Abstract
This study assessed vegetation recovery at Jeongseon Alpine Stadium, Mt. Gariwang, 5 years after the 2018 PyeongChang Winter Olympics to aid in restoration planning. A total of 50 quadrats were surveyed across undisturbed areas, forest edges, and damaged areas at different altitudes. Species occurrences were recorded using a tabulation method to identify characteristic and differential species based on disturbance type. Importance value analysis showed that tree layers were present only in undisturbed areas at all altitudes, while shade-intolerant species, such as Amur choke cherry (P. glandulifolia), had high importance in the subtree layer in low-altitude damaged areas and mid-altitude forest edges. Species diversity was higher in forest edges at medium and high altitudes, whereas control areas exhibited higher diversity at low altitudes. DCA ordination revealed distinct community groupings based on altitude and disturbance type, indicating community heterogeneity. The study found rapid vegetation recovery in damaged areas and forest edges, driven by shade-intolerant species. Restoration efforts should prioritize these species to support successful recovery.
1. Introduction
An ecosystem is a unit comprising biological elements, such as microorganisms, flora, and fauna, interacting with abiotic elements, such as climate, soil, water, and terrain [1,2,3,4], facilitating material production, nutrient cycling, and organism interactions [5,6]. Ecosystems undergo succession, adapting over time to environmental changes brought about by natural and artificial factors, which lead to shifts in community structure and eventually culminate in a climax stage [4,7,8]. However, plant community ecosystems, weather at the climax stage or at various stages of succession, may experience degradation or even destruction of the original structure and functional quality of the natural environment due to severe natural or human activities, such as wildfires and clear-cutting. Degraded ecosystems typically exhibit changes in species composition, alteration of natural landforms, reduction in forest cover, fragmentation of habitats, extinction of species, disruption of wildlife corridors, simplification of habitats, landscape changes, and soil and water pollution [9]. Degraded ecosystems, even if left unmanaged after experiencing severe disturbances, can recover over time through natural regeneration and secondary succession, facilitated by seed banks, seed dispersal, and sprout development at the same location [10,11]. However, if left unattended, it may take a long time for disturbed areas to recover to their original ecosystem, or further degradation may occur due to secondary impacts such as erosion and landslides [11]. Such impacts result in changes in plant species composition, leading to shifts in characteristic species and indicator species of the original vegetation in the area [12]. Ecological restoration aims to mitigate the impacts of secondary damage by reintroducing species into degraded habitats and restoring them to pre-disturbance conditions, thereby enhancing the overall health of the ecosystem [3,13,14,15].
Currently, a focal point for ecological restoration in South Korea is Jeongseon Alpine Stadium in Mt. Gariwang. This stadium, which hosted the alpine/downhill skiing events during the 2018 PyeongChang Winter Olympics, is located on the Habong peak of Mt. Gariwang, which stands at 1381 m and spans across Pyeongchang-gun and Jeongseon-gun in Gangwon Province. The construction of ski slopes for the stadium entailed substantial deforestation, spanning from the Sookam Station (419 m), the gondola’s departure point, to the Habong peak of Mt. Gariwang (1381 m), where the slopes and other facilities are located. This extensive deforestation resulted in major damage at altitudes around 1000 m. After the successful conclusion of the 2018 PyeongChang Winter Olympics, while discussions were ongoing regarding the retention of the gondola lift, natural regeneration and secondary succession primarily involving shade-intolerant species began within the Jeongseon Alpine Stadium area on Mt. Gariwang.
Various studies related to ecosystem degradation include research on restoration approaches for damaged sites [15,16,17,18] and assessments of the current state and conditions of degraded areas [19,20,21]. In terms of secondary succession, studies have explored succession trends and characteristics following shrubland and landslide events [22,23,24] and modeled secondary succession rates in abandoned mountain farmland [25]. However, there is a lack of research on changes in stand structure and vegetation recovery by degradation type in large-scale degraded ecosystems with significant elevation differences.
Therefore, this study aimed to assess the current status of vegetation recovery based on altitude and type of damage in Jeongseon Alpine Stadium, Mt. Gariwang, in 2023, five years after the 2018 PyeongChang Winter Olympics, to provide vegetation and ecological data necessary for the establishment of goals and plans for restoration of the natural environment.
2. Materials and Methods
2.1. Survey Site Location and Site Conditions
The Jeongseon Alpine Stadium, Mt. Gariwang, is located in Sookam-ri, Bukpyeong-myeon, Jeongseon-gun, Gangwon Province, including the north slope on the Habong of Mt. Gariwang (1381.0 m) (Figure 1).
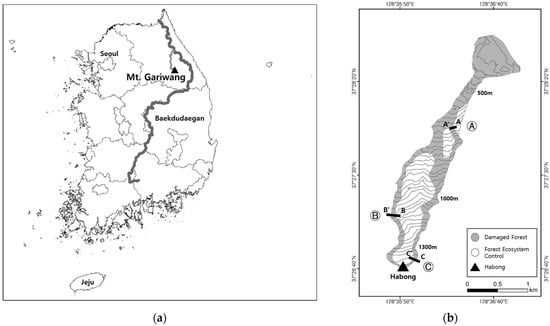
Figure 1.
Maps showing the study location and sites. (a) Mt. Gariwang and Baekdudaegan (a mountain stream extending from Mt. Baekdu, North Korea, to Mt. Jiri, South Korea). (b) Three study sites on the Jeongseon Alpine Stadium slope: Ⓐ low-altitude, Ⓑ mid-altitude, and Ⓒ high-altitude study sites.
The location and environment of the survey sites based on altitude and damage types are shown in Table 1. Low-altitude and mid-altitude areas of the Jeongseon Alpine Stadium are characterized by valleys, whereas the high-altitude area includes the Habong of Mt. Gariwang, connected by a ridgeline linking the Jungbong (1433.0 m) and the Sangbong (1562.0 m). The geographic coordinates of the starting line of the Jeongseon Alpine Stadium’s slope in the Habong of Mt. Gariwang are 37°26′41″ north latitude, 128°35′53″ east longitude. The geology surrounding the survey sites dates back to the Triassic period of the Mesozoic era, predominantly composed of Pyeongan volcanic rocks, with representative rock types including greenish-gray sandstone and grayish shale [26]. A climate diagram based on the work of Walter et al. [27] was constructed using weather data from the past 27 years (1997–2023) obtained from the automatic weather system at Bukpyeong Meteorological Observatory near the survey sites (Figure 2). The study average annual temperature and precipitation were 10.2 °C and 1241.0 mm, respectively, with most of which occurred from May to August [28].

Table 1.
Environmental factors according to altitude and disturbance type in the study site.
