Analyzing the Impact of Simulated Nitrogen Deposition on Stoichiometric Properties and Yield of Ma Bamboo (Dendrocalamus latiflorus Munro) Shoots, Leaves, and Soil Substrate
Abstract
1. Introduction
2. Materials and Methods
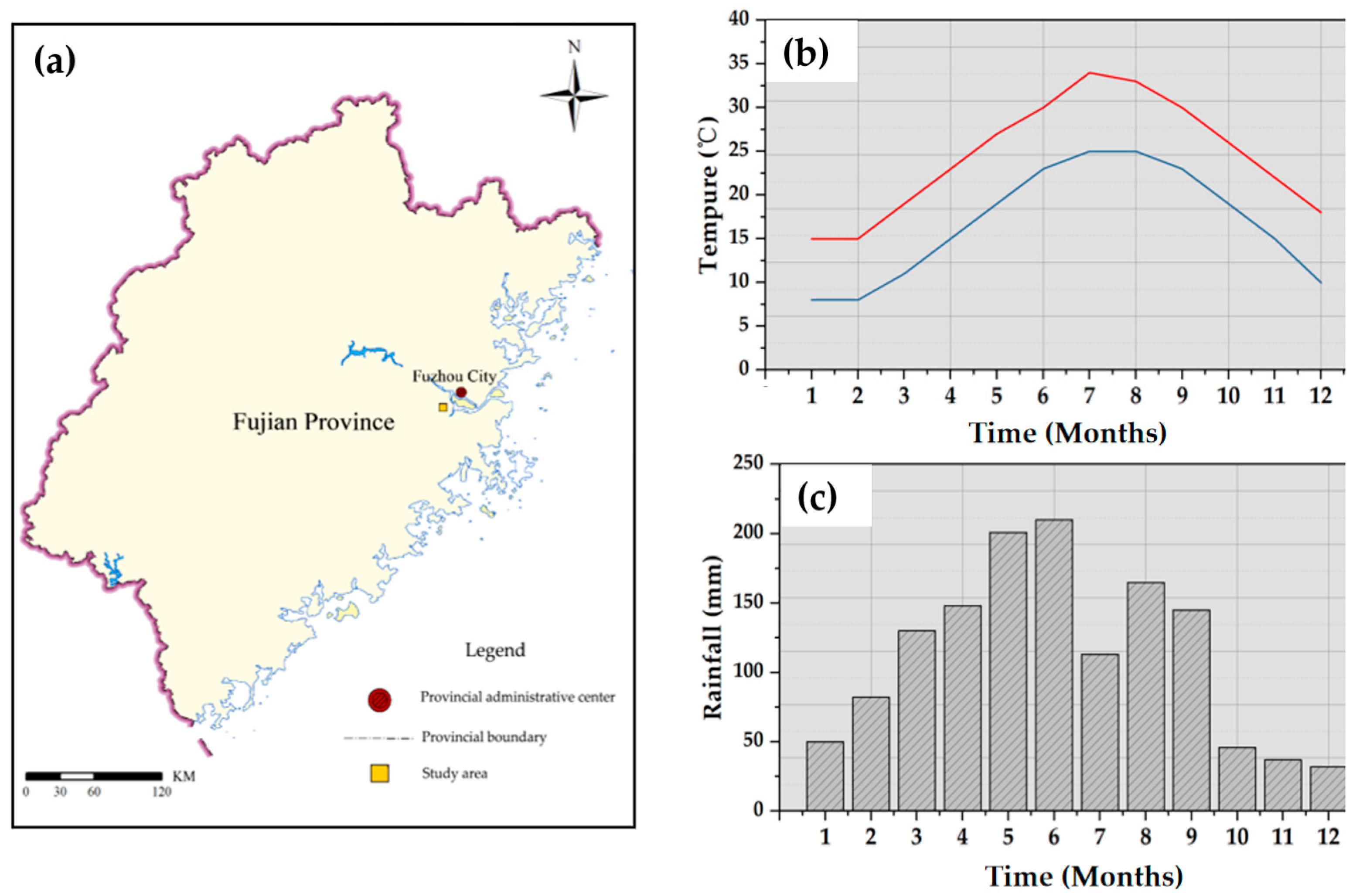
2.1. Experimental Materials
2.2. Nitrogen Deposition Simulation and Sample Collection
2.3. Indicator Determination
2.4. Data Analysis and Processing
2.4.1. Partial Least Squares Structural Equation Model
2.4.2. Other Data Analysis
3. Results
