Abstract
Fire can have negative effects on the ant community by reducing species abundance through direct mortality, changes in resource availability, or foraging activity. Fire can also have positive effects, especially for opportunistic species preferring open or disturbed habitats. We assessed the direct effects of a large-scale fire on ant communities in open habitats (grassland and Carex) and moist forested peatland (birch and alder) sites in Biebrza National Park, testing three hypotheses: (i) the large-scale fire had more significant effects on ant nest density in forests than in open habitats, (ii) the post-fire ant diversity changes within sites are stronger in forests than open habitats, and (iii) ant species preferring disturbed habitats are favoured by the fire event. The fire had negative effects on ant nest density only in the Carex and grassland sites but not in the birch and alder sites, suggesting that fire had a stronger impact in open habitats than in forests. Temporal post-fire ant diversity changes within sites were stronger in forests than in open habitats. We observed higher beta diversity changes between the first and second year of the study in the burned forest sites due to colonisation, indicating a greater fire impact on species community composition followed by a higher recolonisation rate. Ant species preferring disturbed habitats were favoured by the fire. The seed-eating ant species Tetramorium caespitum, a thermophilous and opportunistic species, dominated the burned grassland site. This contrasts with other species, e.g., Lasius alienus, for which nest density decreased after fire, underlining the importance of food resource availability as a major driver of community changes after fire. Our study also underlines the importance of periodic biodiversity monitoring in conservation areas for assessing the recovery of the original status after disturbances and revealing possible habitat changes endangering the survival of local biotic communities.
1. Introduction
Disturbances in natural or anthropogenic environments are central incidents influencing the structuring of populations, communities, and ecosystems [1]. Globally, fire can be regarded both as a natural or anthropogenic source of disturbance in many ecosystems [2]. Fire can have a negative impact on wild arthropod communities through an immediate effect via direct mortality of individuals [3], leading to a reduction of species abundance [4,5,6] and indirectly by changes in resource availability [7,8] and foraging activity [9]. Fire, as an anthropogenic disturbance, changes competition between species from what it would naturally be, and one reason for these changes is altered resources [10].
Disturbances not only alter the ant community structure but also greatly reduce the number of species due to disturbances that affect the whole ecosystem functioning and ecosystem services. This is especially clear after the anthropogenic conversion of primary forest to agricultural land [11]. Although some authors state that ants can survive disturbance better than other soil animals [12,13,14], there is still a general controversy regarding the effect of fire on ant communities [15]. Fire can have positive effects, especially for species that prefer open or disturbed habitats for nesting, foraging, or reproduction [8,16]. Ant communities can be highly resilient when many species nest in the soil and are thus protected from fire [17,18], compared to ant communities with a high number of species linked to the vegetation [8,16,19]. In addition, the extent of habitat modification and the relationship between ant species and habitat characteristics can lead to a situation where some species benefit from post-fire habitat modification or homogenisation, and the modified habitat is not suitable for all original species [20,21]. Ant species that were previously present in the area may not be able to colonise the post-fire habitat in the short or medium term. Nevertheless, all species will be affected either in the short or long term since fire causes significant changes in vegetation structure and composition [8,22,23,24]. Therefore, if differences in vegetation structure between burned and unburned areas are small, the effects of fire are less pronounced [25,26].
To date, there are numerous studies addressing the fire effects on ants and the recovery or succession of ant communities after fire in a variety of open fire-prone environments, e.g., grasslands, shrublands, and heathlands, e.g., [4,25,27,28,29,30,31] and in forested environments [32,33,34,35,36,37,38,39,40]. Fire also results in greater spatial heterogeneity in soil moisture and burn severity in forests, whereas open areas, such as grasslands, show highly uniform patterns of soil moisture, vegetation, and burn severity after fire [41]. Thus, fire leads to more severe consequences in forests than in open habitats [27,42] and can result in two main scenarios in forests. The first one shows a highly resilient forest where the plant composition recovers very quickly, e.g., in forests that burn relatively frequently. Although ant species linked to wood or litter have high mortality in fires, fast recolonisation from the surrounding areas is possible, and the forest returns to its previous state in a relatively short time [42]. The second scenario can occur in more complex habitats, like tropical forests, that seldom burn and require a longer period to recover after fire [43], or the changes in soil properties even lead to the development of a different habitat type [44,45].
Open areas and forests offer different feeding and nesting resources to ants (e.g., trees vs. shrubs, higher temperature, and moisture variations in open areas), and thus, fire effects on ant communities are also different [46]. Forest ant species are more sensitive to the effects of fire than ants in open habitats, as structural complexity, productivity, and moist soil conditions might contribute to a higher proportion of ant species belonging to functional groups that are sensitive to environmental changes (e.g., cold-climate specialists) [47] and dependent on trees and arboreal aphids [48]. Therefore, fire may destroy the previous habitats and lead to drier and less productive vegetation (i.e., open habitats) that are less suitable for the original ant communities [26].
Compared with open areas, forest ecosystems are subject to longer and more complex succession processes. From the beginning of forest succession, species are continuously replaced across time until reaching maturity [49], which can be considered the most stable status. However, disturbances can happen at any time, changing the successional stage of the forest even by promoting the development of early successional species in the case of severe disturbances like hurricanes, drought, or fires [50]. Due to climate change and human activities, disturbances are becoming more frequent [51,52]. Fires are considered as one of the most destructive disturbances in forests due to the changes in habitat characteristics and ecosystem functioning [24,53]. Also, in wetlands, fire can play a significant role in modifying biotic communities and habitats of various plant and animal species [54]. These catastrophic consequences can be considered more harmful in natural habitats where the ecosystem functioning reaches a high ecological balance due to the well-established mature communities of plants and animals, such as some national parks.
