Cloning, Characterization, and Expression Pattern Analysis of the BBM Gene in Tree Peony (Paeonia ostii)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Screening and Cloning of the PoBBM Gene
2.3. Analysis of the Physicochemical Properties and Phylogenetic Tree of PoBBM
2.4. Multiple Sequence Alignment and Analysis of Conserved Domains of PoBBM
2.5. Real-time Fluorescent Quantitative Analysis
2.6. Subcellular Localization of PoBBM Protein
3. Results
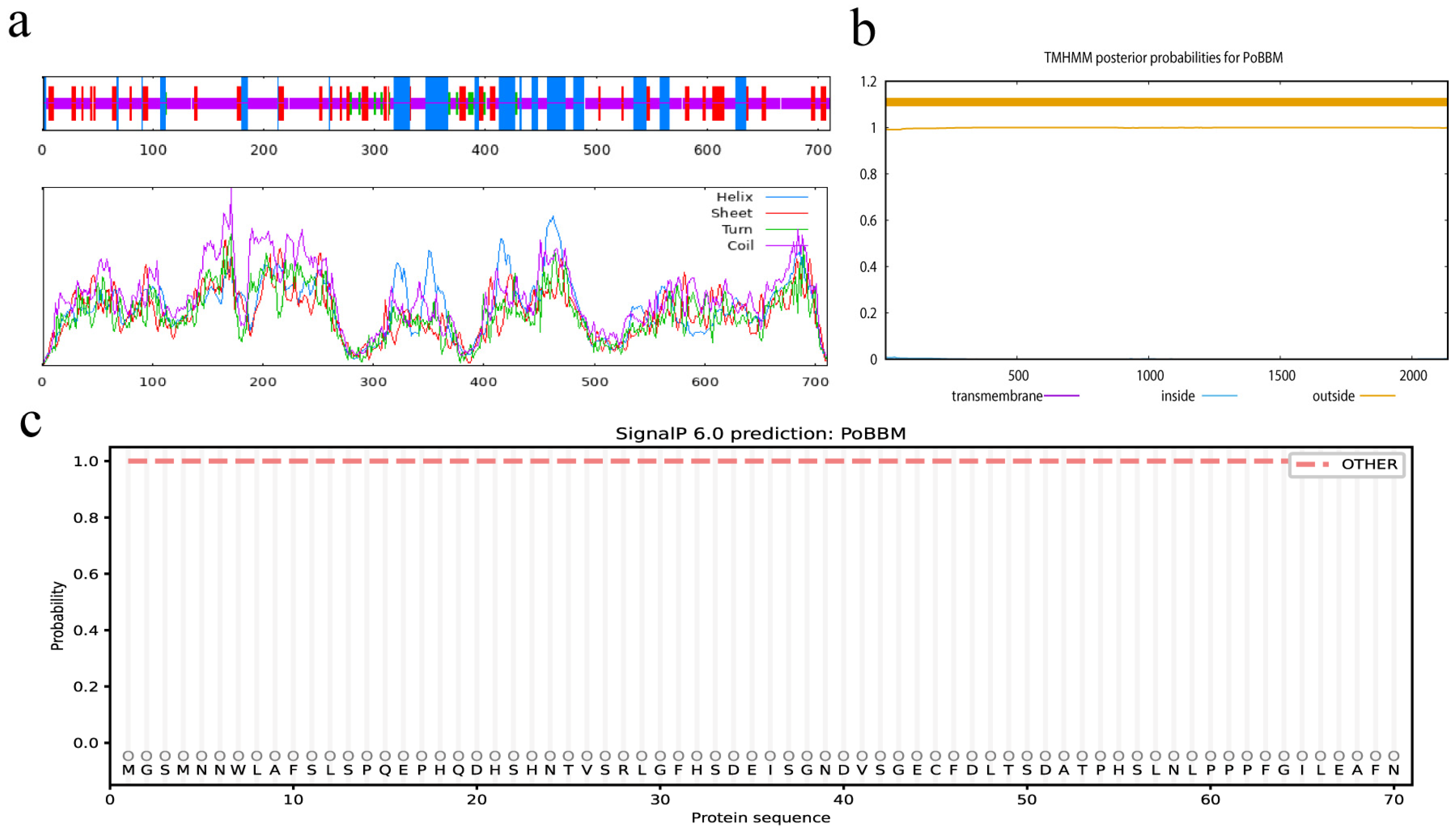
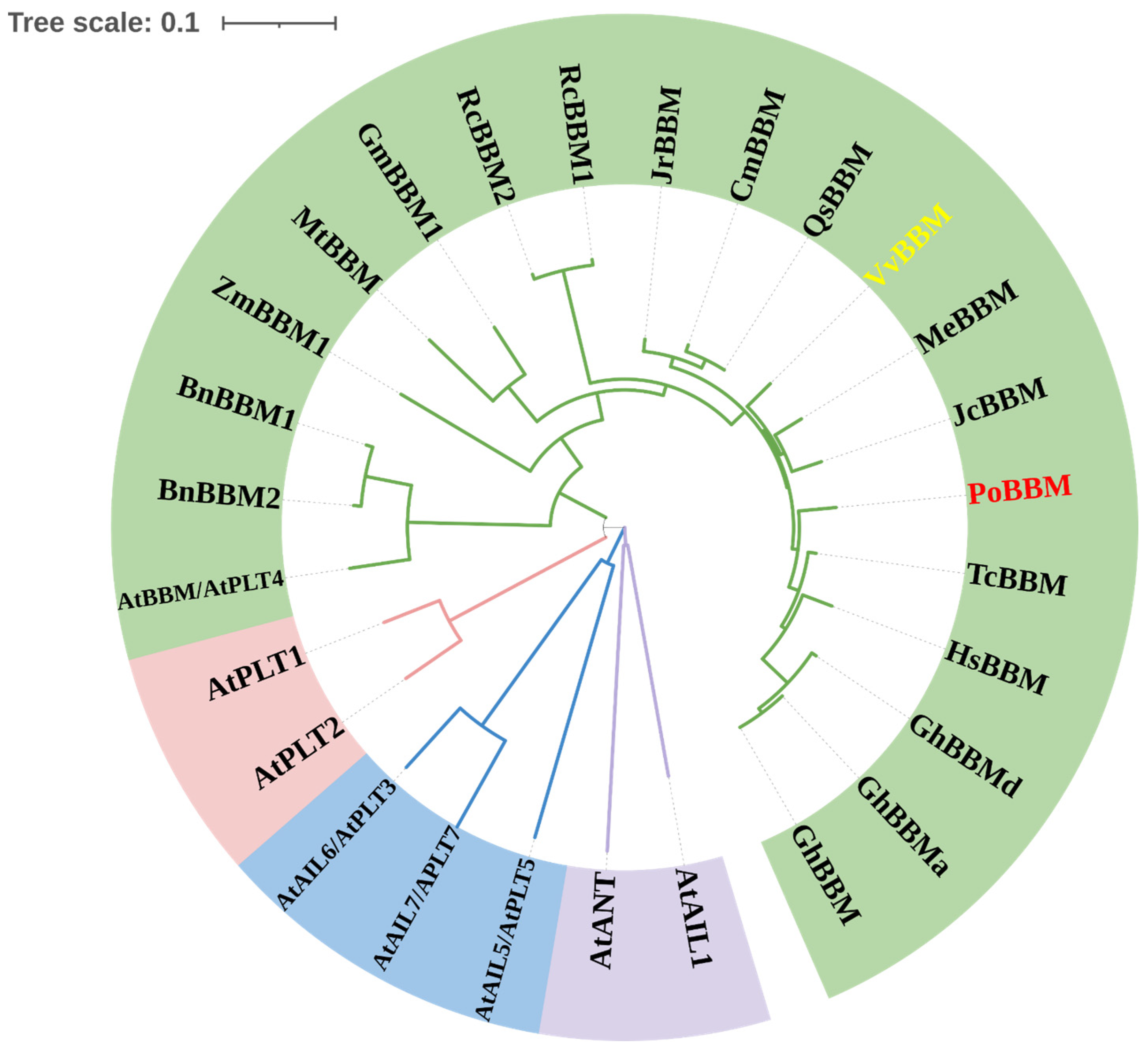
3.1. Basic Physicochemical Properties and Phylogenetic Analysis of PoBBM
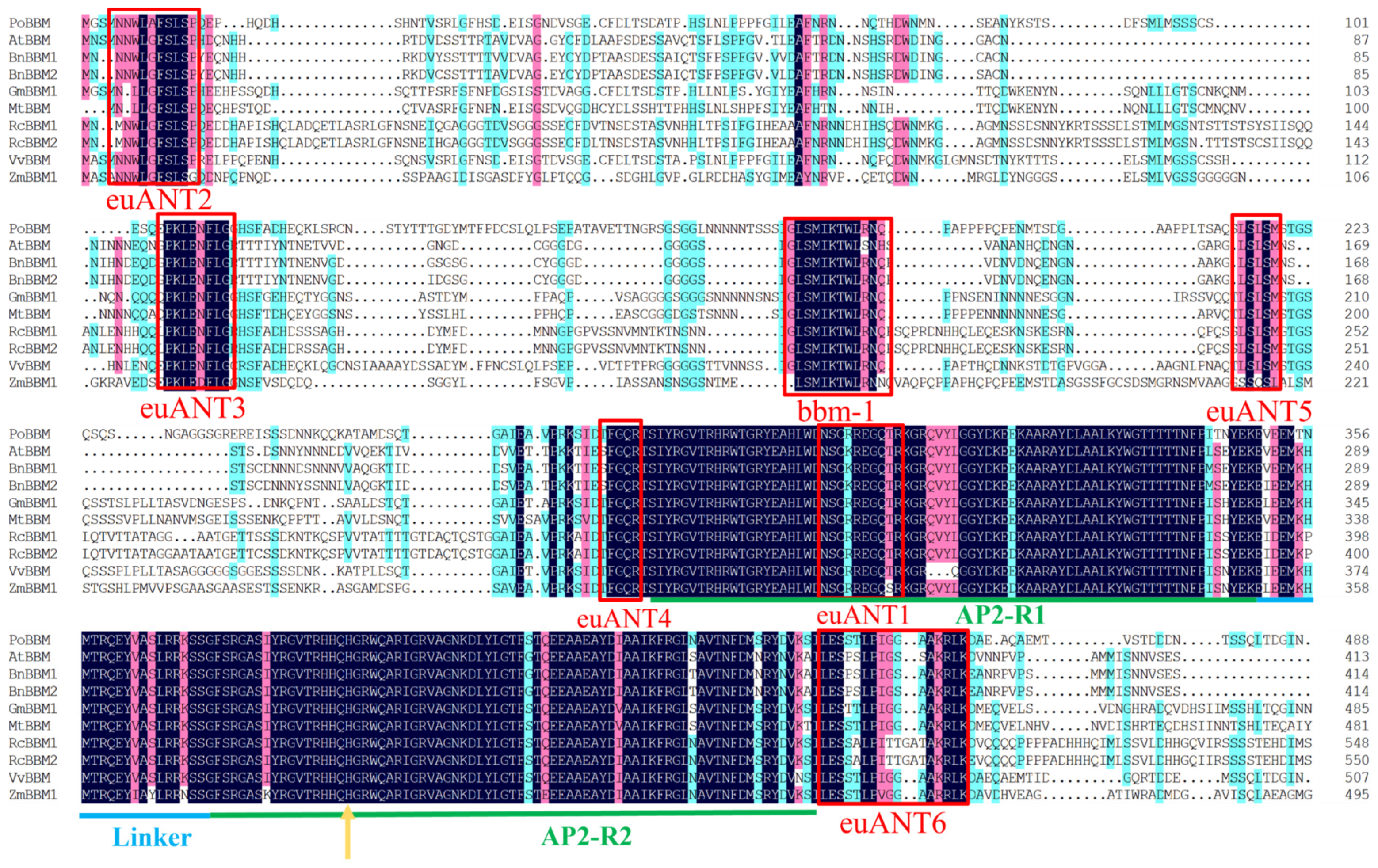
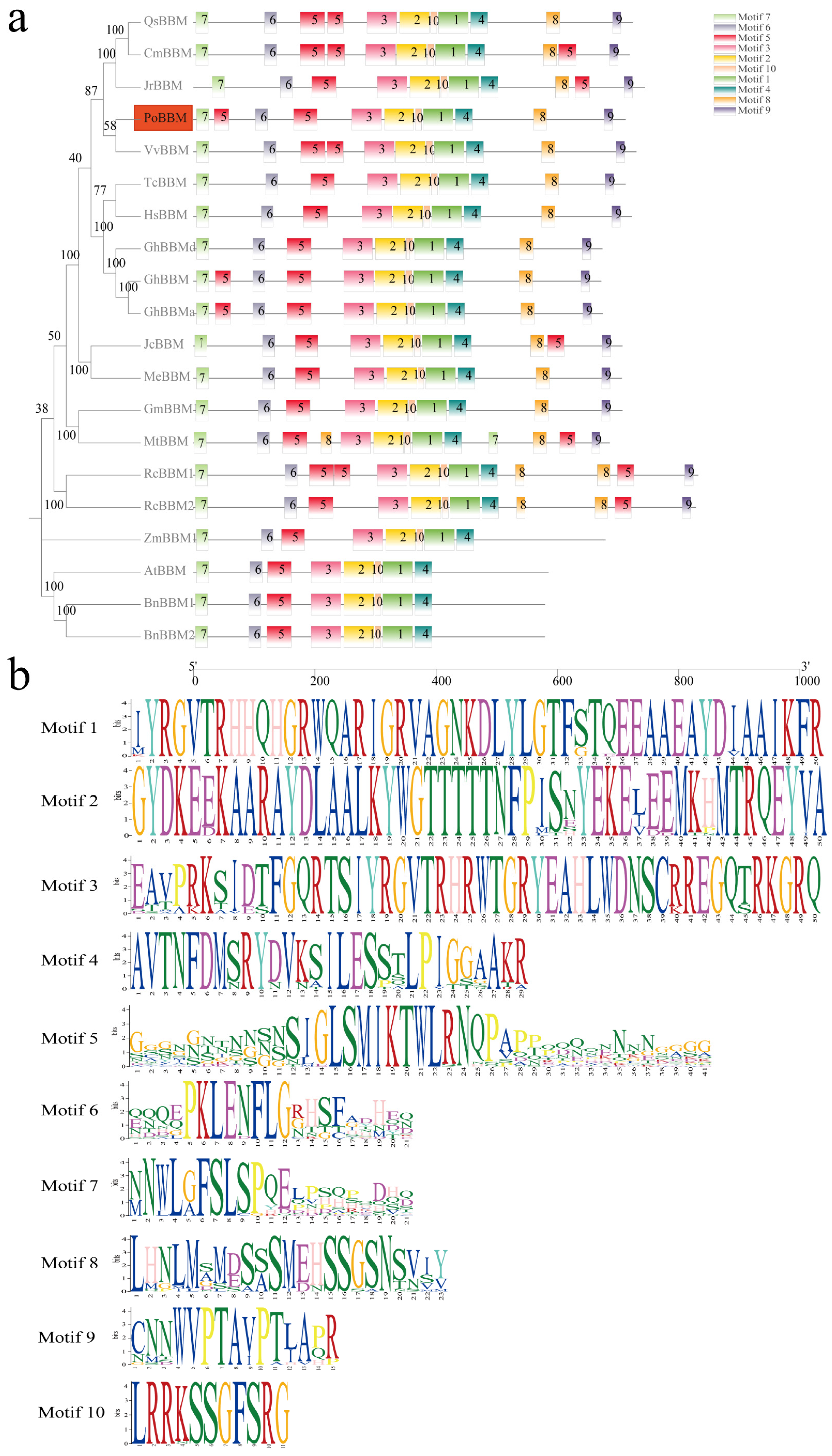
3.2. Multiple Sequence Alignment and Sequence Feature Analysis of the PoBBM Protein
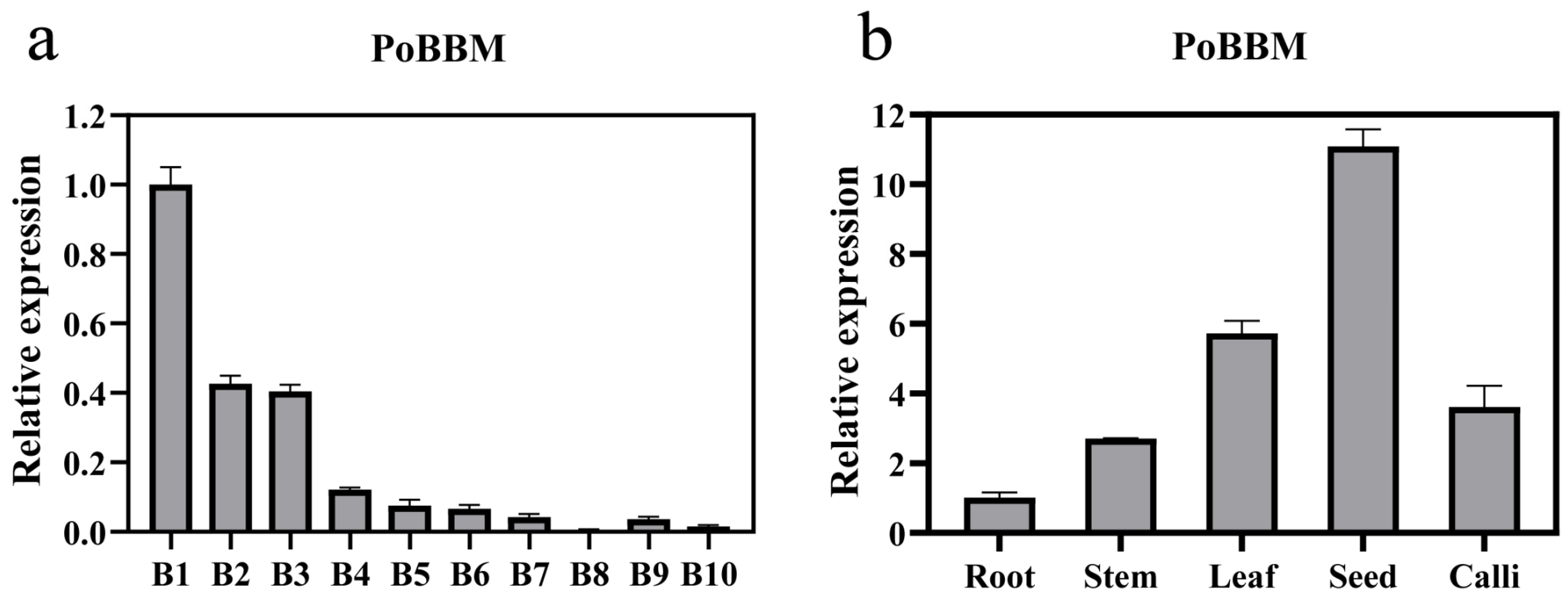
3.3. Analysis of the Expression Pattern of the PoBBM Gene
3.4. Localization Results of PoBBM Protein in N. benthamiana
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, J.; Xue, J.; Xue, Y.; Liu, R.; Ren, X.; Wang, S.; Zhang, X. Transcriptome sequencing and identification of key callus browning-related genes from petiole callus of tree peony (Paeonia suffruticosa cv. Kao) cultured on media with three browning inhibitors. Plant Physiol. Biochem. 2020, 149, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Niu, L.; Zhang, Y.; Jin, M.; Ji, D.; Zhang, X. Pollen Sources Influence the Traits of Seed and Seed Oil in Paeonia ostii ‘Feng Dan’. HortScience 2017, 52, 700–705. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X.; Fang, Z.; Wu, Y.; Tao, J. Physiological and Transcriptomic Analysis of Tree Peony (Paeonia section Moutan DC.) in Response to Drought Stress. Forests 2019, 10, 135. