Simulated Warming Increases Litter Decomposition and Release Rates of Some Metallic Elements and Recalcitrant Components in Different-Aged Chinese Fir Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Litter Collection and Experimental Design
2.3. Litter Mass and Chemical Analysis
2.4. Data Processing and Analyses
3. Results
3.1. Initial Concentrations of Some Metallic Elements and Recalcitrant Components in Needle Litter from Different Chinese Fir Plantation Developmental Stages
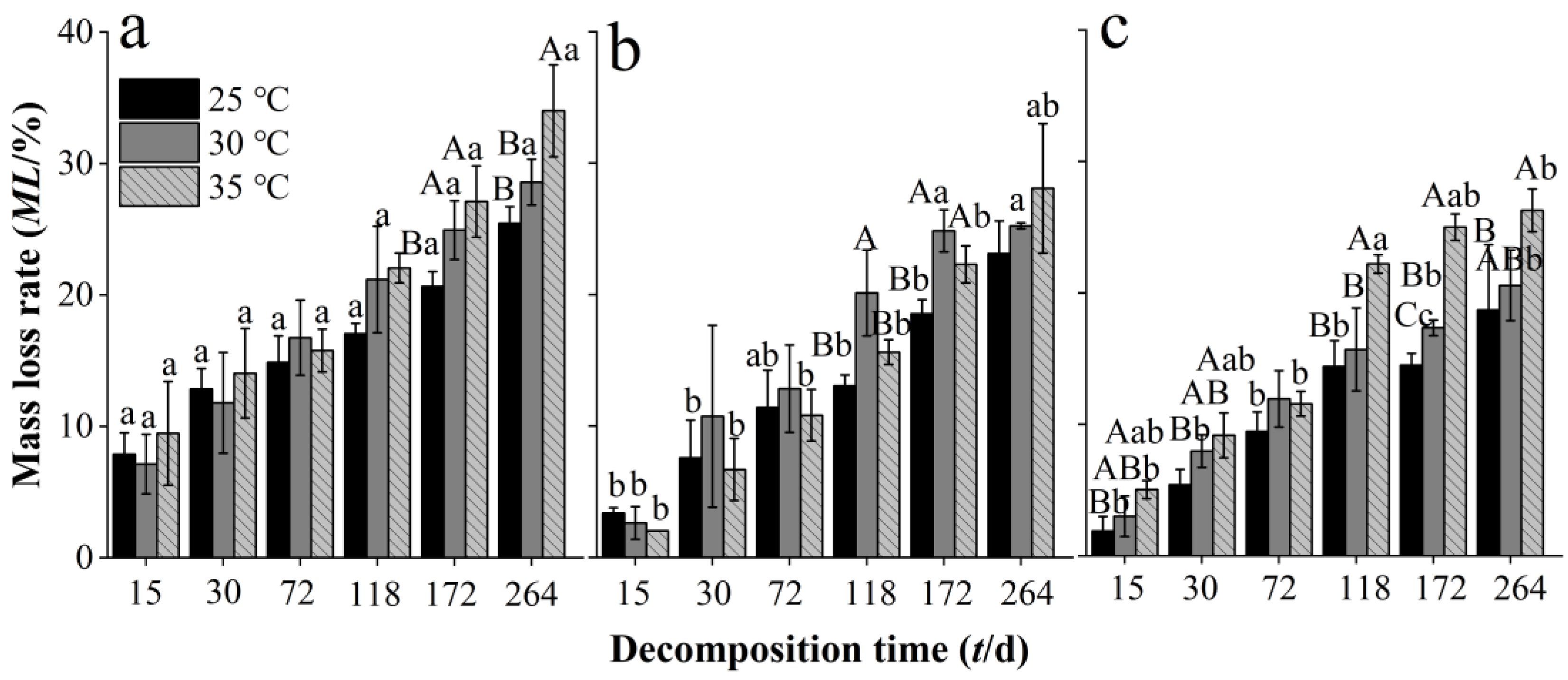
3.2. Effects of Warming on Mass Loss Rate of Needle Litter at Different Chinese Fir Plantation Developmental Stages
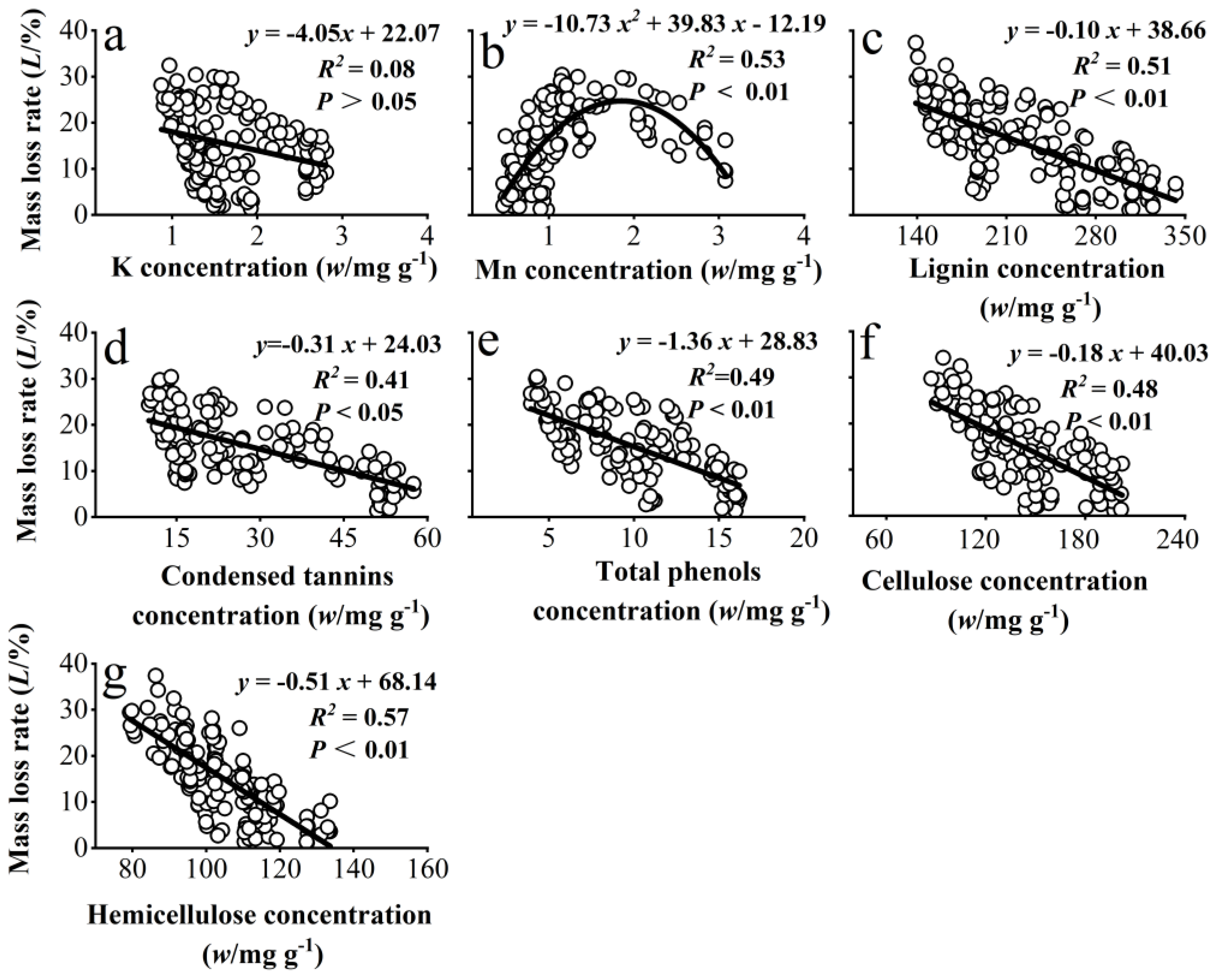
3.3. Correlation Between the Mass Loss Rate of Chinese Needle Litter and the Concentrations of Some Metallic Elements and Recalcitrant Components
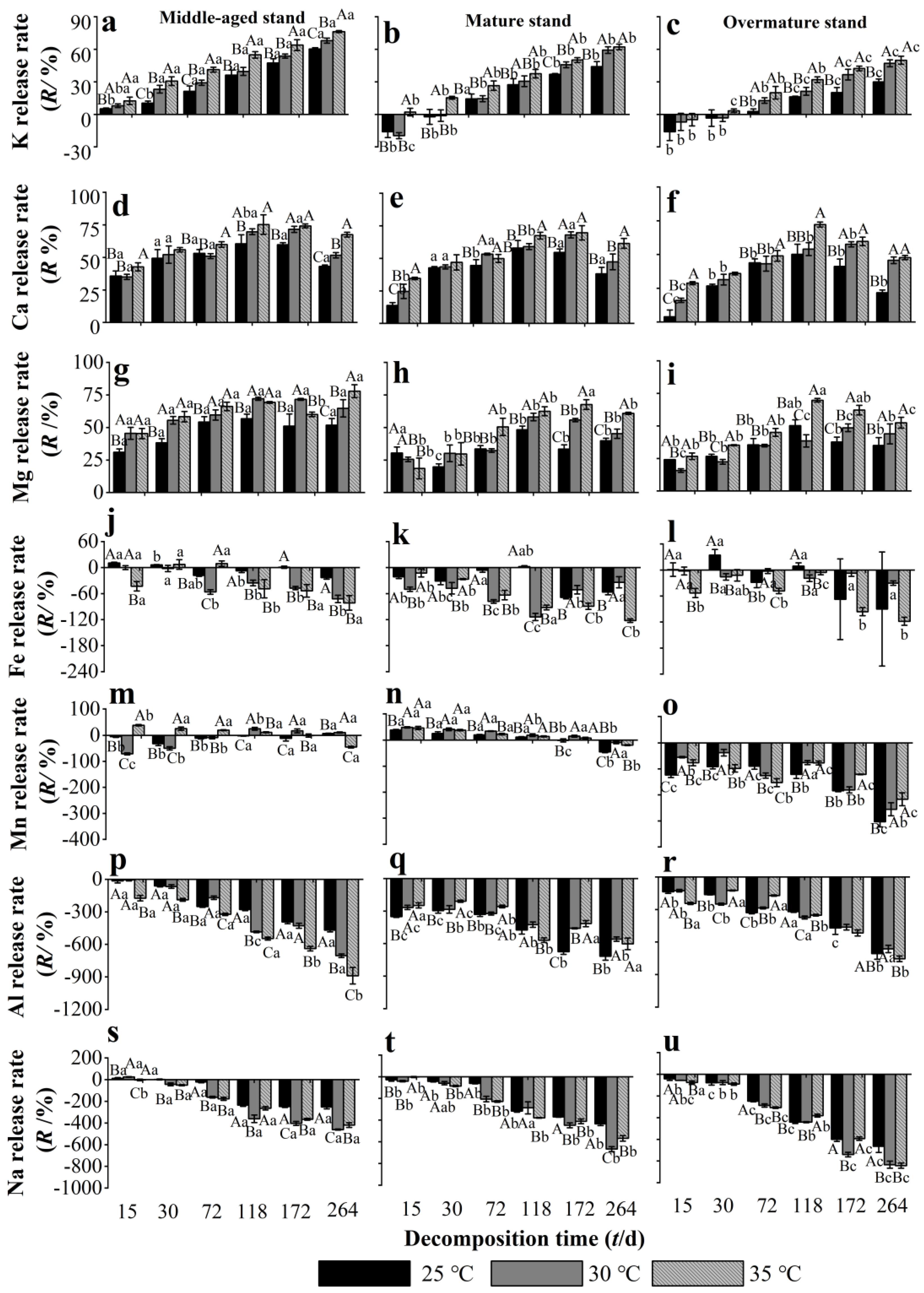
3.4. Effects of Warming on the Release Rate of Some Metallic Elements from Chinese Fir Needle Litter at Different Developmental Stages
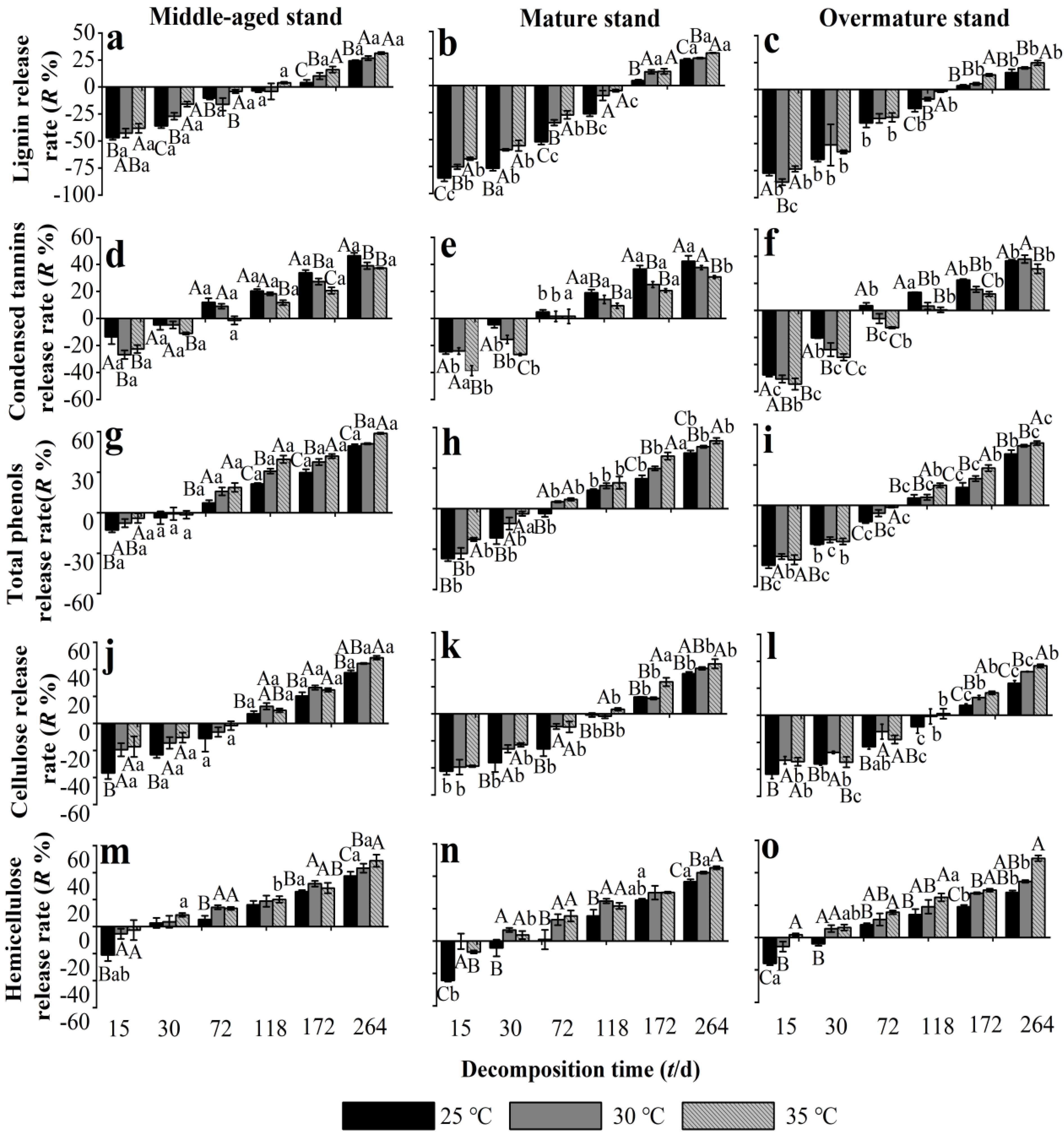
3.5. Effects of Warming on the Release Rate of Recalcitrant Components from Chinese Fir Needle Litter at Different Developmental Stages
4. Discussion
4.1. Warming Increases Chinese Fir Needle Litter Decomposition Rate at Different Developmental Stages
4.2. Warming Promotes the Release of Some Metallic Elements in Chinese Fir Needle Litter and the Enrichment of Other Elements
4.3. Warming Accelerates the Release Rate of Recalcitrant Components from Chinese Fir Needle Litter After 6 Months, Apart from Condensed Tannins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.Q.; Yang, R.; Peng, X.D.; Hou, C.L.; Ma, J.B.; Guo, J.R. Contributions of plant litter decomposition to soil nutrients in ecological tea gardens. Agriculture 2022, 12, 957. [Google Scholar] [CrossRef]
