Abstract
The increasing occurrence of dry and hot summers generates chronic water deficits that negatively affect tree radial growth. This phenomenon has been widely studied in natural stands of native species but not in commercial plantations of exotic tree species. In central Chile, where the species is increasingly planted, the dynamics of stone pine (Pinus pinea L.) growth under drought have been little explored. We studied the impact of drought on four stone pine plantations growing in central Chile. We sampled and cross-dated a total of 112 trees from four sites, measured their tree-ring width (RWL) series, and obtained detrended series of ring width indices (RWIs). Then, we calculated three resilience indices during dry years (Rt, resistance; Rc, recovery; and Rs, resilience), and the correlations between the RWI series and seasonal climate variables. We found the lowest growth rate (1.94 mm) in the driest site (Peñuelas). Wet conditions in the previous winter and current spring favored growth. In the wettest site (Pastene), the growth rates were high (4.87 mm) and growth also increased in response to spring thermal amplitude. Overall, fast-growing trees were less resilient than slow-growing trees. Drought reduced stone pine stem growth and affected tree resilience to hydric deficit. At the stand level, growth rates and resistance were driven by winter and spring precipitation. Fast-growing trees were more resistant but showed less capacity to recover after a drought. In general, stone pine showed a high post-drought resilience due to a high recovery after drought events. The fact that we found high resilience in non-native habitats, opens new perspectives for stone pine cropping, revealing that it is possible to explore new areas to establish the species. We conclude that stone pine shows a good acclimation in non-native, seasonally dry environments.
1. Introduction
Stone pine (Pinus pinea L.) is a conifer native to the Mediterranean basin, where its cones have been harvested for millennia [1]. Stone pine is increasingly used in afforestation both in native and exotic countries due to its multi-functionality and the socioeconomic benefits provided by its pine nuts [2,3]. Most stone pine plantations worldwide are extensively managed, with an increasing interest to apply intensive silviculture, given its impact on productivity [4]. In Chile, stone pine was introduced by European immigrants and exhibits good adaptation and development in the central and southern areas [5].
Climate change is increasing the frequency of extremely dry and hot summers, generating chronic water deficits [6], affecting tree growth and survival [7], and increasing tree susceptibility to pests and pathogens [8]. In Chile, the ecological balance of ecosystems located in the central area of the country is closely related to the presence and resilience of arboreal species. A long-lasting drought known as mega drought [9] has affected both native forests [10] and plantations of several species, including stone pine [11]. The rainfall reduction and temperature increase are negatively impacting stone pine growth and cone production in the European Mediterranean region, challenging management [12]. Climate change is expected to reduce the species’ habitat and shift its geographical distribution [13].
Drought, a climatic stressor of escalating prominence, exerts substantial pressure on the physiological processes of trees, potentially stunting growth and increasing mortality rates within the affected populations [14]. The responses of tree growth to climate variability depend on the species and stand characteristics [15]. Stone pine presents a unique adaptability and tolerance to arid climates characterized by dry and hot conditions in summer and high temporal variability in rainfall [11,16,17]. However, in the stone pine native habitat, the increasing frequency and intensity of drought events have been shown to threaten the vitality and sustainability of these tree populations [15,18,19].
In the face of climate change, understanding the effects of drought on growth dynamics is critical for designing sustainable management strategies [20]. For stone pine trees, growth dynamics have been little explored [21], especially in non-native environments. Diameter growth can be studied from radial ring width sequences measured from cross-sectional cores [22], and is useful to examine growth responses to climatic variability [23].
Relevant questions regarding species resilience in a scenario of prolonged or intensified drought episodes have been posed [24,25]. Mazza et al. [26] suggested that future studies on stone pine plantations should consider climate-driven growth patterns along drought gradients. This study focuses on assessing the impact of drought on stone pine radial growth, measured through tree-ring width variations within plantations along an aridity gradient. Our aims were to assess the dendrochronological response of stone pine in Chile and to investigate growth patterns in response to extreme events by employing the resilience components (resistance, recovery, and resilience). We hypothesize a high degree of growth synchrony within stone pine populations along Chile and a growth reduction in response to drought, particularly in the driest sites.
2. Materials and Methods
2.1. Study Sites
The study sites are located in central Chile. Four plantations with typical Mediterranean climate (Peñuelas, Cahuil, Paredones and Pastene) were selected along a 600 km latitudinal gradient (Figures S1 and S2). Tree density ranged from 400 to 833 trees ha−1 (Table 1). Peñuelas, Cahuil and Paredones sites have a warm-summer climate with winter rainfall, and a long dry season (8 to 9 months). Pastene has a cooler and wetter climate, with only 6 dry months. The soils are acid in all sites.

Table 1.
Characteristics of Pinus pinea L. plantations sampled in central Chile.
