Abstract
Understanding the niche dynamic among distinct populations and delineating the dispersal corridors that exist between them under current climates is critical for elucidating the contemporary forces driving genetic divergence, facilitating population connectivity, and informing targeted conservation efforts, particularly for species exhibiting pronounced intraspecific lineages. In this study, we focus on evaluating the range and niche dynamics of the intraspecific lineages of Emmenopterys henryi and exploring potential patterns of population connectivity both within and across these lineages. Our findings unveiled a significant niche divergence between the two intraspecific lineages, characterized by limited overlap in climatic conditions and suitable ranges. Furthermore, our analysis of population connectivity revealed the presence of dispersal routes with varying degrees of connectivity within each lineage, while low connectivity was observed between the two lineages. Our results highlight the critical role of fine-scale ecological niche models (ENMs) and genetic connectivity analyses in elucidating the complexities of niche evolution and genetic connectivity, particularly for species with discrete intraspecific lineages. In addition, given the fact that rapid genetic erosion of species inhabiting the regions we focus on in this study is often associated with habitat loss and fragmentation, our findings will also offer valuable insights for designing targeted conservation strategies aimed at restoring connectivity and increasing local population sizes for this endangered species.
1. Introduction
The intensifying impacts of climate change are pervasive across multiple levels of ecosystems [1,2], thereby affecting not only species demography and dispersal, which are the cornerstones of short-term recovery, but also genetic diversity and metapopulation structure, which are critical for longer-term recovery and adaptation [3,4]. Empirical evidence has indicated that recurrent climate disturbances might severely affect the reproductive capacity and strength of the genetic connectivity of species or even erode population resilience [5,6,7], potentially leading to local extinction and jeopardizing ecosystem function [8,9,10,11]. In the face of global climate change, ecological niche models (ENMs) have emerged as cost-effective tools for assessing climate change impacts on the range dynamics of species [12,13], guiding conservation interventions for at-risk species [14], testing ecological hypotheses [15], and predicting the maladaptation risk of species [16]. Traditional ENMs usually assume genetic uniformity throughout species’ entire ranges, neglecting the considerable potential for local adaptation to unique biotic and abiotic pressures within different populations [17,18]. However, empirical studies have demonstrated that integrating genetic variability and population structure into ENMs significantly enhances their predictive capacity regarding range dynamics [17,19,20]. Therefore, incorporating insights from population structure and/or phylogenetic relationships into ENMs is crucial and should be a top priority when deciphering niche dynamics and projecting species range shifts in response to anticipated climate change using ENMs [18,20]. Omitting this information can lead to inaccurate assessments of sub-taxon niche variation and biased interpretations, especially for populations or intraspecific lineages occupying distinct habitats [21,22].
Although species attempt to track their optimal climatic niches by shifting their ranges in response to changing climates, climate change has already posed numerous challenges to the genetic connectivity of many organisms [23]. Robust habitat connectivity is paramount in enabling species to track suitable areas that can support large, genetically diverse populations, thereby bolstering their adaptive potential in a changing climate [23,24]. When implementing management strategies for at-risk species, understanding the magnitude of population connectivity is critical. It informs the identification of key habitats and corridors connecting populations, sheds light on the possible impact of human activities on these vital connections, guides reintroduction and translocation efforts, and ultimately aids in formulating substantial strategies aimed at maintaining genetic diversity and species persistence [25,26,27,28]. Within the existing range of a species, strong population connectivity may manifest as climate stability compared to regions with weak or absent linkages, which are known as climate change refugia [29,30]. The magnitude of the threat posed by climate change to a specific linkage can direct conservation efforts towards that linkage, including the maintenance and enhancement of existing connectivity, the implementation of adaptation strategies such as assisted migration, or the establishment of alternative linkages in regions with elevated threat levels [31,32]. It is worth noting that habitat connectivity and dispersal corridors are unique for most species [23,33]. Therefore, deciphering the extent and patterns of population connectivity is paramount in determining tailored conservation actions for a given species.
In this study, we focus on Emmenopterys henryi, a remarkably widespread Tertiary relict and endangered species that predominantly inhabits montane warm-temperate deciduous (WTD) forests in subtropical China [34,35], indicating its remarkable adaptability to diverse environments. This stately canopy tree stands as the sole survivor of a once extensive and species-rich genus that flourished in the boreotropical floras of North America and Eurasia [36]. Empirical studies of E. henryi have predominantly focused on various aspects, including community structure [37], population dynamics [38], genetic differentiation [39], demographic history [40], and reproductive capacity [38]. However, the current distribution of E. henryi in China is becoming increasingly fragmented and diminished, primarily due to anthropogenic factors such as overlogging, tourism, and habitat loss [37]. To date, several ecological studies have been undertaken to comprehend and identify the underlying factors responsible for the endangered status of E. henryi within natural populations [41] and references therein. These investigations aim to shed light on the critical ecological and anthropogenic pressures facing this exceptional tree species, with the ultimate goal of informing conservation efforts and ensuring its long-term persistence. Although a previous study illuminated the existence of two distinctive intraspecific lineages within this species based on three cpDNA markers and then further predicted its range dynamics under alternative climates [40], the niche dynamics and patterns of population connectivity below the species level remain elusive. This lack of clarity poses a considerable hindrance to implementing effective management interventions for this species.
Here, we aim to bridge the gaps by addressing range and niche dynamics and exploring patterns of population connectivity within and between the two intraspecific lineages of E. henryi. Specifically, we harnessed an ensemble modeling approach to approximate the potential ranges of this species and its intraspecific lineages under current climates. Subsequently, we employed the PCA-env method to evaluate the niche dynamics between these closely related lineages. Finally, following the framework of landscape genetics, we evaluated the magnitude of genetic connectivity within and between the two intraspecific lineages. Our findings are anticipated to offer a multifaceted perspective on niche dynamics and population connectivity within E. henryi, thereby providing deeper insights that can inform conservation decision-making and management interventions for this species.
2. Materials and Methods
2.1. Species Occurrences and Bioclimatic Data
In the present study, occurrences of E. henryi were obtained from Zhang et al. [40], resulting in the inclusion of 38 points across its range (Table S1). Subsequently, we allocated these points to their respective intraspecific lineages within this species, using the result of a phylogenetic tree constructed by three cpDNA fragments (e.g., psbA-trnH, trnL-trnF, trnT-trnL) in Zhang et al. [40] as a priori. In consequence, the 38 occurrences were divided into two distinct subsets; 15 occurrences belonged to the northern lineage of E. henryi, while the remaining points were assigned to the southern lineage of this species (also see Figure 1b in Zhang et al. [40]). Although a previous study indicated that a minimum of 13 occurrences were required for accurately assessing the potential ranges of widespread species using ENMs [42], given the significant impact of absences on ENMs results [43,44], we employed the R package biomod2 [45] to generate pseudo-absences for this species and its intraspecific lineages when evaluating their potential ranges under current environmental conditions (see details in Section 2.2).