Biebrza National Park is one of the 23 National Parks in Poland and was created to protect the wetlands of the Biebrza Basin. This National Park includes the largest complex of wetlands in Eastern and Central Europe, called the Biebrza Marshes [55,56]. Small-scale natural fires were present in Biebrza Valley prior to human settlement [57], but in recent decades, fires have started to be one of the major threats to the ecosystems of the National Park due to their increasing frequency and area covered [58]. Monitoring the communities in the park before and after fires is of crucial importance to evaluate the extent of the damages caused by the fires as well as to identify whether the post-fire succession process leads to the restoration of the original habitat or to its transformation into a new habitat type. Therefore, the post-fire monitoring of the biotic communities becomes a valuable tool to evaluate the impact of the disturbance and to assess long-term ecosystem changes [24,59].
In the present study, we focused on the direct effects of large-scale fire on ant communities in open (mineral soil grassland and organic soil Carex) and moist forested peatland (birch and alder) areas in Biebrza National Park and examined how ant communities changed after this disturbance in these areas, one year after the fire. We tested the following hypotheses: (1) the large-scale fire had more significant effects on ant nest density in forests than in open habitats, (2) the post-fire ant diversity changes within sites are stronger in forests than in open habitats, and (3) ant species preferring disturbed habitats are favoured by the fire event.
2. Materials and Methods
2.1. Study Area
The study was conducted in the Biebrza National Park in NE Poland (53°35′30.8″ N 22°53′32.4″ E) in the Biebrza River basin. Biebrza National Park is considered the best-preserved large complex of peatlands in Eastern and Central Europe [56]. The most important parts for bird and wetland protection are the non-forest ecosystems, such as aquatic and open wetland areas. The park covers almost 60,000 ha, dominated by wetland communities [60]. Regularly flooded bogs and wet meadows are dominated by sedges (Carex spp.), and oxbows are covered with reeds [61]. The forests covering the Biebrza National Park are dominated by pine, birch or alder, and dense willow forests and mixed forests can also be found in the area. The climate in this region is continental, with hemiboreal features [62]. The mean annual temperature is 6.6 °C [63], whereas the mean minimum temperature in January is −4.2 °C, and the mean maximum temperature is 17 °C in July [63]. The mean annual precipitation ranges between 536 mm and 610 mm [63].
From 19 to 26 of April 2020, the largest human-caused wildfire in the entire history of Biebrza National Park broke out and burned 5526 ha, mostly over-dried grassland with sedges and reeds of Biebrza marshes [64]. Before the fire, the water level in the Biebrza River was at its lowest April level for 20 years (Institute of Meteorology and Water Management, Poland). As a result of the fire in the open area, more than 90% of the surface vegetation (mainly dead biomass of reeds, tussocks, and grasses from the previous year, but also shrubs and small trees) burned. In the forest, the fire burned the litter and understory and slightly damaged the trees. Soon after the fire, the herbaceous layer began to resprout. By early May, vegetation was still sparse, and a distinct difference could be observed between burned and unburned areas [57]. However, within two months, the green cover had recovered [65]. During the field studies during the summer of 2020, the vegetation in the open burned areas was still sparse, and clear differences between burned and unburned areas could be observed, but in 2021, the differences in vegetation disappeared. In 2021, we observed a regeneration of litter, understory, and trees. The only visible impact of the fire compared to the unburned forested areas was the blackening of the roots and bark of the trees, as well as sporadically dead trees.
2.2. Sampling Methodology
We compared ant communities between burned and unburned control sites to determine the effects of large-scale fires on ant species abundance. Field studies in the area were conducted in July and August 2020 (the first year after the fire) and 2021 (the second year after the fire). The effects of fire on ant communities were studied in habitats representing four plant communities, two open areas: grassland (Sileno otitis-Festucetum) (G) (Figure 1), Carex (Caricetum appropinquatae) (C), and two moist forest types: alder forest (Ribeso nigri-Alnetum) (A) and birch forest (Salici-Betuletum) (B). Additionally, each of the plant communities had two experimental conditions: burned sites (F) and unburned control sites (CTRL, Figure 2). The classification and names of the plant communities’ characteristics of each habitat type are those of Matuszkiewicz [66]. The fieldwork was conducted in eight study sites, each 50 m × 25 m in size. The ant fauna was studied using the manual method of nest searching [67,68]. On each study site, 10 quadratic plots, each with an area of 10 m2 (approximately 3.16 m × 3.16 m; Figure 1), were established on a predefined grid of two lines (with a distance of approx. 10 m to each other) and five plots per line, rendering a total of 80 plots to be searched for ant nests. Nests were searched for in litter and topsoil, in wood fragments, under bark, in sedge tussocks, etc. (Figure 3). To determine the ant species, three to ten workers were taken from each nest, depending on its size. The ants were preserved in 99% ethanol and identified as species using identification keys by Radchenko and Elmes [69], Czechowski et al. [70] and Seifert [71]. We identified the functional groups (opportunistic, cold-climate specialists and cryptic species) according to Hoffman and Andersen [47] and ecological habitats and food preferences according to Radchenko and Elmes [69], Czechowski et al. [70] and Seifert [71]. Cold-climate specialists occur primarily at high latitudes or altitudes, tend to prefer cooler sites, and are sensitive to high levels of disturbance [47]. Note that in this context, the sensitivity to disturbance is of more importance than the climate preference, as we did not study any climatic transects. Opportunists are unspecialised species and well-distributed across environmental gradients and tend to dominate on disturbed sites [47], presumably because they can take advantage of changing resources [72]. Cryptic species are soil-dwelling species occurring widely across environmental gradients, foraging exclusively within soil and litter [47].
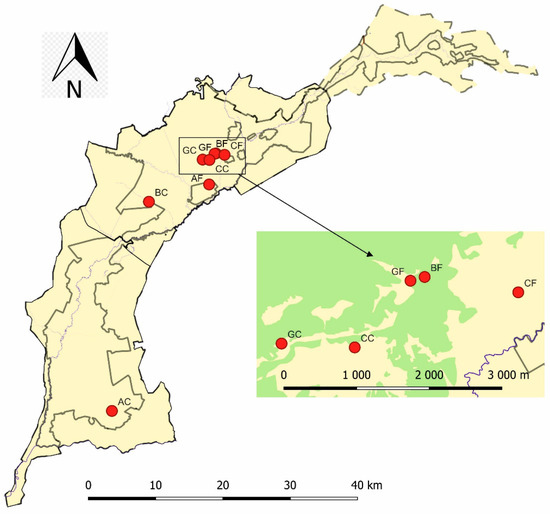
Figure 1.