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, W.; Chang, Y.; Ma, Y.; Deng, Y.; Fan, K.; Zhang, X.; Jiang, Z.; Hu, T. A Preliminary Investigation on the Functional Validation and Interactions of PoWOX Genes in Peony (Paeonia ostii). Horticulturae 2022, 8, 266. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Zhang, Y.; Baskin, J.M.; Baskin, C.C.; Xiong, Z.; Tao, J. A review of the seed biology of Paeonia species (Paeoniaceae), with particular reference to dormancy and germination. Planta 2018, 249, 291–303. [Google Scholar] [CrossRef]
- Guo, Q.; Xue, X.; Hu, X.; He, Y.; Wei, S.; Liu, S.; Zhao, G.; Wang, Z.; Hou, X. Genetic Diversity and Population Structure Analysis of Tree Peony (Paeonia Section Moutan DC.) Germplasm Using Sixteen Functional SSR Markers. Forests 2023, 14, 1945. [Google Scholar] [CrossRef]
- Daler, S.; Cangi, R. Characterization of grapevine (V. vinifera L.) varieties grown in Yozgat province (Turkey) by simple sequence repeat (SSR) markers. Turk. J. Agric. For. 2022, 46, 38–48. [Google Scholar]
- Yazici, K.; Sahin Cevik, M. Development of DNA markers associated with sunburn resistance in pomegranate (Punica granatum L.) using bulk segregant analysis. Turk. J. Agric. For. 2022, 46, 955–965. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Q.; Dong, Z.; Yin, Y.; Teixeira da Silva, J.A.; Yu, X. Advances in molecular biology of Paeonia L. Planta 2019, 251, 23. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, M.; Li, S.; Chen, Q.; Teixeira da Silva, J.A.; Wang, A.; Yu, X.; Wang, L. Germplasm resources and genetic breeding of Paeonia: A systematic review. Hort. Res. 2020, 7, 107. [Google Scholar] [CrossRef]