2.2. Dendrochronological Analyses
In winter 2017, a sample of mature and apparently healthy trees was selected to extract cores in each plantation (n = 28), totaling 112 trees. Two cores (north–south and east–west) were extracted from each tree at 1.3 m height, using a 5.15 mm Pressler increment borer. Cores extend from the bark to the pith of each tree. Each core sample was placed in labeled paper straw tubes and transported to the laboratory.
The cores were air-dried and mounted with glue onto wooden supports. Then, they were sanded with successively finer sandpaper until the annual tree rings were visible [27]. An Epson Perfection V19 scanner was used to scan the cores at a resolution of 1200 dpi. A procedure for scanner calibration to generate comparable data was applied. Tree-ring width (RWL) was measured with a 0.01 mm resolution along two radii per sample using the CooRecorder software version 7.8.1 [28]. Visual crossdating was evaluated using the COFECHA software version 7.8.1 (Laboratory of Tree-Ring Research, University of Arizona, Arizona, USA), which calculates moving correlations between individual tree-ring width series and the mean series of each site [29]. Calendar dates were assigned to rings following the Southern Hemisphere convention, which assigns an annual ring to the calendar year in which ring formation begins [30] (starting in September of the current year and ending in May of the following one). The series of tree-ring width (RWL) difference in percentage from one year versus the previous one along years were calculated. Each RWL series was detrended using a cubic smoothing spline (67% of series length, at 50% cut-off frequency). Then, to account for changes in tree age affecting growth, each series was standardized by dividing the observed by the predicted values [31]. The detrended and standardized series were converted into ring width indices (RWIs). The individual RWIs were averaged into mean site series (chronology) by applying a by-weight robust mean. Negative pointer years, i.e., those when at least 75% of trees show RWIs 40% lower than the average RWIs during the previous three years, were identified as a drought year.
Resilience components were calculated following Lloret et al. [32]: resistance (Rt), i.e., the capacity to sustain growth levels during the drought, represents the decrease in growth during the year of drought with respect to the three-year period before the drought; recovery (Rc), i.e., the capacity of the tree to recover its growth after drought; and resilience (Rs), i.e., the ability of trees to reach growth levels observed before the drought occurred.
where RWID corresponds to the RWIs (standardized, not pre-whitened ring width indices) during the drought year, and the RWIPre−D and RWIPost−D correspond to the average RWIs of the three years before and after the drought, respectively. The resilience components were calculated for every tree in each site every year along a common 30-year period (1987–2017), and separately for each drought year. The series of RWL and RWI have a sample size of 31 year × 4 sites. Rt, Rc and Rs have 1 value per site and drought event; thus, there are different sample sizes across sites. RWIs, negative pointer years and resilience indices were calculated with dplR and pointRes packages of R software (dplR version 1.1.4, PointRes version 2.0.2) [33].
2.3. Climate Data
Climatic data were extracted from “ERA5-Land Daily Aggregated” Copernicus data set [34] and aggregated in months, growth years (defined between 21st June and 20th June of the following year) and seasons (autumn: March 21st to June 20th; winter: June 21st to September 20th; spring: September 21st to December 20th; and summer: December 21st to March 20th), for the following variables: average temperature (AT, °C), minimum temperature (Min T, °C), maximum temperature (Max T, °C), accumulated rainfall (RF, mm), thermal oscillation ([TO = Max T – Min T], °C) and accumulated potential evapotranspiration (PET, mm). A hydric index (HI) was calculated [HI = RF − PET] as a proxy for a hydric balance [35]; lower values of HI indicate a higher water deficit. Meteorological series have sample size of 31 year × 12 months.
2.4. Statistical Analyses
Pearson’s correlation coefficients between RWIs and seasonal climatic variables were calculated for each site. Partial Least Squares (PLS) multivariate regression was used to explore the responses of sites, chronology of ring width series and resilience indices to the significant seasonal climatic variables during negative pointing years at each site. The PLS was calculated using the InfoStat software version 2014 [36].
3. Results
3.1. Dendrochronological Data
The mean DBH within plantations ranged from 18.7 cm in Peñuelas, the driest site with the highest planting density, to 40.9 cm in Pastene, the wettest site with the highest soil water availability and field capacity (Table 1). The age in the sampling year ranged between 30 (Paredones) and 43 years (Peñuelas) (Table 1).
Tree growth (RWL) was the lowest in Peñuelas, the driest site (1.94 mm), with an average of 4.72 mm in the remaining sites. The standard deviation of RWIs, a measure of interannual variability in ring width, ranged between 0.19 in Paredones and 0.41 in Cahuil and Peñuelas, with the latter two sites presenting the highest number of drought events (Table 2). The frequencies of negative pointer years ranged from four in Cahuil to one in Paredones. In Pastene, the wettest site, no negative pointer year was detected; however, a 40% growth decrease was recorded in 50% of trees in 1991, a year that was also considered a drought event (Figure 1).