Due to the critical roles that temperature and precipitation play in species richness and diversity patterns [46], we restricted our focus to temperature- and precipitation-related variables (i.e., 19 bioclimatic variables) at the 1970–2000 time point to assess the contemporary range of E. henryi. All the 19 bioclimatic variables were downloaded from WorldClim v2.1 [47] at a resolution of 2.5 arc minutes (~5 km2), and then we extracted detail values for each of the 38 points associated with E. henryi data. To mitigate the potential impact of strong co-linearity among environmental variables on model accuracy in ENMs, we carefully screened for highly correlated variables (Pearson’s correlations |r| > 0.8) after considering variable contributions towards the ENMs result for our focal species (Figures S1 and S2, Table S2) that were evaluated using all 19 bioclimatic variables via MAXENT v3.3.3k [48]. Finally, we narrowed down our selection to five uncorrelated variables, including isothermality (bio 3), minimum temperature of the coldest month (bio 6), mean temperature of the wettest quarter (bio 8), mean temperature of the warmest quarter (bio 10), and precipitation of the driest month (bio 14).
2.2. Species Distribution Modeling and Bioclimatic Variable Analysis
The ensemble modeling method implemented within the R package biomod2 [45] was employed to assess the potential distribution of E. henryi under contemporary climatic conditions. An ensemble of six widely-used models, including the generalized additive model (GAM), generalized boosted model (GBM), generalized linear model (GLM), maximum entropy (MAXENT), multivariate adaptive regression splines (MARS), and random forest (RF), were created in biomod2. Such an ensemble strategy was chosen in the present study due to its efficacy in capturing variations among different ecological niche model algorithms, thereby enhancing prediction robustness [49,50].
For each of the six used models, we randomly generated 10,000 pseudo-absences across the species’ or lineage’s ranges, assigning equal weights to the presence and absence data [44,51]. While the methodologies employed to generate pseudo-absences exert considerable influence on the model performance of ENMs, it is noteworthy that the widely adopted approach of random sampling may not consistently yield optimal results [52]. Nevertheless, taking into account its simplicity and widespread application in analogous studies for generating pseudo-absences [44,53] and references therein, we opted to utilize the random sampling technique to create pseudo-absences for our focal species. The combined occurrence data were randomly divided into two subsets: 70% for calibration and the remaining 30% for validation. In addition, each model was iterated with five evaluation runs with three permutations, keeping other parameters default. To ensure prediction accuracy, only models with receiver operating characteristic (ROC) [54] greater than 0.8 and true skill statistic (TSS) [55] exceeding 0.6 were retained in the final ensemble model.
For the bioclimatic variable analysis, we employed two approaches to discern environmental disparities between the northern and southern lineages of our focal species. Initially, we utilized the nonparametric Kruskal–Wallis multiple-range test [56] to scrutinize differences in the five uncorrelated environmental variables between the two lineages. Subsequently, a principal component analysis (PCA) was conducted on these five selected variables to ascertain whether the lineages occupy distinct habitats.
2.3. Niche Overlap and Niche Dynamic Tests
The niche dynamics of the northern and southern lineages of E. henryi were compared using the PCA-env method [57]. This approach leverages PCA to transform a complex multivariable environmental space into a more manageable two-dimensional representation. Subsequently, the PCA space, defined by a 100 × 100 grid-cell resolution, is systematically gridded. Within this framework, smoothed densities are calculated using a kernel density function. This computation encompasses both the occurrences of the two lineages in our specific case, the combined data of occurrences and pseudo-absences for both lineages, and the prevalent environmental conditions within the study area. Subsequently, the realized species niche is delineated through a comparative analysis of density functions between lineages and the study area, which takes environmental availability into account within the study region [58].
The measurement of niche overlap between the two intraspecific lineages of our focal species was accomplished using Schöener’s D [59], a metric that ranges from 0 to 1, indicating cases where the niches are completely distinct or fully overlapped in environmental space. This assessment was facilitated by the niche equivalency and niche similarity tests implemented in the R package ecospat [60], which are commonly employed to test hypotheses related to niche divergence and conservatism. The niche equivalency test employs the ecospat.niche.equivalency.test function to assess whether the niches of the two lineages are equivalent. This evaluation is based on random permutations of occurrences between ranges [57,59], providing a robust method to determine niche equivalency. On the other hand, the niche similarity test utilizes the ecospat.niche.similarity.test function to determine whether the niches belonging to the two intraspecific lineages of our focal species are more or less similar than expected by chance. This test involves random shifts of the niches under current climates in the study area [57,59], thereby offering an accurate assessment of niche similarity. In the present study, we aimed to evaluate whether the niches are divergent between the two lineages of E. henryi. To achieve this, we used the parameter alternative = less in the R package ecospat, allowing us to assess whether the observed niche overlap is less than expected by chance. We conducted 1000 permutations to estimate statistical significance levels (α = 5%) for both tests, ensuring robust and reliable results. It is important to note that the niche equivalence and similarity tests can only provide insights into whether niche shifts have occurred; they do not elucidate the underlying causal mechanisms driving these changes.
Additionally, in order to obtain a comprehensive understanding of niche dynamics beyond overlapping regions, we assessed three key indices of niche dynamics (incl. niche stability, niche expansion, and niche unfilling) [58] utilizing the ecospat package. Empirical studies have emphasized the importance of analyzing niche dynamics based on analogous climates between two ranges for meaningful interpretation [58,61]. Consequently, we employed the five bioclimatic variables (i.e., bio 3, bio 6, bio 8, bio 10, and bio 14) to investigate the niche dynamics of the two intraspecific lineages in this study. The aforementioned framework, designed for dissecting niche dynamics and overlap within a species, is uniquely tailored for exploring ecological niche disparities amongst intraspecific lineages inhabiting distinct regions, therefore offering the capability to assess the extent of temporal variation in the environmental niche occupied by intraspecific lineages [57].
