Abstract
Understanding the stability of soil organic matter (SOM) is essential for making accurate predictions regarding carbon release rates. However, there is limited information on the role of chemical composition of dissolved organic matter (DOM) in SOM stability. To address this gap, the peatland soil profile in the discontinuous frozen soil region of Northeast China was selected as the focus of this research, and a comprehensive analysis was conducted on the differences between the molecular composition of DOM and the stability of SOM. The results indicate a significant carbon accumulation phenomenon near the permafrost table. Through analyses using TG-50, δ13C, and δ15N, it was determined that SOM near the permafrost table exhibits high stability, whereas SOM within the permafrost layer demonstrates poor stability. Investigations utilizing UV-vis, 3D-EEM, FT-IR, and 1H-NMR technologies revealed that DOM near the permafrost table is of high quality and highly aromatic. Furthermore, compared to near the permafrost table, humic acid materials in the permafrost layer decreased by 17%, while protein materials increased by 17%. These findings offer a novel perspective on the understanding of SOM stability in peatland soil profiles within discontinuous permafrost regions.
1. Introduction
Permafrost is primarily found in high-latitudinal and high-altitudinal regions [1,2]. The cold conditions in these areas lead to the long-term accumulation of plant debris, resulting in 1000–1300 Pg of carbon (1 Pg = 1015 g) stored as soil organic matter (SOM) in the upper 3 m of soil in permafrost areas [3,4]. Current estimates suggest that between 277 and 800 Pg C is presently contained in permafrost peatlands. In recent decades, the warming of permafrost has occurred at a pace nearly 2–3 times above the global average [5]. The rise in climate temperatures could encourage the release of carbon from soil reservoirs into the atmosphere, which would, in turn, influence both the global carbon cycle and climate change [6]. Therefore, understanding the stability of SOM in permafrost regions can provide scientific basis for predicting future global carbon cycle and climate [7].
The stability of SOM has received increasing attention in recent years [8]. By examining the effects of organic compounds and organic mineral colloids (such as Al and Fe) in soil on element migration, it was elucidated that the enrichment of carbon and metals at the permafrost–mud interface within a Siberian peatland profile is attributed to low-temperature enrichment [9]. Anaerobic cultivation experiments on peatland soil in permafrost areas in Russia have proven that the stability of carbon is stronger than near the permafrost table and permafrost active layer [10]. Through mineralization culture experiments on topsoil with different micro-landforms, we found that micro-relief and temperature are related to the alpha diversity of soil bacteria and proved that the tussock is conducive to the accumulation of soil carbon [11]. However, the stability characteristics of SOM in the soil profile in permafrost peatland soils in northeastern China are poorly understood [12]. Meanwhile, existing studies on the stability of organic matter in permafrost have focused on the active layer of permafrost peatlands and the effects of changes in vegetation communities on the stability of organic matter in surface soils [13,14]. Therefore, a comprehensive understanding of SOM stability in permafrost peatland profiles is needed, and it may be possible to study this through the composition of SOM.
SOM is a complex mixture of different organic substances, and there is a strong link between its chemistry composition and carbon cycle and stability [15]. To further evaluate the stability of SOM, various metrics, including thermal, isotopic, and molecular measurements, have been proposed [16]. Stone et al. applied these techniques to compare the stability of SOM in four tropical forest soils across different layers of the soil profile [17]. High-latitude deep peat reservoirs may remain stable in the face of climate change due to their ultimately lower carbohydrate and higher aromatic content [18]. In the permafrost zone, Hou et al. utilized these techniques to compare the SOM stability in soils from two ecosystems (meadow and shrubland) across different layers of the soil profile on the Tibetan Plateau [19]. An insightful approach to comprehending SOM involves the analysis of the chemical makeup of dissolved organic matter (DOM). To assess the stability of SOM compounds, researchers utilized various molecular techniques, such as three-dimensional fluorescence spectroscopy (3D-EEM) and 1H solution nuclear magnetic resonance (1H-NMR) spectroscopy, to examine the fluorescence characteristics and chemical structure of DOM. DOM is a highly active part of SOM [20,21]. After permafrost thaws, most DOM will be mineralized/photo-oxidized by micro-organisms relatively quickly [22]. Previous studies have confirmed that higher aromatic and lower carbohydrate content indicate more stable DOM. Effective indicators such as soil aromaticity, humic acid content, protein-like content, molecular weight, and C:N ratio can be used to quantify DOM stability [23]. Despite the variety of techniques available for assessing soil organic matter stability, the impact of changes in DOM composition in peatland soils within permafrost regions on SOM stability has not been fully elucidated.
The stability of SOM was investigated using thermal stability indices and stable isotopes. Furthermore, optical measurements were utilized to assess the aromaticity and molecular weight of DOM, while the components of the fluorescent DOM pool were identified through modeling using parallel factor analysis (PARAFAC) and validated by split-half analysis. The primary aim of this study was to use the composition of DOM in permafrost peatland soil profiles to explain changes in SOM stability.
2. Materials and Methods
2.1. Study Area
The sampling locations were situated at the Mohe National Observation and Research Station for the Chinese Forest Ecosystem (53°28.3217′ N, 122°20.3296′ E) in the Greater Khingan Mountains, Northeast China. The research site selected for this study is the Arctic Village, located in the Greater Khingan Mountains, primarily because it lies at the southern boundary of the Eurasian permafrost region. This area is characterized by an uneven distribution of permafrost and demonstrates increased sensitivity to shifts in climate. It experienced a cold-temperate continental monsoon climate, featuring wet summers and long, frigid winters. The average annual temperature was −4.9 °C, with January being the coldest month, averaging −23 °C. Extreme low temperatures could range from −45 to −50 °C, and the highest temperatures throughout the entire year occurred in July, with an average of about 19 °C and extreme highs of more than 37 °C. The annual mean precipitation was 430–550 mm, mainly from May to September. Winter snow cover could last up to 7 months, with an average snow thickness of 30–50 cm and a frost-free period of about 90 days per year. In the study area [24], the predominant soil type identified was peat soil. In the vegetation community, Larix gmelinii was the clearly dominant species, while Betula platyphylla appeared in smaller numbers. The shrub layer primarily consisted of Vaccinium uliginosum, Rhododendron dauricum, and Ledum palustre, whereas the herbaceous layer mainly featured Eriophorum vaginatum, Deyeuxia angustifolia, and Carex Aspen (Figure 1a).
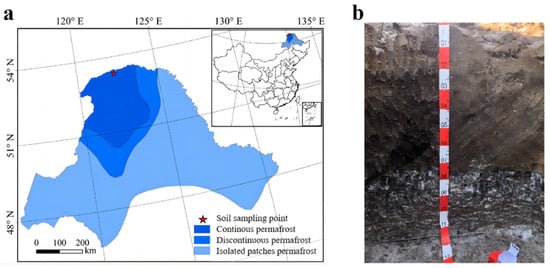
Figure 1.