Spatial location of the study area in Biebrza National Park. Red dots indicate the locations of the study sites (GF: burned grassland, GC: unburned control grassland, CF: burned Carex, CC: unburned control Carex, BF: burned birch forest, BC: unburned control birch forest, AF: burned alder forest, AC: unburned control alder forest). Green areas in the subfigure indicate forest areas and yellowish areas are open areas.
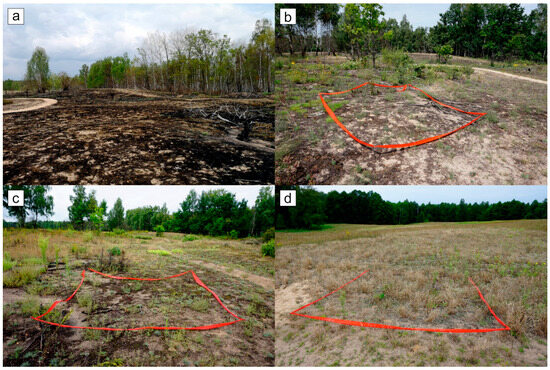
Figure 2.
Example of study plots on grassland communities: the burned grassland site (GF) after 2 weeks (a), after 4 months, coinciding with our first inventory (b), after 16 months, at the time of our second inventory (c), and the unburned control site (GC) (d). The red ribbon delimits the area of one study plot of 10 m2.

Figure 3.
Examples of ant nests on some study plots during the first inventory (4 months after the fire): burned Carex plot (a), burned birch forest plot (b), burned alder forest plot (c) and burned grassland plot (d) during ant sampling.
2.3. Statistical Analyses
The statistical analyses were conducted in several steps, and all statistical testing was conducted in R 4.2.1 [73]. First, we calculated general descriptive results, like nest densities or Shannon diversity indices of nests, separately for each plot and then averaged for each site. Since the Shapiro-Wilk’s test indicated non-normality both for nest density (W = 0.842, p < 0.001) and Shannon diversity (W = 0.840, p < 0.001), all values were sqrt transformed prior to the analyses. We used the ‘lme4’ package in R for conducting linear mixed model analyses and assessing the effects of the three factors site (n = 4), fire (n = 2), and year (n = 2) and their interactions on nest densities and Shannon diversity. The individual plots within each of the eight sites were identified as random effects of the models. Statistically significant interactions between site, fire or year were further analysed with pairwise comparisons using the ‘lsmeans’ command with the Tukey correction.
The temporal changes in beta diversity within a specific treatment (e.g., CTRL grassland in 2020 vs. CTRL grassland in 2021 or burned alder forest in 2020 vs. burned alder forest in 2021) were analysed with the R package ‘ecopart’. This package uses a novel numerical method that additively partitions the temporal changes in multi-site beta diversity into multiple components reflecting the dynamics of beta diversity resulting from local extinctions and colonisations of species [74,75]. We used the ’ecopart.multi’ command with the Whittaker index based on presence-absence data [76].
We then assessed the similarity of sites (habitat types and fire treatments) in terms of ant species assemblages, using the ‘adonis2’ command of the ‘vegan’ package in R and conducting The Nonmetric Multidimensional Scaling (NMDS) analyses of ant species composition intending to reveal differences between sites, separately for 2020 and 2021.
Next, we conducted indicator species analyses (using the ‘indicspecies’ package of R, [77,78]) to identify ant species that were specific for the different habitats and treatments (control and burned ones) separately for 2020 and 2021. The indicator species analysis uses an index called Indicator Value (IndVal), measuring the association between the species and each site group and then looks for the group corresponding to the highest association value and the statistical significance of this relationship is tested with a permutation test. We used the ‘multipatt’ function with 999 permutations without any restrictions for group combinations.
3. Results
3.1. Fire Effects on Changes in Ant Nest Density
A total of 644 nests belonging to 16 ant species were found in the studied areas in 2020 and 2021. In 2020, 372 nests belonging to 16 ant species were found, and in 2021, 272 nests belonging to 10 ant species were found (Table 1). The most abundant ant species in the burned grassland plots was Tetramorium caespitum (31 nests in 2020 and 39 nests in 2021). For the grassland control plots, we found a similar result in 2020 (37 nests of T. caespitum), whereas, in 2021, the largest number of nests belonged to Lasius alienus (67 nests). The burned Carex plots were dominated by Myrmica scabrinodis, whereas the dominant species in the control Carex plots was Myrmica rubra, both in 2020 and 2021. In burned and unburned birch forests, Lasius platythorax was the dominant ant species (Table 1). In the burned alder forest plots, Lasius niger was the predominant species in both years, while in the control alder plots, it was Myrmica rubra (Table 1). The average number of ant species per plot was lower or equal on the burned sites than on the respective control sites (Table 1).

Table 1.
Number of nests and ant species found on the study sites in Biebrza National Park in 2020 and 2021 (burned grassland, unburned control grassland, burned Carex, unburned control Carex, burned birch forest, unburned control birch forest, burned alder forest, unburned control alder forest). Ant species are grouped into functional groups, according to Hoffmann and Andersen [47]. Sums of ten plots per site and plot averages are depicted.
The highest ant nest densities were observed in the control grassland site, being 0.7–1.2 nests per m2, followed by control Carex and control birch forests, where densities were approx. 0.8 and 0.6 nests per m2, respectively (Figure 4A). The lowest nest density was found on burned Carex, as well as birch and alder forests in 2021, being 0.2 nests per m2 or less.
