Abstract
The large-scale dieback of spruce monocultures, especially in the lower alpine, has become a significant problem and has necessitated the restoration of these areas, mainly using seedlings produced in forest nurseries. The primary source of nutrients for seedlings can be slow-release fertilizers and an appropriate dose of fertilizer improves the efficiency of its use and minimizes the negative environmental impact associated with the excessive use of mineral fertilizers. Aims: This study aimed to evaluate the effect of applying different fertilizer dose combinations on the accumulation of macronutrients in different parts of the seedlings (roots, shoots, and leaves) and on the morphology and development of fine roots. Methods: This research was carried out on producing beech seedlings with the application of starter soil fertilization with Yara Mila Complex (YMC) and Osmocote Exact Standard 3-4M (OES) fertilizers in four varying doses. Results: No deficiency of the analyzed macronutrients was noted in any of the tested fertilization variants. The highest content of all analyzed macronutrients was recorded in the leaves of beech seedlings, with values in roots and shoots being several times lower. The mixed fertilization variant OES 1.0 + YMC 1.0 shows a positive correlation with all analyzed elements and the parameters DQI (Dickson Quality Index), SA (Surface Area), RV (Root Volume), and mass. Conclusions: Results confirm the hypothesis that applying a mixture of fast-acting (YMC) and slow-acting (OES) fertilizer positively affects the nutrition and accumulation of macronutrients and the development of root systems in beech seedlings compared to fertilization with a single fertilizer.
1. Introduction
In recent years, the importance of progressive climate change and its impact on the condition of forest ecosystems has been emphasized. Central European conditions are characterized by an uneven precipitation distribution, resulting in seasonal water deficits [1] and an extended growing season [2]. Such conditions have proven particularly unfavorable for spruce monocultures growing in the mountains of Central Europe. Large-scale dieback of spruce stands has made it necessary to use tree seedlings produced in forest nurseries to rebuild the often very poor areas remaining after monocultures and to change the need for the species composition of tree stands. On a European scale, common beech is often used for reconstruction after declining spruce stands [3]. Previous research has shown that the admixture of deciduous trees and the creation of mixed forests that provide a balance of nutrients have a positive impact on higher productivity and lower sensitivity to stress factors [4]. The increase in forest productivity imposes a greater demand on nutrients, which is possible due to the difference in the quality of litter of different tree species and the speed of its decomposition [4,5]. Increasing demand for planting material for forest land restoration makes it necessary to raise good quality seedlings that will show high survival rates after planting [6]. Optimal production conditions are necessary for the proper growth of root systems, which are responsible for the uptake of water and nutrients. In particular, fine roots less than <2 mm in diameter are an important component of plant biomass, which affects biogeochemical cycles by turning the dead roots. In particular, fine roots are the most physiologically active as they respond to environmental changes, such as drought or excessive soil acidity [7]. The distribution of fine roots in the soil profile depends on the nutrient requirements, the adaptive or extensive plants strategy, the morphological characteristics of the roots related to their length and diameter, and changes in the soil’s chemical characteristics [8,9]. Nutrients that are provided are essential in initiating basic plant physiological processes, and a disruption in the availability of any macronutrient can have a major impact on the plant [10]. Even small fluctuations in temperature and precipitation can affect nutrient cycling and availability, particularly nitrogen and phosphorus [11,12]. As one of the most important plant nutrients, nitrogen induces seedling growth, but it must be supplied in a bioavailable form; otherwise, the release rate of this element is higher than the absorption by the plant [13]. A similar problem applies to phosphorus, which is utilized at only 20% [14]. Currently, a decrease in P content is observed in European forests, which may be influenced by an increase in N [6]. The N content in forests leads to its increase but leads to a disturbance in the balance of nutrients in this mainly P [15]. It is known from research that excess N negatively affects the growth of mycorrhizas, which in turn causes a change in the microbial community [16]. Under conditions of nutrient deficiency, morphological features are strongly related to resource acquisition, and root biomass increases in search of additional mineral sources, providing better adaptation under harsh environmental conditions [17].