Table 2.
Characterization of Pinus pinea L. plantations located in central-southern Chile at the tree level and post-drought resilience indices (values are means ± SE).
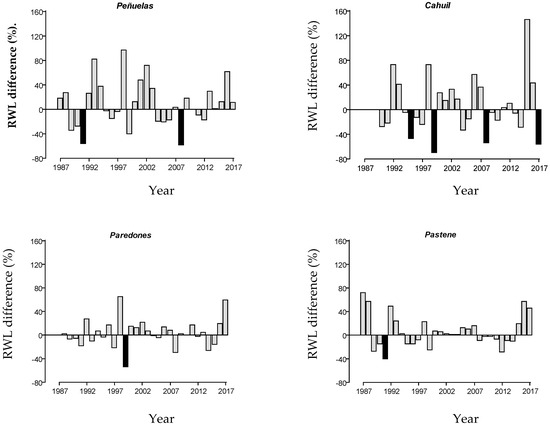
Figure 1.
Series of tree-ring width (RWL) difference in percentage from one year versus the previous one along years. Black bars correspond to negative pointer years, in which a growth deviation above 40% was observed in at least 75% of the trees in each of the three study plantations (Peñuelas, Cahuil, and Paredones) or in at least 50% of the trees in the wettest site (Pastene). RWL deviation was calculated as the ratio between RWL and the average RWL of the previous three years.
3.2. Response to Extreme Events
The correlation coefficients of RWI with monthly climatic variables (Figure S3) were mostly statistically significant in the sites with a high occurrence of droughts. Indeed, in Cahuil, where four droughts occurred, a number of variables of autumn, winter and spring were significant; and in Peñuelas, where two droughts occurred, numerous variables of winter and spring were significant. In Paredones, where one drought was recorded, climatic variables of summer and spring were significant; and in Pastene, the most humid site, climatic variables were significant only in July, the coldest month.
The correlation coefficients of RWIs with seasonal climatic variables were mostly statistically significant. Correlations with climatic variables that were statistically correlated with RWIs in at least one site are presented in Figure 2. Climate affected inter-annual ring width variability in all sites, but mainly in Cahuil, where the highest frequency of droughts was recorded. The correlations of RWIs with spring and winter TO were negative and statistically significant in Peñuelas and Cahuil. The correlation of RWIs with winter AT and Min T was positive and statistically significant for all sites, except for Cahuil; spring Max T was negative and significant only in Cahuil. The correlations of RWIs with spring and winter HI were positive and statistically significant in most sites, except in Pastene. Correlations with spring RF were statistically significant in all sites, whereas correlations with winter RF were significant only in Peñuelas and Cahuil, the driest sites (Figure 2).
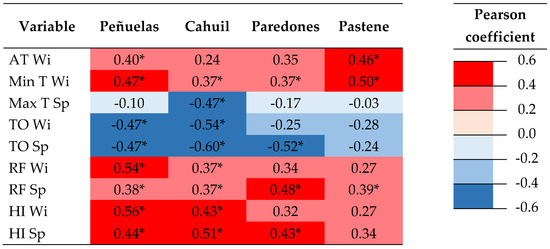
Figure 2.
Heatmap of Pearson’s correlation coefficients between series of ring width indices (RWIs) and seasonal climatic variables that were statistically correlated with RWIs in at least one site. Significant correlations (p < 0.05) are shown with an asterisk. AT Wi: winter average temperature; Min T Wi: winter minimum temperature; Max T Sp: spring maximum temperature; TO Wi: winter thermal oscillation; TO Sp: spring thermal oscillation; RF Wi: winter rainfall; RF Sp: spring rainfall; HI Wi: winter hydric index; HI Sp: spring hydric index.
The RWI and the three resilience components for each year of the chronologies classified by site were simultaneously analyzed using the PLS model to explore the response to seasonal climatic variables. Figure 3 shows the triplot of the two principal latent factors of PLS analysis, which explained 73.7% of the relationship among variables. Figure 3 includes both meteorological and dendrochronological variables in the same space where locations are shown. The angle between one meteorological and one dendrochronological variable is proportional to the capacity of the meteorological variable to predict the dendrochronological one; acute angles represent a positive relationship between the analyzed variables, while obtuse angles represent a negative relationship. Additionally, variables at the plot right side have higher values in the locations in the plot right side than locations in the left side. The triplot shows that Pastene plantation, which is located in an environment characterized by high winter and spring rainfall, and high winter HI, and that had the least severe drought, had the highest RWL and resistance. RWL mostly depended on rainfall, HI and TO; it was favored by high spring and winter RF and HI (lower water deficit), and high spring thermal oscillation. RWL was negatively correlated with tree recovery and positively with tree resistance, but was not correlated with the resilience index. Tree resistance to drought was positively correlated with diameter growth and, consequently, is expected to be correlated with DBH; it depended on winter and spring rainfall (the higher the rainfall during the vegetative growth seasons, the greater the resistance). The triplot also showed that trees with higher RWL were less resilient than trees with lower growth.