(a) Location of the study area and (b) soil sample profile (0–120 cm).
2.2. Experimental Design and Sample Preparation
The sampling sites were selected in permafrost peatlands of the Greater Khingan Mountains, Northeast China. In November 2022, we selected three parallel sample plots in this area, each approximately 20 m apart. At each sample site, five soil samples (four at the apex and one at the center) were collected within 1 m × 1 m sample squares and then combined into one sample. Within each sample point, 4 samples (taken from the same profile) were collected and placed in an incubator. The soil layer was classified into surface-active layers (0–20 cm), subsurface-active layers (20–60 cm), near the permafrost table (60–80 cm), and permafrost layers (80–120 cm) (Figure 1b). To maintain the integrity of the samples during transport, the soil was split at the sampling site and stored in bags in a refrigerator at −20 °C. Prior to laboratory analysis, impurities were removed from the soil samples (impurities include plant roots and gravel). When the sample arrived at the laboratory, all soil samples underwent air drying at room temperature for a duration of 7 days. Subsequently, the samples were passed through a 2 mm sieve and stored at 4 °C for further analysis.
2.3. Sample Analysis
The DOM in the soil was acquired by means of water–soil oscillation. A solid-to-liquid ratio of 1:6 (w:v) was used for the extraction of DOM, which was agitated at 200 rpm for 24 h at ambient temperature (25 °C) [25]. Following this, the suspension underwent centrifugation at 8000 r/min for 3 min and was subsequently filtered using 0.45 μm cellulose acetate membrane filters. DOM extract was freeze-dried at −80 °C for 72 h in a freeze-dryer to obtain DOM powder. Finally, the filtrate and DOM powder were stored at −4 °C for analysis. The Multi N/C 2100 instrument (Analytik-Jena, Jena, Germany) was utilized to measure dissolved organic carbon (DOC) and total organic carbon (TOC). To determine the soil pH, the PHSJ-3F acidity meter was employed, using a soil-to-deionized (DI) water ratio of 1:2.5 (w:v). Total nitrogen (TN) and total carbon (TC) were determined using the dry combustion using Elementar analyzer (Vario EL CNS, elementar, Langenselbold, Germany). The N2 adsorption and desorption method was conducted with a Quantachrome Autosorb iQ device. Prior to the adsorption tests, the samples underwent a degassing process at 120 °C for 6 h using a vacuum system. Thermogravimetric (TG) analysis was executed on a thermal analyzer (STA 449F5, NETZSCH Company, Bavarian, Germany), ranging from room temperature to 800 °C in an oxidizing environment at a rate of 10 °C per minute, utilizing 100 mL min−1 of synthetic air as the oxidizing atmosphere. The TG parameters, such as the 50% burn-off temperature (TG-T50, which indicates the temperature at which 50% of the loss in SOM weight occurs), were determined. Previous studies have indicated that TG-T50 is effective in characterizing the thermal stability of SOM [16]. The total concentrations of Fe and Al, as well as free oxides (Fed, Ald), poorly crystalline oxyhydroxides (Feo, Alo), and organic-complexed oxides (Fep, Alp), were assessed through selective extraction techniques [26,27,28]. Subsamples were pretreated with 37% hydrochloric acid to remove soil inorganic carbon; δ 13C and δ 15N were determined by a stable isotope ratio mass spectrometer (MAT 253, Thermo Fisher, Waltham, MA, USA) [29,30].
2.4. Optical Characterization of DOM
FT-IR spectra were obtained using a Vertax-80 spectrometer (Bruker Company, Billerica, MA, USA), with a KBr matrix, across a range of 400 to 4000 cm−1 [31]. The UV-vis absorbance at a scanning wavelength of 250 to 800 nm was measured using UV spectrophotometer (CARY4000 UV-vis, Thermo Fisher, Waltham, MA, USA). Fluorescence intensity was assessed utilizing a fluorescence spectrometer (Hitachi, F-7000, Tokyo, Japan) with excitation wavelengths spanning from 230 to 500 nm and emission wavelengths from 250 to 550 nm. The scan interval for excitation wavelengths was established at 2 nm, whereas the interval for emission wavelengths was determined to be 5 nm. A consistent scan speed of 1200 nm/min was upheld, and absorbance measurements were conducted to address internal filter effects. To examine the spectral properties of DOM, the subsequent optical indices were computed [32]. The specific ultraviolet absorbance at 254 nm (SUVA) was determined by dividing A254 by the DOC concentration. A higher SUVA254 value generally signifies an increased aromaticity of DOM [33]. The slope ratio (SR) is typically inversely associated with DOM molecular weight and was calculated by taking the ratio of the slope between 275–295 nm and 350–400 nm [34,35]. The fluorescence index (FI) is a useful tool for determining the source of dissolved organic matter in water samples. FI value of approximately 1.8 suggests that the DOM is likely derived from microbial sources, while a value of approximately 1.2 indicates a terrestrial source, which is predominantly plant-derived DOM. This was determined by calculating the Em intensity at 470 nm in relation to that at 520 nm with an excitation wavelength of 370 nm [36,37]. The humification index (HIX) serves as an indicator of the amount of humic substances found in a given sample. It is calculated by taking the peak area under the emission spectra measured at 435–480 nm and dividing it by the peak area within the emission range of 300–345 nm, with an excitation wavelength of 254 nm [38]. In addition, the biological index (BIX), which signifies the fraction of newly generated autochthonous DOM, is determined by the ratio of fluorescent intensity at 380 nm to the maximum intensity detected in the emission range of 420–435 nm when excited at 310 nm [39]. In contexts involving soil-derived DOM, BIX typically shows a correlation with the fluorescence index (FI) and may also reflect microbial contributions to the DOM composition [40].
2.5. Solution-State 1H-NMR Test
Weigh 20 mg of DOM powder and dissolve it in 1100 μL NaO/D2O solution (pH = 14) using ultrasound. After centrifugation, the liquid solution is transferred to a Bruker Bio Spin 5 mm NMR tube [41]. Samples were analyzed by a BioSpin Avance III 400 MHz NMR spectrometer (Bruker Company, Karlsruhe, Germany). The loop delay time and time domain points were set to 2 s and 32 K, respectively, while 256 scans were acquired. Spectra were further processed with a zero-filling factor of 2 and were apodised by multiplication with an exponential decay corresponding to 2.0 Hz line broadening [42]. The integration of four regions was conducted based on the one-dimensional 1H-NMR spectra. These regions comprised (1) MDLT, referring to materials derived from linear terpenoids, with a chemical shift range of 0.6–1.6 ppm; (2) CRAM, representing carboxyl-rich alicyclic molecules, within the range of 1.6–3.2 ppm; (3) carb, indicating carbohydrates and peptides, spanning from 3.2 to 4.5 ppm; and (4) arom, signifying aromatics and phenolics, with a chemical shift range of 6.5–8.4 ppm [43,44,45].