- Liu, X.F.; Chen, S.D.; Li, X.J.; Yang, Z.J.; Xiong, D.C.; Xu, C.; Wanek, W.G.; Yang, Y.S. Soil warming delays leaf litter decomposition but exerts no effect on litter nutrient release in a subtropical natural forest over 450 days. Geoderma 2022, 427, 116139. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Malik, A.A.; Swenson, T.; Weihe, C.; Morrison, E.W.; Martiny, J.B.H.; Brodie, E.L.; Northen, T.R.; Allison, S.D. Drought and plant litter chemistry alter microbial gene expression and metabolite production. SME J. 2020, 14, 2236–2247. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Liu, S.G.; Li, Y.Y.; Liu, S.Z.; Yin, G.C.; Huang, J.; Xu, Y.; Zhou, G.Y. Warming effects on the decomposition of two litter species in model subtropical forests. Plant Soil 2017, 420, 277–287. [Google Scholar] [CrossRef]
- Prieto, I.; Almagro, M.; Bastida, F.; Querejeta, J.I. Altered leaf litter quality exacerbates the negative impact of climate change on decomposition. J. Ecol. 2019, 107, 2364–2382. [Google Scholar] [CrossRef]
- Christiansen, C.T.; Haugwitz, M.S.; Priemé, A.; Nielsen, C.S.; Elberling, B.; Michelsen, A.; Grogan, P.; Blok, D. Enhanced summer warming reduces fungal decomposer diversity and litter mass loss more strongly in dry than in wet tundra. Glob. Chang. Biol. 2016, 23, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Romero-Olivares, A.L.; Allison, S.D.; Treseder, K.K. Decomposition of recalcitrant carbon under experimental warming in boreal forest. PLoS ONE 2017, 12, e0179674. [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Mooney, H.A.; Vitousek, P.M. Principles of Terrestrial Ecosystem Ecology; Springer: New York, NY, USA, 2002. [Google Scholar]
- Du Laing, G.; Van Ryckegem, G.; Tack, F.M.; Verloo, M. Metal accumulation in intertidal litter through decomposing leaf blades, sheaths and stems of Phragmites australis. Chemosphere 2006, 63, 1815–1823. [Google Scholar] [CrossRef]
- Brun, C.B.; Åström, M.E.; Peltola, P.; Johansson, M.B. Trends in major and trace elements in decomposing needle litters during a long-term experiment in Swedish forests. Plant Soil 2008, 306, 199–210. [Google Scholar] [CrossRef]
