Variation and Driving Mechanisms of Bark Thickness in Larix gmelinii under Surface Fire Regimes
Abstract
1. Introduction
2. Materials and Methods
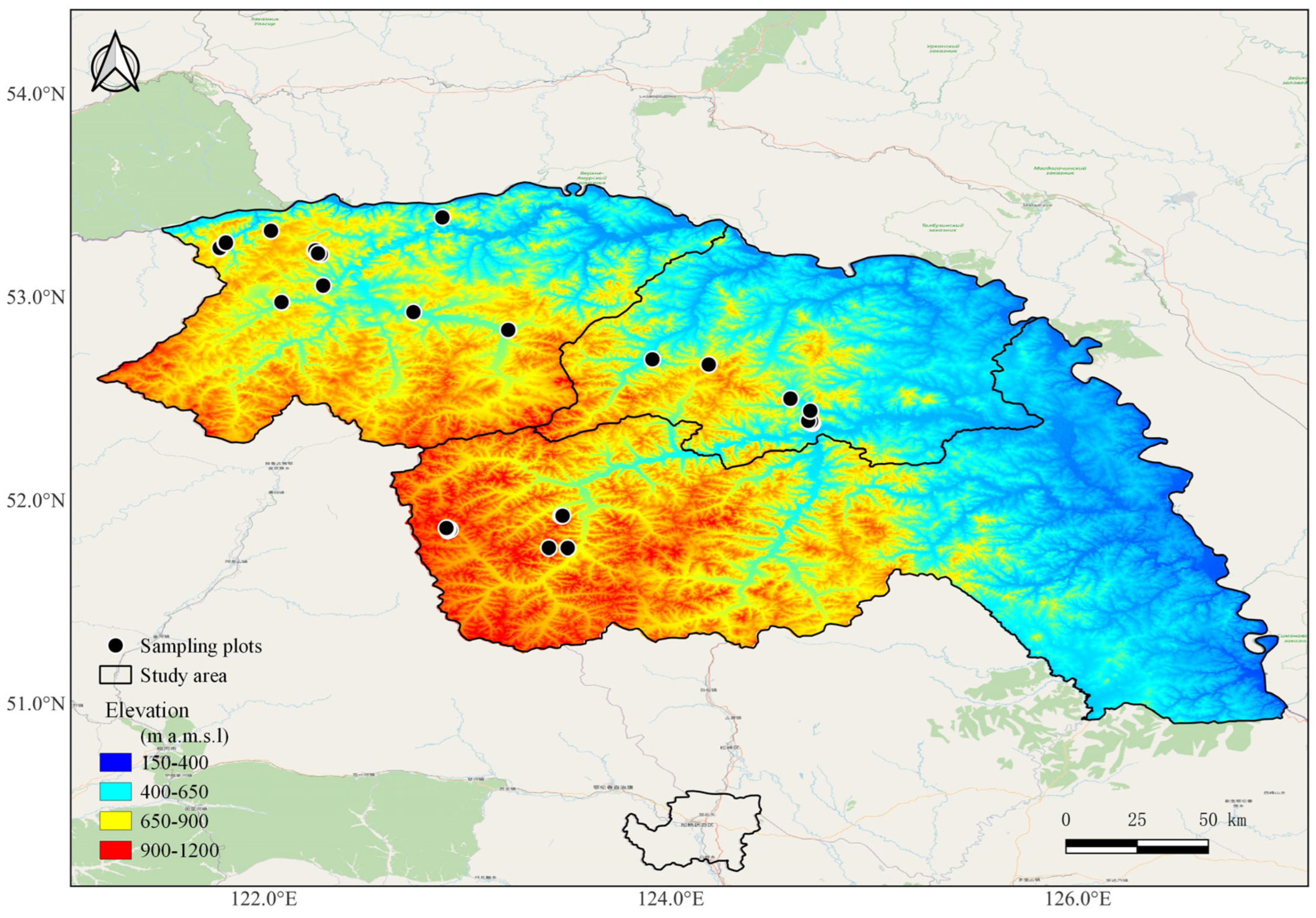
2.1. Study Area and Sites Selection
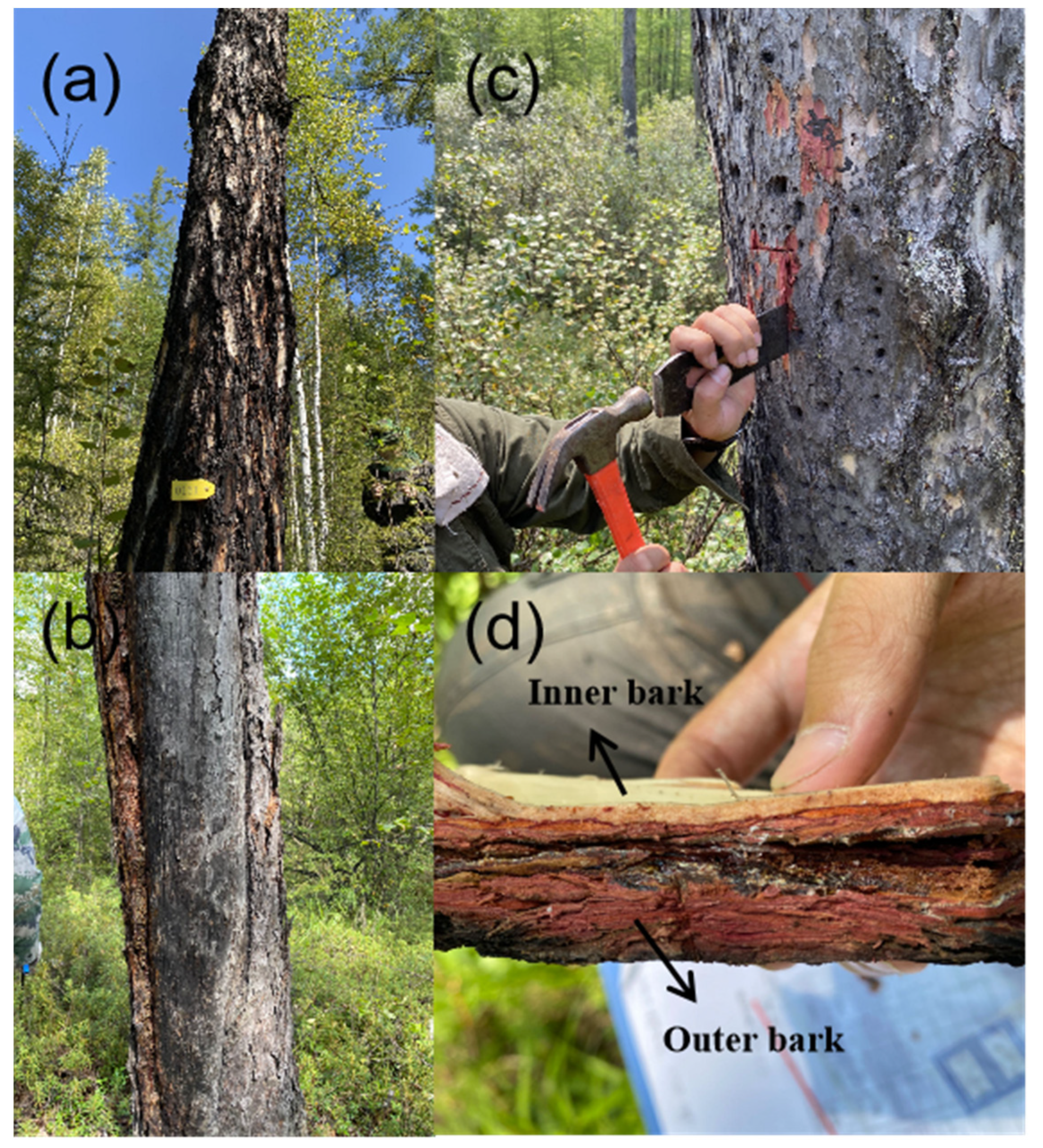
2.2. Field Methods
2.3. Environmental Factor Surveys
2.4. Data Analyses
3. Results
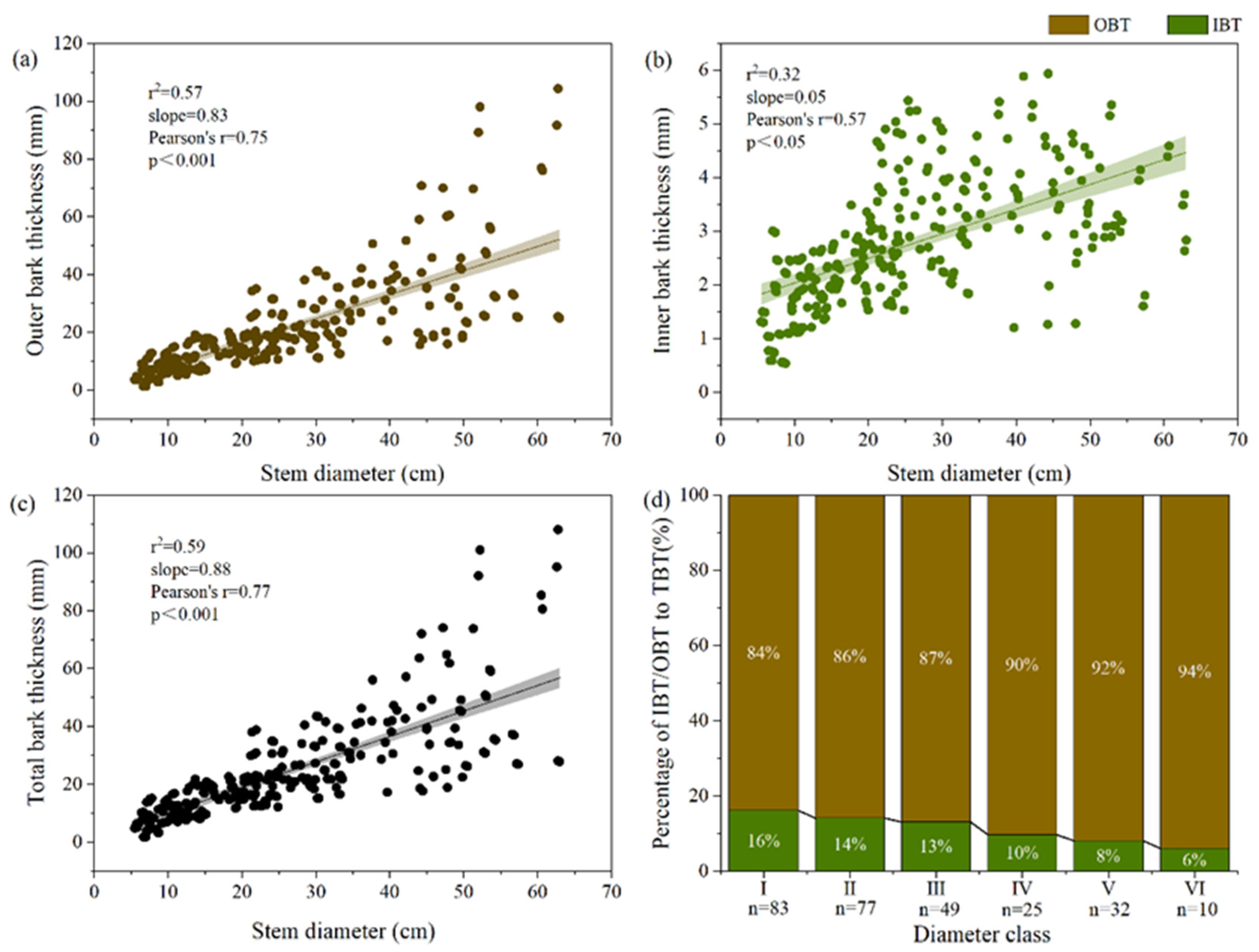
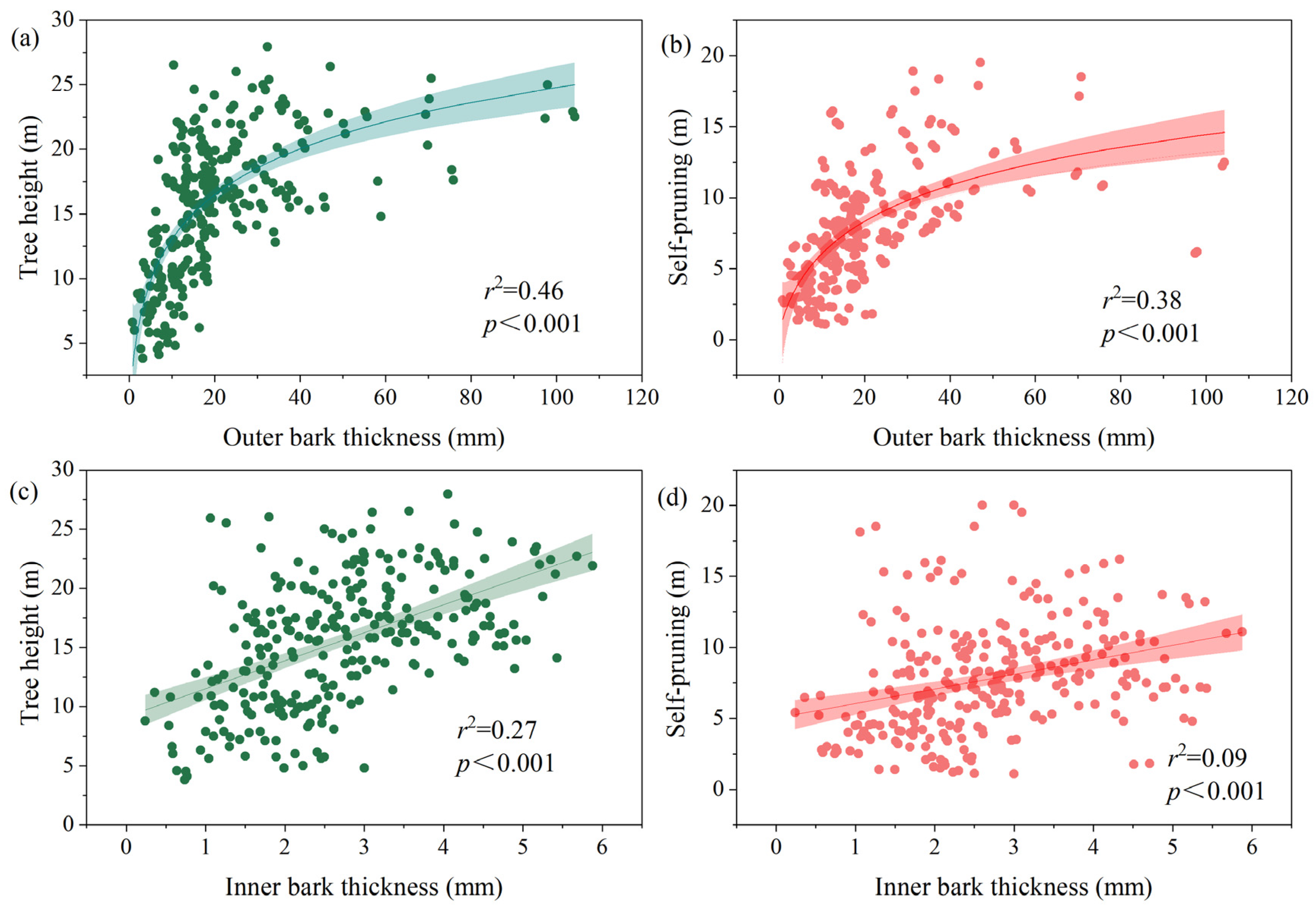
3.1. Associations between Bark Thickness Traits and Plant Size
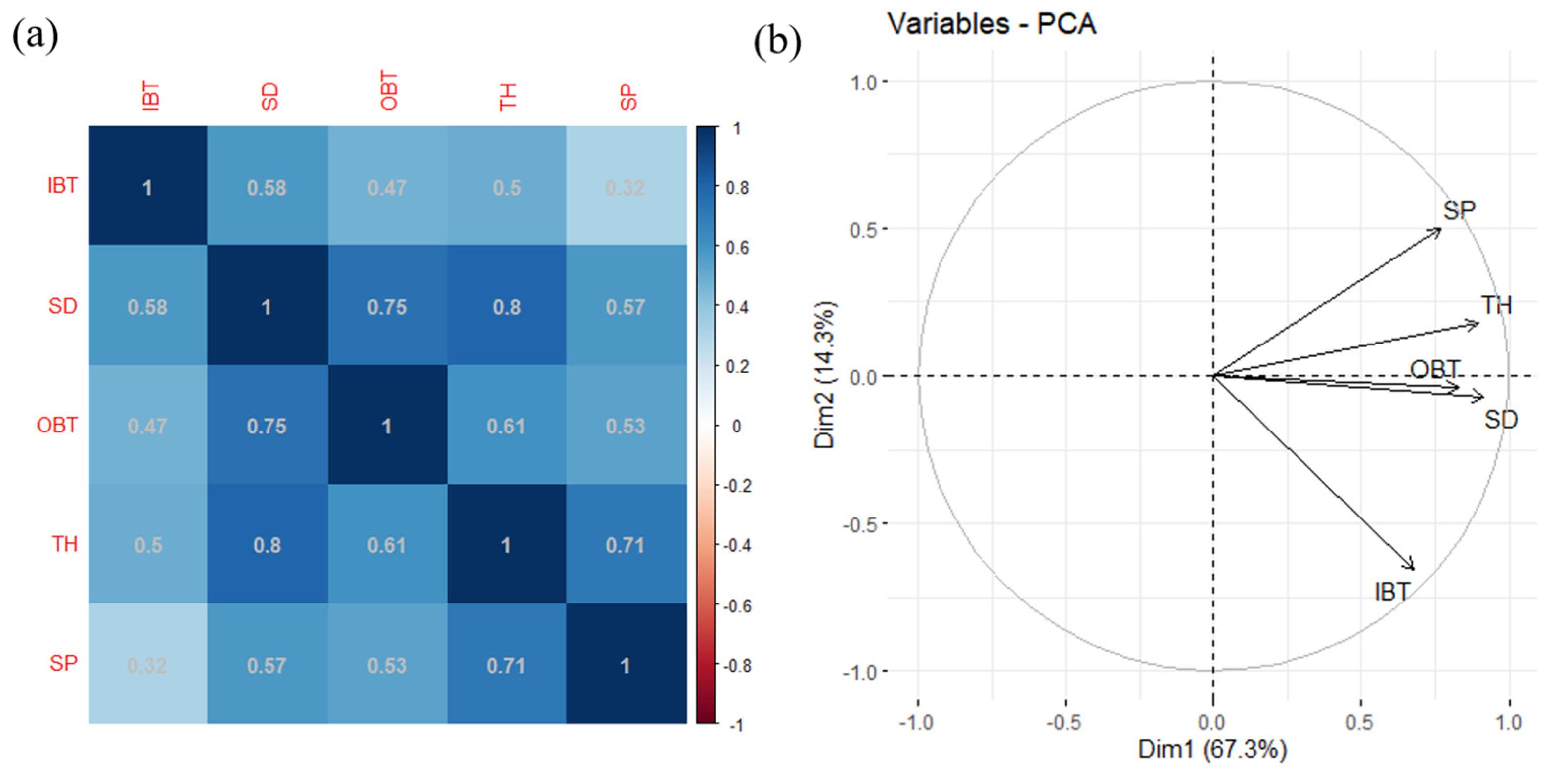
3.2. Covariance of Bark Thickness with Other Fire Resistance Traits
3.3. Roles of Environmental Factors in Explaining Bark Thickness Traits
4. Discussion
4.1. Larix Gmelinii Bark Thickness Was Mainly Driven by Plant Size
4.2. Bark, Tree Height, and Self-Pruning Constitute Defenses against Surface Fire Traits Syndromes
4.3. Environmental Factors Affecting Bark Thickness
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosell, J.A. Bark thickness across the angiosperms: More than just fire. New Phytol. 2016, 211, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Pfanz, H.; Aschan, G. The existence of bark and stem photosynthesis in woody plants and its significance for the overall carbon gain. An eco-physiological and ecological approach. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2001; pp. 477–510. [Google Scholar]
- Ferrenberg, S.; Mitton, J.B. Smooth bark surfaces can defend trees against insect attack: Resurrecting a ‘slippery’ hypothesis. Funct. Ecol. 2014, 28, 837–845. [Google Scholar] [CrossRef]
- Romero, C. Bark: Structure and functional ecology. Adv. Econ. Bot. 2014, 17, 5–25. [Google Scholar]
- Pausas, J.G. Bark thickness and fire regime. Funct. Ecol. 2015, 29, 315–327. [Google Scholar] [CrossRef]