2.6. Statistical Analyses
Statistical analyses and figures drawn from soil data at different depths (0–20 cm, 20–60 cm, 60–80 cm, and 80–120 cm) were using Origin (2021). Two-way analysis of variance (ANOVA) and Tukey’s post hoc test was used for statistical analyses. PARAFAC decomposes the EEM data set into a series of trilinear components using MATLAB software R2019b and DOM Fluor toolbox [46,47].
3. Results
3.1. Soil Physio-Chemical Variables
Table 1 lists soil characteristics at different depths. All soil samples were acidic, and soil pH decreased with the deepening of the soil profile. The soil water content (SWC) ranged from 18.74 to 38.39%. The mean DOC content showed the following order: 60–80 cm > 0–20 cm > 20–60 cm > 80–120 cm. The SOC values showed the same pattern. The δ 15N values increased with the soil profile but decreased at 80–120 cm. The changing trend of δ 13C is similar to that of δ 15N. The C:N value shows a decreasing trend with increasing depth, but there is a turning point at 60-80cm. The total Al content was highest at 60–80 cm, but it plummeted at 80–120 cm, and total Fe content followed the same trend as total Al content (Table 2). The contents of different forms of (Fe, Al) oxides were in the order of Feo > Fed > Fep > Alp >Alo > Ald. The investigated samples exhibited a range of surface area (SA) with values of 31.5, 33.0, 34.2, and 31.7 m2/g from top to bottom of the soil profile (Figure S1).

Table 1.
The characteristics of the soil samples (mean ± standard error).

Table 2.
The total amount of Fe/Al and their oxides in all samples (mean ± standard error).
3.2. Thermal Indices Analysis
Thermal analysis shows that the soil mass loss in the active layer has a similar trend (Figure S2). However, the mass loss of soil near the permafrost table is much greater than the mass loss of soil in the active layer and permafrost layer. Notably, the soil near the permafrost table exhibits a greater mass loss at elevated temperatures. The soil mass loss rate near the permafrost table is higher, which may be the reason for the higher SOM content (Figure S2). Furthermore, the temperature at which half of SOM mass was lost (TG-T50) also had essentially the same value from 0 to 60 cm, with the highest temperature near the permafrost table (60–80 cm) and the lowest temperature in the permafrost layer (80–120 cm). TG-T50 was generally considered a thermal index of SOM stability (Figure 2).
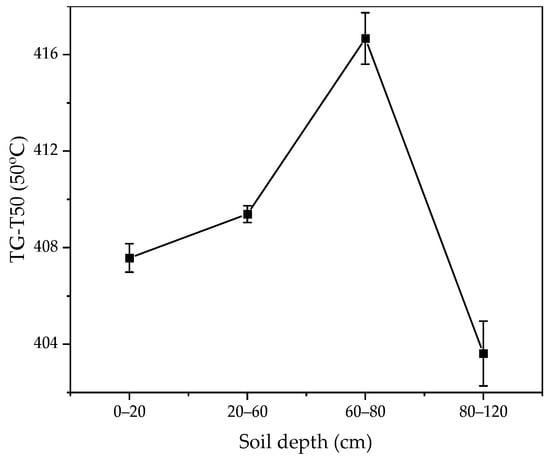
Figure 2.
Temperature at which 50% of the thermogravimetric mass loss appeared (TG-T50) with soil depth.
3.3. Spectral Characteristics of Soils at Different Depths
FT-IR spectra of DOM powders are shown in Figure 3a. The FT-IR spectra of different soil samples have similar peaks, but the peak intensities are obviously different. The extensive hydroxyl band observed at 3430 cm−1 may indicate the presence of carboxyl and hydroxyl groups, typically found in SOM and litter. The sharp band near 1653 cm−1 has been attributed to the vibrations of aromatic C=C bonds, olefinic C=C links, symmetric stretching of COO− groups, and hydrogen-bonded C=O from conjugated ketones [48]. The distinct peaks at 2921 and 2854 cm−1 are associated with C-H stretching. Meanwhile, the band observed at 3700 cm−1 is linked to the stretching and deformation of hydroxyl groups on the inner surface [49]. In particular, the recalcitrance index (ratio rA1635/rA2930) had similar values in the active layer (0–60 cm) [19], with the highest ratio near the permafrost table (60–80 cm) and the lowest ratio in the permafrost layer (80–120 cm), as shown in Figure 3b.
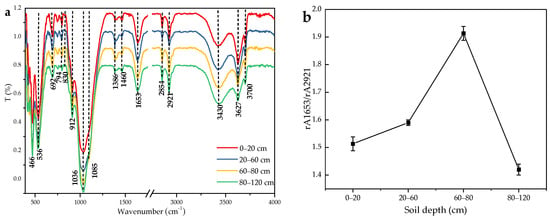
Figure 3.
(a) FT-IR spectra of SOM at soil depths of 0–20, 20–60, 60–80, and 80–120 cm. (b) Changes in soil condensation index (ratio of rA1653/rA2921) of soil based on FT-IR spectroscopy with depth along the profile (rA1653 and rA 2930 are the relative peak areas of 1653 cm−1 and 2921−1).
The SUVA254 value shows a continuous downward trend with the increase in soil depth, but the 60-80 cm soil layer has a higher SUVA254 value. Meanwhile, the HIX value of DOM at 60–80 cm increased significantly, and the value of 80-120 cm is the least (Table 3). It showed that the humification degree of DOM decreased significantly with increasing depth, but the exception was near the permafrost table. The SR values of DOM were the same between 0 and 60 cm, the lowest at 60–80 cm (Table 3), and the highest at 80–120 cm. This shows that the MW of DOM near the permafrost table is higher, while the MW of DOM in the permafrost layer is lower. In contrast, the FI and BIX of the DOM increased as the depth of the profile increased (Table 3), indicating that the degree of humification is progressively more influenced by micro-organisms. Further, PARAFAC extracted four fluorescent components, including three humic-like (C1, C2, and C3) and one protein-like (C4) fluorescent component (Figure 4a–d, Table S1). It was worth noting that the relative abundance of humic-like components and protein-like components hardly changed in the 0–60 cm. In comparison to other components, there had been a significant decrease in the relative abundance of C4 components in the 60–80 cm. Conversely, the largest proportion of C4 components occurred in the 80–120 cm (Figure 4e). The spectroscopic properties altered consistently throughout the soil profile, showing the greatest variation of DOM at a depth of 60–120 cm (Table S2).

Table 3.
Optical indices of DOM at different soil depths (mean ± standard error).
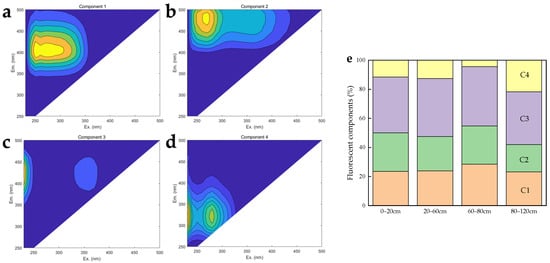
Figure 4.
