, J.D.; Smith, A.P. Post-agricultural tropical forest regeneration shifts soil microbial functional potential for carbon and nutrient cycling. Soil Biol. Biochem. 2020, 145, 107784. [Google Scholar] [CrossRef]
- Lü, X.T.; Hu, Y.Y.; Wolf, A.A.; Han, X.G. Species richness mediates within-species nutrient resorption: Implications for the biodiversity-productivity relationship. J. Ecol. 2019, 107, 2346–2352. [Google Scholar] [CrossRef]
- Jin, W.H.; Tu, J.Y.; Wu, Q.F.; Peng, L.Y.; Xing, J.J.; Liang, C.F.; Shao, S.; Chen, J.H.; Xu, Q.F.; Qin, H. Moso bamboo expansion decreased soil heterotrophic respiration but increased arbuscular mycorrhizal mycelial respiration in a subtropical broadleaved forest. For. Ecosyst. 2023, 10, 100116. [Google Scholar] [CrossRef]
- Hedley, M.J.; White, R.E.; Nye, P.H. Plant-induced changes in the rhizosphere of rape (Brassica napus var. Emerald) seedlings: III. Changes in L value, soil phosphate fractions and phosphatase activity. New Phytol. 1982, 91, 45–56. [Google Scholar] [CrossRef]
- Alt, F.; Oelmann, Y.; Herold, N.; Schrumpf, M.; Wilcke, W. Phosphorus partitioning in grassland and forest soils of Germany as related to land-use type, management intensity, and land use-related pH. J. Plant Nutr. Soil Sci. 2011, 174, 195–209. [Google Scholar] [CrossRef]
- Li, F.R.; Liu, L.L.; Liu, J.L.; Yang, K. Abiotic and biotic controls on dynamics of labile phosphorus fractions in calcareous soils under agricultural cultivation. Sci. Total Environ. 2019, 681, 163–174. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Luo, Y.Q. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef]
- Wan, W.J.; Hao, X.L.; Xing, Y.H.; Liu, S.; Zhang, X.Y.; Li, X.; Chen, W.L.; Huang, Q.Y. Spatial differences in soil microbial diversity caused by pH-driven organic phosphorus mineralization. Land Degrad. Dev. 2021, 32, 766–776. [Google Scholar] [CrossRef]
- Zhu, H.; Bing, H.J.; Wu, Y.H.; Sun, H.Y.; Zhou, J. Low molecular weight organic acids regulate soil phosphorus availability in the soils of subalpine forests, eastern Tibetan Plateau. Catena 2021, 203, 105328. [Google Scholar] [CrossRef]
- Liu, X.Y.; Fang, P.F.; Xiong, Y.; Peng, Q.H.; Yu, Z.P.; Luan, F.G.; Song, Q.N.; Fang, X.; Yang, Q.P.; Liu, J. Assessment of the influence of bamboo expansion on Si pools and fluxes in a disturbed subtropical evergreen broadleaved forest. Catena 2022, 213, 106136. [Google Scholar] [CrossRef]
- Schaller, J.; Frei, S.; Rohn, L.; Gilfedder, B.S. Amorphous silica controls water storage capacity and phosphorus mobility in soils. Front. Environ. Sci. 2020, 8, 94. [Google Scholar] [CrossRef]