2.4. Population Connectivity Analysis
To quantify population connectivity at both the species and intraspecific lineage levels for our focal species, we utilized the least cost path (LCP) method implemented in SDMtoolbox v2.0 [62]. This approach enabled us to map dispersal corridors among populations of E. henryi and its two lineages under current climates (1970–2000). There are two primary prerequisites for population connectivity analysis using LCP [63]: firstly, the presence of highly suitable habitats identified by ENM projections, which facilitate population connectivity across landscapes; secondly, populations sharing haplotypes typically exhibit high probabilities of dispersal among heterogeneous habitats. Given the presence of shared haplotypes across diverse geographical ranges, it is plausible to infer substantial gene flow [63]. By adopting a landscape genetic perspective, we can elucidate the effects of suitable habitats on the genetic connectivity or differentiation of a particular species [64]. Consequently, if comprehensive shared haplotypes between populations and suitable habitats are obtained, it becomes feasible to ascertain the genetic connectivity of a specific species within anticipated climate scenarios using the LCP methodology.
To fulfill these requirements, we initially created a resistance layer for our focal species by inverting the ENM results, which served as a cost distance raster. Subsequently, corridor layers were established between populations based on shared haplotypes obtained from Zhang et al. [40]. We further refined our analysis by applying the categorical LCP approach to account for habitat heterogeneity and its role in dispersal. This was achieved by using percentage of cost path values and classifying each corridor layer into four intervals defined by three cut-off values: 5%, 2%, and 1%. Finally, all pairwise reclassified corridor layers were summarized and standardized, resulting in the identification of explicit dispersal corridors for E. henryi and its two intraspecific lineages across the landscape.
3. Results
3.1. Predicted Ranges of the E. henryi and Its Two Lineages under Current Climates
All individual models obtained relatively high ROC scores when performing the single model projection for our focal species, although there was considerable variation in TSS values among the six selected models (Figure 1). Generally, based on current environmental conditions, the MAXENT model emerged as the most suitable single model for predicting the range of our focal species, while the GLM model proved effective in projecting the ranges of the southern and northern lineages of E. henryi. We conducted separate projections of potential ranges for E. henryi and its two intraspecific lineages; all ensemble models exhibited great discrimination abilities (TSS: 0.876–0.969; ROC: 0.976–0.994, Table S3). Regarding variable significance, bio 6 was the most critical predictor for the northern lineage, while bio 10 emerged as the most important predictor for E. henryi. For the southern lineage, both bio 10 and bio 14 were identified as key predictor variables in the final ensemble ENMs (Table S3).
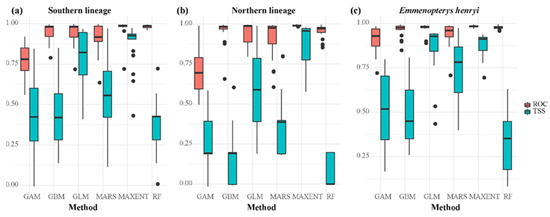
Figure 1.
Evaluation of individual model efficacy based on TSS and ROC values for the potential ranges of (a) the southern lineage, (b) the northern lineage, and (c) Emmenopterys henryi as a whole under current climates.
Under current climatic conditions, our ensemble ENMs revealed distinct distributional ranges for the two intraspecific lineages, despite a high degree of concordance between their predicted ranges and known distributions. Specifically, the projected areas for the southern lineage were predominantly located in central and eastern China, where patchy habitats are prevalent. In contrast, the potential areas for the northern lineage exhibited continuous ranges in western and southwestern China, particularly in regions adjacent to the Sichuan Basin. The overlap areas between the two lineages were primarily concentrated in southwestern China (Figure 2). Utilizing thresholds ranging from 0.5 to 0.75 and 0.75 to 1.0 to delineate moderately and highly suitable ranges for our focal species, respectively, our ENMs results indicated that the moderately suitable range for the northern lineage (ca. 351.7 thousand Km2) was three times larger than that of the southern lineage. Overall, the projected areas with moderate to high suitability scores for E. henryi were ca. 438.9 thousand Km2 (Table S4).
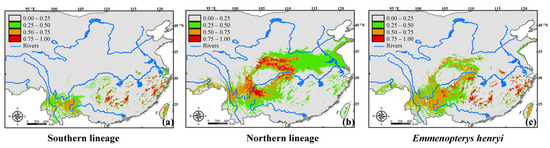
Figure 2.
Ensemble model predictions for the potential ranges of (a) the southern lineage, (b) the northern lineage, and (c) the entire Emmenopterys henryi species under current climatic conditions. Purple and dark red circles indicate the population locations belonging to the southern and northern lineages of our focal species, respectively.
3.2. Bioclimatic Variable Analyses
Our nonparametric Kruskal–Wallis tests identified significant disparities between the two intraspecific lineages in relation to two key variables: a temperature-related factor (bio 6) and a precipitation-related factor (bio 14). Notably, both of these variables were elevated in the southern lineage when compared to their northern counterparts (Figure 3). In contrast, the remaining three predictor variables exhibited no significant variations between the lineages. Additionally, our PCA result based on five selected bioclimatic variables highlighted that the vast majority of the total variance (95.83%) was captured by the first two principal components (PCs). Specifically, PC1 accounted for 89.42% of the variance, while PC2 contributed an additional 6.41% (Figure 3f). The clear separation observed along these two PCs underscores the ecological differentiation that has emerged between the two clusters, with only minimal overlap observed.
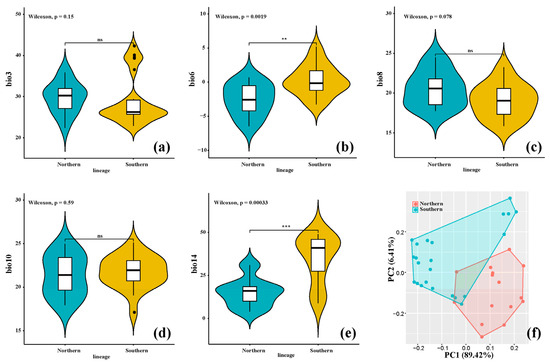
Figure 3.
Results of bioclimatic variable analyses. (a–e) Results of nonparametric Kruskal–Wallis multiple-range tests for five key bioclimatic variables between the two lineages of Emmenopterys henryi. (f) The PCA outcome is based on the same selected bioclimatic variables for the distinct lineages of E. henryi. Significance levels for these tests are indicated (ns: non-significant; ** p < 0.01, *** p < 0.001).