- Morris, J.L.; Cottrell, S.; Fettig, C.J.; Hansen, W.D.; Sherriff, R.L.; Carter, V.A.; Clear, J.L.; Clement, J.; DeRose, R.J.; Hicke, J.A.; et al. Managing bark beetle impacts on ecosystems and society: Priority questions to motivate future research. J. Appl. Ecol. 2017, 54, 750–760. [Google Scholar] [CrossRef]
- Lawes, M.J.; Midgley, J.J.; Clarke, P.J. Costs and benefits of relative bark thickness in relation to fire damage: A savanna/forest contrast. J. Ecol. 2013, 101, 517–524. [Google Scholar] [CrossRef]
- Akashi, N.; Nakashizuka, T. Effects of bark-stripping by Sika deer (Cervus nippon) on population dynamics of a mixed forest in Japan. For. Ecol. Manag. 1999, 113, 75–82. [Google Scholar] [CrossRef]
- Baltzer, J.L.; Day, N.J.; Walker, X.J.; Greene, D.; Mack, M.C.; Alexander, H.D.; Arseneault, D.; Barnes, J.; Bergeron, Y.; Boucher, Y.; et al. Increasing fire and the decline of fire adapted black spruce in the boreal forest. Proc. Natl. Acad. Sci. USA 2021, 118, e2024872118. [Google Scholar] [CrossRef]
- Davis, K.T.; Dobrowski, S.Z.; Higuera, P.E.; Holden, Z.A.; Veblen, T.T.; Rother, M.T.; Parks, S.A.; Sala, A.; Maneta, M.P. Wildfires and climate change push low-elevation forests across a critical climate threshold for tree regeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 6193–6198. [Google Scholar] [CrossRef]
- Morelli, T.L.; Barrows, C.W.; Ramirez, A.R.; Cartwright, J.M.; Ackerly, D.D.; Eaves, T.D.; Ebersole, J.L.; Krawchuk, M.A.; Letcher, B.H.; Mahalovich, M.F.; et al. Climate-change refugia: Biodiversity in the slow lane. Front. Ecol. Environ. 2020, 18, 228–234. [Google Scholar] [CrossRef]
- Hérault, B.; Piponiot, C. Key drivers of ecosystem recovery after disturbance in a neotropical forest. For. Ecosyst. 2018, 5, 2. [Google Scholar] [CrossRef]