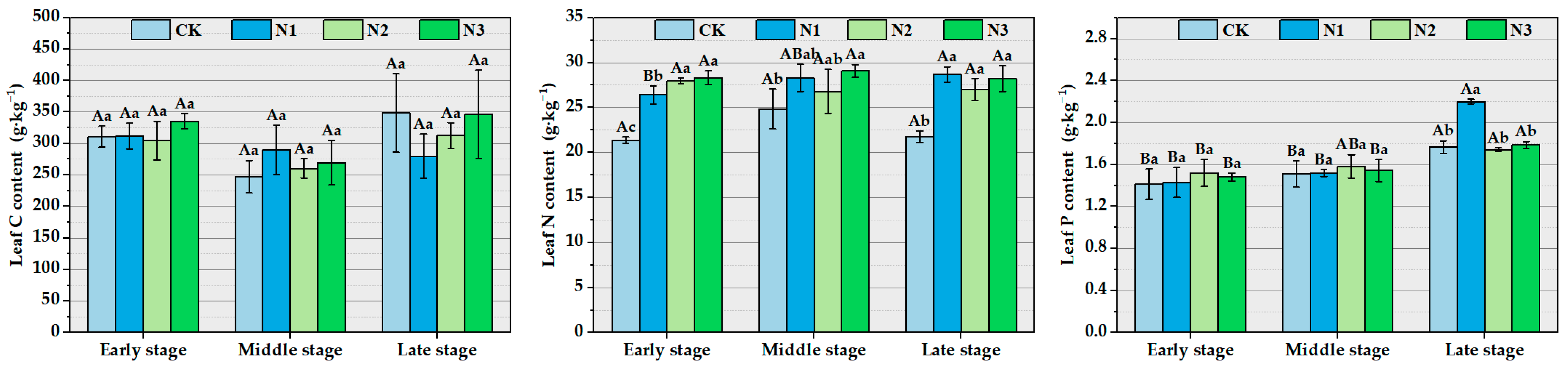
3.1. The Impact of Different Concentrations of Nitrogen Addition on Chemical Stoichiometry Characteristics and Stoichiometric Ratios of Ma Bamboo Leaves
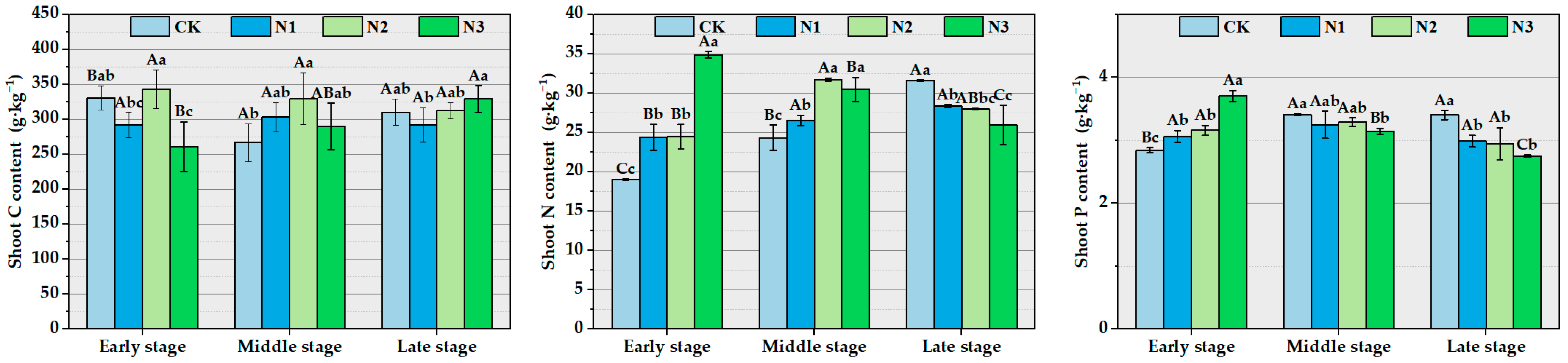
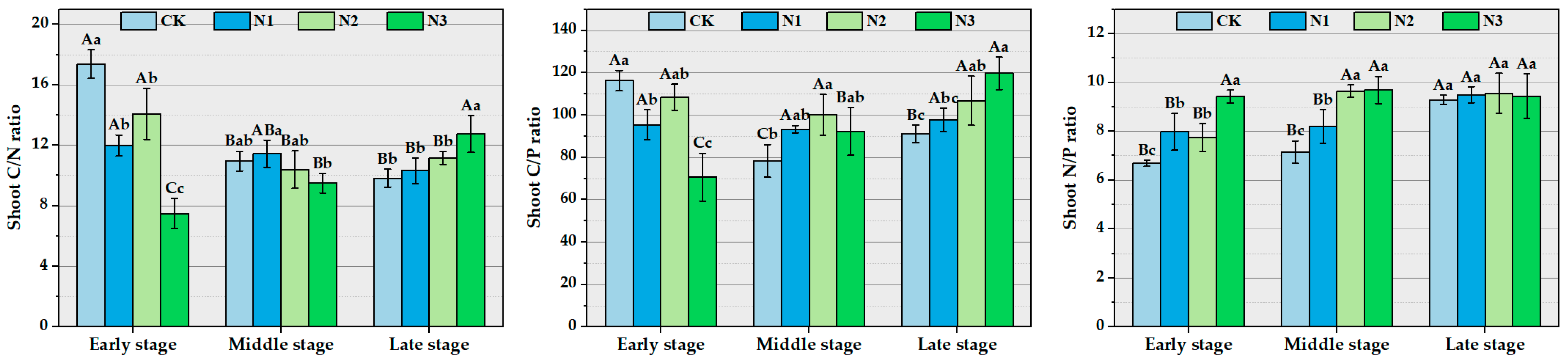
3.2. The Impact of Different Concentrations of Nitrogen Addition on the Stoichiometric Characteristics and Stoichiometric Ratios of Ma Bamboo Shoots
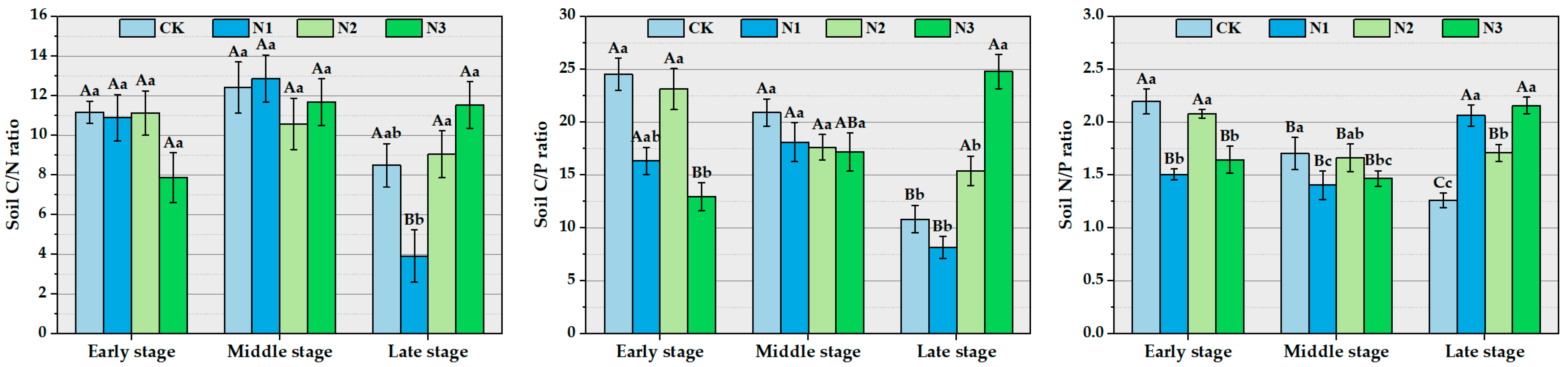
3.3. The Impact of Different Concentrations of Nitrogen Addition on Soil Stoichiometry and Stoichiometric Ratios
