Abstract
Leaf-trait variation has traditionally been focused on both within and among species along environmental gradients, while leaf age has received less attention. By measuring leaf morphological, stomatal, and stoichiometric traits of 40 coexisting woody species in temperate forest in northern China, we analyzed their variation pattern and the correlations among different plant life forms and leaf age. We found that leaf age has significant effects on leaf functional traits. The young leaves of both shrub and tree species revealed a lower stoma density (SD) and a higher stoma length (SL), stoma width (SW), and leaf N content (LNC) than mature leaves. Shrub species have a higher SLA and SD than tree species for both young and mature leaves. The traits of young leaves generally revealed a higher variation than those of mature leaves. Although correlations between traits are similar between young leaves and mature leaves, the slopes of the SLA–SD and SD–LNC relationships were significantly affected by leaf age. These findings elucidate the adaptive changes of leaf traits during leaf maturation and underscore the trade-off between stomatal safety and efficiency, as well as the trade-off between leaf hydraulic and economic traits in temperate woody species during leaf development. We conclude that variation in leaf traits with age may play a potentially important role in understanding the ecological function of woody species in temperate forests.
1. Introduction
Leaf functional traits are regarded as key indicators to understand and predict the abundance and distribution of plant species under climate change at the global and local scale [1,2,3]. A suite of leaf traits related to morphology, stoichiometry, and physiological ecology has been measured to delineate major ecological strategies by integrating global trait data sets [1,4,5]. Within organ types, leaf traits consistently covary in a way described as ‘economics spectra’, which broadly summarize the trade-offs between fast-growing and slow-growing plants [3,6,7]. Leaf development is one of the fundamental processes of plant growth [8], and physiological, anatomical, and morphological traits significantly vary during leaf development [9]. Leaf demography and seasonal differences in leaf age compositions within forest canopy layers are the basis for explaining primary productivity and photosynthetic capacity estimates [10]. Therefore, leaf ontogenetic variations (leaf age) should be considered in studying plant ecological strategies and community assembly based on leaf functional traits.
At the leaf level, some leaf economic traits such as leaf nitrogen concentration (LNC) and specific leaf area (SLA) have been screened to accurately predict the change in maximum photosynthetic rate [11,12] and leaf lifespan for a wide range of species [13]. Regarding the changes in leaf size during leaf development, it not only reflects the light interception efficiency and carbon acquisition ability of plants, but also reflects the trade-off between carbon assimilation and water use efficiency of plants, which is crucial for plant growth regulation [14,15,16]. However, existing studies about changes in leaf functional traits with leaf age only focus on single species and minority traits such as the LNC, SLA, and photosynthetic rate [17,18,19], and the results change depending on plant life forms and growth forms, impeding the formation of common shifting patterns of leaf traits with leaf age. For example, the SLA (specific leaf area) of evergreen species often declines with leaf age because of the increase in leaf tissue density and the accumulation of carbon-rich compounds, while the changing pattern is not unique for deciduous species [20]. A recent meta-analysis synthesized developmental shifts in physical, chemical, and indirect defense traits during leaf maturation across 124 woody plant species [21]. However, the study only focused on defense syndromes during leaf development and the selected species are from different biomes. The trait developmental shifts in species with synchronous leaf flushing were stronger than those in species with asynchronous leaf flushing from different biomes [21]. More species and leaf traits with the same climate condition will provide new insights into the general characteristics of leaf-trait variation.
Leaf stomata is not only an important gateway for plants to balance the relationship between photosynthesis and transpiration, but also a key structure affecting atmospheric carbon and water cycling [22,23]. Stomatal density (SD) and stomatal size (SS) are two important stomatal traits and vary widely between plant species and according to environmental factors, such as temperature, light irradiance, water, soil nutrients, and atmospheric CO2 concentrations, etc. [24,25]. Therefore, stomatal traits play important role in leaf growth. So far, few studies have focused on the changes in stomatal traits with leaf age. Thus, investigating the changes of stomata characteristics during leaf development is crucial to elucidate plant carbon–water fluxes, and understand plant adaptive strategies in the context of ontogenetic shifts.
Here, we measured six continuous leaf traits of different leaf ages of 40 woody species in temperate forest; one trait pertained to leaf morphology, two to leaf stoichiometry, and three to leaf stoma. The major aim was (1) to explore the effects of leaf aging on leaf functional traits, and, further, to understand the difference between different plant growth forms, (2) to investigate the variations between leaf morphological traits, stoichiometric, and stomatal traits, and (3) to examine the relationships among different leaf traits and leaf ages.