- Horstman, A.; Bemer, M.; Boutilier, K. A transcriptional view on somatic embryogenesis. Regeneration 2017, 4, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wrobel-Marek, J.; Heidmann, I.; Horstman, A.; Chen, B.; Reis, R.; Angenent, G.C.; Boutilier, K. Auxin biosynthesis maintains embryo identity and growth during BABY BOOM-induced somatic embryogenesis. Plant Physiol. 2021, 188, 1095–1110. [Google Scholar] [CrossRef] [PubMed]
- Fehér, A. Callus, Dedifferentiation, Totipotency, Somatic Embryogenesis: What These Terms Mean in the Era of Molecular Plant Biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Florez, S.L.; Erwin, R.L.; Maximova, S.N.; Guiltinan, M.J.; Curtis, W.R. Enhanced somatic embryogenesis in Theobroma cacao using the homologous BABY BOOM transcription factor. BMC Plant Biol. 2015, 15, 121. [Google Scholar] [CrossRef] [PubMed]
- Karami, O.; Rahimi, A.; Mak, P.; Horstman, A.; Boutilier, K.; Compier, M.; van der Zaal, B.; Offringa, R. An Arabidopsis AT-hook motif nuclear protein mediates somatic embryogenesis and coinciding genome duplication. Nat. Commun. 2021, 12, 2508. [Google Scholar] [CrossRef] [PubMed]
- Horstman, A.; Li, M.; Heidmann, I.; Weemen, M.; Chen, B.; Muino, J.M.; Angenent, G.C.; Boutilier, K. The BABY BOOM Transcription Factor Activates the LEC1-ABI3-FUS3-LEC2 Network to Induce Somatic Embryogenesis. Plant Physiol. 2017, 175, 848–857. [Google Scholar] [CrossRef]
- Riechmann, J.; Meyerowitz, E. The AP2/EREBP family of plant transcription factors. Biol. Chem. 1998, 379, 633–654. [Google Scholar]
- Stone, S.L.; Kwong, L.W.; Yee, K.M.; Pelletier, J.; Lepiniec, L.; Fischer, R.L.; Goldberg, R.B.; Harada, J.J. LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo developmen. Proc. Natl. Acad. Sci. USA 2001, 98, 11806–11811. [Google Scholar] [CrossRef]