- Schaller, J.; Faucherre, S.; Joss, H.; Obst, M.; Goeckede, M.; Planer-Friedrich, B.; Peiffer, S.; Gilfedder, B.; Elberling, B. Silicon increases the phosphorus availability of Arctic soils. Sci. Rep. 2019, 9, 449. [Google Scholar] [CrossRef] [PubMed]


| Soil Depth | Vegetation | pH | Moisture (%) | NH4+-N (mg kg−1) | NO3−-N (mg kg−1) | SOC (g kg−1) | TN (g kg−1) | TP (g kg−1) | Olsen-P (mg kg−1) | Fed (g kg−1) | Ald(g kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–10 cm | PBF | 5.04 ± 0.04A | 57.98 ± 2.93B | 19.08 ± 0.55A | 2.70 ± 0.52C | 54.95 ± 2.47B | 3.47 ± 0.05B | 0.53 ± 0.04A | 3.21 ± 0.12A | 16.62 ± 0.46A | 6.48 ± 0.08C |
| MLF | 5.03 ± 0.06A | 69.01 ± 3.42A | 18.90 ± 0.58A | 4.60 ± 1.73B | 77.12 ± 5.51A | 3.70 ± 0.12B | 0.48 ± 0.02A | 2.43 ± 0.30B | 17.51 ± 0.24A | 6.74 ± 0.05B | |
| BLF | 4.67 ± 0.11B | 69.99 ± 2.76A | 16.56 ± 0.56B | 6.78 ± 0.23A | 76.93 ± 4.25A | 3.98 ± 0.07A | 0.48 ± 0.03A | 3.37 ± 0.24A | 17.51 ± 0.23A | 7.03 ± 0.10A | |
| 10–20 cm | PBF | 5.07 ± 0.02A | 42.11 ± 2.17B | 9.83 ± 1.01A | 1.19 ± 0.61B | 33.13 ± 2.56B | 1.80 ± 0.08B | 0.47 ± 0.03A | 2.99 ± 0.17A | 17.73 ± 0.48A | 6.65 ± 0.13B |
| MLF | 5.13 ± 0.03A | 55.06 ± 2.16A | 8.93 ± 0.74A | 2.25 ± 0.85A | 43.97 ± 2.24A | 2.43 ± 0.10A | 0.41 ± 0.01A | 2.09 ± 0.31B | 17.96 ± 0.16A | 6.79 ± 0.12B | |
| BLF | 4.63 ± 0.04B | 52.26 ± 1.33A | 7.98 ± 0.81A | 2.48 ± 0.30A | 42.33 ± 2.56A | 2.36 ± 0.12A | 0.41 ± 0.03A | 3.07 ± 0.18A | 18.22 ± 0.11A | 7.21 ± 0.12A | |
| 20–40 cm | PBF | 5.18 ± 0.03A | 42.47 ± 3.13B | 7.05 ± 0.67A | 1.58 ± 0.35B | 23.96 ± 1.64B | 1.36 ± 0.03C | 0.43 ± 0.04A | 3.43 ± 0.11A | 17.60 ± 0.58A | 6.60 ± 0.12B |
| MLF | 5.04 ± 0.06A | 53.26 ± 2.43A | 5.77 ± 0.35A | 1.48 ± 0.47B | 33.37 ± 2.02A | 1.74 ± 0.06B | 0.39 ± 0.02A | 1.93 ± 0.39B | 18.05 ± 0.17A | 6.75 ± 0.11B | |
| BLF | 4.78 ± 0.06B | 48.09 ± 0.84AB | 6.14 ± 0.37A | 2.51 ± 0.40A | 33.19 ± 3.04A | 1.96 ± 0.06A | 0.36 ± 0.03A | 3.14 ± 0.12A | 18.27 ± 0.04A | 7.24 ± 0.06A | |
| Variance analysis of F-statistics | |||||||||||
| Vegetation | 48.54 *** | 18.32 *** | 5.38 ** | 63.62 *** | 19.37 *** | 37.08 *** | 3.54 * | 19.60 *** | 3.60 * | 25.73 *** | |
| Soil depth | 1.90 ns | 46.18 *** | 269.53 *** | 148.88 *** | 129.09 *** | 495.00 *** | 10.27 *** | 1.10 ns | 5.44 ** | 1.46 ns | |
| Soil depth × Vegetation | 1.32 ns | 0.59 ns | 1.07 ns | 15.81 *** | 1.76 ns | 1.92 ns | 0.09 ns | 0.66 ns | 0.28 ns | 0.26 ns | |