2. Material and Methods
2.1. Study Site and Species Selection
The experiment was carried out in Loess Plateau and Qinling Mountains in Shaanxi Province of China, which are the main distribution areas of temperate deciduous forest in China. According to the vegetation distribution, three sites were selected in the study area from south to north for surveying community species composition. Two sites were in the Loess Plateau, and one site was in Qinling Mountains (Figure 1). At each site, one plot of 2500 m2 (50 m × 50 m) was established, and all tree (diameter at breast height > 1 cm) and shrub individuals were identified and their maximum and average height, abundance, and coverage were documented in June–July of 2022.
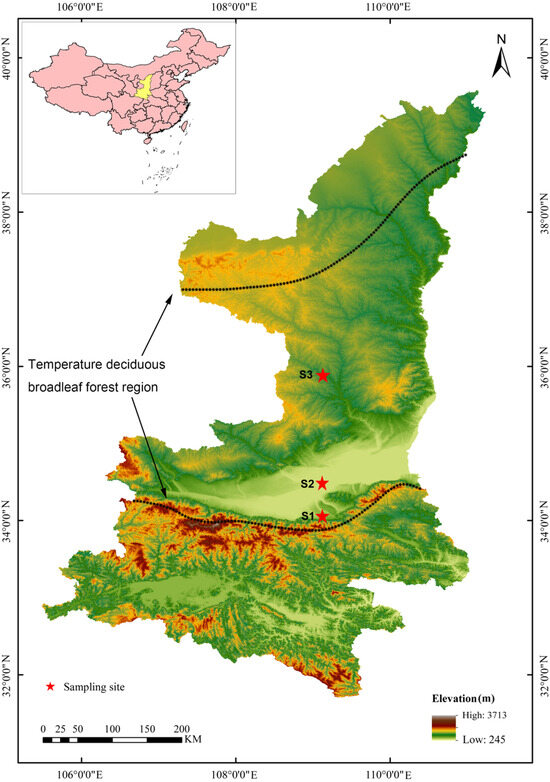
Figure 1.
Map of the study area showing sampling site in the topographic map of the Shannxi Province.
The climate of the sites is a similarly temperate continental monsoon climate. Mean annual rainfall is 550–650 mm and mean annual temperature is 11–14 °C, respectively (Table S1). Air relative moisture is 60%–65%. The soil is yellow-cinnamon soil and yellow-brown soil. In total, 155 mature and healthy individuals of 40 woody species were selected based on the community species composition and vertical structure (tree and shrub layer). The selected species belong to 16 families, and 25 are tree species and 15 are shrub species (Table S2).
2.2. Sampling and Trait Measurements
2.2.1. Morphology and Stoichiometry Traits
A total of 155 mature and healthy individuals of 40 woody species were selected and sampled. In each site, at least three individuals of focal species were selected and four branches from four direction at the middle of the canopy were labeled at early growing season (April). A digital camera was used to take a picture of each branch to record the number and status of leaves. According to the phenological characteristics of deciduous species in the study area, sampling was conducted at the mid-time of the growing season (June–July). For each branch, all leaves were classified into age categories based on Chavana-Bryant et al. [26]. Briefly, independently for each species, leaves were assigned age categories (young and mature) through visual assessment of leaf color, size, and rigidity. ‘Young’ described immature leaves (<2 months old, not fully expanded, and/or not fully green), which is generally at the tip of branch. ‘Mature’ described leaves that had recently reached maturity (fully expanded, green, and >2 months old). A digital camera was used to take a picture of each fresh leaf surface, and the leaf surface area (LA) was measured using Motic Images Plus 2.0. All leaves were placed inside a drying oven for a period of 72 h at 80 °C; then, the dry mass (MD) was measured. The SLA was calculated as the ratio of leaf area to dry mass (cm2•g−1). All leaves were then pooled together and ground to a fine powder using a ball mill. The LCC (leaf carbon concentrations) and LNC (leaf nitrogen concentrations) were then determined with an elemental analyzer (Euro Vector EA3000, Milan, Italy). The EA3000 Series is based on the well-established Dumas principle of dynamic flash combustion followed by gas chromatography separation of the resultant gaseous species (N2, CO2) and TCD detection.
2.2.2. Stomatal Traits
The nail-polish imprint method was used to examine stomatal traits [27]. A coat of transparent nail polish was put on the center of the underside of the leaf, left to dry, removed with tape, and placed on a glass slide. Stomatal prints were photographed by a Classica SK200 Digital light microscope (Motic China Group Co., Ltd., Guangzhou, China) at 200–400× magnification. Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA) software was used to measure SL (μm) and SW (μm). After dividing leaves into grids of 100 × 100 μm, SD (mm−2) was calculated as the number of stomata per unit epidermal area. Over 20 randomly selected fields of view were averaged to obtain the SL and SD.