- Karim, R.; Tan, Y.S.; Singh, P.; Khalid, N.; Harikrishna, J.A. Expression and DNA methylation of SERK, BBM, LEC2 and WUS genes in in vitro cultures of Boesenbergia rotunda (L.) Mansf. Physiol. Mol. Biol. Plants 2018, 24, 741–751. [Google Scholar] [CrossRef]
- Kulinska-Lukaszek, K.; Tobojka, M.; Adamiok, A.; Kurczynska, E.U. Expression of the BBM gene during somatic embryogenesis of Arabidopsis thaliana. Biol. Plant 2012, 56, 389–394. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; Liu, C.; Li, C.; Qiu, J.; Zhao, C.; Xia, H.; Ma, C.; Wang, X.; Li, P. Expression of AtLEC2 and AtIPTs promotes embryogenic callus formation and shoot regeneration in tobacco. BMC Plant Biol. 2019, 19, 314. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, A.M.; Wójcikowska, B.; Gaj, M.D. Current Perspectives on the Auxin-Mediated Genetic Network that Controls the Induction of Somatic Embryogenesis in Plants. Int. J. Mol. Sci. 2020, 21, 1333. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Maas, L.; Figueiredo, D.; Zhong, Y.; Reis, R.; Li, M.; Horstman, A.; Riksen, T.; Weemen, M.; Liu, H.; et al. BABY BOOM regulates early embryo and endosperm development. Proc. Natl. Acad. Sci. USA 2022, 119, e2201761119. [Google Scholar] [CrossRef] [PubMed]
- Passarinho, P.; Ketelaar, T.; Xing, M.; van Arkel, J.; Maliepaard, C.; Hendriks, M.W.; Joosen, R.; Lammers, M.; Herdies, L.; den Boer, B. BABY BOOM target genes provide diverse entry points into cell proliferation and cell growth pathways. Plant Mol. Biol. 2008, 68, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, C.; Tillaboeva, S.; Bakhsh, A. Apprehending the potential of BABY BOOM transcription factors to mitigate cotton regeneration and transformation. J. Cotton Res. 2020, 3, 29. [Google Scholar] [CrossRef]