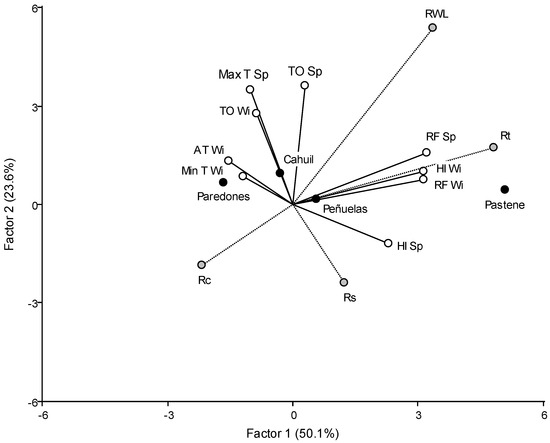
Figure 3.
Triplot from PLS regression showing how tree-ring width (RWL) and resilience components (Rs: resilience index, Rt: resistance index, Rc: recovery index) were related to climate variables (TO Wi: winter thermal oscillation; TO Sp: spring thermal oscillation; HI Wi: winter hydric index; HI Sp: spring hydric index; AT Wi: winter average temperature; Min T Wi: winter minimum temperature; Max T Sp: spring maximum temperature; RF Wi: winter rainfall; RF Sp: spring rainfall). Black dots show plantation sites.
4. Discussion
Knowledge of stone pine growth resilience to drought in a climate-warming scenario is important, especially considering that ecosystem services provided by this species are expected to decrease under aridification [37]. We studied stone pine plantations growing in a latitudinal range in Chile, a non-native habitat for the species. Our results evidenced growth response to the local climate, which is characterized by different drought conditions, and the influence of the stand and tree characteristics on resistance, resilience and recovery indices. We detected responses to environmental factors within the tree–climate–site complex, which highlight local interactions between site and climate conditions, as reported by Calama et al. [38].
4.1. Dendrochronological Response
At the stand level, we observed that growth rates were related to climatic conditions, showing a latitudinal climatic pattern related to rainfall and water deficit. The lowest RWL (1.94 mm) was recorded in Peñuelas, where this value was 2.1 to 2.6 times lower than in the other studied plantations. Peñuelas is characterized by the lowest rainfall, the highest number of dry months, the lowest soil water availability and field capacity, and the highest plantation density. It has been reported that, to cope with water deficit, stone pine modifies the processes regulating growth, including wood formation [13]. The average height of this plantation, 18.7 m at age 43, may have been stimulated by the initial and current high density given the heliophile conditions of stone pine [26]. Indeed, plantation density is an important management decision, especially under drought [39].
4.2. Response to Droughts
In Peñuelas, we found significant correlations of RWI with all winter and spring seasonal climatic variables, except for spring maximum temperature, which is expected since the DBH growth pattern of stone pine in Chile indicates higher growth during winter and spring [11]. This result was also observed in Cahuil, the plantation with the highest number of drought events (4); in fact, except for average winter temperature and rainfall, all correlations were significant in this site. The results suggest the impact of climate on inter-annual ring width variability in all sites, but especially in those where the highest frequency of droughts was recorded (Cahuil and Peñuelas). In sites with extreme drought conditions, water deficit was found to limit the species growth also during early spring and late summer [26].
Two seasonal climatic variables, winter minimum temperature and spring precipitation, showed positive and significant correlations with growth indices in all the study plantations, resulting in a high growth in early spring. This result indicates the relevance of those climatic variables since a large part of the tree-rings are formed during the beginning of the growing season [26]. Our results are in line with those of Mechergui et al. [13], who pointed out that spring rainfall has positive effects on stem growth, and with those of Yu Karpukhin and Yussef [40], who found a significant correlation of growth ring series with annual rainfall. On the other hand, Mazza et al. [26] reported the impact of autumn–winter temperature.
In all sites but Pastene, spring thermal amplitude and hydric index showed significant (negative and positive, respectively) correlations with growth indices. The correlation of growth rates with spring maximum temperature was negative only in Cahuil, the plantations that had undergone several drought events. The correlations of growth indices with winter precipitation and the hydric index were positive in the driest sites (Peñuelas and Cahuil), where two or more drought years were recorded, indicating that tree growth is effectively more controlled by climate on dry sites than on wet sites, as indicated by Lebourgeois et al. [41].