- Yue, K.; Yang, W.Q.; Peng, Y.; Zhang, C.; Huang, C.; Xu, Z.F.; Tan, B.; Wu, F.Z. Dynamics of multiple metallic elements during foliar litter decomposition in an alpine forest river. Ann. For. Sci. 2016, 73, 547–557. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, D.J.; Jian, Z.; Zhou, H.Y.; Zhao, Y.B.; Wei, D.P. Litter decomposition and degradation of recalcitrant components in Pinus massoniana plantations with various canopy densities. J. For. Res. 2019, 30, 1395–1405. [Google Scholar] [CrossRef]
- Loranger, G.; Ponge, J.F.; Imbert, D.; Lavelle, P. Leaf decomposition in two semi-evergreen tropical forests: Influence of litter quality. Biol. Fert. Soils 2002, 35, 247–252. [Google Scholar] [CrossRef]
- Austin, A.T.; Ballaré, C.L. Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2010, 107, 4618–4622. [Google Scholar] [CrossRef]
- Hartzfeld, P.W.; Forkner, R.; Hunter, M.D.; Hagerman, A.E. Determination of hydrolyzable tannins (gallotannins and ellagitannins) after reaction with potassium iodate. J. Agric. Food Chem. 2002, 50, 1785–1790. [Google Scholar] [CrossRef]
- Heil, M.; Baumann, B.; Andary, C.; Linsenmair, E.K.; Mckey, D. Extraction and quantification of "condensed tannins” as a measure of plant anti-herbivore defence? Revisiting an old problem. Naturwissenschaften 2002, 89, 519–524. [Google Scholar] [CrossRef]
- Richardson, S.J.; Press, M.C.; Parsons, A.N.; Hartley, S.E. How do nutrients and warming impact on plant communities and their insect herbivores? A 9-year study from a sub-Arctic heath. J. Ecol. 2002, 90, 544–556. [Google Scholar] [CrossRef]
- Day, T.A.; Ruhland, C.T.; Xiong, F.S. Warming increases aboveground plant biomass and C stocks in vascular-plant-dominated Antarctic tundra. Glob. Chang. Biol. 2008, 14, 1827–1843. [Google Scholar] [CrossRef]