2.3. Data Analyses
Differences in traits among different life forms (trees and shrubs) and ages were analyzed by one-way analyses of variance (ANOVA). Post-hoc tests (Student–Newman–Keuls) were performed when required. We also constructed linear mixed-effects models to detect the effects of leaf age on leaf traits using the lme4 package in R, where study sites were treated as random effects and species; leaf age and their interaction were fixed effects in the models. Multivariate analyses of all traits were conducted by principal component analysis (PCA) for young leaves, mature leaves, and all leaves. Correlations between traits were evaluated using Pearson’s coefficients. The standardized major axis (SMA) estimation method was used to calculate the slope and intercept of the regression equation of any two leaf traits for each leaf age. A likelihood ratio test was used to test the heterogeneity of the regression slope and intercept [28]. All figures were performed by the package ‘ggplot’ in R version 4.1.0 [29].
3. Results
3.1. Variation in Leaf Traits
We found that leaf age has significant effects on leaf functional traits (Figure 2, Table 1). Young leaves of both shrub and tree species revealed a lower stoma density (SD) and a higher stoma length (SL), stoma width (SW), and leaf N content (LNC) than mature leaves (Figure 2). Shrub species had a higher SLA than tree species; moreover, the young leaves of shrub species showed a higher SLA than mature leaves. Shrub species have a higher SD than tree species for both young and mature leaves. The LCC did not show significant differences among different leaf ages and life forms (Figure 2). These results were consistent among all three sites (Figures S1–S3).
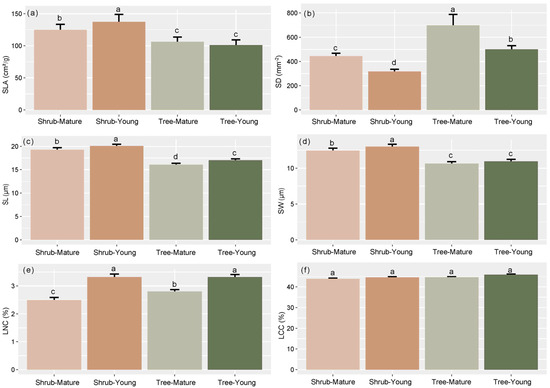
Figure 2.
Differences in specific leaf area (SLA) (a), stomatal density (SD) (b), stomatal length (SL) (c), stomatal width (SW) (d), leaf nitrogen content (LNC) (e), and leaf carbon content (LCC) (f) among different leaf ages and life forms. Different letters correspond to results of post-hoc tests after ANOVA.

Table 1.
Results of linear mixed-effects model analysis presenting the effect of leaf age and species on leaf traits.
For all the species, traits of young leaves generally revealed a higher variation than those of mature leaves (Figure 3). Moreover, the variation of traits among the young leaves of tree species was higher than that of shrub species; the variation among mature leaves did not show significant differences between shrub and tree species (Figure 4).
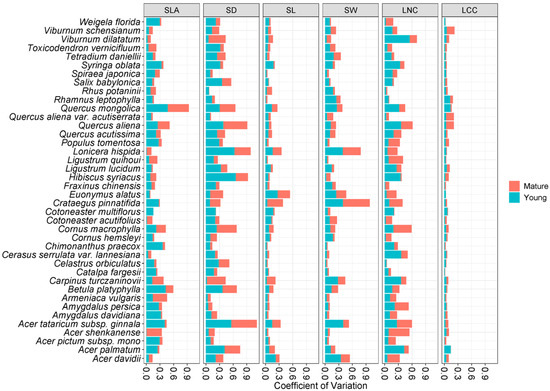
Figure 3.
Variable coefficient within species for mature and young leaves of 40 species. Traits: specific leaf area (SLA), stomatal density (SD), stomatal length (SL), stomatal width (SW), leaf nitrogen content (LNC), and leaf carbon content (LCC).
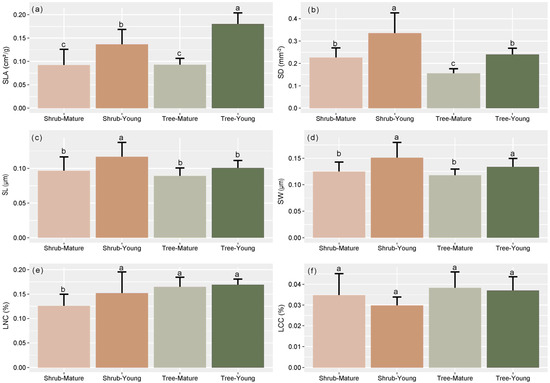
Figure 4.
Differences in variable coefficients of specific leaf area (SLA) (a), stomatal density (SD) (b), stomatal length (SL) (c), stomatal width (SW) (d), leaf nitrogen content (LNC) (e), and leaf carbon content (LCC) (f) among different leaf ages and life forms. Different letters correspond to results of post-hoc tests after ANOVA.