- Ghorbani, R.; Zakipour, Z.; Alemzadeh, A.; Razi, H. Genome-wide analysis of AP2/ERF transcription factors family in Brassica napus. Physiol. Mol. Biol. Plants 2020, 26, 1463–1476. [Google Scholar] [CrossRef]
- Licausi, F.; Ohme-Takagi, M.; Perata, P. APETALA2/Ethylene Responsive Factor (AP2/ERF) transcription factors: Mediators of stress responses and developmental programs. New Phytol. 2013, 199, 639–649. [Google Scholar] [CrossRef]
- Li, K.; Sun, X.; Han, H.; Zhang, S. Isolation, characterization and expression analysis of the BABY BOOM (BBM) gene from Larix kaempferi×L. olgensis during adventitious rooting. Gene 2014, 551, 111–118. [Google Scholar] [CrossRef]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-Wide Analysis of the ERF Gene Family in Arabidopsis and Rice. Plant Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef]
- Sakuma, Y.; Liu, Q.; Dubouzet, J.G.; Abe, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. DNA-Binding Specificity of the ERF/AP2 Domain of Arabidopsis DREBs, Transcription Factors Involved in Dehydration- and Cold-Inducible Gene Expression. Biochem. Biophys. Res. Commun. 2002, 290, 998–1009. [Google Scholar] [CrossRef]
- Shigyo, M.; Ito, M. Analysis of gymnosperm two-AP2-domain-containing genes. Dev. Genes Evol. 2004, 214, 105–114. [Google Scholar] [CrossRef] [PubMed]