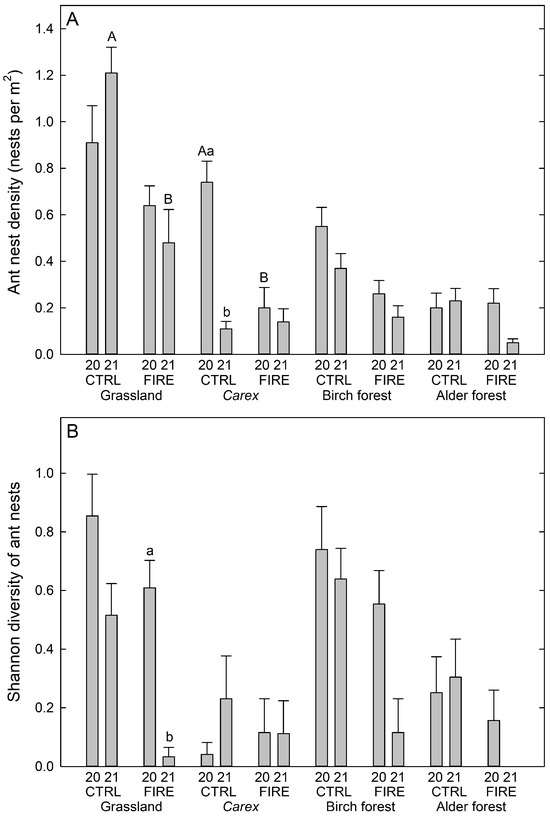
Figure 4.
(A) Ant nest densities (nests per m2), and (B) Shannon diversity index of ant nests for each plot, indicating differences between 2020 and 2021 within the grassland, Carex, birch forest and alder forest sites. Different lowercase letters indicate a statistically significant difference between years and in the same site, and capital letters indicate a statistically significant difference between sites in the same year (p < 0.05). Values are averages of 10 plot-wise values. Note that the value for the Shannon diversity index for the burned alder forest in 2021 is zero (no plots with more than one species).
In addition to significant effects of site (df = 3, 144, F = 3.891, p = 0.010) and year (df = 1, 144, F = 8.183, p = 0.005) for ant nest densities, we also found a significant interaction between site, fire, and year (df = 3, 144, F = 5.676, p = 0.001), although fire alone was not significant (df = 1, 144, F = 0.008, p = 929). More detailed post hoc comparisons revealed that the nest density in 2021 on the burned grassland plots was lower than on the respective control plots (p < 0.001). Furthermore, the nest density was lower on burned Carex plots in 2020 when compared to the respective control plots (p = 0.003, Figure 4A). In addition, the nest density on the control Carex plots was lower in 2021 than in 2020 (p < 0.001, Figure 4A).
3.2. Post-Fire ant Diversity Changes
On average, the highest Shannon diversity indices were found on control grassland and birch forests in 2020 (Figure 4B). We found significant effects of the main factors site (df = 3, 106.4 F = 4.042, p = 0.009) and year (df = 1, 108.5, F = 9.656, p = 0.002) and for the interactions site*year (df = 3, 106.3, F = 4.668, p = 0.004) and fire*year (df = 1, 106.1, F = 4.990, p = 0.034). Fire alone was not significant (df = 1, 106.7, F = 1.408, p = 0.238). Post hoc tests revealed a significant decrease in the Shannon index on burned grassland plots from 2020 to 2021 (p = 0.008, Figure 4B). The average Shannon index was zero for the burned alder forest site in 2021 since there were no plots with more than one ant species.
From 2020 to 2021, beta diversities increased in all burned and the control Carex sites, whereas they decreased in the rest of the control sites (Figure 5). Species extinction (mainly of Myrmica rubra, M. ruginodis and Lasius niger) added to the increases in beta diversities within the sites, except for the control grassland site. Colonisation (mainly of Myrmica ruginodis, Lasius platythorax, and Myrmica rubra) resulted in increased beta diversities within burned sites, whereas it resulted in lower beta diversity in control sites, except the control Carex site, where it also added to a higher diversity within site (Figure 5).
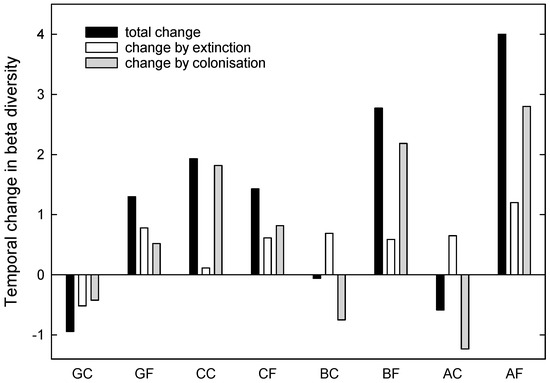
Figure 5.
Temporal changes of beta diversities between 2020 and 2021 in the study sites (more details in Table 2), assessed by presence absence data of ant species (GC: unburned control grassland, GF: burned grassland, CC: unburned control Carex, CF: burned Carex, BC: unburned control birch forest, BF: burned birch forest, AC: unburned control alder forest, AF: burned alder forest). Total changes are additive effects of changes by extinction and colonisation of species.
3.3. Effect of Fire on Ant Species
Similarity analyses for ant species assemblages in 2020 and 2021 suggested significant differences between sites for 2020 (df = 7, R2 = 0.541, F = 10.598, p < 0.001), as well as for 2021 (df = 7, R2 = 0.439, F = 5.687, p < 0.001). The NMDS analyses showed patterns between ant community compositions (Figure 6A,B). The NMDS analyses indicated that, in 2020 and 2021, burned and control grassland sites were overlapping with each other but were separated from all the other sites (Figure 6A,B). In 2020, ant species composition on burned alder and Carex sites was separated from unburned Carex and alder and birch forest sites (CC, AC, BC, and BF) (Figure 6A), but in 2021, they overlapped with the others (Figure 6B).