3.2. Multidimensionality and Bivariate Trait Relationship
The PCA of the multiple traits revealed that the first two components explained >55% of the total variation (Figure 5). The different contributions of the traits of shrub and tree species led to the differences in traits between young and mature leaves (Figure 5, Table S3). However, the correlations between traits were similar among young leaves, mature leaves, and all leaves at an individual level (Figure 5). Overall, a notable inverse correlation was observed between SD and stomatal size (SW and SL) and SLA, as well as between SLA and leaf carbon content (LCC) (Figure 5). Stomatal length and width showed significant negative relationships with stomatal density within species for all the species (Figure 5). A significant positive relationship was found between SW and SL, LCC and SD, and SLA and LNC (Figure 5).
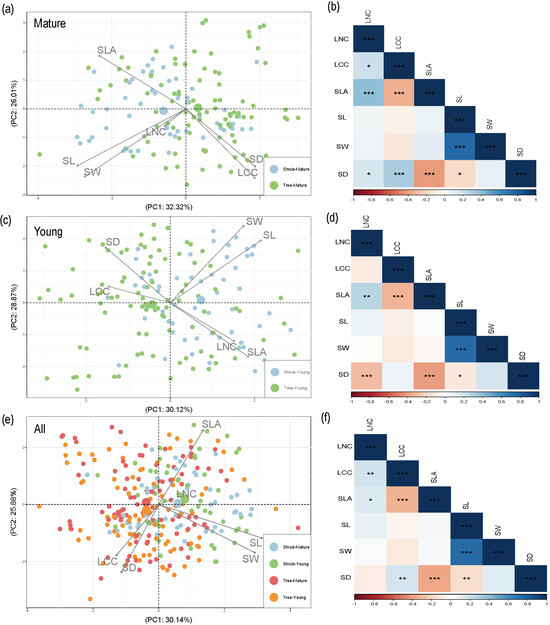
Figure 5.
PCA (a,c,e) and correlation matrix (b,d,f) of leaf traits for different leaf ages. Mature leaves, a and b; Young leaves, c and d; All leaves e and f. Traits: specific leaf area (SLA), stomatal density (SD), stomatal length (SL), stomatal width (SW), leaf nitrogen content (LNC), and leaf carbon content (LCC). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Further SMA analyses showed that the SMA slopes of the SLA–SD and SD–LNC relationships were different for leaves of different leaf ages (Figure 6). The elevation for the LNC–SLA correlation was slightly altered by leaf age (Figure 6).
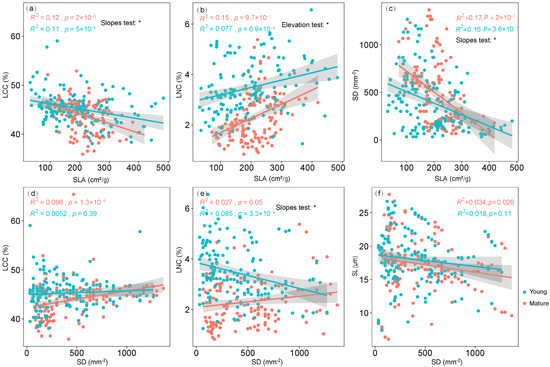
Figure 6.
The bivariate trait relationships of LCC and SLA (a), LNC and SLA (b), SD and SLA (c), LCC and SD (d), LNC and SD (e), SL and SD (f). R2 and p values were estimated from standard major axis (SMA) regression. Significant differences in slopes or elevation between trait relationships were noted by *.
4. Discussion
4.1. Effects of Leaf Age on Leaf Functional Traits of Different Life Forms
By measuring morphological, stomatal, and stoichiometric variations of leaf traits of 40 woody species in temperate forests, we found that leaf age has significant effects on leaf functional traits (Table 1). The young leaves of both shrub and tree species revealed a lower stoma density (SD) and a higher stoma length (SL), stoma width (SW), and leaf N content (LNC) than mature leaves.
Few studies have focused on the changes in stomatal size and density among leaves of different ages. A previous study on six woody species of Sorbus found an increasing stomatal density and stable stomatal size with the leaf area expansion [30], which is partly consistent with our results. However, another study on two shrub species found that the total stomatal density decreased with leaf area expansion, while the size of stomata increased gradually with leaf expansion [31]. Such a divergence may be related to the differences in plant functional groups. During leaf photosynthesis, stomata are turgor-operated valves that control water loss and CO2 uptake, and, thereby, the leaf stomatal functioning of a plant is closely related to water relation and biomass accumulation [32]. Generally, plants with larger stomata and a lower stomata density and showed greater growth rates; thus, the improved growth rate is attributed to a combination of higher metabolic temperatures, lower metabolic costs, and improved water status [33]. At the same time, smaller stomata generally respond faster than larger stomata; this observation was explained in terms of surface-to-volume ratios and the need for transporting solutes to drive movement [34,35]. Therefore, a smaller size and higher density of stomata ensures a fast response of mature leaves to summer heat in temperate zones.