3.4. Correlation Analysis of Nitrogen Application Treatment on C, N and P Contents of Leaves, Bamboo Shoots and Soil at Different Bamboo Shoot Stages
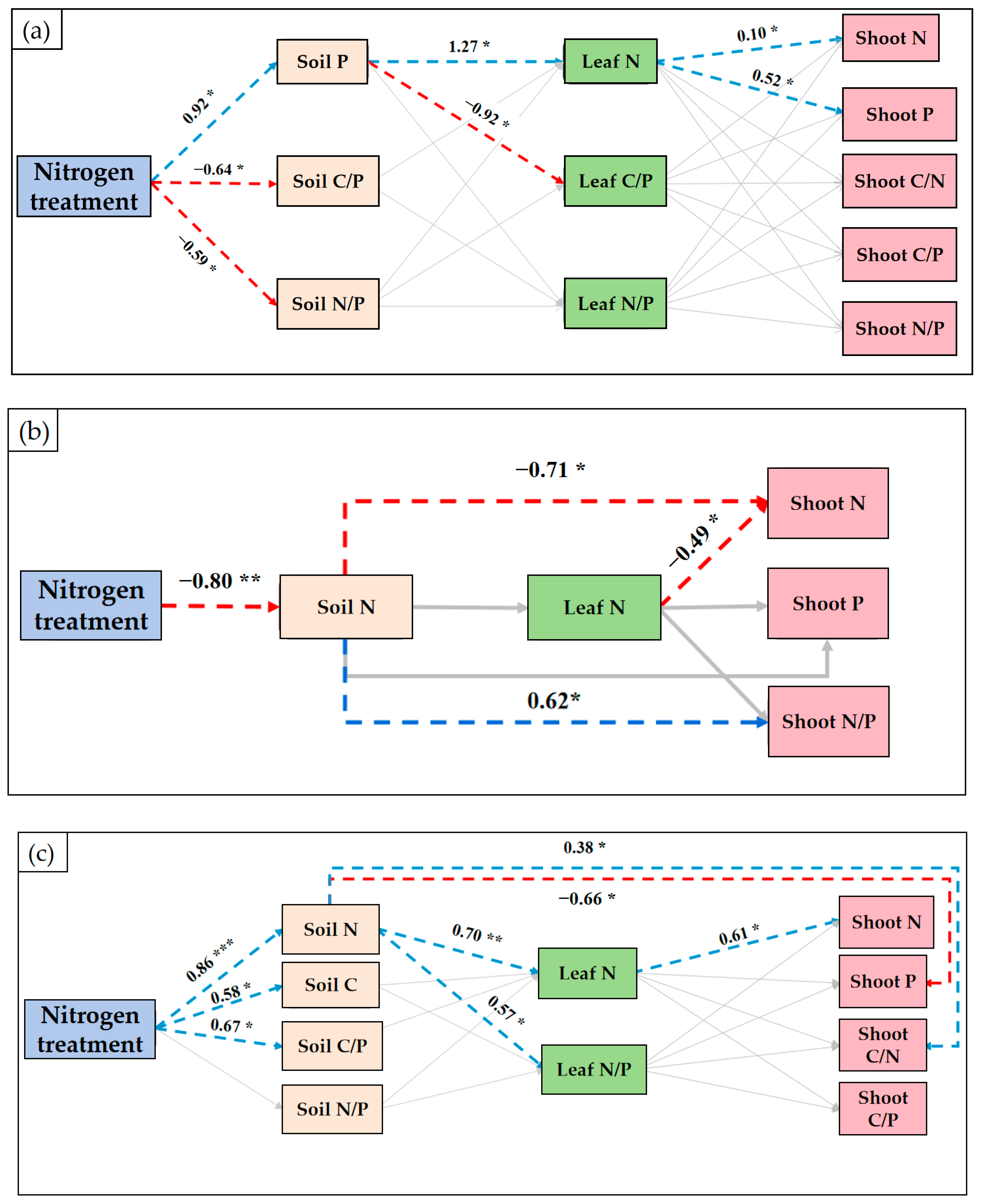
3.5. Direct and Indirect Effects of Nitrogen Treatment on Stoichiometry of Ma Bamboo Leaves, Bamboo Shoots, and Soil
3.6. Response of Shoot Emergence, Shoot Yield, and Shoot Germination Time to Different Nitrogen Treatments
4. Discussion
4.1. The Impact of Nitrogen Addition on Soil Nutrients
4.2. Changes in the Chemical Stoichiometric Characteristics of C, N, and P in Ma Bamboo Leaves and Shoots with Nitrogen Addition
4.3. Response of Bamboo Shoot Emergence Characteristics to Varied Nitrogen Application Levels at Different Shoot Stages
4.4. Limitations and Future Prospects
5. Conclusions
- (1).
- Impact on Nitrogen Content and Stoichiometry
- (2).
- Response of Phosphorus Content and Soil Nutrient Influence
- (3).
- Enhancement of Bamboo Shoot Yield and Emergence Duration
- (4).