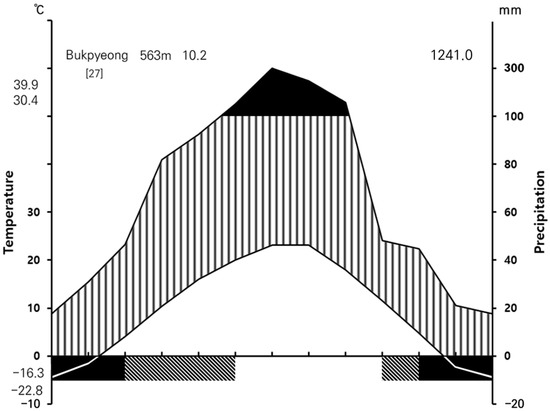
Figure 2.
Climate diagram of Bukpyeong in Jeongseon, located approximately 10 km away from the study site [27].
2.2. Field Survey Method and Analysis
2.2.1. Field Survey Method
The field survey for this study was conducted for approximately 2 months from August to October 2023. Survey equipment included GPS devices, poles, a camera, phytosociological relevé, and writing instruments.
The vegetation survey aimed to determine species composition and structure within the survey area. Dominance and sociability classes of all relevant plant species present in the survey area, which was selected according to uniformity, were measured and recorded following the Z–M School’s phytosociological method [29,30,31]. Environmental factors, such as altitude, bearing, slope, coordinates, terrain, rock exposure, and vegetation cover at each layer, were also measured and recorded.
The survey area was divided based on disturbance types into damaged forest, forest edge, and forest ecosystem control. Quadrat size was standardized at 5 × 5 m (25 m2). In total, 50 quadrats were established, with 13 in the low-altitude area (approximately 650 m), 22 in the mid-altitude area (approximately 1050 m), and 15 in the high-altitude area (approximately 1360 m). Depending on disturbance types, 17 quadrats in damaged areas, 11 in forest edges, and 22 in forest ecosystem control areas (undisturbed areas) were established and surveyed (Table 2).

Table 2.
Number of plots according to altitude and disturbance types.
Plant species were identified and classified using Field Guide [32,33,34,35,36]. Scientific nomenclature and Korean species names were referenced from the National Standard Plant List [37] based on the APG IV guidelines.
2.2.2. Analysis Method
Field survey vegetation data were processed into a phytosociological table based on disturbance types, resulting in a constancy degree table to identify character and differential species in Jeongseon Alpine Stadium.
To assess vegetation structure based on altitude and disturbance types, the survey area was divided into tree layer, subtree layer, shrub layer, and herb layer. The relative importance of each major species in each layer was determined using the method of Curtis and McIntosh [38], combining and averaging relative density, relative coverage, and relative frequency to calculate the importance value.
where RD is relative density; RC is relative coverage; and RF is relative frequency.
Additionally, to visualize vegetation recovery status in the survey area, vegetation cross-sections were created using Adobe Photoshop CS6. These cross-sections, generated separately for low-, mid-, and high-altitude areas, depicted vegetation survey plots (undisturbed, forest edge, damaged forest, and forest edge, undisturbed) in a transect format.
To understand the structural characteristics of forest communities based on altitude and disturbance types, species richness (S) and species diversity (H′) [39] were calculated. Subsequently, maximum species diversity (Hmax′), species evenness (J′), and species dominance (1 − J′) [40] were determined, and the results were averaged based on altitudes and disturbance types.
where s is the number of species.
where Pi is ratio of the individuals of a specific species divided by the total number of individuals in one survey site, Ni is the number of a specific species in one survey site, and N is the total number.
where s is the number of species.
To determine the similarity and quantitative distance between each colony based on altitude and disturbance type, dominance values were averaged and analyzed through DCA using the analysis program PC-ORD v7.10 [41]. Based on hierarchy analysis, survey sites were arranged in a two-dimensional space using two axes with high eigenvalues [42] to identify flexible relationships.
3. Results and Discussion
3.1. Differential Species Groups and Characteristics of Vegetation Units
Comparisons of relative frequencies and minimum–maximum dominance of Clusters i, ii, and iii based on 207 taxonomic groups observed in vegetation survey quadrats in the Jeongseon Alpine Stadium are presented in Table 3. Two pairs of opposed species groups were identified, consisting of species group 2 (Clusters ii and iii) vs. species group 3 (Cluster i), and species group 4 (Clusters i and ii) vs. species group 5 (Cluster iii).

Table 3.
Differentiated constancy table of forest vegetation in forest ecosystem control, forest edge, and damaged forest areas.
The characteristic species group for all three clusters included East Asian ash (Fraxinus rhynchophylla), Korean maple (Acer pseudosieboldianum), Regel’s threesingnut (Tripterygium regelii), Purple reedgrass (Calamagrostis arundinacea), Asian red raspberry (Rubus idaeus var. microphyllus), Korean raspberry (Rubus crataegifolius), Laceshrub (Stephanandra incisa), and Old-fashion weigela (Weigela florida). Notably, East Asian ash (F. rhynchophylla) and Korean raspberry (R. crataegifolius) showed high constancy degree and dominance values. The character species for Clusters ii and iii included Wedding cake tree (Cornus controversa), Korean birch (Betula costata), Amur choke cherry (Prunus glandulifolia), Shrub lespedeza (Lespedeza bicolor), and German rampion (Oenothera biennis). Of these species, Korean birch (B. costata), Amur choke cherry (P. glandulifolia), and Shrub lespedeza (L. bicolor) exhibited high constancy degree and dominance values. In Clusters ii and iii, the differential species Korean birch (B. costata), Amur choke cherry (P. glandulifolia), Korean red pine (Pinus densiflora), Shrub lespedeza (L. bicolor), Leafy lespedeza (Lespedeza cyrtobotrya), and Goat willow (Salix caprea) are shade-intolerant species [43,44], characterized by their preference for open areas with ample sunlight. Additionally, German rampion (O. biennis) is an exotic species, frequently introduced by vehicles using forest roads or human movement [45,46,47,48,49]. Specifically, their seeds travel on the tires or floor of vehicles along with mud, contributing to their introduction into forests [50,51]. Areas affected by slope installation, such as damaged forest areas and forest edges, exhibited distinct environmental characteristics, including sunlight penetration and nutrient availability, differing from forest interiors because of sparse vegetation and reduced competition for moisture and nutrients [52,53]. Compared with previous research findings, the presence of shade-intolerant species, such as Korean birch (Betula costata), Amur choke cherry (Prunus glandulifolia), along with invasive species, such as German rampion (O. biennis) and White heath aster (Symphyotrichum pilosum), indicates that Clusters ii and iii represent a species composition well-adapted to the environmental conditions of forest edges and damaged areas.