The primary source of nutrients for seedlings raised in forest nurseries is mineral fertilizers. Applying an appropriate dose of fertilizer positively affects the physiological and morphological properties and the resistance of seedlings to fungal pathogens [18]. To produce forest tree seedlings using nursery substrates, nutrient-poor components such as high peat, bark, or sawdust are used. Therefore, multi-nutrient, slow-release fertilizers often enrich such a substrate [19]. The use of slow-acting fertilizers, for example, Osmocote Exact Standard (3-4M), reduces the amount of work involved in the implementation of fertilization. Also, it prevents the rapid leaching of macro- and micronutrients necessary for seedling growth. Proper fertilizer application improves the efficiency of fertilizer uptake and the quality of the resulting planting material, minimizing the negative environmental impact of improperly applied fertilizers [20]. The use of a starter fertilizer such as Yara Mila Complex will have an effect on seedlings in the early growth phase and may not be sufficient in terms of seedling nutritional status [21]. Poor fertilizer management by providing too high doses of fertilizer affects the environment’s biogeochemical cycle, changing the soil’s chemical properties, including its pH, cation exchange capacity, and assimilability of macro- and micronutrients [22]. Furthermore, an adverse effect of using one type of fertilizer can be a reduction in the biodiversity of microorganisms available in the soil [23]. A positive aspect of using this type of fertilizer is that the proper dosage can be selected to produce nursery stock suitable for growth under any environmental conditions after planting on the crop.
This study involved growing beech seedlings using starter soil fertilization with Yara Mila Complex (YMC) and Osmocote Exact Standard 3-4M (OES) fertilizers, dosed individually and as a mixture of both fertilizers. The fertilizers used in the nursery experiment are produced by Yara Poland Ltd., Szczecin, Poland (YMC) and ICL Group Ltd., Warszawa, Poland (OES). The study aimed to evaluate the effect of applying different dose combinations of these fertilizers on the nutritional status of beech seedlings. The study tested the following hypothesis: applying a mixture of fast-acting fertilizer (YMC) and slow-acting fertilizer (OES) positively affects the morphology of fine roots, nutrition, and accumulation of macronutrients in common beech seedlings compared to fertilization with a single fertilizer.
2. Material and Methods
2.1. Experiment Design
A pot experiment was established in 2020 under controlled conditions (plastic tent) at the Kopciowa experimental nursery (49°27′3.043″ N; 20°57′17.083″ E). A mixture of fir-spruce sawdust and high peat at a volume ratio of 1:1 was used as a substrate. Before starting the experiment, substrate samples were collected for chemical analyses (Table 1).

Table 1.
The chemical characteristics of the peat–sawdust substrate used for the experiment.
Two fertilizers were used for starter fertilization: Yara Mila Complex and Osmocote Exact Standard 3-4M, which were applied in four variations (Table 2).

Table 2.
Variants of starter fertilization used in the research [24] and the order of treatment according to the decreasing dose of the element (1→4).
The fertilizer variants, YMC 2.5 and OES 2.0, are used in several Polish nurseries when growing forest tree seedlings under controlled conditions. All variants applied differed in the total dose of basic macronutrients and their release rate (availability). The total dose of nitrogen per 1 m3 of nursery medium in variants with mixed fertilization was slightly lower than single fertilization. In contrast, the phosphorus, potassium and magnesium dose decreased linearly from the YMC 2.5 to OES 2.0 variant, but their release time increased.
Yara Mila Complex is a fast-acting, easily dissolved fertilizer due to the presence of nitrogen in the nitrate form and the physical properties of prill-type granules. The second fertilizer used, Osmocote Exact Standard 3-4M, is characterized by a controlled release of mineral nutrients over 3 to 4 months (at 21 °C), resulting from using pellets [25]. The composition of YMC fertilizer includes 12% total nitrogen (N) (consisting of 5% N–NO3 and 7% N–NH4), 18.7% phosphorus (P2O5), 18% potassium (K2O), 2.7% magnesium (MgO), 20% sulfur (SO3), 0.015% boron (B), 0.20% iron (Fe), 0.02% manganese (Mn), and 0.02% zinc (Zn). OES fertilizer composition consists of 16% total nitrogen (N) (including 7% N–NO3 and 9% N–NH4), 3.9% phosphorus (P2O5), 10% potassium (K2O), 1.2% magnesium (MgO), 0.02% boron (B), 0.45% iron (Fe), 0.06% manganese (Mn), 0.05% copper (Cu), 0.02% molybdenum (Mo), and 0.015% zinc (Zn).
Each fertilizer’s dose or mixture was individually added to 30 dm3 of substrate and mixed. Three repetitions were made. The amount of fertilizers was prepared according to the dosage per volume of substrate used (Table 2). The substrate with fertilizer was placed in leak-proof boxes, into which stratified beech seeds were sown (April). The boxes were placed in a tent, from which the sheeting was removed on 5 July. At the end of the experiment in September, 30 seedlings were taken from each replicate (30 seedlings × 3 replicates × 4 experimental variants, 360 seedlings in total) to determine basic seedling growth parameters. These analyses were carried out for individual seedlings. To determine the nutritional status of the seedlings, each replicate was divided into sub-samples, i.e., 5 sub-samples × 6 seedlings (60 sub-samples in total). Laboratory samples of sufficient size were, thus, obtained.