- Optimal Nitrogen Treatment Levels
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, G.R.; Jia, Y.L.; He, N.P.; Zhu, J.X.; Chen, Z.; Wang, Q.F.; Piao, S.L.; Liu, X.J.; He, H.L.; Guo, X.B.; et al. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Botez, F.; Postolache, C. Nitrogen deposition impact on terrestrial ecosystems. Rom. Biotechnol. Lett. 2013, 18, 8723–8742. [Google Scholar]
- Wu, F.; Yang, W.; Lu, Y. Effects of dwarf bamboo (Fargesia denudata) density on biomass, carbon and nutrient distribution pattern. Acta Ecol. Sin. 2009, 29, 192–198. [Google Scholar] [CrossRef]
- Krapp, A.; Castaings, L. Plant adaptation to nitrogen availability. Biol. Aujourd’hui 2012, 206, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.G. Stoichiometric characteristics of woody plant leaves and responses to climate and soil factors in China. PLoS ONE 2023, 18, e0291957. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Wang, Y.X.; Li, J.L. Effects of nitrogen addition on plant-soil carbon dynamics in terrestrial ecosystems of China. Acta Ecol. Sin. 2022, 42, 4823–4833. [Google Scholar] [CrossRef]
- Liang, X.Y.; Zhang, T.; Lu, X.K.; Ellsworth, D.S.; BassiriRad, H.; You, C.M.; Wang, D.; He, P.C.; Deng, Q.; Liu, H.; et al. Global response patterns of plant photosynthesis to nitrogen addition: A meta-analysis. Glob. Change Biol. 2020, 26, 3585–3600. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Du, E.; Jiang, L.; Ma, S.; Zeng, W.; Zou, A.; Feng, C.; Xu, L.; Xing, A.; Wang, W.; et al. Responses of forest ecosystems to increasing N deposition in China: A critical review. Environ. Pollut. 2018, 243, 75–86. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.T.; Chen, H.Y.H.; Ruan, H.H. Responses of C:N stoichiometry in plants, soil, and microorganisms to nitrogen addition. Plant Soil 2020, 456, 277–287. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Xiao, T.H.; Hou, F.J. Different responses of soil C:N:P stoichiometry to stocking rate and nitrogen addition level in an alpine meadow on the Qinghai-Tibetan Plateau. Appl. Soil Ecol. 2021, 165, 103961. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, X.; Sun, Y.; Liu, S.; Hu, H.; Xie, J.; Chen, G.; Xiao, Y.; Tang, Y.; Tu, L. C:N:P stoichiometry responses to 10 years of nitrogen addition differ across soil components and plant organs in a subtropical Pleioblastus amarus forest. Sci. Total Environ. 2021, 796, 148925. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Guo, W.; Liu, J.; Du, N.; Wang, R. Increased nitrogen deposition alleviated the adverse effects of drought stress on Quercus variabilis and Quercus mongolica seedlings. Acta Physiol. Plant. 2015, 37, 107. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Negative effects of fertilization on plant nutrient resorption. Ecology 2015, 96, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Zheng, C.; Tian, D. Impacts of nitrogen addition on plant phosphorus content in forest ecosystems and the underlying mechanisms. Chin. J. Plant Ecol. 2019, 43, 185–196. [Google Scholar] [CrossRef]
- Yue, K.; Yang, W.Q.; Peng, Y.; Peng, C.H.; Tan, B.; Xu, Z.F.; Zhang, L.; Ni, X.Y.; Zhou, W.; Wu, F.Z. Individual and combined effects of multiple global change drivers on terrestrial phosphorus pools: A meta-analysis. Sci. Total Environ. 2018, 630, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Vitousek, P.M.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Zhou, G.; Zou, X.; Bai, E.; Scanlon, T.M.; Hou, E.; et al. Plant acclimation to long-term high nitrogen deposition in an N-rich tropical forest. Proc. Natl. Acad. Sci. USA 2018, 115, 5187–5192. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, P.; Chen, S.; Hang, Z.; Jin, S. Simulated organic-inorganic nitrogen deposition changes the growth rate, leaf stoichiometry, and phenolic content of Cyclocarya paliurus. For. Ecol. Manag. 2023, 546, 121313. [Google Scholar] [CrossRef]