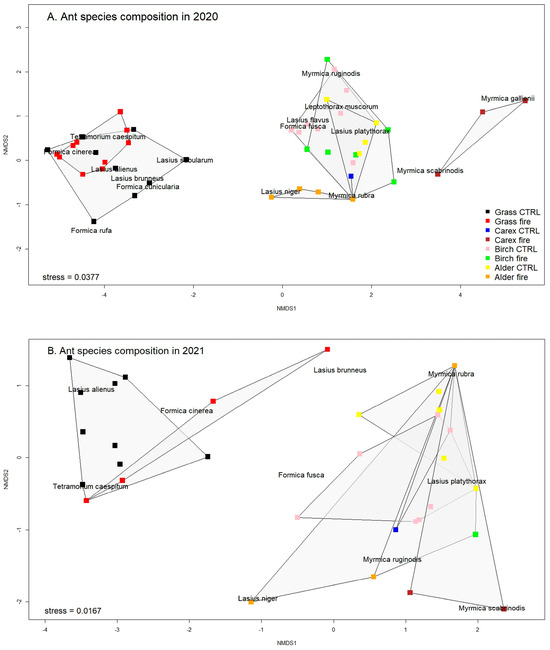
Figure 6.
NMDS plots for ant species compositions (A) for 2020 and (B) for 2021. Different sites are depicted with different symbols. Ant species and stress values are indicated. Stress values equal to or below 0.1 are considered as good, while values equal to or below 0.05 indicate a very good fit. A stress value around 0.2 could still be regarded as fair, but values approaching 0.3 indicate that the ordination is arbitrary.
In total, ten ant species were found to be indicator species in 2020, six species in 2021, and six ant species were indicator species for both years (Table 2). Lasius brunneus was found only on control grassland plots in 2020, and it occurred on half of the plots (Table 2). Myrmica rubra was an indicator species on unburned control plots (CC, BC and AC, Table 2). Formica fusca and Myrmica ruginodis were indicator species for control birch forest plots, but only in 2020 (Table 2). Myrmica scabrinodis and Lasius niger were indicator species for burned Carex and alder forest plots (Table 2). Indicator species for grassland plots (GC and GF) in 2020 and 2021 were Tetramorium caespitum and Formica cinerea (Table 2). Lasius alienus was an indicator species for grassland plots in 2020 (GC and GF), but in 2021, it was an indicator species only for the unburned grassland plots (GC, Table 2). Lasius platythorax was an indicator species for forest habitats (BC, BF and AC, Table 2).

Table 2.
Results of indicator species analysis (ISA) for ant species in 2020 and 2021 in the study area (GF: burned grassland, GC: unburned control grassland, CF: burned Carex, CC: unburned control Carex, BF: burned birch forest, BC: unburned control birch forest, AF: burned alder forest, AC: unburned control alder forest). The table shows only significant indicator species. Generally, the higher the tabulated value (%), the more the species in question is restricted to the respective group: ‘A’ indicates the percentage of a site belonging to the target group given that the species has been found, ‘B’ indicates the percentage of a species occurring on a site belonging in the target group. For example, a value of A = 100 indicates that the species occurs only on the respective site, and a value of B = 100 indicates that the species occurs on all the sites in question. ‘P’ indicates the p-value. Statistical significances: *** < 0.001, ** < 0.01 and * < 0.05.
4. Discussion
4.1. Fire Effects on Ant Nest Density in Forests and in Open Habitats
In both the first and second year after the large-scale fire in Biebrza National Park, we found significantly lower ant nest densities on the open plots, i.e., burned grassland or Carex plots. Ants increase soil fertility and production [44,45] and can thus help to accelerate the recovery of the soil after fire disturbance. Therefore, the post-fire reduction of the number of nests themselves can lead to a slower recovery of the original habitat. Depending on the fire intensity, the disturbance might not result in a total loss of the ant population. Since only 2% of the total population of a mature ant colony is active above ground [79], direct mortality during fire might be limited to a small percentage of foragers, allowing the majority of the colony to survive [16]. At the same time, the insulating properties of the soil can protect most of the colony from high lethal temperatures [33]. These factors can result in situations when a fire has relatively small, although still significant, effects on ant abundance and diversity [16,33,72].
In our data, the difference in nest density between unburned and burned Carex sites was significant in 2020 but not in 2021, which may suggest that other factors, e.g., precipitation or periodic flooding, could have affected nest density. The drastic decrease in nest density in burned Carex plots was connected to one single species, Myrmica rubra. This species is the most hygrophilous Central-European Myrmica species [70], but it can still survive in drier habitats [70,71]. Therefore, the reduction of its population could be related to the fact that the fire reduced soil moisture, and therefore, more drastic density changes (loss of Myrmica rubra) occurred in 2020. In 2021, on the other hand, a high water level could be responsible for the decrease in nest density in the unburned Carex site. The nest density in 2021 on the burned grassland site was significantly lower than on the respective control site. These differences are probably due to the complete disappearance of the Lasius alienus nests in the burned grassland site and the increase in the density of this species in the control site. Altogether, we did not observe any significant fire effects on ant nest densities in forested areas, in contrast, to open habitats, which led us to reject our first hypothesis, stating that the fire had more significant effects on ant nest density in forests than in open habitats.
4.2. Post-Fire Ant Diversity Changes in Forests and in Open Habitats
We observed that in mainly all cases, fire increased beta diversities within sites from 2020 to 2021. Beta diversity defines “the extent of change in community composition, or degree of community differentiation, in relation to a complex gradient of environment, or a pattern of environments” [75]. Thus, beta diversity quantifies the number of different communities in the region or at a site. In our data, increases in beta diversities caused by extinction were mainly caused by the opportunistic species Myrmica rubra, M. ruginodis and the cold-climate specialist Lasius niger. Increases in beta diversity by colonisation occurred mainly in burned sites and were mostly caused by two opportunistic species, Myrmica rubra, M. ruginodis, and the cold-climate specialist, Lasius platythorax. We observed greater beta diversity changes in the burned forest sites due to colonisation, indicating a greater fire effect and, consequently, a higher recolonisation rate. Opportunistic species, unspecialised and occurring widely across environmental gradients and predominating at sites with high levels of disturbance, added both to species colonisation and extinction, whereas more specialised cold-climate species, sensitive to disturbances, added only either to extinction or colonisation. In the latter case, ant species could have escaped from burned sites to search for temporary habitats and food resources and then returned when the original sites were recovered.