Finally, we identified the climate variables that explained some of the components of resilience, and how they impact those components. The highest resistance and resilience were observed in Pastene, the plantation established on the coolest and wettest site, with the highest soil water availability and field capacity. Song et al. [42] reported that under intense drought, growth resistance decreased in several conifer species. Resistance was strongly and positively related to winter rainfall, winter hydric index, and spring rainfall. Peñuelas, the site with most extreme environmental conditions and highest plantation density, showed the lowest resistance and highest recovery, but did not show the lowest resilience. In a meta-analysis including 166 cases assessing the influence of competition level on growth response to drought, Castagneri et al. [43] found that higher competition improved recovery. In Spain, Camarero et al. [44] found that stone pine plantations recovered faster than natural stands. We found that warmer winter and spring reduced stone pine resistance, confirming the species’ sensitivity to climatic conditions during the early growing season. Interestingly, Piraino [24] reported the same effect under warm spring and summer conditions in stone pine natural stands.
All the study plantations had a high recovery, which can be considered a characteristic of drought resilient species [42]. Growth was negatively related to tree recovery and positively to tree resistance, but it was not related to the resilience index. Tree resistance to drought was positively related to diameter growth and, consequently, it is expected to be correlated with DBH. It also depended on winter and spring rainfall, which improves growth and, therefore, boosts tree resistance. The results also showed that trees with higher growth rates were less resilient.
We found that the impact of drought on growth is also affected by tree characteristics. In fact, at the tree level, trees with larger DBH were more resistant but showed less capacity to recover after a drought event. Resilience was lowest in Cahuil, a site with recurrent droughts, high inter-annual growth variability and high recovery, as previously reported [44]. Cahuil is also the plantation showing the lowest tree height, which could be explained by the low density and by the low site quality [45].
The PLS analysis showed that growth depended on rainfall, hydric index and thermal oscillation, being enhanced by high spring and winter precipitation and hydric index (lower water deficit), and by high spring thermal oscillation. During the beginning of the growing period, there is a rapid increase in maximum temperature (superior thermal oscillation) accompanied by greater water availability as a result of winter and spring precipitation, boosting growth. In fact, it has been reported that temperature fluctuations may only have detrimental effects on plant traits including growth beyond a certain threshold [46]. Sites with high precipitation were characterized by a high growth rate, along with a high resistance and low recovery. Pastene had a resilience >1, indicating that drought did not have a strong legacy effect on diameter growth in that plantation.
Tree growth recovery and resilience were reduced with increasing site aridity, as reported by Sohn et al. [47]. In sites with low precipitation, trees exhibited low resistance and high recovery, as reported for fast-growing species [42] such as stone pine [48]. During dry periods, fast-growing species would offset the growth reduction caused by drought by an increased carbon assimilation rates [49] and by an extended multi-layered root system [26].
The adaptation of forest management should be a dynamic process that takes into account the local interactions between climate and the species through a systematic learning process [15]. Adaptive stone pine management tools, including the establishment of mixed stands that enhance the species’ resistance to extreme droughts [15], thinning [50], and genetic selection of drought-resistant trees [19,40], could help in transitioning towards efficient climate adaptation strategies.
In general, our results showed that resilience was not correlated with recovery or resistance in the study area, probably due to the big difference in the frequency of drought years across sites. We suggest performing further studies on plantations subjected to recurrent droughts, which have cumulative stress effects [50]; those studies can provide information about tree resistance and recovery, but not about tree resilience due to the high growth variability.
Stone pine is a species extremely vulnerable to climate change [13]. The increasing aridity and the compounding effects of multiple hot droughts could affect the species’ productivity and increase mortality; these topics should be addressed in future studies. However, due to the way the resilience indices are calculated, differences in growth resilience response to long-term mega droughts are not adequate to assess the impact of droughts on resilience components.
It was found that resilience in non-native habitats offers new insights for understanding the stone pine growth resilience to drought. Beyond the species native habitat, stone pine drought resilience is also present in non-native Mediterranean habitats characterized by longer dry periods than in the species natural distribution. Analyzing growth resilience components at the tree and stand levels allows us to understand stone pine behavior in plantations established in different environments, subjected to increasing drought events that can limit their productive potential and reduce the adaptive capacity of the species, especially in non-native environments. Those analyses can provide valuable information to implement adaptive management techniques to cope with environmental challenging conditions.
5. Conclusions
Drought reduced stone pine stem growth in plantations established in central–southern Chile, affecting tree resistance, recovery and resilience to drought. The impact of drought on these stone pine plantations was related to both stand and tree characteristics. At the stand level, growth rates and resistance were related to winter and spring climatic conditions.
Annual growth showed a latitudinal climatic pattern related to rainfall and water deficit. At the tree level, trees with higher growth rate were more resistant but showed less capacity to recover after a drought year than trees with a lower growth rate. On the other hand, resilience was lower under high inter-annual growth variability. The high recovery of stone pine after drought years revealed a relatively high resilience.
Stone pine showed drought resilience along a non-native latitudinal gradient, being a characteristic inherent of the species that transcends its location. These findings are relevant to the management of the species, particularly with the aim of facing climate change impacts that are expected to include an increased frequency and severity of drought episodes.