2.2. Laboratory Analysis
Ten soil samples were collected before the experiment began. The soil samples were dried at a temperature of 65 °C for 48 h and then sieved through a sieve with a mesh diameter of 2 mm. The chemical properties of the prepared samples were determined. Soil pH was determined using the potentiometric method in water. Total nitrogen and carbon content were determined using a LECO CNS True Mac Analyzer (Leco, St. Joseph, MI, USA). The content of P, K, Ca and Mg was determined after mineralization in a mixture of concentrated nitric and perchloric acids in a 2:1 ratio using the ICP-OES method. For each fertilizer variant, 30 seedlings were taken for analysis. After collection, fresh seedlings were cleaned and measured (height, root length, and diameter at the root collar). Then, the leaves, root system, and shoot were separated, dried, and weighed. These samples were dried at 65 °C for 48 h. P, K, Ca, and Mg concentrations were determined in leaf and root samples after their mineralization in a mixture of HNO3 and HClO4 (3:1) by ICP (ICP-OES Thermo iCAP 6500 DUO, Thermo Fisher Scientific, Cambridge, UK). Carbon (C) and nitrogen (N) were measured in beech leaves and roots using an elemental analyzer in LECO. The results obtained from the prepared material were used for statistical analyses.
In an earlier published paper [24], the results of the analysis of 15 growth traits and three synthetic indices of breeding suitability of seedlings grown in each experimental variant were already presented. In addition, root system fragments were scanned at an 800 dpi resolution and then analyzed using a WinRhizo™ Pro 2021 image analysis system (Regent Instruments Inc., Ville de Québec, QC, Canada) to determine the diameter, length, and root area. After root system analysis, the basic parameters of fine root morphology were determined. In the current study, five parameters were used, including S/R (stem/root ratio based on dry weight), DQI (Dickson Quality Index), weight (weight of fine roots ≤ 2 mm; mg), SA (surface area of fine roots ≤ 2 mm; cm2), and RV (root volume of fine roots ≤ 2 mm; cm3). The DQI was calculated as the ratio of total dry weight of the seedling and the sum of S/R and SQ, i.e., height/root collar diameter ratio [26]. In addition, root parameters, namely root tissue density (RTD; kg m−3), specific root length (SRL; m g−1), and specific root area (SRA; m2 kg−1), were calculated according to the methodology of Ostonen et al. [27]. The amount of root branching was expressed as the number of root tips per 1 mg dry matter [28]. In the second stage of the study, the dried parts of each seedling were ground and used to determine the macronutrient content. To determine the nutritional status, data from Wesoły et al. [29] and from Mellert and Goettlein [30] were used (Table 3).

Table 3.
Ranges [%] of foliar concentrations of macroelements for European beech derived from literature compilation.
2.3. Statistical Analysis
Spearman correlation coefficients were calculated to determine the interrelationships between the different fertilization doses and the nutritional status of the seedlings. This analysis compared different doses of fast and slow-acting fertilizers with the nutritional status of seedlings as well as with growth parameters. Analysis of variance and the Tukey’s test was used to assess differences between mean trait values. Results were considered statistically significant at α < 0.05. All statistical analyses were performed using R statistical software (version 4.1.3) [31] R Studio (RStudio Team, 2020), and Statistica 13 software [32].
3. Results
3.1. Seedling Analysis
Comparing the fertilization variants regarding the macronutrient content in the leaves of beech seedlings, it was revealed that the greatest variation was in P content. The highest percentages among the analyzed elements were determined for N and the lowest for Mg. Moreover, higher N, P, Ca, and K contents was determined in the leaves of beech trees fertilized with the YMC 2.5 variant (Figure 1).
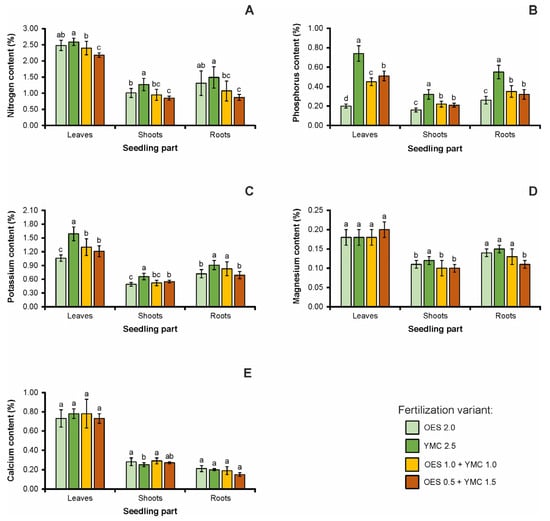
Figure 1.