(a–d) The four fluorescent components for DOM at soil depths of 0–20, 20–60, 60–80, and 80–120 cm ((a): C1; (b): C2; (c): C3; (d): C4). (e) Relative distributions of different fluorescence components (C1–C4) calculated by PARAFAC modeling in 0–120 cm soil profiles.
3.4. DOM Composition by 1H-NMR
According to the analyses conducted using 1H-NMR, the dominant components of soil-derived DOM included materials derived from linear terpenoids (MDLT), carboxyl-rich alicyclic molecules (CRAM), carbohydrates (along with signals contributed by peptides), as well as aromatics and phenolics (Figure 5). The composition of DOM in soil samples from various horizons was quantified utilizing 1H-NMR analyses. The lowest levels of aromatics were found in all samples. Additionally, the relative abundance of these four components showed little variation at 0–60 cm. The content of CRAM and carbohydrates at 60–80 cm was similar, with MDLT being the most abundant. However, permafrost layers (80–120 cm) had a higher proportion of carbohydrates and a significantly lower proportion of CRAM (Table S3).
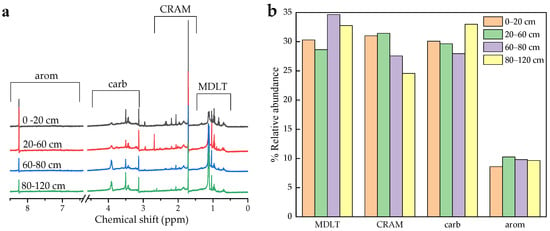
Figure 5.
(a) Solution-state 1H nuclear magnetic resonance spectroscopic characteristics of water at soil depths of 0–20, 20–60, 60–80, and 80–120 cm from the DOM. (b) The relative abundance of MDLT, CRAM, carb, and arom.
4. Discussion
4.1. Properties of Soil
Due to the mineral soil characteristics of peatlands, DOC and TOC values are low and (Fe, Al) oxide content is high [30]. The decrease in TOC values along the profile may stem from microbial action on soil decomposition [50]. In the meantime, the presence of metal (Fe, Al) oxides increased the SA of the soil [51]. The high SA near the permafrost table results in the easy formation of Fep/Alp complexes with SOM, and thus, the area near the permafrost table was richer in C [52]. One additional factor that may contribute is the concurrent freezing of the soil column from both the top and bottom, resulting in a joint freezing process at the mid-point, which in turn concentrates the solute [9]. The reason why SWC at 80–120cm is higher than 0-80 cm may be that the permafrost layer contains a large amount of ice (Figure 1b). Changes in soil pH may be caused by changes in fungal communities in the soil [53].
4.2. Soil SOM Stability
The thermal analysis method proved to be a valuable approach for examining the stability of SOM [54,55]. This research utilized the temperature during which SOM diminishes to half of its weight (TG-T50) as an indicator of its thermal stability. Typically, SOM that combusts at elevated temperatures is associated with more resistant structural components [56]. The area near the permafrost table had the largest percentage of stabilized organic matter (quality share was about 1.23%), indicating obvious organic matter accumulation (Figure S2). As shown in Figure 3, the stability of SOM in the 0–80 cm soil layer increases with depth, and the higher thermal stability of SOM near the permafrost table layer soil may be related to higher Fe and alumina content [57].
The stable isotopes (δ 13C and δ 15N) are helpful to study the stability of SOM [19]. Previous studies have demonstrated that the stability of SOM can be estimated using the principle that δ 15N increases with SOM stability. In this study, the δ 13C value was low, which was in line with the general organic soil rule [58,59]. Krüger et al. reported that in permafrost peatland, there was a positive correlation between the δ 13C value and the C:N ratio, and the δ 13C value first increased and then decreased with increasing depth [60]. Our results indicate that the changes in δ 13C, δ 15N, and C:N in the active layer are not significant; there is a slight trend toward increased stability of SOM. Near the permafrost table, δ 13C, δ 15N, and C:N values increase, demonstrating clear characteristics of enhanced SOM stability. Conversely, the stability of SOM in the permafrost layer appears to be diminished. Heavy plant inputs will lower the value of δ 13C value, and generally, the δ 13C value increases during the microbial transformation of organic matter [61]. However, the low temperatures characteristic of permafrost soils hinder microbial activity and, consequently, the transformation of SOM. Additionally, Fe and Al oxides were enriched at a depth of 60–80 cm, where SOM interacts with soil minerals through active hydroxyl (OH) sites. This interaction enhances the stability of SOM, thereby preventing its degradation near the permafrost table [62,63]. In short, the overall trends of soil δ 13C, δ 15N, and C:N ratio (Table 1) indicated that near the permafrost table, SOM stability was stronger than the permafrost layer, and active layer SOM stability increased mildly with soil depths.
4.3. DOM Characteristics at Different Soil Depths
The optical properties of the DOM vary with the depth of the profile. In this study, the lower C:N and HIX, as well as the higher FI and BIX for the permafrost layer (Table 1 and Table 3), collectively suggested the high production of microbial-derived DOM during soil passage [38,64,65,66]. Compared with the 0–80 cm soil layer, the percentage of protein-like fluorescent components (C4) in the permafrost layer was significantly increased, and the percentage of humus-like components (C1, C2, and C3) was decreased (Figure 4a–d), which also indicated that the input of microbial-derived DOM was large [67,68]. Previous studies have also shown that the accumulation of microbial-derived compounds is higher in permafrost [26,69,70]. The SUVA254 values for DOM in mineral permafrost are lower due to the greater abundance of microbial ex-metabolites and root exudates in mineral soils compared to peatlands with high organic matter content [71]. The values of HIX and SUVA254 are not significantly different in 0–60 cm, indicating similar long-term microbial processing of organic matter [23]. The fluorescence index SUVA254 increased with aromaticity, and the SR is typically inversely associated with DOM molecular weight (MW). In the permafrost layer, these two indices show a negative correlation (Figure S3), indicating that there are many protein-derived substrates in permafrost and the average MW is low. It is worth noting that higher SUVA254 values near the permafrost table correspond to the accumulation of aromatic polymerized compounds in this layer, likely as a result of bacterial consumption of bioavailable carboxylates in the overlying active layer [72]. Components C1, C2, and C3 were linked to humic or fulvic-like substances associated with high-molecular-weight (HMW) aromatic organic compounds that originate from terrestrial sources, including inputs from plants [34,65,73]. Component C4, which resembles tyrosine, was found throughout various terrestrial environments and consists of protein substances resulting from microbial activity, including amino acids, peptide materials, and both free and bound proteins [65]. DOM in permafrost shows unique characteristics, which are characterized by lower molecular weight (LMW), aromaticity, and a higher percentage of protein-based organic compounds in permafrost DOM compared with the active layer and near the permafrost table (Figure 4e). The results showed that HMW aromatic fluorophores from terrestrial sources were abundant in the active layer and near the permafrost table, especially near the permafrost table (95.59% of the total fluorescence). It was believed that simple organic compounds generally have higher decomposition rates and lower activation energies. The reduced molecular mass and molecular structure complexity of SOM in the permafrost layer reduced the energy required for its biochemical reactions, requiring a lower activation energy and resulting in a less stable SOM molecule in the permafrost layer [74,75]. Finally, the 1H-NMR results support our findings by finding an enrichment of low molecular weight compounds such as carbohydrates in the 80–120 cm.