The effects of fire on ant communities are not restricted to their abundance, but fire also affects the densities of their nests. However, most studies concentrated on the results of fire disturbance that are reflected in changes in the community composition, e.g., [22,25]. It must be stated that higher nest density does not necessarily mean more ant species. Our data showed that the fire effect on nest density varied, but the species number was always higher on control plots compared to burned plots, similar to the results of Fagundes et al. [80]. This could be due to the lower availability of food resources after the fire, leading to the coexistence of fewer ant species after disturbance [37].
Considering that ant species can differ in their function in the ecosystem (i.e., seed dispersion by seed-eating ants, wood decomposition by ants nesting in wood or pest control by predators), changes in species composition can play an essential role in the post-fire succession process. Many of the previous studies suggest that habitat changes during the succession after the fire are more important in rebuilding the ant community structure than the effects of fire immediately after its occurrence [27,38]. An immediate consequence of high-intensity fires is the complete removal of vegetation, resulting in the destruction of available food and nesting site resources [23,35,37,81], as well as an increase in temperature and a decrease in soil moisture [17,82]. Overall, our results suggest our second hypothesis, that temporal post-fire ant diversity changes are stronger in forests than in open habitats, to be confirmed.
4.3. Are Ant Species Preferring Disturbed Habitats Favoured by Fire?
The Nonmetric Multidimensional Scaling (NMDS) analyses showed clear patterns of the ant community composition and sites. Unburned and burned grassland habitats overlapped each other. The NMDS analyses suggest that grasslands have a distinctive ant community that is different from the other habitats studied. In the first year of monitoring, we found that after burning, Carex and alder forest sites were separated from the unburned ones. In the second year, all mentioned sites overlapped, but grasslands remained separated. All these changes could relate to the variation in ant composition across time. For example, the significantly higher nest density on unburned grassland than on the burned grassland site seems to be connected with the increase in nest density of Lasius alienus, an intensively trophobiotic species whose diet is highly dependent on aphid honeydew [70,71]. Considering that ants may move their colonies to more suitable places if their original habitat is perturbed [83], the reduction of available food sources due to the fire might be related to the forced migration of the colonies from burned habitats to others with better conditions for survival. Although we did not observe differences in ant nest density and diversity between burned and unburned control plots in the birch and alder forests of our study, changes in the habitat facilitated the colonisation by ant species that are not common in these habitat types. For example, Lasius niger and Myrmica rubra were present in both forests just after the fire. These species are typical for relatively open habitats (such as meadows or semi-dry grasslands [70]), which show a high density of nests during the first year of our study. In the second year after the fire, when the habitat had recovered to pre-fire characteristics, there was a drastic decline in their populations.
It is known that ants are strongly linked to habitat characteristics and could thus be used as bio-indicators for environmental changes [84,85]. We found the dendrophilous species and cold-climate specialist, Lasius brunneus, only in grasslands. Despite this, species mainly occur on sites with broad-leaved trees and dense forests; they can also be found in agricultural areas or human settlements where they can nest under bricks or stones [70,71]. Tetramorium caespitum, Formica cinerea and Lasius alienus are thermophilous species of open habitats or light pine forests [71], which is consistent with their occurrence on the xerothermic grasslands in our study. Nevertheless, the trends of their population change varied across time according to the differences in some functional traits. The population of T. caespitum, an opportunistic seed-eating species, dominated the burned grassland site. Similar results have been found in other studies, showing that the populations of seed-eating species seem to be favoured after fires in grasslands due to a higher availability of its food source [7,8,86]. The fire seems to affect the presence of seeds less than the existence of aphids and, thus, honeydew foraging. Andersen [87] reported that the increase of seed-eating ants increased rapidly after a fire and continued up to two years after the fire. This indicates that after a disturbance like fire, seed-eating ants are at an advantage over ants that are specialised in honeydew. The populations of Formica cinerea and Lasius alienus, the former an opportunistic species and the latter a cold-climate specialist, typically found in this habitat type, were reduced or even extinct. This seems to be related to a low availability of honeydew due to the destruction of surface vegetation and honeydew-producing arthropods, such as aphids. In our study, Formica fusca and Myrmica ruginodis, both opportunistic species, were indicator species for unburned birch forests (in 2020). Formica fusca, a species inhabiting a wide range of habitat types, is mainly found in xerothermous dry, open, or moderately shadowy habitats and generally avoids forests after canopy closure [71]. However, in Poland it can be also found in mid-forest glades, young forests, peatlands, and dense, moist forests with abundant understorey [70]. We observed that in unburned birch forests, Formica fusca built their nests on elevated terrain, e.g., clumps of sedges or grasses. Commonly, its nests are found in the soil, but they can also be found in rotten tree stumps and decaying litter. Myrmica ruginodis is found in all kinds of forests, like scrublands, woodland clearings, and peatlands [71], where nests are commonly found in rotten wood or under bark. Besides the nesting place, F. fusca and M. ruginodis share their nutritional traits, such as honeydew from aphids or floral nectaries [70]. Myrmica rubra, an opportunistic species, was an indicator species for unburned control Carex, birch, and alder forest plots, which is consistent with its occurrence reported by Seifert [71]. This species is typical for humid meadows, and its population suffered a reduction in the second year of our study in Carex and birch plots. As mentioned above, this decrease might not be a direct effect of fire but could be rather connected to the soil moisture level. Finally, two species were indicator species for burned plots, the opportunistic Myrmica scabrinodis for Carex plots and the cold-climate specialist Lasius niger for alder forest plots. Myrmica scabrinodis is typical for grasslands, bogs, and marshes but is also present in open forests and woodlands [69]. Lasius niger, showing more xerothermic preferences, is commonly present in moderately dry to mesophilous soils [71]. Therefore, the higher presence of L. niger in the forest habitats during the first year of study, as well as the reduction of the populations of both species during the second year, reflect the habitat changes due to the succession after the fire. We observed the cold-climate specialist Lasius platythorax to be an indicator species of forest habitats, which is consistent with its ecology and its occurrence in all kinds of forest types [71]. Altogether, our results supported our third hypothesis that ant species preferring disturbed habitats are favoured by fire. Generally, cold-climate specialists, sensitive to high levels of disturbance, occurred more often in control sites than opportunistic species, which occurred both on burned and unburned sites.