The chemical content (mean ± SD in %) of macroelements (per seedling) in the leaves, stems, and roots of European beech seedlings on the fertilization variant; (A)—nitrogen content; (B)—phosphorus content; (C)—potassium content; (D)—magnesium content; (E)—calcium content (abc—small letters mean significant differences in properties between fertilizer doses).
Higher values of N, P, K, Mg, and Ca were recorded in the assimilation apparatus of the seedlings compared to other parts of the plant. The concentration of elements in the seedling leaves differed significantly depending on the fertilizer used (Figure 1). The lowest N concentration was recorded for the OES 0.5 + YMC 1.5 fertilizer, which differs significantly from other variants. A similar relationship was recorded with the P and K contents of the leaves. For the Ca and Mg concentration in leaves, no statistically significant differences were found.
Statistically significant differences were also recorded for shoots and roots of seedlings. The highest N, P, K, and Mg content in shoots was recorded for the YMC 2.5 variant, and this variant differed statistically significantly from the remaining variants in the content of these elements in shoots. In terms of the content of elements in roots, statistically significant differences were found for the content of N and K in YMC 2.5 variant and other fertilization variants. Higher N, P, K, and Mg contents were recorded in roots than in shoots, and the opposite situation was with respect to the Ca content. For the shoots, higher Ca accumulation was found with the mixed variant OES 1.0 + YMC 1.0 and the lowest with the fertilizer YMC 2.5. The variants are significantly different from each other. The P content in shoots was lowest with OES 2.0 fertilizer (Figure 1).
3.2. The Relationship Between Root Traits, Biometric and Fertilizer
The SRL factor with fertilizer OES 1.0 + YMC 1.0 was significantly different from OSE 0.5 + YMC 1.5. SRA shows little variation, without statistically significant differences between the variants. For the RTD parameter, statistically significant differences were found between the YMC 2.5 fertilizer and the other variants. Average values of fine root weight, SA and RV take the highest values for the variants OES 1.0 + YMC 1.0, which differs significantly from the others. The lowest weight and SA values were recorded for the single fertilization variant YMC 2.5 (Table 4).

Table 4.
Basic morphological characteristics of the roots depending on the fertilizer used (treatments sequence according to decreasing dose of total nitrogen).
Spearman’s linear correlation showed that the OES 2.0 fertilizer negatively correlates with P content, whereas it positively correlates with Mg and the S/R ratio. YMC 2.5 shows a positive correlation with P and a negative correlation with Ca and Mg, as well as with individual parameters—DQI, weight, SA and RTD. The mixed fertilization variant OES 1.0 + YMC 1.0 shows a positive correlation with all analyzed elements and the parameters DQI, SA, RV and mass. In addition, a negative correlation was shown with the parameters SRA and SRL. OES 0.5 + YMC 1.5 fertilizer negatively correlates with nitrogen and S/R ratio and RV, and a positive correlation was noted between SRL and RTD. A strong correlation was also noted between various root parameters. The other elements showed no statistically significant differences (Figure 2).
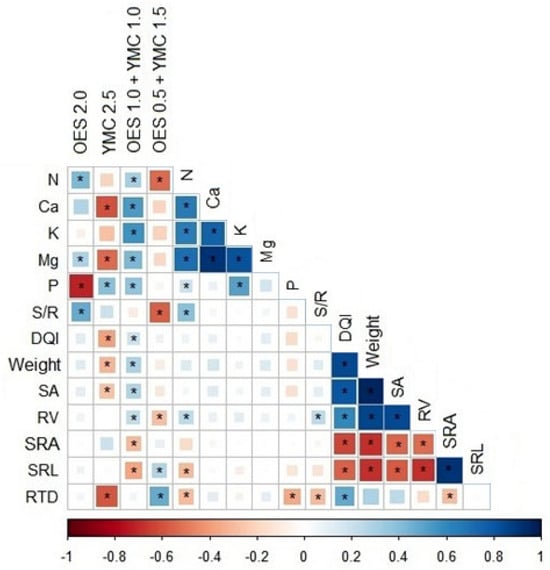
Figure 2.
Correlation between fertilizer concentration, root parameters and the content of individual elements in roots; Significance effect * ≤ 0.05 Traits: S/R—stem/root ratio, DQI—Dickson quality index, SA—surface area of fine roots ≤ 2 mm; RV = root volume of fine roots ≤ 2 mm, RTD—root tissue density, SRA—specific root area and SRL—specific root length.