- Randerson, J.T.; Liu, H.; Flanner, M.G.; Chambers, S.D.; Jin, Y.; Hess, P.G.; Pfister, G.; Mack, M.; Treseder, K.; Welp, L.J.; et al. The impact of boreal forest fire on climate warming. Science 2006, 314, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Bernier, P.; Kuuluvainen, T.; Shvidenko, A.Z.; Schepaschenko, D.G. Boreal forest health and global change. Science 2015, 349, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Loboda, T.V. Surface forcing of non-stand-replacing fires in Siberian larch forests. Environ. Res. Lett. 2018, 13, 045008. [Google Scholar] [CrossRef]
- Keeley, J.E.; Zedler, P.H. Characterization and global distribution of vernal pools. In Ecology, Conservation, and Management of Vernal Pool Ecosystems, Proceedings from 1996 Conference; California Native Plant Society: Sacramento, CA, USA, 1998; p. 14. [Google Scholar]
- Keeley, J.E.; Bond, W.J.; Bradstock, R.A.; Pausas, J.G.; Rundel, P.W. Fire in Mediterranean Ecosystems: Ecology, Evolution and Management; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Jackson, J.F.; Adams, D.C.; Jackson, U.B. Allometry of constitutive defense: A model and a comparative test with tree bark and fire regime. Am. Nat. 1999, 153, 614–632. [Google Scholar] [CrossRef] [PubMed]
- Bond-Lamberty, B.; Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Berrill, J.-P.; O’Hara, K.L.; Kichas, N.E. Bark Thickness in Coast Redwood (Sequoia sempervirens (D.Don) Endl.) Varies According to Tree- and Crown Size, Stand Structure, Latitude and Genotype. Forests 2020, 11, 637. [Google Scholar] [CrossRef]
- Nie, W.; Liu, Y.; Tan, C.; Wang, Y.; Liu, J.; Zhao, X.; Jiang, Z.; Jia, Z. Characteristics and factors driving the variations in bark thickness of major woody plants in China. Ecol. Indic. 2022, 144, 109447. [Google Scholar] [CrossRef]