Characteristic species exclusive to Cluster i, the opposing species group of Clusters ii and iii, included Shield fem (Dryopteris crassirhizoma), Cross holly fem (Polystichum tripteron), Glabrous deutzia (Deutzia glabrata), Five-flavor magnolia vine (Schisandra chinensis), and Oriental ladyfem (Athyrium niponicum). Notably, pteridophytes, comprising the majority of species, showed high constancy degree and dominance values. Pteridophytes tend to thrive in areas with relatively high soil and air humidity within forests, leading to higher species richness and diversity [54]. In contrast, Clusters ii and iii, characterized by relatively higher sunlight exposure, exposed rocks, and low soil moisture, showed lower constancy degree and dominance values. These results suggest that Cluster i reflects the typical habitat conditions of forest ecosystem control areas (undisturbed forest areas).
The characteristic species for Clusters i and ii included Korean mountain magnolia (Magnolia sieboldii), Slender-leaf mock orange (Philadelphus tenuifolius), Tailed-leaf isodon (Isodon excisus), Mongolian oak (Quercus mongolica), and Royal azalea (Rhododendron schlippenbachii), with Korean mountain magnolia (M. sieboldii), Mongolian oak (Q. mongolica), and Royal azalea (R. schlippenbachii), showing high dominance values. Specific to Cluster iii, the opposing species group of Clusters i and ii, were Korean wormwood (Artemisia indica), Fedde’s wormwood (Artemisia lancea), Japanese larch (Larix kaempferi), Korean aspen (Populus tremula var. davidiana), and East Asian white birch (Betula pendula). Among these species, Korean wormwood (A. indica) and Fedde’s wormwood (A. lancea), belonging to Asteraceae family, showed high dominance values. Consistent with the findings of Kim et al. [55] indicating the prevalence of Asteraceae plants near cultivated fields, the absence of species occupying the tree layer and subtree layers in the clear-cut area reflects the environmental characteristics of open areas, which are similar to cultivated fields. The differential species Japanese larch (L. kaempferi), East Asian white birch (B. pendula), and Korean aspen (Populus tremula var. davidiana) in this cluster were considered to act as pioneer species [4,56,57,58].
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3.2. Species Composition Characteristics According to Disturbance Types and Altitude
To understand the species composition of the tree layer (Table 4), subtree layer (Table 5), shrub layer (Table 6), and herb layer (Table 7) in relation to altitude and disturbance types at Jeongseon Alpine Stadium, importance values were determined. Schematic diagrams (Figure 3, Figure 4 and Figure 5) were created to depict the forest vegetation structure and vegetation recovery status in damaged, forest edge, and forest ecosystem control areas (undisturbed areas) at low, mid, and high altitudes, focusing on plants exhibiting a dominance class ≥2 both horizontally and vertically.

Table 4.
Importance values of major species according to altitude and disturbance types in the tree layer.

Table 5.
Importance values of major species according to altitude and disturbance types in the subtree layer.

Table 6.
Importance values of major species according to altitude and disturbance types in the shrub layer.

Table 7.
Importance values of major species according to altitude and disturbance types in the herb layer.
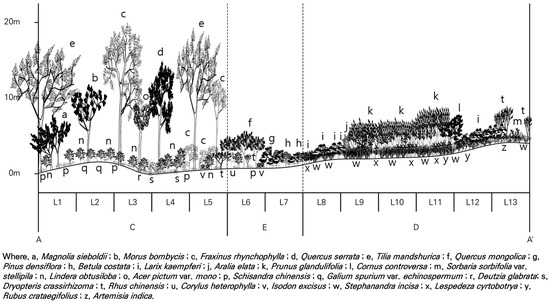
Figure 3.
Cross-section (A–A′ in Figure 1) of the vertical vegetation structure at the low-altitude site.
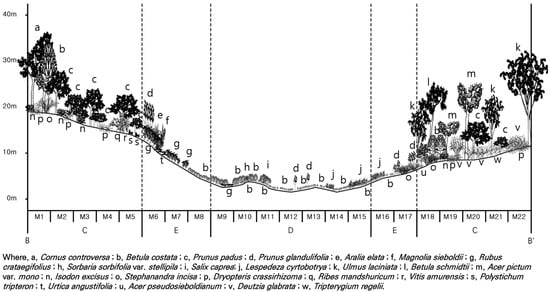
Figure 4.
Cross-section (B–B′ in Figure 1) of the vertical structure at the middle altitude site.
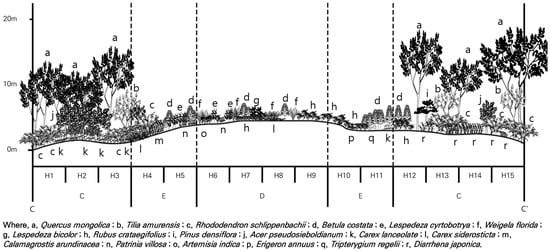
Figure 5.
Cross-section (C–C′ in Figure 1) of the vertical vegetation structure at the high-altitude site.
Comparison of vegetation cross-sections and importance values across altitudes and disturbance types showed that the forest tree layer was solely present in the control areas (undisturbed areas) at all altitudes, whereas the subtree layer and forest edge areas lacked species typical of the tree layer. The absence of tree layer species can be attributed to deforestation for alpine ski slope installation, with pioneer species rapidly colonizing these areas and indicating the initial stages of ecological recovery through secondary succession.
Analysis of vegetation cross-sections and importance values in low-altitude (Figure 3) and mid-altitude (Figure 4) areas revealed dominance patterns. In the control areas (undisturbed areas) at low altitudes (L1–L5), the tree layer was dominated by Manchurian lime (Tilia mandshurica), East Asian ash (F. rhynchophylla), and Jolcham oak (Q. serrata); the subtree layer was dominated by Korean mulberry (Morus bombycis), Korean mountain magnolia (M. sieboldii), and East Asian ash (F. rhynchophylla); the shrub layer was dominated by Blunt-lobe spicebush (Lindera obtusiloba), East Asian ash (F. rhynchophylla), and Glabrous deutzia (D. glabrata); and the herb layer was dominated by Five-flavor magnolia vine (S. chinensis), Shield fem (D. crassirhizoma), and East Asian ash (F. rhynchophylla).