5. Conclusions
To conclude, the habitat disturbance in Biebrza National Park due to the fire in 2020 showed significant effects on ant nest density in open habitats, but already in the second year of our observations, the post-fire habitat changes led to a homogenisation of the ant communities. In burned habitats, the opportunistic, seed-eating species Tetramorium caespitum dominated the ant population, whereas in unburned habitats, the presence of those species increased, which most probably escaped from burned habitats, searching for more suitable conditions for survival. Our study suggested that the role of food source availability could be an important factor in the survival and resilience of the ant species after fire. The successional process of recovery to pre-fire characteristics can also be reflected in the substitution of opportunistic species by specialist species typical for these habitat types during the second year of study. Our study underlines the importance of periodic biodiversity monitoring in conservation areas to assess the recovery of its original status after disturbances. By biodiversity monitoring, it is possible to capture changes in the habitat characteristics that might endanger the survival of the local biotic communities.
Author Contributions
Conceptualization, I.S.; Methodology, I.S.; Formal Analysis, I.S. and T.D.; Investigation, I.S.; Writing—Original Draft Preparation, I.S. and T.D.; Writing—Review and Editing, I.S. and T.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Forest Fund from the State Forests under contract numbers EZ.0290.1.24.2020, EZ.0290.1.4.2021, and EZ.0290.1.3.2022 with the Biebrza National Park.
Data Availability Statement
The data presented in this study are available in the article. Additional data are available on request from the corresponding author.
Acknowledgments
We would like to thank Gema Trigos-Peral for the identification of the ant species and for commenting on an earlier version of the manuscript. We would also like to thank Krzysztof Sućko for his help during the fieldwork and for preparing the map showing the location of the study area, Agnieszka Henel from Biebrza National Park for specialist consultations and two anonymous reviewers for comments on an earlier version of the manuscript.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mouillot, D.; Graham, N.A.J.; Villéger, S.; Mason, N.W.H.; Bellwood, D.R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Bowman, D.M.J.S.; Balch, J.K.; Artaxo, P.; Bond, W.J.; Carlson, J.M.; Cochrane, M.A.; D’Antonio, C.M.; DeFries, R.S.; Doyle, J.C.; Harrison, S.P.; et al. Fire in the Earth System. Science 2009, 324, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.E.; Bock, J.H. Response of grasshoppers (Orthoptera: Acrididae) to wildfire in a southern Arizona grassland. Am. Midl. Nat. 1990, 125, 162–167. [Google Scholar] [CrossRef]
- Andersen, A.N.; Müller, W.J. Arthropod responses to experimental fire regimes in an Australian tropical savannah: Ordinal level analyses. Austral Ecol. 2000, 25, 199–209. [Google Scholar] [CrossRef]
- Sanders, N.J. Immediate effects of the fire on the invasive argentine ant, Linepithema humile. Southwest Natl. 2004, 49, 246–250. [Google Scholar] [CrossRef]
- Verble-Pearson, R.M.; Yanoviak, S.P. Effects of Fire Intensity on Litter Arthropod Communities in Ozark Oak Forests, Arkansas, USA. Am. Midl. Nat. 2014, 172, 14–24. [Google Scholar] [CrossRef]
- Arnan, X.; Rodrigo, A.; Retana, J. Post-fire recovery of Mediterranean grand ant communities follows vegetation and dryness gradients. J. Biogeogr. 2006, 33, 1246–1258. [Google Scholar] [CrossRef]
- Arnan, X.; Cerdá, X.; Rodrigo, A. Do Forest Fires Make Biotic Communities Homogeneous or Heterogeneous? Patterns of Taxonomic, Functional, and Phylogenetic Ant Beta Diversity at Local and Regional Landscape Scales. Front. For. Glob. Change 2020, 3, 67. [Google Scholar] [CrossRef]
- Araújo, M.S.; Della Lucia, M.C.; Picanco, M.C. Impacto da queima da palhaça da cana-de-açúcar no ritmo diário de forrageamento de Atta bisphaerica Forel (Hymenoptera, Formicidae). Rev. Bras. Zool. 2004, 21, 33–38. [Google Scholar] [CrossRef][Green Version]
- Filgueiras, B.K.C.; Peres, C.A.; Melo, F.P.L.; Leal, I.R.; Tabarelli, M. Winner–Loser Species Replacements in Human-Modified Landscapes. Trends Ecol. Evol. 2021, 36, 545–555. [Google Scholar] [CrossRef]
- Floren, A.; Freking, A.; Biehl, M.; Linsenmair, K.E. Anthropogenic disturbance changes the structure of arboreal tropical ant communities. Ecography 2001, 24, 547–554. [Google Scholar] [CrossRef]
- Pryke, J.S.; Samways, M.J. Differential resilience of invertebrates to fire. Austral Ecol. 2012, 37, 460–469. [Google Scholar] [CrossRef]
- Doamba, S.W.M.F.; Savadogo, P.; Nacro, H.B. Effects of burning on soil macrofauna in a savanna-woodland under different experimental fuel load treatments. Appl. Soil Ecol. 2014, 81, 37–44. [Google Scholar] [CrossRef]