- Yin, M.Z.; Guo, W.J.; Zhu, X.M.; Zhang, Z.L.; Liu, Q.; Yin, H.J. Nutrient limiting characteristics of subalpine coniferous forests under conditions of nitrogen deposition in the southwest mountains of China. Chin. J. Appl. Environ. Biol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Du, M.Y.; Fan, S.H.; Liu, G.L.; Feng, H.Y.; Guo, B.H.; Tang, X.L. Stoichiometric characteristics of carbon, nitrogen and phosphorus in Phyllostachys edulis forests of China. Chin. J. Plant Ecol. 2016, 40, 760–774. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, J.S.; Li, Q.; Hänninen, H.; Peng, C.J.; Yao, H.; Song, X.Z.; Ying, Y.Q. Nitrogen Deposition Enhances Photosynthesis in Moso Bamboo but Increases Susceptibility to Other Stress Factors. Front. Plant Sci. 2017, 8, 1975. [Google Scholar] [CrossRef]
- Song, X.; Gu, H.; Wang, M.; Zhou, G.; Li, Q. Management practices regulate the response of Moso bamboo foliar stoichiometry to nitrogen deposition. Sci. Rep. 2016, 6, 24107. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.M.; Wang, Y.J.; Zhuo, R.Y.; Xu, J.; Lu, Z.C.; Fan, H.J.; Huang, B.Y.; Qiao, G.R. TCP Transcription Factors Involved in Shoot Development of Ma Bamboo (Dendrocalamus latiflorus Munro). Front. Plant Sci. 2022, 13, 884443. [Google Scholar] [CrossRef]
- Chen, K.; Hu, K.Q.; Xi, F.H.; Wang, H.H.; Kohnen, M.V.; Gao, P.F.; Liao, J.K.; Wei, W.T.; Liu, X.Q.; Zhang, H.X.; et al. High-Efficient and Transient Transformation of Moso Bamboo (Phyllostachys edulis) and Ma Bamboo (Dendrocalamus latiflorus Munro). J. Plant Biol. 2023, 66, 75–86. [Google Scholar] [CrossRef]
- Fawcett, J.K. The semi-micro Kjeldahl method for the determination of nitrogen. J. Med. Lab. Technol. 1954, 12, 330–340. [Google Scholar]
- He, Y.; Tang, Y.; Lin, L.; Shi, W.; Ying, Y. Differential responses of phosphorus accumulation and mobilization in Moso bamboo (Phyllostachys edulis (Carriere) J. Houz) seedlings to short-term experimental nitrogen deposition. Ann. For. Sci. 2023, 80, 10. [Google Scholar] [CrossRef]
- Guan, F.; Xia, M.; Tang, X.; Fan, S. Spatial variability of soil nitrogen, phosphorus and potassium contents in Moso bamboo forests in Yong’an City, China. CATENA 2017, 150, 161–172. [Google Scholar] [CrossRef]
- Shuai, X.; Yost, R.S.; Wendroth, O. State-space estimation of the intrinsic soil phosphorus pools from soil phosphorus tests. Geoderma 2014, 214, 239–245. [Google Scholar] [CrossRef]
- Lei, Z.; Sun, H.; Li, Q.; Zhang, J.; Song, X. Effects of Nitrogen Deposition on Soil Dissolved Organic Carbon and Nitrogen in Moso Bamboo Plantations Strongly Depend on Management Practices. Forests 2017, 8, 452. [Google Scholar] [CrossRef]
- Nóbrega, G.N.; Ferreira, T.O.; Artur, A.G.; de Mendona, E.S.; Leao, R.A.D.; Teixeira, A.S.; Otero, X.L. Evaluation of methods for quantifying organic carbon in mangrove soils from semi-arid region. J. Soils Sediments 2015, 15, 282–291. [Google Scholar] [CrossRef]
- Sun, T.; Mao, X.L.; Han, K.F.; Wang, X.J.; Cheng, Q.; Liu, X.; Zhou, J.J.; Ma, Q.X.; Ni, Z.H.; Wu, L.H. Nitrogen addition increased soil particulate organic carbon via plant carbon input whereas reduced mineral-associated organic carbon through attenuating mineral protection in agroecosystem. Sci. Total Environ. 2023, 899, 165705. [Google Scholar] [CrossRef]