3.3. Niche Dynamics of the Two Intraspecific Lineages
The kernel density plots mapped within environmental space revealed some variations in the climatic niches inhabited by distinct intraspecific lineages of E. henryi (Figure 4a,b). Our niche dynamic analysis further underscored a low degree of niche overlap (D = 0.079; Figure 4d) between these lineages, as retrieved from environmental data. Within the PCA-env space, the first two principal components collectively accounted for 82.35% of the data variability. The primary PCA axis exhibited positive correlations with several climatic factors, including the minimum temperature of the coldest month (bio 6), mean temperature of the wettest quarter (bio 8), mean temperature of the warmest quarter (bio 10), and precipitation of the driest month (bio 14), while exhibiting a negative relationship with isothermality (bio 3). The secondary PCA axis, meanwhile, was positively aligned with precipitation in the driest month (bio 14) and negatively with isothermality (bio 3) (Figure 4c). Based on the niche equivalence test, we found compelling evidence to accept the hypothesis of niche divergence (p < 0.05) between the two intraspecific lineages. However, in the niche similarity test, no statistically significant differences were observed between the northern and southern lineages (Figure 4e,f).
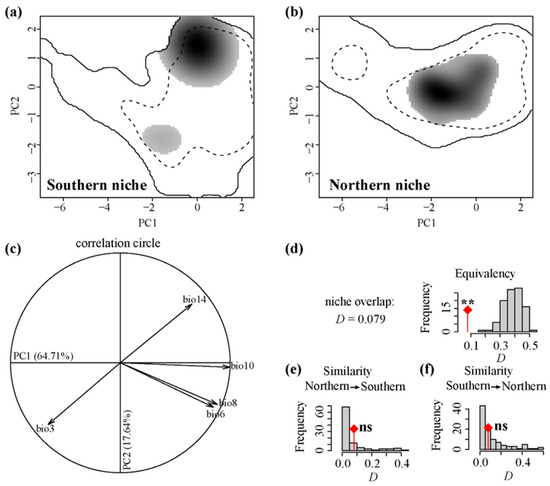
Figure 4.
The niche dynamics of the two lineages of Emmenopterys henryi in climate space were explored via principal component analysis (PCA-env). Panels (a,b) illustrate the niche occupation along the first two principal components for the southern and northern lineages, respectively. Gray-shadowed areas denote the density of occurrences for each lineage per cell. Solid and dashed lines delineate 100% and 50% of the available environmental space, respectively. (c) Details of the contribution of environmental variables to the first two PCA axes and the percentage of inertia explained by these axes. Panels (d–f) depict the observed niche overlap (D) between the two lineages (indicated by diamond-tipped bars) alongside simulated niche overlaps (gray bars) derived from tests of niche equivalency and niche similarity. Significance levels for these tests are indicated (ns: non-significant; ** p < 0.01).
Furthermore, our niche dynamic analyses revealed a pronounced degree of niche expansion (expansion = 0.729) in western and southwestern China, despite detecting moderate niche stability (stability = 0.271) (Figure S3). It is noteworthy that, in these regions, the northern lineage of E. henryi occupies a substantial area within the PCA space (Figure S3), indicating its adaptive capability to a wide array of climatic conditions. The observed niche shifts were predominantly attributed to the processes of niche expansion and niche unfilling (unfilling = 0.658).
3.4. Patterns of Population Connectivity between and within Two Lineages
Under current climates, the putative dispersal corridors between and within the two lineages were visualized based on the shared haplotypes derived from the concatenated cpDNA dataset. Our result indicated the existence of dispersal corridors between and within the two intraspecific lineages (Figure 5). Specifically, for the southern lineage, moderate dispersal routes were observed for populations situated in southwestern and central China. Populations located in the easternmost regions appeared to have one or more low-resistance dispersal routes among them (Figure 5a). Regarding the northern lineage, it was apparent that most populations exhibited strong connectivity, particularly those situated near the Sichuan Basin. However, for some peripheral populations occupying the northern lineage’s range edge, their dispersal routes encountered relatively higher resistance (Figure 5b). Furthermore, our analysis identified only one dispersal route with moderate resistance between the northern and southern lineages. This route connected some southern populations of the northern lineage to three populations located at the northwestern-most fringe of the southern lineage’s range (Figure 5c).
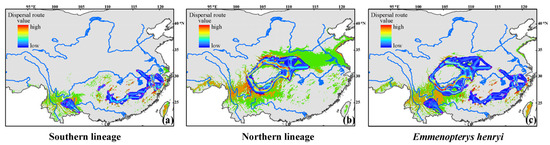
Figure 5.
Potential dispersal routes within (a) the southern lineage, (b) the northern lineage, and (c) the entire range of Emmenopterys henryi. Purple circles indicate the geographic locations of our focal species populations.
4. Discussion
4.1. Distinct Trajectories of Climate Divergence between the Intraspecific Lineages
Species frequently harbor distinct lineages across their ranges, and the adaptation potentials to future climate change can vary substantially due to their unique responses to local climates, leading to distinctly evolutionary trajectories [22,65,66,67]. Thus, modeling the ecological niches of intraspecific lineages can reveal that they deviate significantly from projections made at the species level [22]. Some landmark studies have advocated for the integration of evolutionary information, derived from neutral or adaptive genetic markers, into niche modeling [17,18]. However, despite this progress, there remains a scarcity of split models that incorporate intraspecific variation at the lineage level into ENMs, highlighting the need for more such studies to enhance our understanding of how species respond to climate change.
In the present study, we employed split models to evaluate the suitable habitats for each lineage of E. henryi. The notable differences in climate niches and geographic distributions between the two intraspecific lineages likely resulted in their niche divergence (Figure 2 and Figure 4). In addition, the first two axes of the PCA, accounting for ca. 96% of the variation in climate between the two lineages, underscored their climate inconsistency (Figure 3). These findings suggested that the two lineages have adapted differently to anticipated climate change, aligning with previous studies indicating that distinct lineages or ecotypes occupy different climate regimes [17,20,67]. Empirical studies have indicated that niche divergence often evolves early as divergent selection pressures drive organisms to adapt to distinct ecological niches, ultimately leading to reproductive isolation and speciation [68,69]. Considering the repeated climate changes and the availability of suitable habitats for the two lineages during the Quaternary, which might have imposed divergent selection pressures [40], it is plausible that if these trends persist in the face of future climate change, the lineages will continue on independent trajectories of genetic and climatic divergence, potentially leading to the emergence of new species.
Our results also demonstrated that the potential range based on all occurrences of E. henryi was smaller than the combined potential ranges of the southern and northern lineages under current climates (Figure 2, Table S4), thus supporting the idea that lump and split models generate different distribution estimates when performing ENMs [22]. In line with our findings, previous studies indeed reported that suitable habitats predicted by combined split models tend to be larger than those predicted by lump models, as split models often predict suitable areas that are geographically peripheral to those predicted by lump models [19,21,70]. Although it has been suggested that neglecting intraspecific variation in ENMs can lead to overly pessimistic forecasts of range dynamics for species [19], in the absence of evidence for niche divergence between intraspecific lineages, it is recommended to include all available occurrences when performing ENMs based on the lump model [70]. However, we caution the readers here that the projections of range dynamics under current climates for our focal species are only based on low present occurrences. Previous studies had demonstrated that model performance was strongly impacted by sample size, with increased sample sizes leading to enhanced SDM performance and reduced variability in model outcomes [42,71]. While predictions of range dynamics can be highly stable when sample sizes reach a certain threshold, if they fall below a specific value, the predicted ranges may be insufficient for generating reliable models [71].