4. Discussion
4.1. Chemical Properties of Seedlings
The content of elements in leaves provide information about the nutritional status of trees [33,34]. The nutritional state is determined by the supply of elements from the substrate, captured from the atmosphere, and leached from the assimilation apparatus [35]. Depending on the fertilizer applied in the experiment, differences were noted in the nutrition of beech seedlings produced under controlled conditions. Plant organs vary in predictable nutrient concentrations. Typically, leaves also have higher concentrations of N, P, and K responsible for metabolism and lower concentrations of Ca than woody shoots, while intermediate values were recorded in roots [36].
The highest content of all analyzed macronutrients was recorded in the leaves of beech seedlings, with values in roots and shoots being several times lower. The application of different combinations of slow-release fertilizers affected the nutritional status of beech seedlings produced under controlled conditions. No deficiency of the analyzed macronutrients, according to Wesoły et al. [29], was noted in any of the tested fertilization variants. For all fertilization variants, Ca, Mg, and N in leaves were within the ranges presented in Table 3. In the case of P, average values above the optimal ranges were recorded in leaves for OES 0.5 + YMC 1.5 fertilization (0.51%), OES 1.0 + YMC 1.0 fertilization (0.45%), and YMC 2.5 fertilization (0.74%). Moreover, above optimal values were recorded for K for OES 1.0 + YMC 1.0 fertilization (1.30%) and YMC 2.5 fertilization (1.59%). Slightly narrower ranges of normal nutrition (Table 3) for beech stands are given in the work of Mellert and Goettlein [30]. These values were created on the basis of stands of different ages and constitute an integrated criterion for assessing habitat conditions and environmental factors, including the assessment of susceptibility to diseases and pathogens. Comparing the obtained results with these ranges created on the basis of Van den Burg’s work, nitrogen in the leaves for variants OES 2, YMC 2.5 and OES 1.0 + YMC 1.0 were in the luxury surplus range. In the case of phosphorus and potassium, the variants for which the standards according to Wesoły [29] were exceeded (YMC 2.5, OES 1.0 + YMC 1.0 and OES 0.5 + YMC 1.5) were in the extreme surplus range.
4.2. Biometric Analysis of Seedlings
Analysis of growth parameters and root morphological traits by Banach et al. [24] showed that the OES 1.0 + YMC 1.0 fertilizer mixture caused the highest seedling growth among the other tested fertilizers. The lowest was found in YMC 2.5. A similar relationship was observed for shoot volume and total root length (TRLen). This study found the highest concentration of analyzed elements in beech leaves using YMC 2.5 fertilizer. The observed inverse relationship may be due to the higher concentration of some compounds in fertilizers, such as about five times more P [24]. Seemingly well-nourished beech seedlings were grown with YMC 2.5 fertilizer, but they exhibited much lower growth parameters, potentially resulting from higher P concentrations. Additionally, higher P content in leaves may reduce the content of chlorophyll and the content of some proteins, thereby disturbing the seedling’s stability during unfavorable conditions [37,38]. As a result, the photosynthetic process and chemical transformations are regulated, which will affect growth and primary productivity [39].
Banach et al. [24] found that the total length of the roots was comparable when using the YMC 2.5 variant and the mixed variant OES 1.0 + YMC 1.0. Furthermore, root length (RLen) was comparable when using the YMC 2.5 variant and the mixed variant OES 1.0 + YMC 1.0. In addition, this relationship was confirmed by analysis of basic root parameters SRA and SRL. The content of N and P affects root morphology and the root-to-shoot ratio [38,40], which is confirmed by previous studies considering the S/R ratio [24]. These elements affecting root elongation and the amount of branching improve the availability of other nutrients, which may explain the higher accumulation of other macronutrients in the leaves [38]. Thus, longer and thinner roots may exhibit higher exploitation efficiency when competition is intense [38,41].