In addition, the high nutrient content in young leaves is important for leaf development. A previous study also found that the LNC decreased with increasing leaf age and leaf area expansion for evergreen woody species [36]. Developing leaves are regarded as strong sinks, requiring carbohydrates and nutrients to construct the leaf anatomical structure and to improve high metabolic rates for leaf differentiation and expansion [21,37]. Even so, the young leaves of some plant species have been found to have a low nitrogen content, resulting in the ‘delayed greening’ syndrome, which is a syndrome found especially in tropical species that flush leaves synchronously [38]. The high LNC in our study may be due to the fact that the metabolism in leaves is at a high level at the young stage; in addition, growth requires high amounts of protein and nucleic acid, which increases the N concentrations [39]. By contrast, in mature leaves, most of the nutrients acquired by the plant are transported to other organs to meet the reproduction requirement; as a result, the N content in the leaves of the adult plants decreases.
4.2. Variation of Leaf Functional Traits among Different Life Forms
In general, shrub species have a higher SLA than tree species; moreover, the young leaves of shrub species showed a higher SLA than those of mature leaves. A higher SLA generally indicates a quick nutrient-obtaining strategy, shorter leaf lifespans, and a higher plant growth rate [40,41]. Light source availability declined in the understory of forest. Therefore, the high SLA of shrub leaves allows them to maintain a high photosynthetic efficiency [42]. The young leaves of shrub species showed a higher SLA than those of mature leaves, reflecting a faster increase in leaf area than leaf mass for shrub species. Some previous studies have also found that the SLA decreases with increasing leaf age for evergreen woody species [17,18,20,36]. However, in deciduous tree species, there is not a certain pattern found in the previous studies [20]. Therefore, the differentiation of leaf traits in leaf-age adaptive strategy shows functional differentiation because of heterogeneity of light resources.
Shrub species have a lower SD and higher stoma size than tree species for both young and mature leaves. Some previous studies have compared the interspecific differences in stomatal density, distribution, stomatal index for fully mature leaves [32,43]. A previous study on the mature leaves of species of Sorbus found that stomata lengths and densities were significantly larger in the shrub than that in the tree species [30]. The variation among species indicates a long-term response depending on the genetic background of each species and a reaction to short-term environmental changes [44,45]. Our study focused on both young and mature leaves. The lower stomata density of shrub species may indicate a, adaption to more humid conditions in the understory, and the higher stomatal size is a compensation to achieve a higher photosynthetic capacity [33].
4.3. Effects of Leaf Age on Trait Relationships
Although the correlations between traits are similar across different leaf ages, leaf age tends to affect the slope or elevation of the relationships between these traits.
Firstly, we found a significantly negative relationship between the SLA and SD and the LCC. Moreover, the SMA slopes of the SLA–SD relationships were different among leaves of different ages. Stomatal density decreases allometrically with increasing SLA, suggesting that the balance between leaf area and its matter may be associated with the guard cell number [46,47]. This result reflects a general link between the SD and morphological leaf traits. However, the covariation between the SLA and SD can respond to selection over short evolutionary periods because of environmental changes [47]. For example, the SLA was higher and the SD lower for shade leaves compared to sun leaves [48]. Changing irradiance (as well as other environmental conditions) induced changes in guard cell length and, therefore, stomatal density [49]. In our study, a higher slope of SLA-SD was found in mature leaves. This could come from an individual plasticity that allows the leaves to adapt quickly to their environment. It has been shown that the SD of young leaves depends on the state of stomatal conductance of older leaves [50]. Thus, the negative correlation between the SD and SLA is unlikely to be a result of a greater cell size via expansion of leaf cells in the bud after the end of the cell division.
Secondly, although there is a consistent positive relationship between the SLA and LNC for both young leaves and mature leaves, the elevation for the LNC–SLA correlation was slightly altered by leaf age because of the higher LNC of young leaves. The positive relationships between LNC and SLA have been confirmed in many previous studies at both global and region scales [3,5]. At the same time, the slopes in the interspecific trait relationships hold stable under environmental changes, while only their elevations vary. The elevation changes regarding trait relationships are mainly driven by asymmetrically interspecific responses contrary to the direction of the leaf economic spectrum [51]. In our study, the change in the elevation of trait relationships is probably because the structural traits (SLA) and chemical traits (N) have different strategies for adapting to changes in leaf aging [1,20].
Negative relationships between SD and stomatal size have been found in many studies of mature leaves at species level, indicating a stomatal safety–efficiency trade-off [27]. Our results further prove this trade-off relationship in leaves of different ages. Moreover, leaf age did not show significant effects on the relationship between slope and elevation. Therefore, there is a robust trade-off between stomatal size and density both across species and within species under leaf aging.