In the forest ecosystem control areas (undisturbed areas) of the low-altitude region (M1–M5 and M18–M22), dominant tree species in the tree layer included Mono maple (Acer pictum var. mono), Wedding cake tree (C. controversa), and Korean birch (B. costata). In the subtree layer, Bird cherry (Prunus padus), Glabrous deutzia (D. glabrata), and Slender-leaf mock orange (P. tenuifolius) were prominent, whereas in the shrub layer, Blunt-lobe spicebush (L. obtusiloba), East Asian ash (F. rhynchophylla), and Glabrous deutzia (D. glabrata) were prevalent. In the herb layer, Shield fem (D. crassirhizoma), Laceshrub (S. incisa), and Tailed-leaf isodon (I. excisus) were dominant, exhibiting high importance values. The prevalence of moisture-demanding species, such as Manchurian lime (T. mandshurica), East Asian ash (F. rhynchophylla), Wedding cake tree (C. controversa), and Mono maple (A. pictum var. mono), in the control areas (undisturbed areas) of the low- and mid-altitude regions suggested that their abundance may be attributed to the topographical characteristics of valley terrain or the high humidity environment in these areas of Jeongseon Alpine Stadium [59].
Analysis of vegetation cross-sections (Figure 5) and importance values revealed that Mongolian oak (Q. mongolica) was the dominant species in the tree layer of forest ecosystem control areas (undisturbed areas) at high altitudes (H1–H3 and H12–H15). Most of Korea’s alpine areas are categorized as subalpine zones. The high-altitude region in Mt. Gariwang belongs to this zone, characterized by tall shrubs and canopy trees [60]. The main tree species in the subalpine zone include Mongolian oak (Q. mongolica), Korean maple (A. pseudosieboldianum), Royal azalea (R. schlippenbachii), Bearded maple (Acer barbinerve), Silvery mountain ash (Sorbus commixta), Khingan fir (Abies nephrolepis), Erman’s birch (Betula ermanii), Rigid-branch yew (Taxus cuspidate), and Korean pine (Pinus koraiensis) [60,61,62]. Among these species, Mongolian oak (Q. mongolica) exhibited low importance values in the low-altitude area, with values tending to increase with increasing altitude [63,64]. These results highlight Mongolian oak (Q. mongolica) as a key species mainly distributed in the subalpine region of Korea. Its importance value of 100 in the tree layer of the control area (undisturbed area) at high altitude in the Jeongseon Alpine Stadium confirms its dominance in such environments.
In low-, mid-, and high-altitude areas, forest edges and damaged areas predominantly featured shrubs and herbaceous plants. Particularly in low-altitude damaged areas, the importance value of Amur choke cherry (P. glandulifolia) was the highest at 66.5. In the shrub layer, the importance value followed the order Laceshrub (S. incisa), Korean birch (B. costata), Leafy lespedeza (L. cyrtobotrya), and Korean raspberry (R. crataegifolius), whereas in the herb layer, importance values were in the order Korean birch (B. costata), Korean raspberry (R. crataegifolius), and East Asian ash (F. rhynchophylla). Shade-intolerant species were distributed in damaged areas and along forest edges at all altitudes. Notably, Amur choke cherry (P. glandulifolia) had a high importance value in low-altitude damaged areas and forest edges in the subtree layer, whereas Korean birch (B. costata) dominated the shrub layer in low-, mid-, and high-altitude damaged areas and forest edges. In the herb layer, Korean wormwood (A. indica) showed high importance values in low-, mid-, and high-altitude damaged areas and forest edges. The prevalence of shade-intolerant species, such as Amur choke cherry (P. glandulifolia), Korean birch (B. costata), Korean wormwood (A. indica), Goat willow (S. caprea), Leafy lespedeza ( L. cyrtobotrya), Laceshrub (S. incisa), and Korean raspberry (R. crataegifolius), in damaged areas and forest edges across all altitudes can be attributed to the absence of occupying species in the tree layer due to deforestation for slope installation, allowing light to reach the ground surface. The presence and growth of shade-intolerant species, including Amur choke cherry (P. glandulifolia), in the subtree layer indicate that secondary succession by pioneer species is progressing in the damaged areas and forest edges, suggesting that the vegetation recovery process has reached the initial stage of forest stand development [4,65].
The presence of Japanese larch (L. kaempferi) and Korean red pine (P. densiflora) in damaged areas and forest edges at lower and middle altitudes prior to slope installation can be explained by natural seed dispersal near damaged areas via wind, as most areas below an altitude of 1000 m were forested with deciduous species, including Japanese larch (L. kaempferi) and Korean red pine (P. densiflora), even before slope installation [66]. Over time, seeds of Japanese larch (L. kaempferi) and Korean red pine (P. densiflora) near damaged areas were likely dispersed naturally by wind, as reported in various studies [56,67,68]. Considering that Korean red pine (P. densiflora) is a shade-intolerant tree species with a short fruiting cycle, it is assumed that species introgression into the shrub and herb layers is progressing rapidly.
Figure 3 shows the vegetation structure of the low-altitude area, where valley terrain tree species are prevalent in forest ecosystem control areas (undisturbed areas) because of valley topography. Forest edges and damaged areas comprise open spaces with shade-intolerant species, including Korean red pine (Pinus densiflora), Korean birch (Betula costata), Japanese larch (Larix kaempferi), and Amur choke cherry (Prunus glandulifolia). Species growing in the control areas (undisturbed areas) reach heights of up to 18 m, whereas Mono maple (Acer pictum var. mono) and other tree species in the subtree layer reach 8 m, with plants in the shrub layer reaching 2.5 m. Notably, Amur choke cherry (Prunus glandulifolia) in the damaged area grew to a height of 6 m, extending into the subtree layer.
Figure 4 shows the vegetation structure of the mid-altitude area, revealing its valley topography even prior to slope installation. In forest ecosystem control areas (undisturbed areas) at this altitude, species such as Wedding cake tree (Cornus controversa), Bird cherry (Prunus padus), and Manchurian elm (Ulmus laciniata) dominate the tree layer, reaching heights of 18 m. Forest edges and damaged areas show the introduction and renewal of shade-intolerant species, such as Amur choke cherry (Prunus glandulifolia), Korean raspberry (Rubus crataegifolius), and Korean birch (Betula costata), with heights reaching 5 m. Notably, in the damaged area, these species are confined to the herb layer.
Figure 5 shows the vegetation structure of the high-altitude areas, characterized by ridge topography. In forest ecosystem control areas (undisturbed areas) at high altitude, Mongolian oak (Quercus mongolica) predominated in the tree layer, forming a multi-layered structure with tree heights of 14–16 m. The shrub layer was dominated by Royal azalea (Rhododendron schlippenbachii), whereas the herb layer was dominated by Lanceolate sedge (Carex lanceolate). Species observed in forest edges and damaged areas were distributed only in the herb layer, indicating the presence of shade-intolerant species, such as Korean brich (Betula costata), Leafy lespedeza (Lespedeza cyrtobotrya), Old-fashion weigela (Weigela florida), and Korean raspberry (Rubus crataegifolius), likely distributed via translocation.