Trees growing in temperate climates show a seasonal tendency to grow root systems. At the beginning of the growing season, root systems grow faster than shoots, followed by stunted root growth and intense shoot development [42]. Applying a fast-acting fertilizer like YMC could affect the intensive growth of fine roots. At the same time, the amount of nutrients was insufficient at the time of the highest demand for the construction of shoot biomass. Using a mixture of both fertilizers allows the supply of needed nutrients throughout the growing season, thus, securing elemental reserves after the seedling stops growing or when planting a forest crop [43]. An adequate state of seedling revival can influence faster root system development in the first year after planting and better adaptation [44]. A well-developed root system and an appropriate balanced S/R ratio indicate a good chance of proper adaptation to difficult conditions and to minimize subsequent stress. As a result, the root system grows faster and the seedling becomes less susceptible to water balance fluctuations during summer droughts [43]. A greater share of roots in the seedling’s biomass increases the adaptive potential of seedlings grown in conditions with a high share of water stress [45,46]. Taking into account that the survival of seedlings to be greater with lower S/R, especially under droughty field conditions [47], the best potential has seedlings of European beech grown with YMC 0.5 + OSE 1.5 fertilization. This was also confirmed by the analysis of the size of Dickson’s quality index (DQI), which indicates the adaptation potential of seedlings after planting in the forest crop [46,47] and is additionally well correlated with the height and root collar diameter of seedlings [48]. In the variant with OES fertilization and in both variants of mixed fertilization with this fertilizer, the DQI was high and comparable (same homogeneous group), confirming the better suitability of mixed fertilization (OES + YMC) for growing European beech seedlings.
5. Conclusions
Our study showed that the nutritional status of beech seedlings also changes depending on the fertilization regime. The results support our hypothesis that applying a mixture of a fast-acting fertilizer (YMC) and a slow-acting fertilizer (OES) positively affects the nutrition and accumulation of macronutrients in beech seedlings compared to fertilization with a single fertilizer. Optimal fertilization enables the production of seedlings with a developed root system, as indicated by improved SRA and SRL parameters. A well-developed root system allows for proper nutrition of the seedlings and together with S/R ratio is a crucial element in forest regeneration. To summarize, using a mixture of OES 0.5 + YMC 1.5 fertilizers ensures a constant supply of nutrients, increases the density of the root system, total root length, and ensures a good S/R ratio, which may contribute to better adaptation of European beech seedlings to cultivation.
Author Contributions
M.J.: Conceptualization, Investigation, Methodology, Writing—original draft. K.S.-S.: Investigation, Methodology, visualization, Writing—original draft. S.M.: Conceptualization, Methodology, Writing—review and editing. J.B.: Conceptualization, Investigation, Methodology, Visualization, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of the Republic of Poland (SUB/040012/D019).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Trnka, M.; Olesen, J.E.; Kersebaum, A.C.; Skjelvåg, A.O.; Eitzinger, J.; Seguin, B.; Dubrovský, M. Agrolimatic conditions in Europe under climate change. Glob. Chang. Biol. 2011, 17, 2298–2318. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Roy, D.B. Altered geographic and temporal variability in phenology in response to climate change. Glob. Ecol. Biogeogr. 2006, 15, 498–504. [Google Scholar] [CrossRef]
- Jurásek, A.; Bartoš, J.; Nárovcová, J. Intensively fertilised seedlings of the beech (Fagus sylvatica L.) for artificial regeneration of the spruce stands in the process of conversion. J. For. Sci. 2008, 54, 452–458. [Google Scholar] [CrossRef]
- Jonard, M.; Fürst, A.; Verstraeten, A.; Thimonier, A.; Timmermann, V.; Potočić, N.; Rautio, P. Tree mineral nutrition is deteriorating in Europe. Glob. Chang. Biol. 2015, 21, 418–430. [Google Scholar] [CrossRef]
- Lukac, M.; Calfapietra, C.; Lagomarsino, A.; Loreto, F. Global climate change and tree nutrition: Effects of elevated CO2 and temperature. Tree Physiol. 2010, 30, 1209–1220. [Google Scholar] [CrossRef]
- Sierota, Z. Czy wprowadzanie w odnowieniach sadzonek z zakrytym systemem korzeniowym wszędzie znajduje uzasadnienie—Punkt widzenia fitopatologa (Is the introduction of covered root seedlings in every renewal reasonable—Phytopathological point of view). Sylwan 2019, 163, 989–996. [Google Scholar] [CrossRef]
- Hirano, Y.; Mizoguchi, T.; Brunner, I. Root parameters of forest trees as sensitive indicators of acidifying pollutants: A review of research of Japanese forest trees. J. For. Res. 2007, 12, 134–142. [Google Scholar] [CrossRef]