5. Conclusions
By quantifying morphological, stomatal, and stoichiometric traits for 40 coexisting woody species (including tree and shrub species), we found that leaf age significantly influences the variation and relationships of functional traits. The young leaves from both shrub and tree species exhibited a lower stomatal density (SD) but a higher stomatal length (SL), stomatal width (SW), and leaf nitrogen content (LNC) compared to mature leaves, illustrating how leaf traits adapt during maturation. The correlations between traits highlight the trade-offs between stomatal safety and efficiency, as well as between hydraulic and economic traits, throughout leaf development. Moreover, leaf age modulated the slope of SD–SLA and the elevation of LNC–SD. Therefore, we conclude that the variation in leaf traits of different ages may play a potentially important role in exploring the ecological function of woody species in temperate forests. Our results improve the understanding of age-dependent plasticity changes during leaf development for coexisting species in temperate forests.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15101803/s1, Table S1. Detailed information and sampling number of 40 woody species. Table S2. Detailed information and sampling number of 40 woody species. Table S3. Contribution of different traits in PC1 and PC2. Figure S1. Differences in specific leaf area (SLA) (a), stomatal density (SD) (b), stomatal length (SL) (c), stomatal width (SW) (d), leaf nitrogen content (LNC) (e), and leaf carbon content (LCC) (f) among different leaf ages and growth forms in Site 1. Different letters correspond to results of post-hoc tests after ANOVA. Figure S2. Differences in specific leaf area (SLA) (a), stomatal density (SD) (b), stomatal length (SL) (c), stomatal width (SW) (d), leaf nitrogen content (LNC) (e), and leaf carbon content (LCC) (f) among different leaf ages and growth forms in Site 2. Different letters correspond to results of post-hoc tests after ANOVA. Figure S3. Differences in specific leaf area (SLA) (a), stomatal density (SD) (b), stomatal length (SL) (c), stomatal width (SW) (d), leaf nitrogen content (LNC) (e), and leaf carbon content (LCC) (f) among different leaf ages and growth forms in Site 3. Different letters correspond to results of post-hoc tests after ANOVA.
Author Contributions
L.W. conceived the ideas and wrote the paper; X.Z., G.L., Q.W., F.W. and Y.L. completed the field work. All authors have read and agreed to the published version of the manuscript.
Funding
The study was financially supported by the Shaanxi Academy of Science Research Funding Project (2022-k14), Shaanxi Academy of Fundamental Science (2022JQ-189).
Data Availability Statement
The datasets supporting the conclusions of this article will be archived in Figshare upon acceptance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Niinemets, Ü.; Keenan, T.F.; Hallik, L. A Worldwide Analysis of Within-Canopy Variations in Leaf Structural, Chemical and Physiological Traits across Plant Functional Types. New Phytol. 2015, 205, 973–993. [Google Scholar] [CrossRef] [PubMed]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.-L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant Functional Markers Capture Ecosystem Properties during Secondary Succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Colin Prentice, I.; et al. The Global Spectrum of Plant Form and Function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Wang, H.; Prentice, I.C.; Wright, I.J.; Warton, D.I.; Qiao, S.; Xu, X.; Zhou, J.; Kikuzawa, K.; Stenseth, N.C. Leaf Economics Fundamentals Explained by Optimality Principles. Sci. Adv. 2023, 9, eadd5667. [Google Scholar] [CrossRef]
- Siefert, A.; Violle, C.; Chalmandrier, L.; Albert, C.; Taudiere, A.; Fajardo, A.; Aarssen, L.; Baraloto, C.; Carlucci, M.; Cianciaruso, M.; et al. A Global Meta-Analysis of the Relative Extent of Intraspecific Trait Variation in Plant Communities. Ecol. Lett. 2015, 18, 1406–1419. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Zanne, A.E. Towards a Worldwide Wood Economics Spectrum. Ecol. Lett. 2010, 12, 351–366. [Google Scholar] [CrossRef]
- Volkenburgh, E.V. Leaf Expansion—An Integrating Plant Behaviour. Plant Cell Environ. 1999, 22, 1463–1473. [Google Scholar] [CrossRef]