One possible explanation for the change in DOM quality is the selective mineral adsorption during the infiltration process, in which Al oxides play a key role (Figure S3). In addition, we found that the metal oxide of Al has a significant negative correlation with C4 and carb. This phenomenon should be studied in future experiments. The multivariate statistical analyses based on absorbance and fluorescence indices demonstrated that permafrost DOM showed significant differences in optical characteristics as depth increases (Figure 4). Long-term microbial processing and transformation can promote the production of microbial-derived molecules by breaking down plant-derived DOM and root exudates. For example, microbial conversion of lignin-like substances into lipid compounds [76] and other water-soluble substances [77] in permafrost can serve as an important source of microbial-derived molecules such as CRAMs or amino sugars. This was consistent with the higher carbohydrate content of DOM in 1H-NMR in permafrost (Figure 5b). Tyrosine-like components of DOM (amino acids and oligopeptides) became utilized as aliphatic compounds almost immediately after the thawing of permafrost and were, thus, preferentially mineralized by soil micro-organisms [22,78,79]. In addition, CRAM contributed more to DOM in 0–60 cm soil, indicating the presence of more carboxylic acid functional molecules [80]. Although there is a decrease in CRAM content near the permafrost table, the layer still contains high levels of Fe-Al metal oxides. The cation bridging interactions between CRAM and soil minerals are stronger in this layer, leading to a more stable soil organic matter near the permafrost table [81,82].
FT-IR spectroscopy was a powerful tool for studying soil organic molecules [29,83]. The overall trend in soil FT-IR spectra varied non-significantly along the profile, indicating that the differences between DOM compounds at different depths were not significant (Figure 3a). Changes in peak intensity indicated changes in substance content. The peak at 1036, 1085 cm−1 (carbohydrate) between the 0–80 cm soil layers gradually diminishes. The result might be the result of microbial use of carbohydrates during transformation. In permafrost, the increase in carbohydrate content can be attributed to the weak activity of micro-organisms at low temperatures and the low utilization rate of carbohydrates. This was consistent with the results of 1H-NMR performance. At the same time, this also shows that the permafrost in the mineral peatlands of the Greater Khingan Mountains may contain a higher proportion of biodegradable DOM. The aliphatic peak area in the soil at 2921 (3010–2800 cm−1) and aromatic peaks at 1653 (1660–1580 cm−1) correspond to the more labile and stable SOM compounds. In this study, the ratio of relative peak areas of 1653 and 2921 cm−1 (rA1653/rA2921) was used as an index for assessing the molecular stability of DOM [31]. This trend in Figure 4b shows that DOM stability (or recalcitrance) gradually increases in the 0–80 cm soil layer, and DOM stability is greatly reduced in permafrost. The above studies on the composition of DOM can indicate that the SOM near the permafrost table is the most stable, while the stability of the permafrost layer is poor.
5. Conclusions
The study observed an increasing trend in thermal, molecular, and isotopic indices of organic matter stability with soil depth in the active layer. The DOM in the topsoil changes from high quality and high aromaticity to low quality and low aromaticity in the subsoil. The findings indicate that the perennial near the permafrost table contains a significant amount of carbon, with soil organic matter being more stable at this interface than in the permafrost layer. Since SOM in permafrost is less stable, it is more easily decomposed by micro-organisms. Therefore, when temperatures rise, the region’s carbon emissions are likely to increase. Additionally, the consistency in the stability of DOM and SOM with changes in soil depth enhances the predictive capability of permafrost zones in response to climate change and the feedback to SOM. This study aimed to better understand SOM in permafrost peatland soil profiles by quantifying the chemical composition of constituents DOM to more accurately assess changes in SOM stability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15101797/s1, Figure S1: Adsorption and desorption isotherms of soil samples (0–20, 20–60, 60–80, and 80–120 cm); Figure S2: TG at soil depths of 0–20, 20–60, 60–80, and 80–120 cm; Figure S3: Correction analysis of DOC content by various soil parameters and DOM component content. The dimensions of the circles represent the magnitude of the correlation coefficient, with darker hues indicating a stronger relationship. A red circle signifies a positive correlation, while blue denotes a negative correlation, and the figures associated with the various colors reflect the correlation coefficients. Table S1: Comparison of the four fluorescent components identified by parallel factor analysis (PARAFAC) with previously reported components; Table S2: percentages of five fluorescent components in water samples at soil depths of 0–20 cm, 20–60 cm, 60–80cm, and 80–140 cm; Table S3: The relative abundance of MDLT, CRAM, carb, and arom in the 1H-NMR spectra of DOM.
Author Contributions
Conceptualization, L.S. and S.Z. (Shuying Zang); data curation, J.Z. and Q.L.; methodology, X.W. and R.X.; writing—original draft, S.Z. (Siyuan Zou). All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Key Joint Program of the National Natural Science Foundation of China (NSFC) and Heilongjiang Province for Regional Development (U20A2082), the National Natural Science Foundation of China (42271135), and the Doctoral student innovation project of Harbin Normal University (HSDBSCX2024-01).
Data Availability Statement
The original contributions discussed in this research are available in the article/Supplementary Material; any further questions can be directed to the corresponding author.