- Hoffmann, W.A.; Orthen, B.; Do Nascimento, P.K.V. Comparative fire ecology of tropical savanna and forest trees. Funct. Ecol. 2003, 17, 720–726. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Ackerly, D.D.; Baum, D.; Bazzaz, F.A. Phylogenetic overdispersion in Floridian oak communities. Am. Nat. 2004, 163, 823–843. [Google Scholar] [CrossRef]
- Richardson, S.J.; Laughlin, D.C.; Lawes, M.J.; Holdaway, R.J.; Wilmshurst, J.M.; Wright, M.; Curran, T.J.; Bellingham, P.J.; McGlone, M.S. Functional and environmental determinants of bark thickness in fire-free temperate rain forest communities. Am. J. Bot. 2015, 102, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Stängle, S.M.; Sauter, U.H.; Dormann, C.F. Comparison of models for estimating bark thickness of Picea abies in southwest Germany: The role of tree, stand, and environmental factors. Ann. For. Sci. 2017, 74, 16. [Google Scholar] [CrossRef]
- Su, Z.; Hu, H.; Tigabu, M.; Wang, G.; Zeng, A.; Guo, F. Geographically weighted negative Binomial regression model predicts wildfire occurrence in the Great Xing’an Mountains better than negative Binomial Model. Forests 2019, 10, 377. [Google Scholar] [CrossRef]
- Harris, I.; Osborn, T.J.; Jones, P.; Lister, D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data 2020, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Z.; Ma, D.; Zhang, M.; Fu, L. Diversity and Distribution Characteristics of Soil Microbes across Forest–Peatland Ecotones in the Permafrost Regions. Int. J. Environ. Res. Public Health 2022, 19, 14782. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Liu, Y.; Zhang, Y.; Qin, Q.; Bai, Y.; Sun, X. Population dynamics and individual growth dynamics of Larix gmelinii under non-stand replacing fire. For. Ecol. Manag. 2023, 538, 120951. [Google Scholar] [CrossRef]