3.3. Species Diversity Depending on Disturbance Types
Table 8 presents S, H′, J′, and 1 − J′ values according to altitude and disturbance types. The range of H′ was 1.446–2.110, highest in forest ecosystem control areas (undisturbed areas) at low-altitude regions, whereas mid- and high-altitude regions showed the highest H′ in forest edges. In damaged areas, the overall low H′ can be attributed to the ongoing introduction of pioneer plant species. High H′ in the control areas (undisturbed areas) at low altitudes occurred because of the valley topography present even before slope installation, fostering the growth of numerous species, including valley-specific plant species and pteridophytes, thereby increasing S and elevating H′. Forest edges in mid- and high-altitude areas showed high H′ owing to increased sunlight exposure, influence of wind, and dryness, creating unique microclimate conditions [69]. In such environments, light-loving plants, early pioneer species, or those well-adapted to disturbances thrive [70], resulting in high S and H′. This finding is consistent with the previous studies of Erdős et al. [71], who reported that H' was highest at forest edges compared with that in grasslands and forest interiors, and Krebs [72], who indicated that H′ increases in heterogeneous and complex environments or during localized disturbances.

Table 8.
Species diversity index according to altitude and disturbance types.
J′, which indicates the degree of community stability, with a maximum value of 1 representing full stability [73], ranged from 0.474 to 0.655 across all disturbance types in this study. In damaged areas, J′ values were 0.545, 0.474, and 0.614 according to altitude, likely due to the dominance of shade-intolerant species in the upper layer of these areas as secondary succession progresses. However, given that J′ was <0.7 for all types of disturbances in the present study, the community is not in a stable state. Additionally, 1 − J′ was 0.346–0.526 across all altitudes and disturbance types, with higher 1 − J′ values observed in damaged areas at low and middle altitudes compared with higher 1 − J′ values in the control areas (undisturbed areas) at high altitudes. The dominance of shade-intolerant species contributed to higher dominance in damaged areas at lower and middle altitudes, whereas the dominance of Mongolian oak (Q. mongolica) in the tree layer led to higher dominance in the control areas (undisturbed areas) at high altitudes. Where 1 − J′ is <0.3, only a few dominant species are present [74]. In comparison, 1 − J′ exceeded 0.4 in damaged areas at lower and middle altitudes in the present study, indicating the dominance of a few species in these areas.
3.4. Detrended Correspondence Analysis Based on Disturbance Types
Detrended correspondence analysis (DCA) ordination, depending on altitude and disturbance types, revealed eigenvalues for the DCA axes as follows: Axis 1 = 0.8556, Axis 2 = 0.5565, and Axis 3 = 0.4400, totaling 1.8520 across the three axes. Axis 1 and Axis 2 combined explained approximately 76.2% of the total variance, indicating a high concentration of variance.
According to hierarchical analysis of species composition across different disturbance types, damaged areas and forest edges at all altitudes appeared centrally located with respect to Axis 1 and Axis 2. Forest ecosystem control areas (undisturbed areas) in the low- and mid-altitude regions were clustered toward the top on Axis 1, whereas the control areas (undisturbed areas) in the high-altitude region tended to cluster toward the bottom, within the densely populated region of damaged areas and forest edges based on Axis 1. Additionally, based on Axis 2, the control areas (undisturbed areas) in the low-altitude region were positioned to the right, whereas those in the mid-altitude region were positioned to the left (Figure 6).
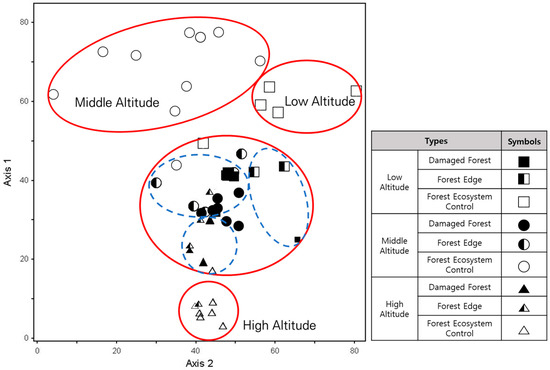
Figure 6.
Detrended correspondence analysis ordination according to altitude and disturbance types.
The dense clustering of the control areas (undisturbed areas) in the low- and mid-altitude regions toward the top based on Axis 1 is attributed to the even distribution of species, such as Korean mountain magnolia (M. sieboldii), Mono maple (A. pictum var. mono), Laceshrub (S. incisa), and Nutgall tree (R. chinensis), in both regions. The discrepancy in clustering of the control areas (undisturbed areas) between low and middle altitudes based on Axis 2 is due to differences in species composition in the tree and subtree layers. Furthermore, the clustering of the control areas (undisturbed areas) in the high-altitude region toward the bottom based on Axis 1 is due to the sole presence of Mongolian oak (Q. mongolica) in the tree layer of the control areas (undisturbed areas) in the region.
The clustering of damaged areas and forest edges toward the center on Axis 1 and Axis 2 is attributed to the absence of tree layers in all surveyed sites for those areas as well as the presence of subtree and shrub layers dominated by shade-intolerant species. Additionally, within the clustered group of damaged areas and forest edges, they were also clustered according to altitudes, showing a distribution pattern similar to that in forest ecosystem control areas (undisturbed areas).
4. Conclusions
This study aimed to assess vegetation recovery across various altitudes and disturbance types within the Jeongseon Alpine Stadium as of 2023, five years after the conclusion of the 2018 PyeongChang Winter Olympics. This study was conducted to provide vegetation and ecological data necessary for establishing restoration goals and future plans for the stadium site. Analyses encompassing constancy degree, importance values, species diversity, and DCA ordination were performed. The Constancy degree and importance value analysis results indicated that shade-intolerant plant species predominated in damaged areas and forest edges. This suggests that ecological recovery is in its early stage of secondary succession [65]. As shown in Figure 7, species such as Bc (Betula costata), Bs (Betula schmidtii), Fr (Fraxinus rhynchophylla), Lk (Larix kaempferi), Pd (Pinus densiflora), Pg (Prunus glandulifolia), and Sc (Salix caprea) in adjacent forest edges and damaged areas are considered to have regenerated through seed banks and sprouts, or seed dispersal from external sources [11].
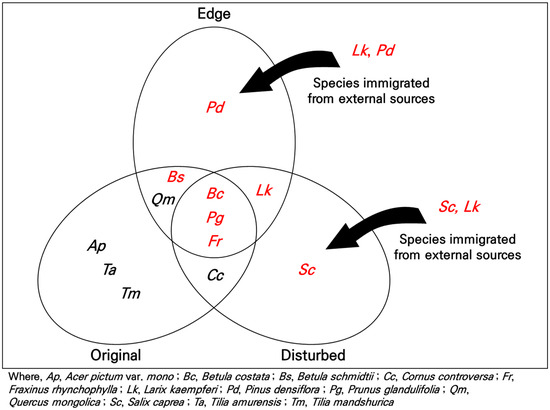
Figure 7.