- BI, J.; Blanco, J.A.; Seely, B.; Kimmins, J.P.; Ding, Y.; Welham, C. Yield decline in Chinese-fir plantations: A simulation investigation with implications for model complexity. Can. J. For. Res. 2007, 37, 1615–1630. [Google Scholar] [CrossRef]
- Li, A.G.; Fan, Y.X.; Chen, S.L.; Song, H.W.; Lin, C.F.; Yang, Y.S. Soil warming did not enhance leaf litter decomposition in two subtropical forests. Soil Biol. Biochem. 2022, 170, 108716. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.R.; An, S.S. Ecological stoichiometry in leaves, roots, litters and soil among different plant communities in a desertified region of Northern China. CATENA 2018, 166, 328–338. [Google Scholar] [CrossRef]
- Zhang, W.D.; Wang, X.F.; Wang, S.L. Fate of Chinese-fir litter during decomposition as a result of inorganic N additions. Appl. Soil Ecol. 2014, 74, 30–36. [Google Scholar] [CrossRef]
- Wang, Q.K.; Wang, S.L.; Huang, Y. Comparisons of litterfall, litter decomposition and nutrient return in a monoculture Cunninghamia lanceolata and a mixed stand in southern China. For. Ecol. Manag. 2008, 255, 1210–1218. [Google Scholar] [CrossRef]
- Wu, Z.L. Chinese Fir; China Forestry Publishing House: Beijing, China, 1984. (In Chinese) [Google Scholar]
- Zhou, L.L.; Shalom, A.D.D.; Wu, P.F.; Li, S.B.; Jia, Y.Y.; Ma, X.Q. Litterfall production and nutrient return in different-aged Chinese fir (Cunninghamia lanceolata) plantations in South China. J. For. Res. 2015, 26, 79–89. [Google Scholar] [CrossRef]
- Ma, X.Q.; Liu, C.J.; Hannu, I.; Westman, C.J.; Liu, A.Q. Biomass, litterfall and the nutrient fluxes in Chinese fir stands of different age in subtropical China. J. For. Res. 2002, 13, 165–170. [Google Scholar]
- Yan, G.Y.; Dong, X.D.; Huang, B.B.; Wang, H.L.; Hong, Z.M.; Zhang, J.H.; Xing, Y.J.; Wang, Q.G. Effects of nitrogen deposition on litter decomposition and nutrient release mediated by litter types and seasonal change in a temperate forest. Can. J. Soil Sci. 2019, 100, 11–25. [Google Scholar] [CrossRef]