- Marchi, S.; Tognetti, R.; Minnocci, A.; Borghi, M.; Sebastiani, L. Variation in Mesophyll Anatomy and Photosynthetic Capacity during Leaf Development in a Deciduous Mesophyte Fruit Tree (Prunus persica) and an Evergreen Sclerophyllous Mediterranean Shrub (Olea europaea). Trees 2008, 22, 559–571. [Google Scholar] [CrossRef]
- Menezes, J.; Garcia, S.; Grandis, A.; Nascimento, H.; Domingues, T.F.; Guedes, A.V.; Aleixo, I.; Camargo, P.; Campos, J.; Damasceno, A.; et al. Changes in Leaf Functional Traits with Leaf Age: When Do Leaves Decrease Their Photosynthetic Capacity in Amazonian Trees? Tree Physiol. 2022, 42, 922–938. [Google Scholar] [CrossRef]
- Shipley, B.; Vile, D.; Garnier, E.; Wright, I.J.; Poorter, H. Functional Linkages between Leaf Traits and Net Photosynthetic Rate: Reconciling Empirical and Mechanistic Models. Funct. Ecol. 2005, 19, 602–615. [Google Scholar] [CrossRef]
- Walker, A.P.; Beckerman, A.P.; Gu, L.; Kattge, J.; Cernusak, L.A.; Domingues, T.F.; Scales, J.C.; Wohlfahrt, G.; Wullschleger, S.D.; Woodward, F.I. The Relationship of Leaf Photosynthetic Traits—Vcmax and Jmax—To Leaf Nitrogen, Leaf Phosphorus, and Specific Leaf Area: A Meta-Analysis and Modeling Study. Ecol. Evol. 2014, 4, 3218–3235. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Miyajima, Y. Relationships between Leaf Life Span, Leaf Mass per Area, and Leaf Nitrogen Cause Different Altitudinal Changes in Leaf δ13C between Deciduous and Evergreen Species. Botany 2008, 86, 1233–1241. [Google Scholar] [CrossRef]
- Pantin, F.; Simonneau, T.; Muller, B. Coming of Leaf Age: Control of Growth by Hydraulics and Metabolics during Leaf Ontogeny. New Phytol. 2012, 196, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Waring, R.; Landsberg, J.; Linder, S. Tamm Review: Insights Gained from Light Use and Leaf Growth Efficiency Indices. For. Ecol. Manag. 2016, 379, 232–242. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The Relationship between Leaf Area Growth and Biomass Accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef]
- Gratani, L.; Bonito, A. Leaf Traits Variation during Leaf Expansion in Quercus ilex L. Photosynthetica 2009, 47, 323–330. [Google Scholar] [CrossRef]
- Gratani, L.; Ghia, E. Changes in Morphological and Physiological Traits during Leaf Expansion of Arbutus Unedo. Environ. Exp. Bot. 2002, 48, 51–60. [Google Scholar] [CrossRef]
- Catoni, R.; Bracco, F.; Gratani, L.; Granata, M.U. Physiological, Morphological and Anatomical Leaf Traits Variation across Leaf Development in Corylus Avellana. Mediterr. Bot. 2019, 40, 185–192. [Google Scholar] [CrossRef]
- Niinemets, Ü. Leaf Age Dependent Changes in Within-Canopy Variation in Leaf Functional Traits: A Meta-Analysis. J. Plant Res. 2016, 129, 313–338. [Google Scholar] [CrossRef]
- Barton, K.E.; Edwards, K.F.; Koricheva, J. Shifts in Woody Plant Defence Syndromes during Leaf Development. Funct. Ecol. 2019, 33, 2095–2104. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The Role of Stomata in Sensing and Driving Environmental Change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Wolz, K.J.; Wertin, T.M.; Abordo, M.; Wang, D.; Leakey, A.D.B. Diversity in Stomatal Function Is Integral to Modelling Plant Carbon and Water Fluxes. Nat. Ecol. Evol. 2017, 1, 1292–1298. [Google Scholar] [CrossRef]
- Dow, G.J.; Bergmann, D.C.; Berry, J.A. An Integrated Model of Stomatal Development and Leaf Physiology. New Phytol. 2014, 201, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Brendel, O.; Buré, C.; Le Thiec, D. Changes in Irradiance and Vapour Pressure Deficit under Drought Induce Distinct Stomatal Dynamics between Glasshouse and Field-Grown Poplars. New Phytol. 2020, 227, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Chavana-Bryant, C.; Malhi, Y.; Wu, J.; Asner, G.P.; Anastasiou, A.; Enquist, B.J.; Cosio Caravasi, E.G.; Doughty, C.E.; Saleska, S.R.; Martin, R.E.; et al. Leaf Aging of Amazonian Canopy Trees as Revealed by Spectral and Physiochemical Measurements. New Phytol. 2017, 214, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wang, L.; Lei, M.; Dang, H.; Quan, J.; Tian, T.; Chai, Y.; Yue, M. The Relationships between Leaf Economics and Hydraulic Traits of Woody Plants Depend on Water Availability. Sci. Total Environ. 2018, 621, 245–252. [Google Scholar] [CrossRef]
- Warton, D.I.; Duursma, R.A.; Falster, D.S.; Taskinen, S. Smatr 3—An R Package for Estimation and Inference about Allometric Lines: The Smatr 3—An R Package. Methods Ecol. Evol. 2012, 3, 257–259. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: http://www.R-project.org (accessed on 6 April 2024).