The mechanisms of secondary succession following five years after an ecosystem disturbance. The red text represents shade-intolerant species.
This rapid regeneration of dominant shade-intolerant species should be considered a crucial factor in ecological restoration planning. The rapid regeneration observed in damaged areas, where slopes were installed for Olympic competitions, primarily with shade-intolerant species, including Japanese larch (L. kaempferi), Amur choke cherry (P. glandulifolia), and Korean birch (B. costata), through seed dispersal from neighboring areas, indicates a process of stable vegetation recovery, serving as foundational data for ecological restoration efforts.
Species diversity analysis results showed a tendency for S values to increase with altitude in the damaged areas. The H' index indicated higher diversity due to secondary succession following disturbances caused by slope installation, with the migration of shade-intolerant species contributing to this trend (Figure 7). The J′ and 1 − J′ values showed that vegetation in damaged areas of Jeongseon Alpine Stadium is generally dominated by a few species, indicating overall vegetation instability in these plant communities. Furthermore, DCA analysis revealed that damaged areas and forest edges at all altitudes were clustered in the central part of the Axis 1 × Axis 2 coordinates. These results imply that vegetation recovery in Jeongseon Alpine Stadium on Mt. Gariwang primarily favors shade-intolerant species, deviating from the original status across all types of damaged areas. Nonetheless, over the next few decades, damaged areas and forest edges are expected to undergo secondary succession and progress toward a stable ecological structure similar to forest ecosystem control areas (undisturbed areas). Additionally, periodic monitoring of vegetation recovery within the Jeongseon Alpine Stadium in Mt. Gariwang using UAV and spectrometer equipment is deemed necessary.
In summary, considering the vegetation recovery situation over the past 5 years, characterized by the proliferation of shade-intolerant species as part of the secondary succession process from damaged areas and forest edges, it is advisable to prioritize the selection of native shade-intolerant species as target species for ecological restoration.
Author Contributions
Conceptualization, C.-W.Y.; methodology, J.-H.S. and C.-W.Y.; software, S.-W.L. and J.-E.L.; validation, S.-W.L., J.-E.L., J.-H.S. and C.-W.Y.; formal analysis, S.-W.L. and J.-E.L.; investigation, S.-W.L., J.-E.L., J.-H.S. and C.-W.Y.; resources, J.-H.S. and C.-W.Y.; data curation, S.-W.L. and J.-E.L.; writing—original draft preparation, S.-W.L.; writing—review and editing, S.-W.L. and C.-W.Y.; visualization, S.-W.L.; supervision, C.-W.Y.; project administration, J.-E.L. and C.-W.Y.; funding acquisition, C.-W.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Korea Forest Service’s and Research on Measures to Maximize Forest Utility, including Preservation and Effective Use of Gariwangsan Cultural Heritage and Korea Arboreta and Gardens Institute Long-term ecological monitoring of the changes of forest edge in forest damaged area (Project No.: 2022-KS-OB-02-03).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors acknowledge the Korea Forest Service for leading this study. We especially thank Byung Bae, Park who was the principal investigator of the research project for the Korea Forest Service’s Research on Measures to Maximize Forest Utility, including Preservation and Effective Use of Gariwangsan Cultural Heritage. The authors would like to thank the staff of the Forest Ecology Research Laboratory, Department of Forest Science, Kongju National University for their considerable efforts in the survey. Authors also acknowledge the residents of Jeongseon-gun and Jeongseon County Installation Management Corporation for their strong support and interest in this research, with which we were be able to carry out the survey more efficiently.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tansley, A.G. The use and abuse of vegetational concepts and terms. Ecology 1935, 16, 284–307. [Google Scholar] [CrossRef]
- Golley, F.B. The ecosystem concept: A search for order. Ecol. Res. 1991, 6, 129–138. [Google Scholar] [CrossRef]
- Bradshaw, A.D. Ecological principles and land reclamation practice. Landsc. Plan. 1984, 11, 35–48. [Google Scholar] [CrossRef]
- Shon, Y.H.; Koo, C.D.; Kim, C.S.; Park, P.S.; Yun, C.W.; Lee, K.H. Forest Ecology, 3rd ed.; Hyangmonsa Publishing Company: Seoul, Republic of Korea, 2024; p. 366. [Google Scholar]
- Likens, G.E. The Ecosystem Approach: Its Use and Abuse; Ecology Institute: Oldendorf-Luhe, Germany, 1992; p. 166. [Google Scholar]
- Begon, M.; Townsend, C.R.; Harper, J.L. Ecology: From Individuals to Ecosystems, 5th ed.; Blackwell, Wiley: Hoboken, NJ, USA, 2020; p. 864. [Google Scholar]
- Cowls, H. The ecological relations of the vegetation on the sand dunes of Lake Michigan. Bot. Gaz. 1899, 27, 167–202. [Google Scholar] [CrossRef]
- Clements, F.E. Plant Succession: An Analysis of the Development of Vegetation; Caregie Institution of Washington: Washington, DC, USA, 1916; p. 512. [Google Scholar]
- Kim, G.H.; Sung, H.C.; Choi, J.Y.; Heo, Y.J. Ecology Restoration, Korean ed.; Kimoondang: Seoul, Republic of Korea, 2018; p. 232. [Google Scholar]
- Bill, F. Environmental Ecology: The Ecological Effects of Pollution, Disturbance, and Other Stresses, 2nd ed.; Academic Press: San Diego, CA, USA, 1995; p. 5. [Google Scholar]
- Cho, D.G. Ecological Restoration Planning and Design Vol. 1—Theories, Law, and Institutions in Ecological Restoration, 2nd ed.; Nexus Environmental Design Institute Press: Seoul, Republic of Korea, 2017; p. 583. [Google Scholar]
- Song, J.H.; Yun, C.W.; Cho, Y.H.; Kang, H.K. A study vegetation structure changes between natural land and damaged land in Regional Ecological Network at Chungnam Province. J. Korean Soc. Environ. Restor. Technol. 2017, 20, 13–35. [Google Scholar] [CrossRef][Green Version]
- Bradshaw, A.D.; Chadwick, J. The Restoration of Land: The Ecology and Reclamation of Derelict and Degraded Land; Blackwell, Wiley: Hoboken, NJ, USA, 1980; p. 317. [Google Scholar]
- SER (Society for Ecological Restoration). Available online: https://www.ser.org/ (accessed on 15 December 2023).