4.2. Population Connectivity under Current Climates
Ecological corridors, which exert a beneficial influence on biodiversity and species dispersal, play a pivotal role in enabling species to track optimal climatic conditions [24]. These corridors have been extensively utilized as a significant management intervention to increase habitat connectivity and advance biodiversity conservation efforts [72]. Traditionally, a spatially explicit framework, rooted in habitat resistance models and approximations of the LCP, has been the predominant method for delineating ecological corridors and population connectivity patterns [73]. In the present study, by employing ENMs, molecular data, and the LCP approach, our study seeks to gain a deeper understanding of population connectivity within E. henryi.
Our findings indicated that, within the Hengduan Mountains region, a dispersal route encircling the Sichuan Basin connected a significant portion of the populations belonging to the northern lineage (Figure 5b), implying the high-elevation mountains and intricate river drainages may not impede population dispersal in this region. This could be attributed to the favorable niche suitability for the northern lineage populations in many of the high-altitude mountains (Figure 2b). In alignment with our results, recent studies have also unveiled notable population connectivity adjacent to the Sichuan Basin [74,75], thereby corroborating our findings. Intuitively, when considering population connectivity in the southern lineage, the landscape characteristics of central and eastern China, dominated by vast plains and smaller mountain ranges [76], appear to facilitate population dispersals in these areas. However, our analysis indicated that some moderate dispersal routes seemed to provide connectivity among populations in these areas (Figure 2a), which could be attributed to the fragmented habitats and the associated high costs of resistance encountered by populations residing in these regions.
It is important to note that our study primarily focused on elucidating population connectivity patterns within and between intraspecific lineages in current climates. Nevertheless, a recent study highlights that anticipated climate change may significantly alter the availability of suitable habitats and subsequently impact the viability of dispersal routes necessary to access newly suitable habitats [23]. However, relying solely on neutral molecular markers to assess genetic connectivity within a given species may underestimate non-neutral genetic diversity and adaptive variance [77,78], leading to unrealistic population connectivity within the species under alternative climate scenarios. Future studies focusing on its genetic connectivity should incorporate adaptive molecular markers to obtain a more holistic understanding of population connectivity patterns within this species.
4.3. Implications for Conservation
Appropriate delineation of conservation units (CUs) is paramount for effective management aimed at safeguarding endangered species. This is because CUs serve as the foundation for conservation efforts, guiding strategies to bolster population recovery and optimize evolutionary potential [79,80]. Among the most widely utilized CUs are those based on the evolutionarily significant unit (ESU) or evolutionarily sustaining conservation unit (ESCU), which represents essential components of intraspecific variability on the basis of genetic, phenotypic, ecological, geographical, and life history attributes [81,82]. Given compelling evidence indicating that two distinct intraspecific lineages of E. henryi have persisted for ca. 5.06 million years (95% HPD: 1.68–8.91 million years) [40], coupled with niche divergence observed in our study, it is imperative to recognize these lineages as separate CUs when conducting conservation interventions.
Furthermore, we propose that priority actions should be directed towards the populations situated in the northeastern-most areas of the northern lineage’s range, as well as populations of the southern lineage located in central regions. This recommendation stems from the limited genetic connectivity observed among these populations (Figure 5). Additionally, the southwestern-most populations within the southern lineage necessitate urgent conservation attention due to their prolonged isolation from other southern lineage populations, despite exhibiting moderate genetic connectivity among themselves. To bolster genetic diversity and mitigate the risk of maladaptation to anticipated climate change, a promising conservation strategy may involve carefully planned translocations aimed at enhancing genetic connectivity among the above populations [26,83]. Indeed, efforts to increase population connectivity are instrumental in bolstering the adaptive capacity for forthcoming climate change [23]. Moreover, it has been proposed that contact zones often function as reservoirs of genetic diversity, thereby playing critical roles in species resilience amidst climate change [84]. Consequently, the three populations situated at the northwestern-most fringe of the southern lineage’s range also warrant conservation concerns.
5. Conclusions
In this study, we evaluated niche dynamics and genetic connectivity within E. henryi while also unveiling the potential ranges of its two lineages under current climates. Our findings revealed that the two intraspecific lineages occupy divergent niches, with minimal overlap observed in terms of climatic conditions and suitable ranges. This lack of overlap further substantiates the niche divergence between the two lineages. Furthermore, our analysis of population connectivity demonstrated the existence of dispersal routes within each lineage, albeit with varying degrees of connectivity, while the connectivity between the two lineages was limited. Our study underscores the importance of employing fine-scale ENMs and genetic connectivity analyses to enhance our understanding of niche evolution and genetic connectivity, especially for species exhibiting discrete intraspecific lineages. Given the seemingly rapid genetic erosion linked to habitat loss and fragmentation, which is commonly observed in species inhabiting the regions we focus on here, our findings provide valuable insights for conservation practitioners and policymakers to develop targeted conservation interventions aimed at restoring connectivity and increasing local population sizes for this endangered species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15020316/s1. Figure S1: The correlated outcomes of 19 bioclimatic variables. The upper-right diagonal matrix indicates the correlation coefficients, where the size of each coefficient is proportionally scaled to its |r|. The bivariate scatter plots and histograms of 19 bioclimatic variables are presented below and on diagonals, respectively. Figure S2: The gain values of the jackknife test based on 38 occurrences of the Emmenopterys henryi for (a) training and (b) test data when performing ENMs via Maxent. (c) Results showing the AUC values of the jackknife test for each bioclimatic variable. Figure S3: Niches of the two lineages of Emmenopterys henryi in climatic space. Unfilled and expanded niches are represented by green and red shades, respectively. The blue shade revealed by a red arrow represents the stable niches shared by two lineages. Table S1: Geographic information and haplotypes of 38 Emmenopterys henryi populations used in this study. Table S2: Variable contributions for ENMs using Maxent based on 38 occurrences of Emmenopterys henryi. Table S3: Variable importance and values of TSS and ROC for ensemble modeling at the lineage and species levels. Table S4: Areas of good and high suitability for Emmenopterys henryi at the lineage and species levels.