- Mengku, Z.; Lichun, J.J. Prediction of bark thickness for Larix gmelinii based on machine learning. J. Beijing For. Univ. 2022, 44, 54–62. [Google Scholar]
- Walker, X.J.; Baltzer, J.L.; Cumming, S.G.; Day, N.J.; Johnstone, J.F.; Rogers, B.M.; Solvik, K.; Turetsky, M.R.; Mack, M.C. Soil organic layer combustion in boreal black spruce and jack pine stands of the Northwest Territories, Canada. Int. J. Wildland Fire 2018, 27, 125–134. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Z.; Lv, S.; Huang, H.; Hu, J. Detection of soil total nitrogen, phosphorus and potassium content based on the spectral information of citrus canopy. Am. J. Biochem. Biotechnol. 2020, 16, 177–183. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Williams, V.L.; Witkowski, E.T.F.; Balkwill, K. Relationship between bark thickness and diameter at breast height for six tree species used medicinally in South Africa. S. Afr. J. Bot. 2007, 73, 449–465. [Google Scholar] [CrossRef][Green Version]
- Paine, C.E.T.; Stahl, C.; Courtois, E.A.; Patiño, S.; Sarmiento, C.; Baraloto, C. Functional explanations for variation in bark thickness in tropical rain forest trees. Funct. Ecol. 2010, 24, 1202–1210. [Google Scholar] [CrossRef]
- Schafer, J.L.; Breslow, B.P.; Hohmann, M.G.; Hoffmann, W.A. Relative bark thickness is correlated with tree species distributions along a fire frequency gradient. Fire Ecol. 2015, 11, 74–87. [Google Scholar] [CrossRef]