- Olson, J.S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Hobbie, S.E. Temperature and plant species control over litter decomposition in Alaskan tundra. Ecol. Monogr. 1996, 66, 503–522. [Google Scholar] [CrossRef]
- Hong, J.T.; Lu, X.Y.; Ma, X.X.; Wang, X.D. Five-year study on the effects of warming and plant litter quality on litter decomposition rate in a Tibetan alpine grassland. Sci. Total Environ. 2021, 750, 142306. [Google Scholar] [CrossRef]
- Robinson, C.H. Controls on decomposition and soil nitrogen availability at high latitudes. Plant Soil 2002, 242, 65–81. [Google Scholar] [CrossRef]
- Fierer, N.; Craine, J.M.; McLauchlan, K.; Schimel, J.P. Litter quality and the temperature sensitivity of decomposition. Ecology 2005, 86, 320–326. [Google Scholar] [CrossRef]
- Sanchez, F.G. Loblolly pine needle decomposition and nutrient dynamics as affected by irrigation, fertilization, and substrate quality. For. Ecol. Manag. 2001, 152, 85–96. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Decomposition as a Process—Some Main Features. In Plant Litter—Decomposition, Humus Formation, Carbon Sequestration; Springer: Cham, Switzerland, 2020; pp. 13–43. [Google Scholar] [CrossRef]
- Edmonds, R.L.; Tuttle, K.M. Red alder leaf decomposition and nutrient release in alder and conifer riparian patches in western Washington, USA. For. Ecol. Manag. 2010, 259, 2375–2381. [Google Scholar] [CrossRef]
- Laskowskim, R.; Niklinskam, M.; Maryanski, M. The dynamics of chemical elements in forest litter. Ecology 1995, 76, 1393–1406. [Google Scholar] [CrossRef]
- Yue, K.; Ni, X.Y.; Fornara, D.A.; Peng, Y.; Liao, S.; Tan, S.Y.; Wang, D.Y.; Wu, F.Z.; Yang, Y.S. Dynamics of calcium, magnesium, and manganese during litter decomposition in alpine forest aquatic and terrestrial ecosystems. Ecosystems 2021, 24, 516–529. [Google Scholar] [CrossRef]