Author Contributions
L.F., Y.-H.Z. and Y.-H.W. planned and designed the research; L.F., Z.-Y.W. and T.Z. analyzed the data; L.F. and Z.-Y.W. reviewed the data and edited the manuscript. Y.-H.W. improved the English language and grammatical editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-supported by the National Natural Science Foundation of China, grant numbers 32271550 and 32371911.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank Xu-Mei Wang and Li Yang for their constructive feedback on an earlier draft.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Malhi, Y.; Franklin, J.; Seddon, N.; Solan, M.; Turner, M.G.; Field, C.B.; Knowlton, N. Climate change and ecosystems: Threats, opportunities and solutions. Philos. T. R. Soc. B 2020, 375, 20190104. [Google Scholar] [CrossRef]
- Bernatchez, L.; Ferchaud, A.-L.; Berger, C.S.; Venney, C.J.; Xuereb, A. Genomics for monitoring and understanding species responses to global climate change. Nat. Rev. Genet. 2023, in press. [Google Scholar] [CrossRef]
- Exposito-Alonso, M.; Booker, T.R.; Czech, L.; Gillespie, L.; Hateley, S.; Kyriazis, C.C.; Lang, P.L.M.; Leventhal, L.; Nogues-Bravo, D.; Pagowski, V.; et al. Genetic diversity loss in the Anthropocene. Science 2022, 377, 1431–1435. [Google Scholar] [CrossRef]
- Gaitán-Espitia, J.D.; Hobday, A.J. Evolutionary principles and genetic considerations for guiding conservation interventions under climate change. Glob. Chang. Biol. 2021, 27, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Kerry, J.T.; Baird, A.H.; Connolly, S.R.; Chase, T.J.; Dietzel, A.; Hill, T.; Hoey, A.S.; Hoogenboom, M.O.; Jacobson, M.; et al. Global warming impairs stock–recruitment dynamics of corals. Nature 2019, 568, 387–390. [Google Scholar] [CrossRef]
- Inoue, K.; Berg, D.J. Predicting the effects of climate change on population connectivity and genetic diversity of an imperiled freshwater mussel, Cumberlandia monodonta (Bivalvia: Margaritiferidae), in riverine systems. Glob. Chang. Biol. 2017, 23, 94–107. [Google Scholar] [CrossRef]
- Guyennon, A.; Reineking, B.; Salguero-Gomez, R.; Dahlgren, J.; Lehtonen, A.; Ratcliffe, S.; Ruiz-Benito, P.; Zavala, M.A.; Kunstler, G. Beyond mean fitness: Demographic stochasticity and resilience matter at tree species climatic edges. Glob. Ecol. Biogeogr. 2023, 32, 573–585. [Google Scholar] [CrossRef]
- Pillet, M.; Goettsch, B.; Merow, C.; Maitner, B.; Feng, X.; Roehrdanz, P.R.; Enquist, B.J. Elevated extinction risk of cacti under climate change. Nat. Plants 2022, 8, 366–372. [Google Scholar] [CrossRef]
- Rumpf, S.B.; Hülber, K.; Wessely, J.; Willner, W.; Moser, D.; Gattringer, A.; Klonner, G.; Zimmermann, N.E.; Dullinger, S. Extinction debts and colonization credits of non-forest plants in the European Alps. Nat. Commun. 2019, 10, 4293. [Google Scholar] [CrossRef] [PubMed]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211. [Google Scholar] [CrossRef] [PubMed]
- Poppenwimer, T.; Mayrose, I.; DeMalach, N. Revising the global biogeography of annual and perennial plants. Nature 2023, 624, 109–114. [Google Scholar] [CrossRef]
- Loiseau, N.; Mouquet, N.; Casajus, N.; Grenié, M.; Guéguen, M.; Maitner, B.; Mouillot, D.; Ostling, A.; Renaud, J.; Tucker, C.; et al. Global distribution and conservation status of ecologically rare mammal and bird species. Nat. Commun. 2020, 11, 5071. [Google Scholar] [CrossRef]
- Faurby, S.; Araújo, M.B. Anthropogenic range contractions bias species climate change forecasts. Nat. Clim. Chang. 2018, 8, 252–256. [Google Scholar] [CrossRef]
- Casazza, G.; Abeli, T.; Bacchetta, G.; Dagnino, D.; Fenu, G.; Gargano, D.; Minuto, L.; Montagnani, C.; Orsenigo, S.; Peruzzi, L.; et al. Combining conservation status and species distribution models for planning assisted colonisation under climate change. J. Ecol. 2021, 109, 2284–2295. [Google Scholar] [CrossRef]
- Liu, C.; Wolter, C.; Xian, W.; Jeschke, J.M. Most invasive species largely conserve their climatic niche. Proc. Natl. Acad. Sci. USA 2020, 117, 23643. [Google Scholar] [CrossRef]
- Razgour, O.; Forester, B.; Taggart, J.B.; Bekaert, M.; Juste, J.; Ibáñez, C.; Puechmaille, S.J.; Novella-Fernandez, R.; Alberdi, A.; Manel, S. Considering adaptive genetic variation in climate change vulnerability assessment reduces species range loss projections. Proc. Natl. Acad. Sci. USA 2019, 116, 10418. [Google Scholar] [CrossRef]
- Ikeda, D.H.; Max, T.L.; Allan, G.J.; Lau, M.K.; Shuster, S.M.; Whitham, T.G. Genetically informed ecological niche models improve climate change predictions. Glob. Chang. Biol. 2017, 23, 164–176. [Google Scholar] [CrossRef]
- Smith, A.B.; Godsoe, W.; Rodríguez-Sánchez, F.; Wang, H.-H.; Warren, D. Niche estimation above and below the species level. Trends Ecol. Evol. 2019, 34, 260–273. [Google Scholar] [CrossRef]
- Valladares, F.; Matesanz, S.; Guilhaumon, F.; Araújo, M.B.; Balaguer, L.; Benito-Garzón, M.; Cornwell, W.; Gianoli, E.; van Kleunen, M.; Naya, D.E.; et al. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 2014, 17, 1351–1364. [Google Scholar] [CrossRef]
- Maguire, K.C.; Shinneman, D.J.; Potter, K.M.; Hipkins, V.D. Intraspecific niche models for ponderosa pine (Pinus ponderosa) suggest potential variability in population-level response to climate change. Syst. Biol. 2018, 67, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Pearman, P.B.; D’Amen, M.; Graham, C.H.; Thuiller, W.; Zimmermann, N.E. Within-taxon niche structure: Niche conservatism, divergence and predicted effects of climate change. Ecography 2010, 33, 990–1003. [Google Scholar] [CrossRef]
- Hällfors, M.H.; Liao, J.; Dzurisin, J.; Grundel, R.; Hyvärinen, M.; Towle, K.; Wu, G.C.; Hellmann, J.J. Addressing potential local adaptation in species distribution models: Implications for conservation under climate change. Ecol. Appl. 2016, 26, 1154–1169. [Google Scholar] [CrossRef]