Acknowledgments
Crucial support in the lab was provided by Tong-Jiao Yin.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brown, J.; Sidlauskas, F.J.; Delinski, G. Circum-Arctic Map of Permafrost and Ground Ice Conditions; US Geological Survey: Reston, VA, USA, 1997. [Google Scholar]
- Obu, J.; Westermann, S.; Bartsch, A.; Berdnikov, N.; Christiansen, H.H.; Dashtseren, A.; Delaloye, R.; Elberling, B.; Etzelmüller, B.; Kholodov, A. Northern Hemisphere permafrost map based on TTOP modelling for 2000–2016 at 1 km2 scale. Earth-Sci. Rev. 2019, 193, 299–316. [Google Scholar] [CrossRef]
- Hugelius, G.; Strauss, J.; Zubrzycki, S.; Harden, J.W.; Schuur, E.A.; Ping, C.-L.; Schirrmeister, L.; Grosse, G.; Michaelson, G.J.; Koven, C.D. Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences 2014, 11, 6573–6593. [Google Scholar] [CrossRef]
- Mishra, U.; Hugelius, G.; Shelef, E.; Yang, Y.; Strauss, J.; Lupachev, A.; Harden, J.W.; Jastrow, J.D.; Ping, C.-L.; Riley, W.J. Spatial heterogeneity and environmental predictors of permafrost region soil organic carbon stocks. Sci. Adv. 2021, 7, eaaz5236. [Google Scholar] [CrossRef] [PubMed]
- Biskaborn, B.K.; Smith, S.L.; Noetzli, J.; Matthes, H.; Vieira, G.; Streletskiy, D.A.; Schoeneich, P.; Romanovsky, V.E.; Lewkowicz, A.G.; Abramov, A. Permafrost is warming at a global scale. Nat. Commun. 2019, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Elberling, B.; Michelsen, A.; Schädel, C.; Schuur, E.A.; Christiansen, H.H.; Berg, L.; Tamstorf, M.P.; Sigsgaard, C. Long-term CO2 production following permafrost thaw. Nat. Clim. Chang. 2013, 3, 890–894. [Google Scholar] [CrossRef]
- Tesi, T.; Muschitiello, F.; Smittenberg, R.H.; Jakobsson, M.; Vonk, J.; Hill, P.; Andersson, A.; Kirchner, N.; Noormets, R.; Dudarev, O. Massive remobilization of permafrost carbon during post-glacial warming. Nat. Commun. 2016, 7, 13653. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Lim, A.G.; Loiko, S.V.; Kuzmina, D.M.; Krickov, I.V.; Shirokova, L.S.; Kulizhsky, S.P.; Vorobyev, S.N.; Pokrovsky, O.S. Dispersed ground ice of permafrost peatlands: Potential unaccounted carbon, nutrient and metal sources. Chemosphere 2021, 266, 128953. [Google Scholar] [CrossRef]
- Pastukhov, A.; Kovaleva, V.; Kaverin, D. Microbial community structure in ancient European Arctic Peatlands. Plants 2022, 11, 2704. [Google Scholar] [CrossRef]
- Dong, X.; Liu, C.; Wu, X.; Man, H.; Wu, X.; Ma, D.; Zang, S. Linking soil organic carbon mineralization with soil variables and bacterial communities in a permafrost-affected tussock wetland during laboratory incubation. Catena 2023, 221, 106783. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Deep soil organic matter—A key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Li, M.; Min, Y.; Zhan, F.; Wang, Y.; Sheng, L.; Bian, H. Changes in soil dissolved organic matter optical properties during peatland succession. Ecol. Indic. 2022, 143, 109386. [Google Scholar] [CrossRef]
- Cong, J.; Gao, C.; Ji, S.; Li, X.; Han, D.; Wang, G. Changes in organic matter properties and carbon chemical stability in surface soils associated with changing vegetation communities in permafrost peatlands. Biogeochemistry 2023, 163, 139–153. [Google Scholar] [CrossRef]
- Shaver, G.R.; Giblin, A.E.; Nadelhoffer, K.J.; Thieler, K.; Downs, M.; Laundre, J.; Rastetter, E.B. Carbon turnover in Alaskan tundra soils: Effects of organic matter quality, temperature, moisture and fertilizer. J. Ecol. 2006, 94, 740–753. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. Analytical approaches for characterizing soil organic matter. Org. Geochem. 2000, 31, 609–625. [Google Scholar] [CrossRef]
- Stone, M.M.; Plante, A.F. Relating the biological stability of soil organic matter to energy availability in deep tropical soil profiles. Soil Biol. Biochem. 2015, 89, 162–171. [Google Scholar] [CrossRef]
- Hodgkins, S.B.; Richardson, C.J.; Dommain, R.; Wang, H.; Glaser, P.H.; Verbeke, B.; Winkler, B.R.; Cobb, A.R.; Rich, V.I.; Missilmani, M. Tropical peatland carbon storage linked to global latitudinal trends in peat recalcitrance. Nat. Commun. 2018, 9, 3640. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, Y.; Chen, X.; He, K.; Zhu, B. Changes in soil organic matter stability with depth in two alpine ecosystems on the Tibetan Plateau. Geoderma 2019, 351, 153–162. [Google Scholar] [CrossRef]
- Kalbitz, K.; Solinger, S.; Park, J.-H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Drake, T.W.; Wickland, K.P.; Spencer, R.G.; McKnight, D.M.; Striegl, R.G. Ancient low–molecular-weight organic acids in permafrost fuel rapid carbon dioxide production upon thaw. Proc. Natl. Acad. Sci. USA 2015, 112, 13946–13951. [Google Scholar] [CrossRef] [PubMed]
- Fouché, J.; Christiansen, C.; Lafrenière, M.; Grogan, P.; Lamoureux, S. Canadian permafrost stores large pools of ammonium and optically distinct dissolved organic matter. Nat. Commun. 2020, 11, 4500. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zang, S.; Zhang, K.; Sun, D.; Sun, L. Occurrence, sources and potential risks of polycyclic aromatic hydrocarbons in a permafrost soil core, northeast China. Ecotoxicology 2021, 30, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jia, X.; Li, M.; Wu, H. Insight into the vertical characteristics of dissolved organic matter in 5-m soil profiles under different land-use types on the Loess Plateau. Sci. Total Environ. 2019, 692, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Steinbeiss, S.; Temperton, V.; Gleixner, G. Mechanisms of short-term soil carbon storage in experimental grasslands. Soil Biol. Biochem. 2008, 40, 2634–2642. [Google Scholar] [CrossRef]
- Mikutta, R.; Lorenz, D.; Guggenberger, G.; Haumaier, L.; Freund, A. Properties and reactivity of Fe-organic matter associations formed by coprecipitation versus adsorption: Clues from arsenate batch adsorption. Geochim. Cosmochim. Acta 2014, 144, 258–276. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.; Wang, X.; Song, C.; Song, Y.; Liu, Z.; Wang, S.; Gao, S.; Ma, G. Mineral protection controls soil organic carbon stability in permafrost wetlands. Sci. Total Environ. 2023, 869, 161864. [Google Scholar] [CrossRef]