- Norton, B.J.; Strube, M.J. Guide for the interpretation of one-way analysis of variance. Phys. Ther. 1985, 65, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Z.; Tang, X.; Liu, L.; Lai, Y.; Lei, J.; Zeng, C.; Ma, X.; Du, M.; Cai, C.; et al. Differential Response of Soil Respiration and Total Belowground Carbon Allocation to Simulated Nitrogen and Phosphorus Deposition in Moso Bamboo Forests. Forests 2022, 13, 1860. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, J.; Li, Q.; Ying, Y.; Peng, C.; Song, X. Effects of nitrogen deposition and management practices on leaf litterfall and N and P return in a Moso bamboo forest. Biogeochemistry 2017, 134, 115–124. [Google Scholar] [CrossRef]
- Feng, J.G. Effects of nitrogen and phosphorus addition on soil organic carbon: Review and prospects. Chin. J. Plant Ecol. 2022, 46, 855–870. [Google Scholar] [CrossRef]
- Sardar, M.F.; Younas, F.; Farooqi, Z.U.R.; Li, Y.L. Soil nitrogen dynamics in natural forest ecosystem: A review. Front. For. Glob. Change 2023, 6, 1144930. [Google Scholar] [CrossRef]
- Huang, C.Y.; Jien, S.H.; Chen, T.H.; Tian, G.L.; Chiu, C.Y. Soluble organic C and N and their relationships with soil organic C and N and microbial characteristics in moso bamboo (Phyllostachys edulis) plantations along an elevation gradient in Central Taiwan. J. Soils Sediments 2014, 14, 1061–1070. [Google Scholar] [CrossRef]
- Gu, D.X.; Chen, H. Effects of soil nitrogen and phosphonium on leaf nitrogen and phosphonium stoichiometric characteristics and chlorophyll content of Oligostachyum lubricum. Chin. J. Plant Ecol. 2011, 35, 1219–1225. [Google Scholar] [CrossRef]
- Fan, L.; Tarin, M.W.K.; Han, Y.; Li, B.; Rong, J.; Chen, L.; He, T.; Zheng, Y. Effects of soil exogenous nitrogen on bamboo (dendrocalamus latiflorus munro) shoots, photosynthetic characteristics, and nitrogen metabolism. Appl. Ecol. Environ. Res. 2022, 20, 3911–3926. [Google Scholar] [CrossRef]
- Padgurschi, M.C.G.; Vieira, S.A.; Stefani, E.J.F.; Nardoto, G.B.; Joly, C.A. Nitrogen input by bamboos in neotropical forest: A new perspective. PeerJ 2018, 6, e6024. [Google Scholar] [CrossRef]
- Su, W.H.; Fan, S.H.; Zhao, J.C.; Cai, C.J. Effects of various fertilization placements on the fate of urea-15N in moso bamboo forests. For. Ecol. Manag. 2019, 453, 117632. [Google Scholar] [CrossRef]
- Yang, K.B.; Zhu, C.L.; Zhang, J.B.; Li, Z.Y.; Liu, Y.; Song, X.Z.; Gao, Z.M. Nitrogen fertilization in bamboo forest accelerates the shoot growth and alters the lignification process in shoots. Ind. Crops Prod. 2022, 187, 115368. [Google Scholar] [CrossRef]


| Stage | Total Nitrogen/(g·kg−1) | Hydrolyzable Nitrogen /(mg·kg−1) | Total Phosphorus /(g·kg−1) | Effective Phosphorus /(mg·kg−1) | Total Potassium /(g·kg−1) | Rapidly Available Potassium /(mg·kg−1) | pH | Electric Conductivity | Organic Carbon /(g·kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| Early | 1.12 | 181.11 | 0.63 | 4.75 | 16.49 | 5.18 | 6.00 | 85.71 | 8.73 |
| Middle | 1.15 | 180.56 | 0.59 | 5.9 | 16.10 | 5.73 | 6.05 | 87.07 | 8.73 |
| Late | 1.13 | 180.98 | 0.60 | 5.34 | 16.29 | 5.45 | 5.98 | 86.79 | 8.69 |
| Early Stage. | Leaf | Bamboo Shoot | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | N | P | C:N | C:P | N:P | C | N | P | C:N | C:P | N:P | ||
| Soil | C | −0.09 | −0.03 | 0.08 | −0.01 | −0.11 | −0.09 | 0.83 * | −0.41 | −0.31 | 0.59 ** | 0.65 ** | −0.44 |
| N | 0.04 | 0.47 | 0.30 | −0.41 | −0.10 | 0.26 | 0.23 | 0.31 | 0.38 | −0.12 | −0.03 | 0.23 | |
| P | 0.46 | 0.87 * | 0.07 | −0.63 ** | 0.24 | 0.84 * | −0.50 | 0.88 * | 0.80 * | −0.87 * | −0.73 * | 0.90 * | |
| C:N | −0.11 | −0.25 | −0.05 | 0.20 | −0.02 | −0.23 | 0.75 * | −0.58 ** | −0.52 | 0.67 ** | 0.715 * | −0.56 | |
| C:P | −0.24 | −0.45 | 0.01 | 0.34 | −0.16 | −0.48 | 0.84 * | −0.71 * | −0.59 ** | 0.84 * | 0.826 * | −0.75 * | |