- Littlefield, C.E.; Krosby, M.; Michalak, J.L.; Lawler, J.J. Connectivity for species on the move: Supporting climate-driven range shifts. Front. Ecol. Environ. 2019, 17, 270–278. [Google Scholar] [CrossRef]
- McGuire, J.L.; Lawler, J.J.; McRae, B.H.; Nuñez, T.A.; Theobald, D.M. Achieving climate connectivity in a fragmented landscape. Proc. Natl. Acad. Sci. USA 2016, 113, 7195–7200. [Google Scholar] [CrossRef]
- Lloyd-Jones, L.R.; Brien, M.L.; Feutry, P.; Lawrence, E.; Beri, P.; Booth, S.; Coulson, S.; Baylis, S.M.; Villiers, K.; Taplin, L.E.; et al. Implications of past and present genetic connectivity for management of the saltwater crocodile (Crocodylus porosus). Evol. Appl. 2023, 16, 911–935. [Google Scholar] [CrossRef]
- Frankham, R. Genetic rescue of small inbred populations: Meta-analysis reveals large and consistent benefits of gene flow. Mol. Ecol. 2015, 24, 2610–2618. [Google Scholar] [CrossRef]
- Jangjoo, M.; Matter, S.F.; Roland, J.; Keyghobadi, N. Connectivity rescues genetic diversity after a demographic bottleneck in a butterfly population network. Proc. Natl. Acad. Sci. USA 2016, 113, 10914–10919. [Google Scholar] [CrossRef]
- Lowe, W.H.; Allendorf, F.W. What can genetics tell us about population connectivity? Mol. Ecol. 2010, 19, 3038–3051. [Google Scholar] [CrossRef] [PubMed]
- Keppel, G.; Van Niel, K.P.; Wardell-Johnson, G.W.; Yates, C.J.; Byrne, M.; Mucina, L.; Schut, A.G.T.; Hopper, S.D.; Franklin, S.E. Refugia: Identifying and understanding safe havens for biodiversity under climate change. Glob. Ecol. Biogeogr. 2012, 21, 393–404. [Google Scholar] [CrossRef]
- Morelli, T.L.; Daly, C.; Dobrowski, S.Z.; Dulen, D.M.; Ebersole, J.L.; Jackson, S.T.; Lundquist, J.D.; Millar, C.I.; Maher, S.P.; Monahan, W.B.; et al. Managing climate change refugia for climate adaptation. PLoS ONE 2016, 11, e0159909. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, J.S.; Hellmann, J.J.; Schwartz, M.W. A framework for debate of assisted migration in an era of climate change. Conserv. Biol. 2007, 21, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Hällfors, M.H.; Aikio, S.; Schulman, L.E. Quantifying the need and potential of assisted migration. Biol. Conserv. 2017, 205, 34–41. [Google Scholar] [CrossRef]
- Marrotte, R.R.; Bowman, J.; Brown, M.G.C.; Cordes, C.; Morris, K.Y.; Prentice, M.B.; Wilson, P.J. Multi-species genetic connectivity in a terrestrial habitat network. Mov. Ecol. 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Jin, J. Red List of Endangered Plants in China; Science Press: Beijing, China, 1992. [Google Scholar]
- Chen, T.; Taylor, C.M.P. Rubiaceae-Emmenopterys. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2013; Volume 19, pp. 1989–2013. [Google Scholar]
- Manchester, S.R.; Chen, Z.-D.; Lu, A.-M.; Uemura, K. Eastern Asian endemic seed plant genera and their paleogeographic history throughout the Northern Hemisphere. J. Syst. Evol. 2009, 47, 1–42. [Google Scholar] [CrossRef]
- Ma, M.; Wu, Y.; Zhang, Y.; Kang, H.; Chen, Z.; Liu, P. Sprouting as a survival strategy for non-coniferous trees: Relation to population structure and spatial pattern of Emmenopterys henryi (Rubiales). Acta Ecologica Sinica 2019, 39, 1–8. [Google Scholar] [CrossRef]
- Guo, L.; Shao, X.; Xue, P.; Tian, Y.; Xiao, Z.; Wu, Y. Root sprouting ability and growth dynamics of the rootsuckers of Emmenopterys henryi, a rare and endangered plant endemic to China. Forest Ecol. Manag. 2017, 389, 35–45. [Google Scholar] [CrossRef]
- Xu, W.-Q.; Comes, H.P.; Feng, Y.; Zhang, Y.-H.; Qiu, Y.-X. A test of the centre–periphery hypothesis using population genetics in an East Asian Tertiary relict tree. J. Biogeogr. 2021, 48, 2853–2864. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Wang, I.J.; Comes, H.P.; Peng, H.; Qiu, Y.-X. Contributions of historical and contemporary geographic and environmental factors to phylogeographic structure in a Tertiary relict species, Emmenopterys henryi (Rubiaceae). Sci. Rep. 2016, 6, 24041. [Google Scholar] [CrossRef]
- Niu, Y.; Bhatt, A.; Peng, Y.; Chen, W.; Gao, Y.; Zhan, X.; Zhang, Z.; Hu, W.; Song, M.; Yu, Z. Genetic diversity and population structure analysis of Emmenopterys henryi Oliv., an endangered relic species endemic to China. Genet. Resour. Crop Evol. 2021, 68, 1135–1148. [Google Scholar] [CrossRef]
- van Proosdij, A.S.J.; Sosef, M.S.M.; Wieringa, J.J.; Raes, N. Minimum required number of specimen records to develop accurate species distribution models. Ecography 2016, 39, 542–552. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Hortal, J. The uncertain nature of absences and their importance in species distribution modelling. Ecography 2010, 33, 103–114. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Thuiller, W.; Bruno, L.; Robin, E.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Antonelli, A.; Kissling, W.D.; Flantua, S.G.A.; Bermúdez, M.A.; Mulch, A.; Muellner-Riehl, A.N.; Kreft, H.; Linder, H.P.; Badgley, C.; Fjeldså, J.; et al. Geological and climatic influences on mountain biodiversity. Nat. Geosci. 2018, 11, 718–725. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.B.; Anderson, R.P.; Márcia Barbosa, A.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B.; et al. Standards for distribution models in biodiversity assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef]
- Bellard, C.; Thuiller, W.; Leroy, B.; Genovesi, P.; Bakkenes, M.; Courchamp, F. Will climate change promote future invasions? Glob. Chang. Biol. 2013, 19, 3740–3748. [Google Scholar] [CrossRef]