- Ernakovich, J.G.; Wallenstein, M.D.; Calderón, F. Chemical indicators of cryoturbation and microbial processing throughout an Alaskan permafrost soil depth profile. Soil Sci. Soc. Am. J. 2015, 79, 783–793. [Google Scholar] [CrossRef]
- Harris, D.; Horwáth, W.R.; Van Kessel, C. Acid fumigation of soils to remove carbonates prior to total organic carbon or carbon-13 isotopic analysis. Soil Sci. Soc. Am. J. 2001, 65, 1853–1856. [Google Scholar] [CrossRef]
- Demyan, M.S.; Rasche, F.; Schulz, E.; Breulmann, M.; Müller, T.; Cadisch, G. Use of specific peaks obtained by diffuse reflectance Fourier transform mid-infrared spectroscopy to study the composition of organic matter in a Haplic Chernozem. Eur. J. Soil Sci. 2012, 63, 189–199. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Q.; Huang, W.-L.; Feng, L.; Wang, Y.-H.; Xie, Z.; Yong, S.-S.; Zhang, S.; Jiang, B.; Zheng, Y. Spectroscopic and molecular-level characteristics of dissolved organic matter in the Pearl River Estuary, South China. Sci. Total Environ. 2020, 710, 136307. [Google Scholar] [CrossRef] [PubMed]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.A.; Amon, R.M.; Stedmon, C.A. Variations in high-latitude riverine fluorescent dissolved organic matter: A comparison of large Arctic rivers. J. Geophys. Res. Biogeosci. 2013, 118, 1689–1702. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Cory, R.M.; McKnight, D.M. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ. Sci. Technol. 2005, 39, 8142–8149. [Google Scholar] [CrossRef] [PubMed]
- Gabor, R.S.; Eilers, K.; McKnight, D.M.; Fierer, N.; Anderson, S.P. From the litter layer to the saprolite: Chemical changes in water-soluble soil organic matter and their correlation to microbial community composition. Soil Biol. Biochem. 2014, 68, 166–176. [Google Scholar] [CrossRef]
- Ohno, T. Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef]
- Wilson, H.F.; Xenopoulos, M.A. Effects of agricultural land use on the composition of fluvial dissolved organic matter. Nat. Geosci. 2009, 2, 37–41. [Google Scholar] [CrossRef]
- Wang, J.-J.; Dahlgren, R.A.; Erşan, M.S.; Karanfil, T.; Chow, A.T. Temporal variations of disinfection byproduct precursors in wildfire detritus. Water Res. 2016, 99, 66–73. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Simpson, A.J.; Soong, R.; Oren, A.; Chefetz, B.; Simpson, M.J. Solution-state NMR investigation of the sorptive fractionation of dissolved organic matter by alkaline mineral soils. Environ. Chem. 2013, 10, 333–340. [Google Scholar] [CrossRef][Green Version]
- Simpson, A.J.; Brown, S.A. Purge NMR: Effective and easy solvent suppression. J. Magn. Reson. 2005, 175, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.; Baer, A.; Alaee, M.; Lefebvre, B.; Moser, A.; Williams, A.; Simpson, A.J. Major structural components in freshwater dissolved organic matter. Environ. Sci. Technol. 2007, 41, 8240–8247. [Google Scholar] [CrossRef] [PubMed]
- Woods, G.C.; Simpson, M.J.; Pautler, B.G.; Lamoureux, S.F.; Lafrenière, M.J.; Simpson, A.J. Evidence for the enhanced lability of dissolved organic matter following permafrost slope disturbance in the Canadian High Arctic. Geochim. Cosmochim. Acta 2011, 75, 7226–7241. [Google Scholar] [CrossRef]
- Wang, J.-J.; Lafreniere, M.J.; Lamoureux, S.F.; Simpson, A.J.; Gelinas, Y.; Simpson, M.J. Differences in riverine and pond water dissolved organic matter composition and sources in Canadian high Arctic watersheds affected by active layer detachments. Environ. Sci. Technol. 2018, 52, 1062–1071. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Z.; Ye, Q.; Liang, Y.; Liu, M.; Dang, Z.; Wang, Y.; Liu, C. Chemodiversity of soil dissolved organic matter. Environ. Sci. Technol. 2020, 54, 6174–6184. [Google Scholar] [CrossRef]
- Hu, Z.; Li, C.; Kang, S.; Li, X.; Zhang, C.; Yan, F.; Chen, P. Dissolved organic carbon fractionation in wet deposition and its potential impact on radiative forcing in the central Tibetan Plateau. Res. Cold Arid Reg. 2023, 15, 171–178. [Google Scholar] [CrossRef]
- Ma, H.; Allen, H.E.; Yin, Y. Characterization of isolated fractions of dissolved organic matter from natural waters and a wastewater effluent. Water Res. 2001, 35, 985–996. [Google Scholar] [CrossRef]
- Choe, E.; van der Meer, F.; Rossiter, D.; van der Salm, C.; Kim, K.-W. An alternate method for Fourier transform infrared (FTIR) spectroscopic determination of soil nitrate using derivative analysis and sample treatments. Water Air Soil Pollut. 2010, 206, 129–137. [Google Scholar] [CrossRef]
- Gentsch, N.; Mikutta, R.; Alves, R.J.E.; Barta, J.; Čapek, P.; Gittel, A.; Guggenberger, G. Storage and transformation of organic matter fractions in cryoturbated permafrost soils across the Siberian Arctic. Biogeosciences 2015, 12, 4525–4542. [Google Scholar] [CrossRef]
- Jiang, C.; Séquaris, J.-M.; Wacha, A.; Bóta, A.; Vereecken, H.; Klumpp, E. Effect of metal oxide on surface area and pore size of water-dispersible colloids from three German silt loam topsoils. Geoderma 2014, 235, 260–270. [Google Scholar] [CrossRef]
- Szymański, W.; Drewnik, M.; Stolarczyk, M.; Musielok, Ł.; Gus-Stolarczyk, M.; Skiba, M. Occurrence and stability of organic intercalation in clay minerals from permafrost-affected soils in the High Arctic–A case study from Spitsbergen (Svalbard). Geoderma 2022, 408, 115591. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, N.F.; Liu, H.Y.; Zhang, Y.Q.; Yu, L. Soil pH is a key determinant of soil fungal community composition in the Ny-Ålesund Region, Svalbard (High Arctic). Front. Microbiol. 2016, 7, 227. [Google Scholar] [CrossRef]
- Plante, A.F.; Fernández, J.M.; Leifeld, J. Application of thermal analysis techniques in soil science. Geoderma 2009, 153, 1–10. [Google Scholar] [CrossRef]
- Peltre, C.; Fernández, J.M.; Craine, J.M.; Plante, A.F. Relationships between biological and thermal indices of soil organic matter stability differ with soil organic carbon level. Soil Sci. Soc. Am. J. 2013, 77, 2020–2028. [Google Scholar] [CrossRef]
- Kučerík, J.; Tokarski, D.; Demyan, M.S.; Merbach, I.; Siewert, C. Linking soil organic matter thermal stability with contents of clay, bound water, organic carbon and nitrogen. Geoderma 2018, 316, 38–46. [Google Scholar] [CrossRef]