- Windham, L.M.; Weis, J.S.; Weis, P. Metal dynamics of plant litter of Spartina alterniflora and Phragmites australis in Metal-Contaminated salt marshes. Part 1: Patterns of decomposition and metal uptake. Environ. Toxicol. Chem. 2004, 23, 1520–1528. [Google Scholar] [CrossRef]
- Bockheim, J.G.; Jepsen, E.A.; Heisey, D.M. Nutrient dynamics in decomposing leaf litter of four tree species on a sandy soil in northwestern Wisconsin. Can. J. For. Res. 1991, 21, 803–812. [Google Scholar] [CrossRef]
- Gray, D.M.; Dighton, J. Mineralization of forest litter nutrients by heat and combustion. Soil Biol. Biochem. 2006, 38, 1469–1477. [Google Scholar] [CrossRef]
- Berg, B.; Steffen, K.T.; McClaugherty, C. Litter decomposition rate is dependent on litter Mn concentrations. Biogeochemistry 2007, 82, 29–39. [Google Scholar] [CrossRef]
- Maie, N.; Behrens, A.; Knicker, H.; Kögel-Knabner, I. Changes in the structure and protein binding ability of condensed tannins during decomposition of fresh needles and leaves. Soil Biol. Biochem. 2003, 35, 577–589. [Google Scholar] [CrossRef]
- Schädel, C.; Blöchl, A.; Richter, A.; Hoch, G. Quantification and monosaccharide composition of hemicelluloses from different plant functional types. Plant Physiol. Biochem. 2010, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Osono, T. Ecology of ligninolytic fungi associated with leaf litter decomposition. Ecol. Res. 2007, 22, 955–974. [Google Scholar] [CrossRef]
- Cox, P.; Wilkinson, S.P.; Anderson, J.M. Effects of fungal inocula on the decomposition of lignin and structural polysaccharides in Pinus sylvestris litter. Biol. Fertil. Soils 2001, 33, 246–251. [Google Scholar] [CrossRef]
- Fioretto, A.; Di Nardo, C.; Papa, S.; Fuggi, A. Lignin and cellulose degradation and nitrogen dynamics during decomposition of three leaf litter species in a Mediterranean ecosystem. Soil Biol. Biochem. 2005, 37, 15. [Google Scholar] [CrossRef]
- Berg, B.; Hannus, K.; Popoff, T.; Theander, O. Changes in organic chemical components of needle litter during decomposition. Long-term decomposition in a Scots pine forest. I. Can. J. Bot. 1982, 60, 1310–1319. [Google Scholar] [CrossRef]