- Iturbide, M.; Bedia, J.; Herrera, S.; del Hierro, O.; Pinto, M.; Gutiérrez, J.M. A framework for species distribution modelling with improved pseudo-absence generation. Ecol. Model. 2015, 312, 166–174. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Glob. Ecol. Biogeogr. 2012, 21, 498–507. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 2002, 24, 38–49. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.-J.; Randin, C.; Zimmermann, N.E.; et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Guisan, A.; Petitpierre, B.; Broennimann, O.; Daehler, C.; Kueffer, C. Unifying niche shift studies: Insights from biological invasions. Trends Ecol. Evol. 2014, 29, 260–269. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef] [PubMed]
- Cola, V.D.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Fitzpatrick, M.C.; Hargrove, W.W. The projection of species distribution models and the problem of non-analog climate. Biodivers. Conserv. 2009, 18, 2255. [Google Scholar] [CrossRef]
- Brown, J.L. SDMtoolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Chan, L.M.; Brown, J.L.; Yoder, A.D. Integrating statistical genetic and geospatial methods brings new power to phylogeography. Mol. Phylogenet. Evol. 2011, 59, 523–537. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Liu, L.; Qi, W.; Li, S.; Hu, Z. Combining the least cost path method with population genetic data and species distribution models to identify landscape connectivity during the late Quaternary in Himalayan hemlock. Ecol. Evol. 2015, 5, 5781–5791. [Google Scholar] [CrossRef]
- Shaw, R.G.; Etterson, J.R. Rapid climate change and the rate of adaptation: Insight from experimental quantitative genetics. New Phytol. 2012, 195, 752–765. [Google Scholar] [CrossRef]
- Prasad, A.M.; Potter, K.M. Macro-scale assessment of demographic and environmental variation within genetically derived evolutionary lineages of eastern hemlock (Tsuga canadensis), an imperiled conifer of the eastern United States. Biodivers. Conserv. 2017, 26, 2223–2249. [Google Scholar] [CrossRef]
- Theodoridis, S.; Patsiou, T.S.; Randin, C.; Conti, E. Forecasting range shifts of a cold-adapted species under climate change: Are genomic and ecological diversity within species crucial for future resilience? Ecography 2018, 41, 1357–1369. [Google Scholar] [CrossRef]
- Arnegard, M.E.; McGee, M.D.; Matthews, B.; Marchinko, K.B.; Conte, G.L.; Kabir, S.; Bedford, N.; Bergek, S.; Chan, Y.F.; Jones, F.C. Genetics of ecological divergence during speciation. Nature 2014, 511, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Wiens, J.J. How does climate influence speciation? Am. Nat. 2013, 182, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Collart, F.; Hedenäs, L.; Broennimann, O.; Guisan, A.; Vanderpoorten, A. Intraspecific differentiation: Implications for niche and distribution modelling. J. Biogeogr. 2021, 48, 415–426. [Google Scholar] [CrossRef]
- Tessarolo, G.; Rangel, T.F.; Araújo, M.B.; Hortal, J. Uncertainty associated with survey design in Species Distribution Models. Divers. Distrib. 2014, 20, 1258–1269. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Damschen, E.I.; Evans, D.M.; Johnson, B.L.; Levey, D.J.; Orrock, J.L.; Resasco, J.; Sullivan, L.L.; Tewksbury, J.J.; et al. Potential negative ecological effects of corridors. Conserv. Biol. 2014, 28, 1178–1187. [Google Scholar] [CrossRef]
- Storfer, A.; Murphy, M.A.; Spear, S.F.; Holderegger, R.; Waits, L.P. Landscape genetics: Where are we now? Mol. Ecol. 2010, 19, 3496–3514. [Google Scholar] [CrossRef]
- Rana, S.K.; Luo, D.; Rana, H.K.; O’Neill, A.R.; Sun, H. Geoclimatic factors influence the population genetic connectivity of Incarvillea arguta (Bignoniaceae) in the Himalaya–Hengduan Mountains biodiversity hotspot. J. Syst. Evol. 2021, 59, 151–168. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, X.-J.; Jiang, X.-L.; Li, C.; Li, X.-P.; Li, W.-P.; Tian, D.-K. Spatial genetic patterns and distribution dynamics of Begonia grandis (Begoniaceae), a widespread herbaceous species in China. Front. Plant Sci. 2023, 14, 1178245. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Deng, T.; Zhou, Z.; Sun, H. Is the East Asian flora ancient or not? Natl. Sci. Rev. 2018, 5, 920–932. [Google Scholar] [CrossRef]
- Holderegger, R.; Kamm, U.; Gugerli, F. Adaptive vs. neutral genetic diversity: Implications for landscape genetics. Landsc. Ecol. 2006, 21, 797–807. [Google Scholar] [CrossRef]
- Reed, D.H.; Frankham, R. How closely correlated are molecular and quantitative measures of genetic variation? A meta-analysis. Evolution 2001, 55, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Funk, W.C.; McKay, J.K.; Hohenlohe, P.A.; Allendorf, F.W. Harnessing genomics for delineating conservation units. Trends Ecol. Evol. 2012, 27, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, S.; Mestre, F.; White, T.A.; Paupério, J.; Alves, P.C.; Searle, J.B. Integrative approaches to guide conservation decisions: Using genomics to define conservation units and functional corridors. Mol. Ecol. 2018, 27, 3452–3465. [Google Scholar] [CrossRef] [PubMed]
- Hoelzel, A.R. Where to now with the evolutionarily significant unit? Trends Ecol. Evol. 2023, 38, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Forester, B.R.; Murphy, M.; Mellison, C.; Petersen, J.; Pilliod, D.S.; Van Horne, R.; Harvey, J.; Funk, W.C. Genomics-informed delineation of conservation units in a desert amphibian. Mol. Ecol. 2022, 31, 5249–5269. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, A.R.; Fitzpatrick, S.W.; Funk, W.C.; Tallmon, D.A. Genetic rescue to the rescue. Trends Ecol. Evol. 2015, 30, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, H.M.; Evans, L.M.; Hersch-Green, E.I.; Woolbright, S.A.; Allan, G.J.; Whitham, T.G. Genetic data improves niche model discrimination and alters the direction and magnitude of climate change forecasts. Ecol. Appl. 2021, 31, e02254. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).