- Fang, K.; Qin, S.; Chen, L.; Zhang, Q.; Yang, Y. Al/Fe mineral controls on soil organic carbon stock across Tibetan alpine grasslands. J. Geophys. Res. Biogeosci. 2019, 124, 247–259. [Google Scholar] [CrossRef]
- Guillaume, T.; Damris, M.; Kuzyakov, Y. Losses of soil carbon by converting tropical forest to plantations: Erosion and decomposition estimated by δ13C. Glob. Chang. Biol. 2015, 21, 3548–3560. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Ou, Y.; Jia, H.; Li, J.; Shi, C.; Liu, Y. Variations in soil δ13C with alpine meadow degradation on the eastern Qinghai–Tibet Plateau. Geoderma 2019, 338, 178–186. [Google Scholar] [CrossRef]
- Krüger, J.; Leifeld, J.; Alewell, C. Degradation changes stable carbon isotope depth profiles in palsa peatlands. Biogeosciences 2014, 11, 3369–3380. [Google Scholar] [CrossRef]
- Schnecker, J.; Borken, W.; Schindlbacher, A.; Wanek, W. Little effects on soil organic matter chemistry of density fractions after seven years of forest soil warming. Soil Biol. Biochem. 2016, 103, 300–307. [Google Scholar] [CrossRef]
- Kramer, M.G.; Sanderman, J.; Chadwick, O.A.; Chorover, J.; Vitousek, P.M. Long-term carbon storage through retention of dissolved aromatic acids by reactive particles in soil. Glob. Chang. Biol. 2012, 18, 2594–2605. [Google Scholar] [CrossRef]
- Kramer, M.G.; Chadwick, O.A. Climate-driven thresholds in reactive mineral retention of soil carbon at the global scale. Nat. Clim. Chang. 2018, 8, 1104–1108. [Google Scholar] [CrossRef]
- Lobbes, J.M.; Fitznar, H.P.; Kattner, G. Biogeochemical characteristics of dissolved and particulate organic matter in Russian rivers entering the Arctic Ocean. Geochim. Cosmochim. Acta 2000, 64, 2973–2983. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; Spencer, R.G. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- D’Andrilli, J.; Cooper, W.T.; Foreman, C.M.; Marshall, A.G. An ultrahigh-resolution mass spectrometry index to estimate natural organic matter lability. Rapid Commun. Mass Spectrom. 2015, 29, 2385–2401. [Google Scholar] [CrossRef]
- Podgorski, D.C.; Zito, P.; McGuire, J.T.; Martinovic-Weigelt, D.; Cozzarelli, I.M.; Bekins, B.A.; Spencer, R.G. Examining natural attenuation and acute toxicity of petroleum-derived dissolved organic matter with optical spectroscopy. Environ. Sci. Technol. 2018, 52, 6157–6166. [Google Scholar] [CrossRef]
- Wu, X.; Wu, L.; Liu, Y.; Zhang, P.; Li, Q.; Zhou, J.; Hess, N.J.; Hazen, T.C.; Yang, W.; Chakraborty, R. Microbial interactions with dissolved organic matter drive carbon dynamics and community succession. Front. Microbiol. 2018, 9, 1234. [Google Scholar] [CrossRef]
- Kaiser, K.; Guggenberger, G.; Haumaier, L. Changes in dissolved lignin-derived phenols, neutral sugars, uronic acids, and amino sugars with depth in forested Haplic Arenosols and Rendzic Leptosols. Biogeochemistry 2004, 70, 135–151. [Google Scholar] [CrossRef]
- Ohno, T.; Parr, T.B.; Gruselle, M.C.I.; Fernandez, I.J.; Sleighter, R.L.; Hatcher, P.G. Molecular composition and biodegradability of soil organic matter: A case study comparing two new England forest types. Environ. Sci. Technol. 2014, 48, 7229–7236. [Google Scholar] [CrossRef]
- Raudina, T.V.; Loiko, S.V.; Lim, A.G.; Krickov, I.V.; Shirokova, L.S.; Istigechev, G.I.; Kuzmina, D.M.; Kulizhsky, S.P.; Vorobyev, S.N.; Pokrovsky, O.S. Dissolved organic carbon and major and trace elements in peat porewater of sporadic, discontinuous, and continuous permafrost zones of western Siberia. Biogeosciences 2017, 14, 3561–3584. [Google Scholar] [CrossRef]
- Kuzmina, D.M.; Lim, A.G.; Loiko, S.V.; Shefer, N.; Shirokova, L.S.; Julien, F.; Rols, J.-L.; Pokrovsky, O.S. Dispersed ice of permafrost peatlands represents an important source of labile carboxylic acids, nutrients and metals. Geoderma 2023, 429, 116256. [Google Scholar] [CrossRef]
- Mann, P.J.; Spencer, R.G.; Hernes, P.J.; Six, J.; Aiken, G.R.; Tank, S.E.; McClelland, J.W.; Butler, K.D.; Dyda, R.Y.; Holmes, R.M. Pan-Arctic trends in terrestrial dissolved organic matter from optical measurements. Front. Earth Sci. 2016, 4, 25. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Bracho, R.; Natali, S.; Pegoraro, E.; Crummer, K.G.; Schädel, C.; Celis, G.; Hale, L.; Wu, L.; Yin, H.; Tiedje, J.M. Temperature sensitivity of organic matter decomposition of permafrost-region soils during laboratory incubations. Soil Biol. Biochem. 2016, 97, 1–14. [Google Scholar] [CrossRef]
- Khatami, S.; Deng, Y.; Tien, M.; Hatcher, P.G. Formation of water-soluble organic matter through fungal degradation of lignin. Org. Geochem. 2019, 135, 64–70. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Farrell, M.; Hill, P.W.; Farrar, J.; DeLuca, T.H.; Roberts, P.; Kielland, K.; Dahlgren, R.; Murphy, D.V.; Hobbs, P.J.; Bardgett, R.D. Oligopeptides represent a preferred source of organic N uptake: A global phenomenon? Ecosystems 2013, 16, 133–145. [Google Scholar] [CrossRef]
- Textor, S.R.; Wickland, K.P.; Podgorski, D.C.; Johnston, S.E.; Spencer, R.G. Dissolved organic carbon turnover in permafrost-influenced watersheds of interior Alaska: Molecular insights and the priming effect. Front. Earth Sci. 2019, 7, 275. [Google Scholar] [CrossRef]
- Kalbitz, K.; Schmerwitz, J.; Schwesig, D.; Matzner, E. Biodegradation of soil-derived dissolved organic matter as related to its properties. Geoderma 2003, 113, 273–291. [Google Scholar] [CrossRef]
- Newcomb, C.J.; Qafoku, N.P.; Grate, J.W.; Bailey, V.L.; De Yoreo, J.J. Developing a molecular picture of soil organic matter–mineral interactions by quantifying organo–mineral binding. Nat. Commun. 2017, 8, 396. [Google Scholar] [CrossRef]
- Qin, J.; Ding, Y.; Han, T.; Chang, Y.; Shi, F.; You, Y. The hydrothermal changes of permafrost active layer and their impact on summer rainfall-runoff processes in an alpine meadow watershed, northwest China. Res. Cold Arid Reg. 2022, 14, 361–369. [Google Scholar] [CrossRef]
- Djukic, I.; Zehetner, F.; Tatzber, M.; Gerzabek, M.H. Soil organic-matter stocks and characteristics along an Alpine elevation gradient. J. Plant Nutr. Soil Sci. 2010, 173, 30–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).