This study of growth responses to climate variability elucidated vulnerabilities and potential adaptive strategies of stone pine plantations in response to drought, providing insights into management strategies for the species. The findings of this research may contribute to the discussion about the resilience of stone pine, an emergent crop in central Chile, in the face of escalating climatic uncertainties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15101775/s1, Figure S1. Location of stone pine plantations studied in central Chile; Figure S2. Climodiagram of the four study sites in central Chile. Rainfall, blue line; mean temperature, red line; Figure S3. Heatmap of Pearson’s correlation coefficients between series of ring width indices (RWI) and monthly climatic variables. Significant correlations (p < 0.05) are shown with an asterisk.
Author Contributions
V.L.-M. and M.B. designed this study. C.D. and R.D.R. prepared the material and collected the data. M.B. led the statistical analysis. R.N.-C. and J.J.C. contributed to the result analyses and discussion. A.M.C.-V. performed the dendrochronological measurements and validation. V.L.-M. wrote the first draft of this manuscript and all authors commented on successive versions. All authors read, edited, and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Project ANID BASAL FB210015 (CENAMAD) and program “Development and contributions for the use of high value forest and fruit-forest species for Chile”, Ministry of Agriculture.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to institutional guidelines, but are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank Aldo Salinas and Luis Barrales for their assistance in core collection.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Cortés-Sánchez, M.; Morales-Muñiz, A.; Simón-Vallejo, M.D.; Lozano-Francisco, M.C.; Vera-Peláez, J.L.; Finlayson, C.; Rodríguez-Vidal, J.; Delgado-Huertas, A.; Jiménez-Espejo, F.J.; Martínez-Ruiz, F.; et al. Earliest Known Use of Marine Resources by Neanderthals. PLoS ONE 2011, 6, e24026. [Google Scholar] [CrossRef] [PubMed]
- Sülüsoglu, M. The Management of Villagers Owned Stone Pine (Pinus pinea L.) Plantations in Kozak Region, Turkey, a Case Study; FAO Working Paper; FAO: Rome, Italy, 2004. [Google Scholar]
- Awan, H.; Pettenella, D. Pine Nuts: A review of recent sanitary conditions and market development. Forests 2017, 8, 367. [Google Scholar] [CrossRef]
- Pinno, B.D.; Hossain, K.L.; Gooding, T.; Lieffers, V.J. Opportunities and Challenges for Intensive Silviculture in Alberta, Canada. Forests 2021, 12, 791. [Google Scholar] [CrossRef]
- Loewe, V.; Delard, C.; Balzarini, M.; Álvarez, A.; Navarro, R. Impact of climate and management variables on stone pine (Pinus pinea L.) growing in Chile. Agric. For. Meteorol. 2015, 214–215, 106–116. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2022—Impacts, Adaptation and Vulnerability; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; pp. 3–34. [Google Scholar]
- Truettner, C.; Anderegg, W.R.L.; Biondi, F.; Koch, G.W.; Ogle, K.; Schwalm, C.; Litvak, M.E.; Shaw, J.D.; Ziaco, E. Conifer radial growth response to recent seasonal warming and drought from the southwestern USA. For. Ecol. Manag. 2018, 418, 55–62. [Google Scholar] [CrossRef]
- Trowbridge, A.M.; Adams, H.D.; Collins, A.; Dickman, L.T.; Grossiord, C.; Hofland, M.; Malone, S.; Weaver, D.K.; Sevanto, S.; Stoy, P.C.; et al. Hotter droughts alter resource allocation to chemical defenses in piñon pine. Oecologia 2021, 197, 921–938. [Google Scholar] [CrossRef]
- Garreaud, R.D.; Boisier, J.P.; Rondanelli, R.; Montecinos, A.; Sepúlveda, H.H.; Veloso-Aguila, D. The Central Chile Mega Drought (2010–2018): A climate dynamics perspective. Int. J. Climatol. 2020, 40, 421–439. [Google Scholar] [CrossRef]
- Santelices-Moya, R.; Gibson-Carpintero, S.; Cabrera-Ariza, A.; Santini-Junior, L.; Venegas-González, A. Reduced Rainfall Variability Reduces Growth of Nothofagus alessandrii Espinosa (Nothofagaceae) in the Maule Region, Chile. Forests 2022, 13, 1184. [Google Scholar] [CrossRef]
- Loewe-Muñoz, V.; del Río, R.; Delard, C.; Balzarini, M. Short-term stem diameter variations in irrigated and non-irrigated stone pine (Pinus pinea L.) trees in a xeric non-native environment. Ann. For. Sci. 2021, 78, 99. [Google Scholar] [CrossRef]