| N:P | −0.34 | −0.53 | 0.11 | 0.38 | −0.29 | −0.62 ** | 0.60 ** | −0.59 ** | −0.48 | 0.71 * | 0.64 ** | −0.66 ** | |
| Middle Stage | Leaf | Bamboo Shoot | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | N | P | C:N | C:P | N:P | C | N | P | C:N | C:P | N:P | ||
| Soil | C | −0.08 | −0.69 ** | −0.09 | 0.44 | −0.01 | −0.50 | 0.03 | −0.41 | 0.64 ** | 0.52 | −0.18 | −0.52 |
| N | −0.49 | −0.71 * | −0.03 | 0.07 | −0.44 | −0.55 | −0.13 | −0.31 | 0.62 ** | 0.20 | −0.33 | −0.44 | |
| P | −0.13 | −0.67 ** | 0.46 | 0.38 | −0.33 | −0.80 * | 0.13 | −0.38 | 0.43 | 0.58 ** | −0.02 | −0.45 | |
| C:N | 0.18 | −0.34 | −0.10 | 0.427 | 0.24 | −0.20 | 0.13 | −0.27 | 0.40 | 0.49 | −0.01 | −0.34 | |
| C:P | −0.04 | −0.47 | −0.31 | 0.31 | 0.12 | −0.19 | −0.01 | −0.28 | 0.53 | 0.34 | −0.18 | −0.38 | |
| N:P | −0.43 | −0.23 | −0.37 | −0.23 | −0.23 | 0.01 | −0.23 | −0.04 | 0.31 | −0.21 | −0.32 | −0.12 | |
| Late Stage | Leaf | Bamboo Shoot | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | N | P | C:N | C:P | N:P | C | N | P | C:N | C:P | N:P | ||
| Soil | C | 0.35 | 0.11 | −0.53 | 0.12 | 0.46 | 0.60 ** | 0.48 | −0.64 ** | −0.46 | 0.763 * | 0.63 ** | −0.20 |
| N | −0.05 | 0.66 ** | −0.24 | −0.44 | 0.04 | 0.87 * | 0.30 | −0.77 * | −0.78 * | 0.68 ** | 0.73 * | 0.13 | |
| P | 0.31 | −0.45 | −0.74 * | 0.43 | 0.52 | 0.23 | 0.21 | 0.22 | 0.27 | −0.04 | −0.06 | −0.07 | |
| C:N | 0.45 | −0.14 | −0.56 | 0.34 | 0.55 | 0.37 | 0.44 | −0.41 | −0.22 | 0.599 ** | 0.44 | −0.26 | |
| C:P | 0.29 | 0.21 | −0.37 | 0.03 | 0.35 | 0.54 * | 0.48 | −0.73 * | −0.54 | 0.832 * | 0.68 ** | −0.217 | |
| N:P | −0.31 | 0.88 * | 0.45 | −0.71 * | −0.42 | 0.45 | 0.06 | −0.78 * | −0.82 * | 0.567 * | 0.60 ** | 0.153 | |
| Nitrogen Treatment | Number of Bamboo Shoots | ||
|---|---|---|---|
| Early Stage | Middle Stage | Late Stage | |
| CK | 3 | 8 | 6 |
| N1 | 5 | 11 | 7 |
| N2 | 5 | 12 | 7 |
| N3 | 6 | 16 | 13 |
| Nitrogen treatment | Bamboo shoot yield (g) | ||
| Early stage | Middle stage | Late stage | |
| CK | 23.95 | 141.87 | 78.00 |
| N1 | 50.64 | 166.27 | 85.63 |
| N2 | 35.73 | 165.65 | 121.33 |
| N3 | 51.70 | 220.79 | 122.61 |
| Nitrogen treatment | Bamboo shoot germination time (day) | ||
| Early stage | Middle stage | Late stage | |
| CK | 39 | 37 | 23 |
| N1 | 42 | 43 | 24 |
| N2 | 52 | 45 | 33 |
| N3 | 53 | 46 | 29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Zheng, S.; Su, J.; Rong, J.; He, T.; Zheng, Y.; Chen, L. Analyzing the Impact of Simulated Nitrogen Deposition on Stoichiometric Properties and Yield of Ma Bamboo (Dendrocalamus latiflorus Munro) Shoots, Leaves, and Soil Substrate. Forests 2024, 15, 151. https://doi.org/10.3390/f15010151
Lin Y, Zheng S, Su J, Rong J, He T, Zheng Y, Chen L. Analyzing the Impact of Simulated Nitrogen Deposition on Stoichiometric Properties and Yield of Ma Bamboo (Dendrocalamus latiflorus Munro) Shoots, Leaves, and Soil Substrate. Forests. 2024; 15(1):151. https://doi.org/10.3390/f15010151
Chicago/Turabian StyleLin, Yuwei, Suyun Zheng, Jianlin Su, Jundong Rong, Tianyou He, Yushan Zheng, and Liguang Chen. 2024. "Analyzing the Impact of Simulated Nitrogen Deposition on Stoichiometric Properties and Yield of Ma Bamboo (Dendrocalamus latiflorus Munro) Shoots, Leaves, and Soil Substrate" Forests 15, no. 1: 151. https://doi.org/10.3390/f15010151
APA StyleLin, Y., Zheng, S., Su, J., Rong, J., He, T., Zheng, Y., & Chen, L. (2024). Analyzing the Impact of Simulated Nitrogen Deposition on Stoichiometric Properties and Yield of Ma Bamboo (Dendrocalamus latiflorus Munro) Shoots, Leaves, and Soil Substrate. Forests, 15(1), 151. https://doi.org/10.3390/f15010151