- Finér, L.; Ohashi, M.; Noguchi, K.; Hirano, Y. Fine root production and turnover in forest ecosystems in relation to stand and environmental characteristics. For. Ecol. Manag. 2011, 262, 2008–2023. [Google Scholar] [CrossRef]
- Staszel, K.; Błońska, E.; Lasota, J. Fine root morphology and soil properties under influence of different tree stands along an altitudinal climosequence in the Carpathian mountains. For. Ecosyst. 2022, 9, 100066. [Google Scholar] [CrossRef]
- Soares, J.C.; Santos, C.S.; Carvalho, S.M.P. Preserving the nutritional quality of crop plants under a changing climate: Importance and strategies. Plant Soil 2019, 443, 1–26. [Google Scholar] [CrossRef]
- Matías, L.; Castro, J.; Zamora, R. Soil-nutrient availability under a global-change scenario in a Mediterranean mountain ecosystem. Glob. Chang. Biol. 2011, 17, 1646–1657. [Google Scholar] [CrossRef]
- Arndal, M.F.; Merrild, M.P.; Michelsen, A.; Schmidt, I.K.; Mikkelsen, T.N.; Beier, C. Net root growth and nutrient acquisition in response to predicted climate change in two contrasting heathland species. Plant Soil 2013, 369, 615–629. [Google Scholar] [CrossRef]
- Pang, W.; Hou, D.; Wang, H. Preparation of Microcapsules of Slow-Release NPK Compound Fertilizer and the Release Characteristics. J. Braz. Chem. Soc. 2018, 29, 2397–2404. [Google Scholar] [CrossRef]
- Hasan, M.M.; Teixeira da Silva, J.A.; Li, X. Regulation of phosphorus uptake and utilization: Transitioning from current knowledge to practical strategies. Cell. Mol. Biol. Lett. 2016, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Talkner, U.; Meiwes, K.J.; Potočić, N.; Seletković, I.; Cools, N.; de Vos, B.; Rautio, P. Phosphorus nutrition of beech (Fagus sylvatica L.) is decreasing in Europe. Ann. For. Sci. 2015, 72, 919–928. [Google Scholar] [CrossRef]
- Nilsson, L.O.; Wallander, H. Production of external mycelium by ectomycorrhizal fungi in a Norway spruce forest was reduced in response to nitrogen fertilization. New Phytol. 2003, 158, 409–416. [Google Scholar] [CrossRef]
- Mašková, T.; Herben, T. Root: Shoot ratio in developing seedlings: How seedlings change their allocation in response to seed mass and ambient nutrient supply. Ecol. Evol. 2018, 8, 7143–7150. [Google Scholar] [CrossRef]
- Trubat, R.; Cortina, J.; Vilagrosa, A. Nursery fertilization affects seedling traits but not field performance in Quercus suber L. J. Arid Environ. 2010, 74, 491–497. [Google Scholar] [CrossRef]
- Bosiacki, M.; Golcz-Polaszewska, M.; Kozik, E. Slow-release fertilizers in the production of horticultural plants. Part I. Effect of Osmocote Exact Standard fertilizer on the growth and condition of nourishing of selected taxons of ornamental trees and shrubs. J. Res. Appl. Agric. Eng. 2009, 54, 29–35. [Google Scholar]
- Wu, L.; Liu, M. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr. Polym. 2008, 72, 240–247. [Google Scholar] [CrossRef]
- Qiao, D.; Liu, H.; Yu, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Zhu, Z.; Jiang, Y. Long-term impact of fertilization on soil pH and fertility in an apple production system. J. Soil Sci. Plant Nutr. 2018, 18, 282–293. [Google Scholar] [CrossRef]
- Zhou, J.; Guan, D.W.; Zhou, B.K.; Zhao, B.S.; Ma, M.C.; Qin, J.; Jiang, X.; Chen, S.; Gao, F.; Shen, D. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Banach, J.; Kempf, M.; Skrzyszewska, K.; Olejnik, K. The effect of starter fertilization on the growth of seedlings of European beech Fagus sylvatica L. Sylwan 2021, 165, 565–576. [Google Scholar] [CrossRef]
- Szołtyk, G.; Zajączkowski, P. Nawożenie doglebowe. In Szkółkarstwo Leśne od, A. do Z: Praca Zbiorowa; Wesoły, W., Hauke, M., Eds.; Centrum Informacyjne Lasów Państwowych: Warszawa, Poland, 2009; pp. 233–241. ISBN 978-83-89744-81-4. [Google Scholar]
- Banach, J.; Kormanek, M.; Małek, S.; Durło, G.; Skrzyszewska, K. Effect of the changing seedlings density of Quercus robur L. grown in nursery containers on their morphological traits and planting suitability. Sylwan 2023, 167, 1–12. [Google Scholar] [CrossRef]
- Ostonen, I.; Lõhmus, K.; Lasn, R. The role of soil conditions in fine root ecomorphology in Norway spruce (Picea abies (L.) Karst.). Plant Soil 1999, 208, 283–292. [Google Scholar] [CrossRef]
- Staszel, K.; Lasota, J.; Błońska, E. Effect of drought on root exudates from Quercus petraea and enzymatic activity of soil. Sci. Rep. 2022, 12, 7635. [Google Scholar] [CrossRef]
- Wesoły, W.; Hauke, M.; Sienkiewicz, A. Nawożenie Dolistne oraz Stosowanie Nawozów Wieloskładnikowych o długim Okresie Działania w Szkółkach Kontenerowych i Otwartych (Foliar Fertilization and the Use of Compound Fertilizers with a Long Period of Action in Container and Open Nurseries); Wesoły, W., Ed.; Szkółkarstwo leśne od, A. do Z. Warszawa; Centrum Informacyjne Lasów Państwowych: Warszawa, Poland, 2009; pp. 241–254. (In Polish)
- Mellert, K.H.; Göttlein, A. Comparison of new foliar nutrient thresholds derived from VAN DEN BURG’s literature compilation with established central European references. Eur. J. For. Res. 2012, 131, 1461–1472. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 15 December 2023).