- Kain, G.; Güttler, V.; Barbu, M.-C.; Petutschnigg, A.; Richter, K.; Tondi, G. Density related properties of bark insulation boards bonded with tannin hexamine resin. Eur. J. Wood Wood Prod. 2014, 72, 417–424. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Amarasekare, P.; Araújo, M.S.; Bürger, R.; Levine, J.M.; Novak, M.; Rudolf, V.H.; Schreiber, S.J.; Urban, M.C.; Vasseur, D.A. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 2011, 26, 183–192. [Google Scholar] [CrossRef]
- Seifert, T.; Meincken, M.; Odhiambo, B.O. The effect of surface fire on tree ring growth of Pinus radiata trees. Ann. For. Sci. 2017, 74, 34. [Google Scholar] [CrossRef]
- Ibanez, T.; Curt, T.; Hely, C. Low tolerance of N ew C aledonian secondary forest species to savanna fires. J. Veg. Sci. 2013, 24, 177–188. [Google Scholar] [CrossRef]
- Schulte, L.; Li, C.; Lisovski, S.; Herzschuh, U. Forest-permafrost feedbacks and glacial refugia help explain the unequal distribution of larch across continents. J. Biogeogr. 2022, 49, 1825–1838. [Google Scholar] [CrossRef]
- Lara, A.R.; Fraver, S.; Aravena, J.C.; Wolodarsky-Franke, A. Fire and the dynamics of Fitzroya cupressoides (alerce) forests of Chile’s Cordillera Pelada. Ecoscience 1999, 6, 100–109. [Google Scholar] [CrossRef]
- Stephenson, N.L. Reference conditions for giant sequoia forest restoration: Structure, process, and precision. Ecol. Appl. 1999, 9, 1253–1265. [Google Scholar] [CrossRef]
- Beaty, R.M.; Taylor, A.H. Fire disturbance and forest structure in old-growth mixed conifer forests in the northern Sierra Nevada, California. J. Veg. Sci. 2007, 18, 879–890. [Google Scholar]
- Keeley, J.E. Ecology and evolution of pine life histories. Ann. For. Sci. 2012, 69, 445–453. [Google Scholar] [CrossRef]
- Rosas, T.; Mencuccini, M.; Batlles, C.; Regalado, Í.; Saura-Mas, S.; Sterck, F.; Martínez-Vilalta, J.J.F.E. Are leaf, stem and hydraulic traits good predictors of individual tree growth? Funct. Ecol. 2021, 35, 2435–2447. [Google Scholar] [CrossRef]