- Natalini, F.; Alejano, R.; Pardos, M.; Calama, R.; Vázquez-Piqué, J. Declining Trends in Long-Term Pinus pinea L. Growth Forecasts in Southwestern Spain. Dendrochronologia 2024, 88, 126252. [Google Scholar] [CrossRef]
- Mechergui, K.; Saleh Altamimi, A.; Jaouadi, W.; Naghmouchi, S. Climate change impacts on spatial distribution, tree-ring growth, and water use of stone pine (Pinus pinea L.) forests in the Mediterranean region and silvicultural practices to limit those impacts. iForest—Biogeosciences For. 2021, 14, 104–112. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Vergarechea, M.; Calama, R.; Pretzsch, H.; Alday, J.G.; del Río, M. Short- and long-term growth response to climate in mixed and monospecific forests of Pinus pinea and Pinus pinaster. Eur. J. For. Res. 2021, 140, 387–402. [Google Scholar] [CrossRef]
- Cutini, A. Pinus pinea L. In Pines of Silvicultural Importance; CABI, Ed.; CABI Publishing: New York, NY, USA, 2002; pp. 329–343. ISBN 0 85199 539 X. [Google Scholar]
- Perdiguero, P.; Soto, Á.; Collada, C. Comparative analysis of Pinus pinea and Pinus pinaster dehydrins under drought stress. Tree Genet. Genomes 2015, 11, 70. [Google Scholar] [CrossRef]
- Valeriano, C.; Gutiérrez, E.; Colangelo, M.; Gazol, A.; Sánchez-Salguero, R.; Tumajer, J.; Shishov, V.; Bonet, J.A.; Martínez de Aragón, J.; Ibáñez, R.; et al. Seasonal precipitation and continentality drive bimodal growth in Mediterranean forests. Dendrochronologia 2023, 78, 126057. [Google Scholar] [CrossRef]
- Balekoglu, S.; Caliskan, S.; Dirik, H.; Rosner, S. Response to drought stress differs among Pinus pinea provenances. For. Ecol. Manag. 2023, 531, e120779. [Google Scholar] [CrossRef]
- Graham, S.I.; Rokem, A.; Hille Ris Lambers, J. forestexplorR: An R package for the exploration and analysis of stem-mapped forest stand data. Ecography 2022, 2022, e06223. [Google Scholar] [CrossRef]
- Barbato, M. Construction of New Site Index Curves for the Spatial Tree Growth Model PineaFits: A Holistic Study Approach with Stem Analysis. Master’s Thesis, University of Naples, Naples, Italy, 2015; p. 61. [Google Scholar]
- Divya, K.; Kaur, S. A Study on Tree Rings: Dendrochronology using Image Processing. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1022, 012115. [Google Scholar] [CrossRef]
- Newton, P.F. Wood quality attribute models and their utility when integrated into density management decision-support systems for boreal conifers. For. Ecol. Manag. 2019, 438, 267–284. [Google Scholar] [CrossRef]
- Piraino, S. Assessing Pinus pinea L. resilience to three consecutive droughts in central-western Italian Peninsula. iForest—Biogeosci. For. 2020, 13, 246–250. [Google Scholar] [CrossRef]
- Albrich, K.; Rammer, W.; Turner, M.G.; Ratajczak, Z.; Braziunas, K.H.; Hansen, W.D.; Seidl, R. Simulating forest resilience: A review. Glob. Ecol. Biogeogr. 2020, 29, 2082–2096. [Google Scholar] [CrossRef]
- Mazza, G.; Cutini, A.; Manetti, M.C. Site-specific growth responses to climate drivers of Pinus pinea L. tree rings in Italian coastal stands. Ann. For. Sci. 2014, 71, 927–936. [Google Scholar] [CrossRef]
- Smiley, T. An Introduction to Tree-Ring Dating; University of Arizona Press: Tucson, AR, USA, 1968. [Google Scholar]
- Larsson, L.; Larsson, P. CDendro and CooRecorder; Cybis Elektronik and Data AB: Saltsjöbaden, Sweden, 2018. [Google Scholar]
- Holmes, R. Computer assisted quality control in tree ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Schulman, E. Dendroclimatic Changes in Semiarid America; University of Arizona Press: Tucson, AR, USA, 1956. [Google Scholar]
- Fritts, H. Tree Rings and Climate; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Muñoz-Sabater, J.; Dutra, E.; Agustí-Panareda, A.; Albergel, C.; Arduini, G.; Balsamo, G.; Boussetta, S.; Choulga, M.; Harrigan, S.; Hersbach, H.; et al. ERA5-Land: A State-of-the-Art Global Reanalysis Dataset for Land Applications. Earth Syst. Sci. Data 2021, 13, 4349–4383. [Google Scholar] [CrossRef]
- Ashaolu, E.D.; Iroye, K.A. Rainfall and Potential Evapotranspiration Patterns and Their Effects on Climatic Water Balance in the Western Lithoral Hydrological Zone of Nigeria. Ruhuna J. Sci. 2018, 9, 92. [Google Scholar] [CrossRef]
- Di Rienzo, J.; Casanoves, F.; Balzarini, M.; Gonzalez, L.; Tablada, M.; Robledo, C. InfoStat Version 2024. 2024. Available online: http://www.infostat.com.ar (accessed on 14 February 2024).