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13. 2017. Available online: https://docs.tibco.com/products/spotfire-statistica/archive (accessed on 12 December 2023).
- Mellert, K.H.; Prietzel, J.; Straussberger, R.; Rehfuess, K.E.; Kahle, H.P.; Perez, P.; Spiecker, H. Relationships between long-term trends of air temperature, precipitation, nitrogen nutrition and growth of coniferous stands in Central Europe and Finland. Eur. J. For. Res. 2008, 127, 507–524. [Google Scholar] [CrossRef]
- Sardans, J.; Alonso, R.; Janssens, I.A.; Carnicer, J.; Vereseglou, S.; Rillig, M.C.; Fernandez-Martinez, M.; Sanders, T.G.M.; Penuelas, J. Foliar and soil concentrations and stoichiometry of nitrogen and phosphorous across European Pinus sylvestris forests: Relationships with climate, N deposition and tree growth. Funct. Ecol. 2016, 30, 676–689. [Google Scholar] [CrossRef]
- Talkner, U.; Riek, W.; Dammann, I.; Kohler, M.; Göttlein, A.; Mellert, K.H.; Meiwes, K.J. Nutritional status of major forest tree species in Germany. In Status and Dynamics of Forests in Germany: Results of the National Forest Monitoring; Wellbrock, N., Bolte, A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 261–293. [Google Scholar]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology; Springer: New York, NY, USA, 2008; Volume 2, pp. 11–99. [Google Scholar]
- Xiulan, L.; Yingchun, L.; Hai, N.; Lixia, X. The effect of low phosphorus stress on main physiological traits of different maize genotypes. Zuo Wu Xue Bao 2005, 31, 667–669. [Google Scholar]
- Razaq, M.; Zhang, P.; Shen, H.L. Salahuddin Influence of nitrogen and phosphorus on the growth and root morphology of Acer mono. PLoS ONE 2017, 12, e0171321. [Google Scholar] [CrossRef] [PubMed]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Chang. Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Q.; Guo, D.L.; Xu, X.L. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Eissenstat, D.M. Costs and benefits of constructing roots of small diameter. J. Plant Nutr. 1992, 15, 763–782. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Uscola, M.; Jacobs, D.F. The role of stored carbohydrates and nitrogen in the growth and stress tolerance of planted forest trees. New For. 2015, 46, 813–839. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; Ivetić, V. Root system development and field establishment: Effect of seedling quality. New Forests 2022, 53, 1021–1067. [Google Scholar] [CrossRef]
- Luoranen, J.; Rikala, R. Nutrient loading of Norway spruce seedlings hastens bud burst and enhances root growth after out planting. Silva Fenn. 2011, 45, 319–329. [Google Scholar] [CrossRef]
- Bernier, P.Y.; Lamhamedi, M.S.; Simpson, D.G. Shoot:root ratio is of limited use in evaluating the quality of container conifer stock. Tree Plant. Notes 1995, 46, 102–106. [Google Scholar]
- Tsakaldimi, M.; Ganatsas, P.; Jacobs, D.F. Prediction of planted seedling survival of five Mediterranean species based on initial seedling morphology. New For. 2012, 44, 327–339. [Google Scholar] [CrossRef]
- Ivetić, V.; Grossnickle, S.; Škorić, M. Forecasting the field performance of Austrian pine seedlings using morphological attributes. iForest 2016, 10, 99–107. [Google Scholar] [CrossRef]
- Guimarães, Z.T.M.; Da Silva, D.C.; Ferreira, M.J. Seedling quality and short-term field performance of three Amazonian forest species as affected by site conditions. iForest 2024, 17, 80–89. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).