| Soil Depth | Vegetation | Citrate-P/CaCl2-P | Enzyme-P/CaCl2-P | HCl-P/CaCl2-P | Citrate-P/Enzyme-P | Citrate-P/HCl-P | Enzyme-P/HCl-P |
|---|---|---|---|---|---|---|---|
| 0–10 cm | PBF | 22.92 ± 1.46B | 1.98 ± 0.17B | 68.92 ± 4.46B | 11.78 ± 0.95A | 0.34 ± 0.03AB | 0.03 ± 0.00B |
| MLF | 43.06 ± 3.16A | 4.91 ± 0.43A | 136.07 ± 8.66A | 8.96 ± 0.78B | 0.32 ± 0.03B | 0.04 ± 0.00AB | |
| BLF | 22.07 ± 1.67B | 2.35 ± 0.21B | 53.77 ± 6.80B | 9.49 ± 0.41AB | 0.42 ± 0.04A | 0.05 ± 0.00A | |
| 10–20 cm | PBF | 7.65 ± 0.52B | 1.24 ± 0.03B | 39.72 ± 2.29B | 6.21 ± 0.52A | 0.19 ± 0.01AB | 0.03 ± 0.00C |
| MLF | 10.60 ± 0.67A | 2.64 ± 0.31A | 59.97 ± 3.73A | 4.13 ± 0.30B | 0.18 ± 0.01B | 0.04 ± 0.00B | |
| BLF | 7.93 ± 1.10B | 1.86 ± 0.17B | 31.65 ± 2.42B | 4.38 ± 0.75B | 0.25 ± 0.04A | 0.06 ± 0.00A | |
| 20–40 cm | PBF | 3.22 ± 0.56B | 0.59 ± 0.07B | 19.46 ± 1.68B | 5.39 ± 0.50A | 0.16 ± 0.01B | 0.03 ± 0.00B |
| MLF | 10.55 ± 1.68A | 4.87 ± 1.12A | 65.78 ± 10.91A | 2.30 ± 0.23B | 0.16 ± 0.01B | 0.07 ± 0.01A | |
| BLF | 2.81 ± 0.55B | 1.07 ± 0.10B | 11.28 ± 1.29B | 2.69 ± 0.58B | 0.24 ± 0.03A | 0.10 ± 0.02A | |
| Variance analysis of F-statistics | |||||||
| Vegetation | 47.14 *** | 38.45 *** | 79.66 *** | 17.22 *** | 11.30 *** | 23.62 *** | |
| Soil depth | 222.46 *** | 6.10 ** | 75.60 *** | 100.66 *** | 45.34 *** | 17.08 *** | |
| Soil depth × Vegetation | 12.54 *** | 3.97 ** | 6.72 *** | 0.23 ns | 0.15 ns | 4.67 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Shi, F.; Fang, X.; Zhang, R.; Shi, J.; Zhang, Y. Effect of the Moso Bamboo Pyllostachys edulis (Carrière) J.Houz. on Soil Phosphorus Bioavailability in a Broadleaf Forest (Jiangxi Province, China). Forests 2024, 15, 328. https://doi.org/10.3390/f15020328
Yang D, Shi F, Fang X, Zhang R, Shi J, Zhang Y. Effect of the Moso Bamboo Pyllostachys edulis (Carrière) J.Houz. on Soil Phosphorus Bioavailability in a Broadleaf Forest (Jiangxi Province, China). Forests. 2024; 15(2):328. https://doi.org/10.3390/f15020328
Chicago/Turabian StyleYang, Dou, Fuxi Shi, Xiangmin Fang, Ruoling Zhang, Jianmin Shi, and Yang Zhang. 2024. "Effect of the Moso Bamboo Pyllostachys edulis (Carrière) J.Houz. on Soil Phosphorus Bioavailability in a Broadleaf Forest (Jiangxi Province, China)" Forests 15, no. 2: 328. https://doi.org/10.3390/f15020328
APA StyleYang, D., Shi, F., Fang, X., Zhang, R., Shi, J., & Zhang, Y. (2024). Effect of the Moso Bamboo Pyllostachys edulis (Carrière) J.Houz. on Soil Phosphorus Bioavailability in a Broadleaf Forest (Jiangxi Province, China). Forests, 15(2), 328. https://doi.org/10.3390/f15020328