- Choi, J.Y.; Lee, S.H.; Ji, S.Y.; Lee, S.H. Evaluation method development for ecological restorations by damaged types. J. Korean Soc. Environ. Restor. Technol. 2016, 19, 121–133. [Google Scholar] [CrossRef]
- Jeong, S.J.; Oh, K.K.; Oh, J.G. A study on restoration measures of vegetation for devastated ridge line area in national park, Korea. Korean J. Environ. Ecol. 2001, 15, 69–78. [Google Scholar]
- Lee, H.J.; Kim, D.K.; Oh, J.H.; Cha, D.S. Development of soil bioengineering technique for natural recovering of artificial earthworks (Ⅱ). J. Korea Soc. Eng. Technol. 2006, 4, 208–215. [Google Scholar]
- Lee, S.H.; Lee, S.H.; Lee, S.A.; Choi, J.Y. Development of evaluation indices for ecological restoration of degraded environments near DMZ in the Republic of Korea. J. Korean Soc. Environ. Restor. Technol. 2015, 18, 135–151. [Google Scholar] [CrossRef][Green Version]
- Jang, G.S.; Jeon, S.W.; Kim, S.S. Analysis characteristics of forest damage within the Geum-buk mountain range. J. Korean Inst. Landsc. Archit. 2008, 36, 55–63. [Google Scholar]
- Lee, Y.S.; Lee, D.G.; Yu, Y.G.; Lee, H.J. Application of drone photogrammetry for current state analysis of damage in forest damage areas. J. Korean Soc. Geospat. Inf. Sci. 2016, 24, 49–58. [Google Scholar] [CrossRef]
- Lee, S.H.; Yun, J.U.; Choi, I.Y. Analysis of damage status to the trail in Unmunsan County Park. J. Natl. Park Res. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Guariguata, M.; Ostertag, R. Neotropical secondary forest succession: Changes in structural and functional characteristics. For. Ecol. Manag. 2001, 148, 158–206. [Google Scholar] [CrossRef]
- Calvo, L.; Tárrega, R.; Luis, E.D. Secondary succession after perturbations in a shrubland community. Acta Oecol. 2002, 23, 393–404. [Google Scholar] [CrossRef]
- Li, B.; Zeng, T.; Ran, J.; Yue, B.; Zhang, M.; Shang, T.; Zhu, D. Characteristics of the early secondary succession after landslides in a broad-leaved deciduous forest in the south Minshan Mountains. For. Ecol. Manag. 2017, 405, 238–245. [Google Scholar] [CrossRef]
- Pueyo, Y.; Beguería, S. Modelling the rate of secondary succession after farmland abandonment in a Mediterranean mountain area. Landsc. Urban Plan. 2007, 83, 245–254. [Google Scholar] [CrossRef]
- KIGMR (Korea Institute of Geoscience and Mineral Resources). Available online: https://data.kigam.re.kr/ (accessed on 26 February 2024).
- Walter, H.; Harnickell, E.; Müller-Dombois, D. Climate-Diagram Maps of the Individual Continents and the Ecological Climatic Regions of the Earth; Springer: Berlin, Germany, 1975; p. 37. [Google Scholar]
- KMA (Korea Meteorological Administration). Available online: https://data.kma.go.kr/ (accessed on 23 January 2024).
- Ellenberg, H. Aufgaben und Methoden der Vegetationskunde; Verlag Eugen Ulmer: Stutthart, Germany, 1956; p. 136. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie Grundzüge der Vegetation der Vegetation, 3rd ed.; Springer: Wien, Austria, 1964; p. 631. [Google Scholar]
- Kim, J.W.; Lee, Y.K. Classification and Assessment of Plant Communities; Worldscience Pulishing Company: Seoul, Republic of Korea, 2006; p. 240. [Google Scholar]
- Lee, C.B. Coloured Flora of Korea, 3rd ed.; Hyangmonsa Publishing Company: Seoul, Republic of Korea, 2003; p. 1828. [Google Scholar]
- Cho, Y.H.; Kim, J.H.; Park, S.H. Grasses and Sedges in South Korea; Geobook Publishing Company: Seoul, Republic of Korea, 2016; p. 528. [Google Scholar]
- Lee, C.S.; Lee, K.H. Pteridophytes of Korea: Lycophytes & Ferns; Geobook Publishing Company: Seoul, Republic of Korea, 2018; p. 492. [Google Scholar]
- KNA(Korea National Arboretum). Checklist of Alien Plants in Korea; Korea National Arboretum: Pocheon, Republic of Korea, 2019; p. 225. [Google Scholar]
- Yun, C.W. Field Guide to Trees and Shrubs; Geobook Publishing Company: Seoul, Republic of Korea, 2023; p. 704. [Google Scholar]
- KNA (Korea National Arboretum). Available online: http://www.nature.go.kr/main/Main.do (accessed on 20 December 2023).