- Pardos, M.; Calama, R.; Maroschek, M.; Rammer, W.; Lexer, M.J. A model-based analysis of climate change vulnerability of Pinus pinea stands under multiobjective management in the Northern Plateau of Spain. Ann. For. Sci. 2015, 72, 1009–1021. [Google Scholar] [CrossRef]
- Calama, R.; Conde, M.; De-Dios-García, J.; Madrigal, G.; Vázquez-Piqué, J.; Gordo, F.J.; Pardos, M. Linking Climate, Annual Growth and Competition in a Mediterranean Forest: Pinus pinea in the Spanish Northern Plateau. Agric. For. Meteorol. 2019, 264, 309–321. [Google Scholar] [CrossRef]
- Sohn, J.A.; Hartig, F.; Kohler, M.; Huss, J.; Bauhus, J. Heavy and frequent thinning promotes drought adaptation in Pinus sylvestris forests. Ecol. Appl. 2016, 26, 2190–2205. [Google Scholar] [CrossRef]
- Yu Karpukhin, M.; Yussef, A.M. Electing drought-resistant Pinus pinea L. (stone pine) using dendroclimatology. IOP Conf. Ser. Earth Environ. Sci. 2021, 699, 012051. [Google Scholar] [CrossRef]
- Lebourgeois, F.; Mérian, P.; Courdier, F.; Ladier, J.; Dreyfus, P. Instability of climate signal in tree-ring width in Mediterranean mountains: A multi-species analysis. Trees 2012, 26, 715–729. [Google Scholar] [CrossRef]
- Song, Y.; Sterck, F.; Sass-Klaassen, U.; Li, C.; Poorter, L. Growth resilience of conifer species decreases with early, long-lasting and intense droughts but cannot be explained by hydraulic traits. J. Ecol. 2022, 110, 2088–2104. [Google Scholar] [CrossRef]
- Castagneri, D.; Vacchiano, G.; Hacket-Pain, A.; DeRose, R.J.; Klein, T.; Bottero, A. Meta-analysis Reveals Different Competition Effects on Tree Growth Resistance and Resilience to Drought. Ecosystems 2022, 25, 30–43. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Linares, J.C.; Fajardo, A.; Colangelo, M.; Valeriano, C.; Sánchez-Salguero, R.; Sangüesa-Barreda, G.; Granda, E.; Gimeno, T.E. Differences in temperature sensitivity and drought recovery between natural stands and plantations of conifers are species-specific. Sci. Total Environ. 2021, 796, 148930. [Google Scholar] [CrossRef]
- Aguirre, A.; Moreno-Fernández, D.; Alberdi, I.; Hernández, L.; Adame, P.; Cañellas, I.; Montes, F. Mapping forest site quality at national level. For. Ecol. Manage. 2022, 508, 120043. [Google Scholar] [CrossRef]
- Aslam, M.A.; Ahmed, M.; Hassan, F.U.; Afzal, O.; Mehmood, M.Z.; Qadir, G.; Asif, M.; Komal, S. Impact of Temperature Fluctuations on Plant Morphological and Physiological Traits. In Building Climate Resilience in Agriculture; Jatoi, W.N., Mubeen, M., Ahmad, A., Cheema, M.A., Lin, Z., Hash-mi, M.Z., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Sohn, J.A.; Saha, S.; Bauhus, J. Potential of forest thinning to mitigate drought stress: A meta-analysis. For. Ecol. Manag. 2016, 380, 261–273. [Google Scholar] [CrossRef]
- Montero, G. El Pino Piñonero (Pinus pinea L.) en Andalucía: Ecología, Distribución y Selvicultura; Consejería de Medio Ambiente; Junta de Andalucía: Sevilla, Spain, 2004; ISBN 84-95785-94-3. [Google Scholar]
- Fu, Z.; Ciais, P.; Bastos, A.; Stoy, P.C.; Yang, H.; Green, J.K.; Wang, B.; Yu, K.; Huang, Y.; Knohl, A.; et al. Sensitivity of gross primary productivity to climatic drivers during the summer drought of 2018 in Europe. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190747. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Cachinero-Vivar, A.M.; Pérez-Priego, Ó.; Aspizua Cantón, R.; Begueria, S.; Julio Camarero, J. Developing alternatives to adaptive silviculture: Thinning and tree growth resistance to drought in a Pinus species on an elevated gradient in Southern Spain. For. Ecol. Manag. 2023, 537, 120936. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).