- El Ouakfaoui, S.; Schnell, J.; Abdeen, A.; Colville, A.; Labbé, H.; Han, S.; Baum, B.; Laberge, S.; Miki, B. Control of somatic embryogenesis and embryo development by AP2 transcription factors. Plant Mol. Biol. 2010, 74, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.F.; Kou, Y.P.; Gao, B.; Soliman, T.M.A.; Xu, K.D.; Ma, N.; Cao, X.; Zhao, L.J. Identification and functional analysis of BABY BOOM genes from Rosa canina. Biol. Plant 2014, 58, 427–435. [Google Scholar] [CrossRef]
- Xie, Z.; Nolan, T.M.; Jiang, H.; Yin, Y. AP2/ERF Transcription Factor Regulatory Networks in Hormone and Abiotic Stress Responses in Arabidopsis. Front. Plant Sci. 2019, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, K.; Offringa, R.; Sharma, V.K.; Kieft, H.; Ouellet, T.; Zhang, L.; Hattori, J.; Liu, C.M.; van Lammeren, A.A.; Miki, B.L.; et al. Ectopic Expression of BABY BOOM Triggers a Conversion from Vegetative to Embryonic Growth. Plant Cell 2002, 14, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, C.; Liu, Z.; Heidmann, I.; Supena, E.D.J.; Fukuoka, H.; Joosen, R.; Lambalk, J.; Angenent, G.; Scorza, R.; Custers, J.B.M.; et al. Heterologous expression of the BABY BOOM AP2/ERF transcription factor enhances the regeneration capacity of tobacco (Nicotiana tabacum L.). Planta 2006, 225, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tomes, S.; Gleave, A.P.; Hall, W.; Luo, Z.; Xu, J.; Yao, J.-L. Significant improvement of apple (Malus domestica Borkh.) transgenic plant production by pre-transformation with a Baby boom transcription factor. Hort. Res. 2022, 9, uhab014. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein Identification and Analysis Tools in the ExPASy Server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar]