- Parfitt, R.L.; Newman, R.H. 13C NMR study of pine needle decomposition. Plant Soil 2000, 219, 273–278. [Google Scholar] [CrossRef]
- Polyakova, O.; Billor, N. Impact of deciduous tree species on litterfall quality, decomposition rates and nutrient circulation in pine stands. For. Ecol. Manag. 2007, 253, 11–18. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Jørgensen, H.B. Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J. Ecol. 2010, 98, 754–763. [Google Scholar] [CrossRef]
| Developmental Stage | Metallic Element Concentrations (mg g−1) | ||||||
|---|---|---|---|---|---|---|---|
| K | Ca | Mg | Fe | Mn | Al | Na | |
| Middle-aged | 2.67 ± 0.10 a | 15.74 ± 1.98 a | 1.56 ± 0.06 a | 0.28 ± 0.01 c | 1.66 ± 0.08 a | 0.13 ± 0.02 b | 0.26 ± 0.01 a |
| Mature | 1.54 ± 0.09 b | 13.73 ± 0.62 ab | 1.37 ± 0.02 b | 0.31 ± 0.01 b | 0.98 ± 0.03 b | 0.15 ± 0.02 ab | 0.21 ± 0.00 b |
| Overmature | 1.18 ± 0.07 c | 11.40 ± 0.27 b | 1.15 ± 0.01 c | 0.33 ± 0.00 a | 0.41 ± 0.01 c | 0.17 ± 0.01 a | 0.19 ± 0.01 c |
| Recalcitrant Component Concentrations (mg g−1) | |||||||
| Lignin | Condensed Tannins | Total Phenols | Cellulose | Hemicellulose | |||
| Middle-aged | 122.1 ± 3.19 c | 12.33 ± 1.42 c | 5.22 ± 0.60 b | 92.74 ± 0.58 c | 87.54 ± 3.18 b | ||
| Mature | 149.9 ± 11.46 b | 20.45 ± 1.50 b | 7.78 ± 0.25 b | 106.1 ± 8.97 b | 103.0 ± 2.48 a | ||
| Overmature | 172.6 ± 6.11 a | 34.04 ± 0.76 a | 10.76 ± 0.08 a | 135.5 ± 4.13 a | 107.8 ± 5.33 a | ||
| Developmental Stage | Temperature | Regression Equation | Decomposition Coefficient k | Time of 50% Decomposition T50%/y | Time of 95% Decomposition T95%/y | Regression Coefficient R2 |
|---|---|---|---|---|---|---|
| Middle-aged | 25 °C | y = 90.82e−0.276t | 0.28 ± 0.03 Ca | 2.52 ± 0.25 Aa | 10.91 ± 1.08 Aa | 0.93 ** |
| 30 °C | y = 91.02e−0.366t | 0.36 ± 0.04 Ba | 1.95 ± 0.23 Ba | 8.42 ± 0.99 Aa | 0.86 ** | |