- Čaňová, I.; Ďurkovič, J.; Hladká, D.; Lukáčik, I. Changes in Stomatal Characteristics and Photochemical Efficiency during Leaf Development in Six Species of Sorbus. Photosynthetica 2012, 50, 635–640. [Google Scholar] [CrossRef]
- Wu, B.-J.; Chow, W.S.; Liu, Y.-J.; Shi, L.; Jiang, C.-D. Effects of Stomatal Development on Stomatal Conductance and on Stomatal Limitation of Photosynthesis in Syringa oblata and Euonymus japonicus Thunb. Plant Sci. 2014, 229, 23–31. [Google Scholar] [CrossRef]
- Camargo, M.A.B.; Marenco, R.A. Density, Size and Distribution of Stomata in 35 Rainforest Tree Species in Central Amazonia. Acta Amaz. 2011, 41, 205–212. [Google Scholar] [CrossRef]
- Doheny-Adams, T.; Hunt, L.; Franks, P.J.; Beerling, D.J.; Gray, J.E. Genetic Manipulation of Stomatal Density Influences Stomatal Size, Plant Growth and Tolerance to Restricted Water Supply across a Growth Carbon Dioxide Gradient. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Beerling, D.J. Maximum Leaf Conductance Driven by CO2 Effects on Stomatal Size and Density over Geologic Time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343–10347. [Google Scholar] [CrossRef] [PubMed]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, Faster Stomata: Scaling of Stomatal Size, Rate of Response, and Stomatal Conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jin, G.; Liu, Z. Importance of Organ Age in Driving Intraspecific Trait Variation and Coordination for Three Evergreen Coniferous Species. Ecol. Indic. 2021, 121, 107099. [Google Scholar] [CrossRef]
- Turgeon, R. The Sink-Source Transition in Leaves. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 119–138. [Google Scholar] [CrossRef]
- Kursar, T.A.; Coley, P.D. Convergence in Defense Syndromes of Young Leaves in Tropical Rainforests. Biochem. Syst. Ecol. 2003, 31, 929–949. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and Consequences of Variation in Leaf Mass per Area (LMA): A Meta-Analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Chai, Y.; Liu, X.; Yue, M.; Guo, J.; Wang, M.; Wan, P.; Zhang, X.; Zhang, C. Leaf Traits in Dominant Species from Different Secondary Successional Stages of Deciduous Forest on the Loess Plateau of Northern China. Appl. Veg. Sci. 2015, 18, 50–63. [Google Scholar] [CrossRef]
- Tomlinson, K.W.; Poorter, L.; Sterck, F.J.; Borghetti, F.; Ward, D.; de Bie, S.; van Langevelde, F. Leaf Adaptations of Evergreen and Deciduous Trees of Semi-Arid and Humid Savannas on Three Continents. J. Ecol. 2013, 101, 430–440. [Google Scholar] [CrossRef]
- Onoda, Y.; Wright, I.J.; Evans, J.R.; Hikosaka, K.; Kitajima, K.; Niinemets, Ü.; Poorter, H.; Tosens, T.; Westoby, M. Physiological and Structural Tradeoffs Underlying the Leaf Economics Spectrum. New Phytol. 2017, 214, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Tay, A.-C.; Furukawa, A. Variations in Leaf Stomatal Density and Distribution of 53 Vine Species in Japan. Plant Species Biol. 2008, 23, 2–8. [Google Scholar] [CrossRef]
- Qi, X.; Torii, K.U. Hormonal and Environmental Signals Guiding Stomatal Development. BMC Biol. 2018, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of Stomatal Density and Morphology on Water-Use Efficiency in a Changing World. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of Leaf Stomatal Density to Water Status and Its Relationship with Photosynthesis in a Grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef]
- Loranger, J.; Shipley, B. Interspecific Covariation between Stomatal Density and Other Functional Leaf Traits in a Local Flora. Botany 2010, 88, 30–38. [Google Scholar] [CrossRef]
- Afas, N.A.; Marron, N.; Ceulemans, R. Variability in Populus Leaf Anatomy and Morphology in Relation to Canopy Position, Biomass Production, and Varietal Taxon. Ann. For. Sci. 2007, 64, 521–532. [Google Scholar] [CrossRef]
- Lomax, B.H.; Woodward, F.I.; Leitch, I.J.; Knight, C.A.; Lake, J.A. Genome Size as a Predictor of Guard Cell Length in Arabidopsis thaliana Is Independent of Environmental Conditions. New Phytol. 2009, 181, 311–314. [Google Scholar] [CrossRef]
- Miyazawa, S.-I.; Livingston, N.J.; Turpin, D.H. Stomatal Development in New Leaves Is Related to the Stomatal Conductance of Mature Leaves in Poplar (Populus trichocarpa × P. deltoides). J. Exp. Bot. 2006, 57, 373–380. [Google Scholar] [CrossRef]
- Cui, E.; Weng, E.; Yan, E.; Xia, J. Robust Leaf Trait Relationships across Species under Global Environmental Changes. Nat. Commun. 2020, 11, 2999. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).