- Geourjon, C.; Deléage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Bioinformatics 1995, 11, 681–684. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; Von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Chou, K.-C.; Shen, H.-B. Plant-mPLoc: A Top-Down Strategy to Augment the Power for Predicting Plant Protein Subcellular Localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wei, X.R. Cloning and Analysis of PoWUS and PoAGL15, Somatic Embryogenesis and Related Genes in Peony; Chinese Academy of Forestry Sciences: Beijing, China, 2019. (In Chinese) [Google Scholar]
- Aida, M.; Beis, D.; Heidstra, R.; Willemsen, V.; Blilou, I.; Galinha, C.; Nussaume, L.; Noh, Y.S.; Amasino, R.; Scheres, B. The PLETHORA Genes Mediate Patterning of the Arabidopsis Root Stem Cell Niche. Cell 2004, 119, 109–120. [Google Scholar] [CrossRef]
- Santuari, L.; Sanchez-Perez, G.F.; Luijten, M.; Rutjens, B.; Terpstra, I.; Berke, L.; Gorte, M.; Prasad, K.; Bao, D.; Timmermans-Hereijgers, J.L.; et al. The PLETHORA Gene Regulatory Network Guides Growth and Cell Differentiation in Arabidopsis Roots. Plant Cell 2016, 28, 2937–2951. [Google Scholar] [CrossRef]
- Yuan, J.; Jiang, S.; Jian, J.; Liu, M.; Yue, Z.; Xu, J.; Li, J.; Xu, C.; Lin, L.; Jing, Y.; et al. Genomic basis of the giga-chromosomes and giga-genome of tree peony Paeonia ostii. Nat. Commun. 2022, 13, 7328. [Google Scholar] [CrossRef]
- Nole-Wilson, S.; Tranby, T.L.; Krizek, B.A. AINTEGUMENTA-like (AIL) genes are expressed in young tissues and may specify meristematic or division-competent states. Plant Mol. Biol. 2005, 57, 613–628. [Google Scholar] [CrossRef]

| Primers Name | Primers Sequence (5′–3′) |
|---|---|
| PoBBM-F | ATGGGTTCTATGAACAACTGGT |
| PoBBM-R | TTAAGTATCATTCCACACTGTGAAAGT |
| Q-PoBBM-F | GGAAGCAGCAGAAGCATA |