- Alavi, S.J.; Ahmadi, K.; Dormann, C.F.; Serra-Diaz, J.M.; Nouri, Z.J.B. Assessing the dominant height of oriental beech (Fagus orientalis L.) in relation to edaphic and physiographic variables in the Hyrcanian Forests of Iran. BASE 2020, 24, 262–273. [Google Scholar] [CrossRef]
- Figueroa, J.; Armesto, J.J. Community-wide germination strategies in a temperate rainforest of Southern Chile: Ecological and evolutionary correlates. Aust. J. Bot. 2001, 49, 411–425. [Google Scholar] [CrossRef]
- Rosbakh, S.; Römermann, C.; Poschlod, P. Specific leaf area correlates with temperature: New evidence of trait variation at the population, species and community levels. Alp. Bot. 2015, 125, 79–86. [Google Scholar] [CrossRef]
- Martin, A.R.; Rapidel, B.; Roupsard, O.; Van den Meersche, K.; de Melo Virginio Filho, E.; Barrios, M.; Isaac, M.E. Intraspecific trait variation across multiple scales: The leaf economics spectrum in coffee. Funct. Ecol. 2017, 31, 604–612. [Google Scholar] [CrossRef]
| Random Effects: OBT | Random Effects: IBT | ||||
|---|---|---|---|---|---|
| Random Effects | Variance | Standard Deviation | Random Effects | Variance | Standard Deviation |
| SD.CLASS (Intercept) | 254.4 | 15.95 | SD.CLASS (Intercept) | 0.6002 | 0.7747 |
| Residual | 135.3 | 11.63 | Residual | 0.8192 | 0.9051 |
| Relative contribution of the random effect: 65.3% | Relative contribution of the random effect: 42.8% | ||||
| SITE (Intercept) | 45.1 | 6.716 | SITE (Intercept) | 0.08332 | 0.2887 |
| Residual | 241.8 | 15.549 | Residual | 1.36059 | 1.1664 |
| Relative contribution of the random effect: 15.7% | Relative contribution of the random effect: 5.5% | ||||