| 35 °C | y = 90.86e−0.446t | 0.45 ± 0.02 Aa | 1.56 ± 0.07 Ba | 6.73 ± 0.30 Ba | 0.93 ** | |
| Mature | 25 °C | y = 95.75e−0.313t | 0.31 ± 0.07 Aa | 2.21 ± 0.56 Aa | 9.92 ± 2.40 Aa | 0.91 ** |
| 30 °C | y = 93.58e−0.367t | 0.37 ± 0.05 Aa | 1.91 ± 0.25 Aa | 8.27 ± 1.08 Aa | 0.75 ** | |
| 35 °C | y = 97.75e−0.440t | 0.44 ± 0.08 Aa | 1.61 ± 0.32 Aa | 6.97 ± 1.37 Aa | 0.93 ** | |
| Overmature | 25 °C | y = 96.46e−0.256t | 0.26 ± 0.08 Ba | 2.89 ± 0.89 Aa | 12.47 ± 3.87 Aa | 0.80 ** |
| 30 °C | y = 94.52e−0.265t | 0.27 ± 0.05 Ba | 2.68 ± 0.49 Ab | 11.57 ± 2.12 Ab | 0.82 ** | |
| 35 °C | y = 93.59e−0.384t | 0.38 ± 0.01 Aa | 1.80 ± 0.04 Aa | 7.78 ± 0.19 Aa | 0.85 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Guo, W.; Chen, Y.; Li, Z.; Liu, Q.; Heal, K.V.; Li, S.; Zhou, L. Simulated Warming Increases Litter Decomposition and Release Rates of Some Metallic Elements and Recalcitrant Components in Different-Aged Chinese Fir Plantations. Forests 2024, 15, 2151. https://doi.org/10.3390/f15122151
Zhang L, Guo W, Chen Y, Li Z, Liu Q, Heal KV, Li S, Zhou L. Simulated Warming Increases Litter Decomposition and Release Rates of Some Metallic Elements and Recalcitrant Components in Different-Aged Chinese Fir Plantations. Forests. 2024; 15(12):2151. https://doi.org/10.3390/f15122151
Chicago/Turabian StyleZhang, Lixian, Wenjuan Guo, Yulong Chen, Zhihao Li, Qi Liu, Kate V. Heal, Shubin Li, and Lili Zhou. 2024. "Simulated Warming Increases Litter Decomposition and Release Rates of Some Metallic Elements and Recalcitrant Components in Different-Aged Chinese Fir Plantations" Forests 15, no. 12: 2151. https://doi.org/10.3390/f15122151
APA StyleZhang, L., Guo, W., Chen, Y., Li, Z., Liu, Q., Heal, K. V., Li, S., & Zhou, L. (2024). Simulated Warming Increases Litter Decomposition and Release Rates of Some Metallic Elements and Recalcitrant Components in Different-Aged Chinese Fir Plantations. Forests, 15(12), 2151. https://doi.org/10.3390/f15122151