- Curtis, J.T.; McIntosh, R.P. An Upland Forest Continuum in the Prairie-Forest Border Region of Wisconsin. Ecology 1951, 32, 476–496. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1998; p. 144. [Google Scholar]
- Brower, J.E.; Zar, J.H. Field and Laboratory Methods for General Ecology; Wm C Brown Company: Dubuque, IA, USA, 1977; p. 288. [Google Scholar]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software: Eugene, OR, USA, 2002; p. 300. [Google Scholar]
- Hill, M.O. DECORANA—A FORTRAN Program for Detrended Correspondence Analysis and Reciprocal Averaging; Cornell University: Ithaca, NY, USA, 1979; p. 52. [Google Scholar]
- Choung, Y.S.; Lee, J.S.; Cho, S.Y.; Noh, J.S. Review on the succession process of Pinus densiflora forests in South Korea: Progressive and disturbance-driven succession. J. Ecol. Environ. 2020, 44, 16. [Google Scholar] [CrossRef]
- Kondratenko, L.; Gura, D.; Shaidullina, V.; Rogulin, R.; Kondrashev, S. Restoration of vegetation around mining enterprises. Saudi J. Biol. Sci. 2022, 29, 1881–1886. [Google Scholar] [CrossRef]
- Western, L.; Juvik, J.O. Roadside plant communities on Mauna Loa, Hawaii. J. Biogeogr. 1983, 10, 307–316. [Google Scholar] [CrossRef]
- Tyser, R.W.; Worley, C.A. Alien flora in grasslands adjacent to road and trail corridors in Glacier National Park, Montana (USA). Conserv. Biol. 1992, 6, 253–262. [Google Scholar] [CrossRef]
- Wein, R.W.; Wein, G.; Bahret, S.; Cody, W.J. Northward invading non-native vascular plant species in and adjacent to Wood Buffalo national park, Canada. Can. Field-Nat. 1992, 106, 216–224. [Google Scholar] [CrossRef]
- Greenberg, C.H.; Crownover, S.H.; Gordon, D.R. Roadside soil: A corridor for invasion of xeric scrub by nonindigenous plants. Nat. Areas J. 1997, 17, 99–109. [Google Scholar]
- Birdsall, J.L.; McCaughey, W.; Runyon, J.B. Roads impact the recruitment and distribution of noxious weeds more than restoration treatments in a lodgepole pine forest in Montana, USA. Restor. Ecol. 2012, 20, 517–523. [Google Scholar] [CrossRef]
- Clifford, H.T. Seed dispersal by motor vehicles. J. Ecol. 1959, 47, 311–315. [Google Scholar] [CrossRef]
- Schmidt, W. Plant dispersal by motor cars. Vegetatio 1989, 80, 147–152. [Google Scholar] [CrossRef]
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invisibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef]
- Parendes, L.A.; Jones, J.A. Role of light availability and dispersal in exotic plant invasion along roads and streams in the H.J. Andrews experimental Forest, Oregon. Conserv. Biol. 2000, 14, 64–75. [Google Scholar] [CrossRef]
- Lee, C.H. Characters and mass propagation of Pteridophyta native to Korea. Korean J. Plant Resour. 2000, 13, 1–10. [Google Scholar]
- Kim, M.H.; Choi, S.K.; Kim, M.K.; Choe, L.J.; Hong, S.C.; Jung, G.B.; Cho, K.J.; Han, D.U.; Oh, Y.J.; Lee, W.J.; et al. Characteristics of flora on dry field margins in Korean peninsula. Korean J. Environ. Agric. 2015, 34, 77–90. [Google Scholar] [CrossRef]
- Kondo, T.; Tsuyuzaki, S. Natural regeneration patterns of the introduced larch, Larix kaempferi (Pinaceae), on the volcano Mount Koma, northern Japan. Divers. Distrib. 1999, 5, 223–233. [Google Scholar] [CrossRef]
- Telenius, B.F. Stand growth of deciduous pioneer tree species on fertile agricultural land in southern Sweden. Biomass Bioenergy 1999, 16, 13–23. [Google Scholar] [CrossRef]
- Hynynen, J.; Niemistö, P.; Viherä-Aarnio, A.; Brunner, A.; Hein, S.; Velling, P. Silviculture of birch (Betula pendula Roth and Betula pubescens Ehrh.) in northern Europe. Forestry 2010, 83, 103–119. [Google Scholar] [CrossRef]
- Byeon, S.Y.; Yun, C.W. Stand structure of actual vegetation in the natural forests and plantation area of Mt. Janggunbong, Bonghwa-gun. Korean J. Environ. Ecol. 2016, 30, 1032–1046. [Google Scholar] [CrossRef]
- Kong, W.S. Species composition and distribution of Korean alpine plants. J. Korean Geogr. Soc. 2002, 37, 357–370. [Google Scholar]
- Kim, Y.S.; Chon, S.H.; Kang, K.H. Floristic study of Odaesan National Park. Korean J. Environ. Ecol. 1996, 9, 77–98. [Google Scholar]
- An, J.H.; Park, H.J.; Nam, G.H.; Lee, B.Y.; Park, C.H.; Kim, J.H. Vertical distribution of vascular plant species along an elevational gradients in the Gyebangsan area of Odaesan National Park. Korean J. Ecol. Environ. 2017, 50, 381–402. [Google Scholar] [CrossRef]
- Yu, J.E.; Lee, J.H.; Kwon, K.W. An analysis of forest community and dynamics according to elevation in Mt. Sokri and Odae. Korean J. Agric. For. Meteorol. 2003, 5, 238–246. [Google Scholar]
- Han, S.H.; Han, S.H.; Yun, C.W. Classification and stand characteristics of subalpine forest vegetation at Hyangjeukbong and Jungbong in Mt. Deogyusan. J. Korean For. Soc. 2016, 105, 48–62. [Google Scholar] [CrossRef]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics (Biological Resource Management Series); McGraw-Hill Publishing Company: New York, NY, USA, 1990; p. 467. [Google Scholar]
- Byun, J.G.; Jang, J.W.; Yang, J.C.; Lee, Y.M.; Jung, S.Y.; Ji, S.J.; Jang, J.; Lee, H.J.; Hwang, H.S.; Oh, S.H. The flora of vascular plants in Mt. Gariwang protected area for forest genetic resource conservation, South Korea. Korean J. Plant Resour. 2013, 26, 566–588. [Google Scholar] [CrossRef][Green Version]
- Zhang, M.; Kim, J.S.; Cho, Y.C.; Bae, S.W.; Yun, C.W.; Byun, B.K.; Bae, K.H. Initial responses of understory vegetation to 15% aggregated retention Harvest in mature oak (Quercus mongolica) forest in Gyunsangbukdo. J. Korea Soc. For. Sci. 2013, 102, 239–246. [Google Scholar]
- Kim, H.H.; Lee, J.E.; Lee, S.Y.; Park, D.E.; Yun, C.W. Vegetation structure of urban forest on Mt. Goehwa, Sejong-si. J. Korea Soc. For. Sci. 2024, 113, 51–65. [Google Scholar] [CrossRef]
- Forman, R.T.T.; Sperling, D.; Bissonette, J.A.; Clevenger, A.P.; Cutshall, C.D.; Dale, V.H. Road Ecology: Science and Solutions; Island Press: Washington, DC, USA, 2003; p. 504. [Google Scholar]
- Mehrhoff, L.A. Reproductive vigor and environmental factors in populations of an endangered North American orchid, Isotria medeoloides (Pursh) Rafinesque. Biol. Conserv. 1989, 47, 281–296. [Google Scholar] [CrossRef]
- Erdős, L.; Gallé, R.; Körmöczi, L.; Bátori, Z. Species composition and diversity of natural forest edges: Edge responses and local edge species. Community Ecol. 2013, 14, 48–58. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecology: The Experimental Analysis of Distribution and Abundance, 3rd ed.; Haber and Row Publishing Company: New York, NY, USA, 1985; p. 672. [Google Scholar]
- Cho, M.G.; Chung, J.M.; Im, H.I.; Noh, I.; Kim, T.W.; Kim, C.Y.; Moon, H.S. Ecological characteristics of sub-alpine coniferous forest on Banyabong in Mt. Jiri. J. Clim. Change Res. 2016, 7, 465–476. [Google Scholar] [CrossRef]
- Whittaker, R.H. Dominance and diversity in land plant communities: Numerical relations of species express the importance of competition in community function and evolution. Science 1965, 147, 250–260. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).