| Q-PoBBM-R | TGGTGTTGTCGTCATCAG |
| Q-Poubiquitin-F | TCCTCCACCTCCTACCTTCCGACTC |
| Q-Poubiquitin-R | CGATCCTCCTGAGCCAAGCGTCAT |
| PHG-PoBBM-F | TCTCTCTCTCAAGCTTATGGGTTCTATGAACAACTGGT |
| PHG-PoBBM-R | CGGGTCATGAGCTCCTGCAGAGTATCATTCCACACTGTGAAAGT |
| Basic Physicochemical Properties | PoBBM |
|---|---|
| GenBank Accession | OR711905 |
| Open Reading Frame (bp) | 2136 |
| Protein Length (aa) | 711 |
| Molecular Weight (kDa) | 77.92892 |
| Isoelectric Point (PI) | 5.90 |
| Instability Coefficient (II) | 47.81 |
| Aliphatic Index | 50.14 |
| GRAVY | −0.794 |
| Subcellular Localization | Nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, W.; Chang, Y.; Ma, Y.; Deng, Y.; Zhang, N.; Bai, Y.; Jiang, Z.; Hu, T. Cloning, Characterization, and Expression Pattern Analysis of the BBM Gene in Tree Peony (Paeonia ostii). Forests 2024, 15, 36. https://doi.org/10.3390/f15010036
Zhang X, Zhang W, Chang Y, Ma Y, Deng Y, Zhang N, Bai Y, Jiang Z, Hu T. Cloning, Characterization, and Expression Pattern Analysis of the BBM Gene in Tree Peony (Paeonia ostii). Forests. 2024; 15(1):36. https://doi.org/10.3390/f15010036
Chicago/Turabian StyleZhang, Xue, Wenbo Zhang, Yanting Chang, Yanjun Ma, Yayun Deng, Na Zhang, Yiwei Bai, Zehui Jiang, and Tao Hu. 2024. "Cloning, Characterization, and Expression Pattern Analysis of the BBM Gene in Tree Peony (Paeonia ostii)" Forests 15, no. 1: 36. https://doi.org/10.3390/f15010036
APA StyleZhang, X., Zhang, W., Chang, Y., Ma, Y., Deng, Y., Zhang, N., Bai, Y., Jiang, Z., & Hu, T. (2024). Cloning, Characterization, and Expression Pattern Analysis of the BBM Gene in Tree Peony (Paeonia ostii). Forests, 15(1), 36. https://doi.org/10.3390/f15010036