| Random Effects: OBT | Random Effects: IBT | ||||||
|---|---|---|---|---|---|---|---|
| Predictors | Estimates | CI | p | Predictors | Estimates | CI | p |
| (Intercept) | 83.51 | 44.8–122.21 | <0.001 | (Intercept) | 3.01 | 0.14–5.88 | 0.04 |
| slope | −0.38 | −0.67–0.08 | 0.734 | slope | 0.01 | −0.02–0.03 | 0.52 |
| Residual thickness | −0.39 | −0.84–0.07 | 0.413 | Residual thickness | 0.03 | −0.01–0.07 | 0.098 |
| Mean temperature | 0.27 | 0.14–0.4 | <0.001 | Mean temperature | 0.05 | 0.03–0.08 | <0.001 |
| Annual precipitation | 0.06 | 0.01–0.11 | 0.016 | Annual precipitation | 0.00 | −0.01–0.01 | 0.201 |
| Soil TP content | 0.16 | −11.16–11.48 | 0.191 | Soil P content | 0.47 | −0.82–1.75 | 0.185 |
| Soil TN content | 4.9 | −1.6–11.53 | 0.043 | Soil N content | 0.47 | −0.82–1.75 | 0.071 |
| NSDCLASS:6 Marginal R2 = 0.044 | Conditional R2 = 0.723 | NSDCLASS:6 Marginal R2 = 0.084 | Conditional R2 = 0.498 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Liu, Y.; Wu, Y.; Guo, L. Variation and Driving Mechanisms of Bark Thickness in Larix gmelinii under Surface Fire Regimes. Forests 2024, 15, 96. https://doi.org/10.3390/f15010096
Zhu Q, Liu Y, Wu Y, Guo L. Variation and Driving Mechanisms of Bark Thickness in Larix gmelinii under Surface Fire Regimes. Forests. 2024; 15(1):96. https://doi.org/10.3390/f15010096
Chicago/Turabian StyleZhu, Qiang, Yanhong Liu, Yingda Wu, and Lijun Guo. 2024. "Variation and Driving Mechanisms of Bark Thickness in Larix gmelinii under Surface Fire Regimes" Forests 15, no. 1: 96. https://doi.org/10.3390/f15010096
APA StyleZhu, Q., Liu, Y., Wu, Y., & Guo, L. (2024). Variation and Driving Mechanisms of Bark Thickness in Larix gmelinii under Surface Fire Regimes. Forests, 15(1), 96. https://doi.org/10.3390/f15010096