- Buckingham, S.; Murphy, N.; Gibb, H. Effects of fire severity on the composition and functional traits of litter-dwelling macroinvertebrates in a temperate forest. For. Ecol. Manag. 2019, 434, 279–288. [Google Scholar] [CrossRef]
- He, T.; Lamont, B.B.; Pausas, J.G. Fire as a key driver of Earth’s biodiversity. Biol. Rev. 2019, 94, 1983–2010. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Turschak, G.; Brehme, C.; Rochester, C.; Mitrovich, M.; Fischer, R. Effects of large-scale wildfires on ground foraging ants (Hymentoptera: Formicidae) in Southern California. Environ. Entomol. 2011, 40, 204–216. [Google Scholar] [CrossRef]
- Andersen, A.N.; Yen, A.L. Immediate effects of fire on ants in the semi-arid mallee region of northwestern Victoria. Austral Ecol. 1985, 10, 25–30. [Google Scholar] [CrossRef]
- Neumann, F.G. Responses of foraging ant populations to high-intensity wildfire, salvage logging and natural regeneration processes in Eucalyptus regnans regrowth forest of the Victorian Central Highlands. Aust. For. 1992, 55, 29–38. [Google Scholar] [CrossRef]
- Rosa, T.F.; Camarota, F.; Zuanon, L.A.; Tito, R.; Maravalhas, J.B.; Powell, S.; Vasconcelos, H.L. The effects of high-severity fires on the arboreal ant community of a Neotropical savanna. Oecologia 2021, 196, 951–961. [Google Scholar] [CrossRef]
- Neumann, F.G.; Tolhurst, K. Effects of fuel reduction burning on epigeal arthropods and earthworms in dry sclerophyll eucalypt forest of west-central Victoria. Austral Ecol. 1991, 16, 315–330. [Google Scholar] [CrossRef]
- York, A. The long-term effects of fire on forest ant communities: Management implications for the conservation of biodiversity. Mem. Qld. Mus. 1994, 36, 231–239. [Google Scholar]
- Ratchford, J.S.; Wittman, S.E.; Jules, E.S.; Ellison, A.M.; Gotelli, N.J.; Sanders, N.J. The effects of fire, local environment, and time on ant assemblages in fens and forests. Divers. Distrib. 2005, 11, 487–497. [Google Scholar] [CrossRef]
- York, A. Long-term effects of frequent low-intensity burning on ant communities in coastal blackbutt forests of southeastern Australia. Austral Ecol. 2000, 25, 83–98. [Google Scholar] [CrossRef]
- Vidal-Cordero, J.M.; Arnan, X.; Rodrigo, A.; Cerdá, X.; Boulay, R. Four-year study of arthropod taxonomic and functional responses to a forest wildfire: Epigeic ants and spiders are affected differently. For. Ecol. Manag. 2022, 520, 120379. [Google Scholar] [CrossRef]
- Parr, C.L.; Robertson, H.G.; Biggs, H.C.; Chown, S.L. Response of African savanna ants to long-term fire regimens. J. Appl. Ecol. 2004, 41, 630642. [Google Scholar] [CrossRef]
- Arruda, F.V.; Izzo, T.J.; Teresa, F.B.; Camarota, F. Different burning intensities affect cavity utilization patterns by arboreal ants in a tropical savanna canopy. Ecol. Indic. 2020, 116, 106493. [Google Scholar] [CrossRef]
- Ahlgren, I.F. The effect of fire on soil organisms. In Fire and Ecosystems; Kozlowski, T.T., Ahlgren, C.E., Eds.; Academic Press: New York, US, USA, 1974; pp. 7–72. [Google Scholar]
- Lyon, L.J.; Crawford, H.S.; Czuhai, E.; Fredriksen, R.L.; Harlow, R.F.; Metz, L.J.; Pearson, H.A. Effects of fire on fauna: A state-of-knowledge review. In General Technical Report WO-6; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1978. [Google Scholar]
- Warren, S.D.; Scifres, C.J.; Teel, P.D. Response of grassland arthropods to burning: A review. Agric. Ecosyst. Environ. 1987, 19, 105–130. [Google Scholar] [CrossRef]
- Hoffmann, B.D. Responses of ant communities to experimental fire regimes on rangelands in the Victoria River District of the Northern Territory. Austral Ecol. 2003, 28, 182–195. [Google Scholar] [CrossRef]
- Underwood, E.C.; Christian, C.E. Consequences of Prescribed Fire and Grazing on Grassland Ant Communities. Community Ecol. 2009, 38, 325–332. [Google Scholar] [CrossRef]
- Whitford, W.G.; Gentry, J.B. Ant communities of southeastern longleaf pine plantations. Environ. Entomol. 1981, 10, 183–185. [Google Scholar] [CrossRef]
- Andersen, A.N. Response of ground-foraging ant communities to three experimental regimes in a savanna forest of tropical Australia. Biotropica 1991, 23, 575–585. [Google Scholar] [CrossRef]
- MacKay, W.P.; Rebeles, A.M.; Arredondo, H.C.B.; Rodriguez, A.D.R.; Gonzalez, D.A.; Vinson, S.B. Impact of the slashing and burning of a tropical rain forest on the native ant fauna (Hymenoptera: Formicidae). Sociobiology 1991, 18, 257–268. [Google Scholar]
- Punttila, P.; Haila, Y.; Pajunen, T.; Tukia, H. Colonisation of clearcut forests by ants in the southern Finnish taiga: A quantitative survey. Oikos 1991, 61, 250–262. [Google Scholar] [CrossRef]
- Punttila, P.; Haila, Y. Colonisation of a burned forest by ants in the southern Finnish boreal forest. Silva Fenn. 1996, 30, 421–435. [Google Scholar] [CrossRef]
- Rodrigo, A.; Retana, J. Post-fire recovery of ant communities in Submediterranean Pinus nigra forests. Ecography 2006, 29, 231–239. Available online: https://www.jstor.org/stable/3683484 (accessed on 10 October 2022). [CrossRef]
- Santos, J.C.; Delabie, J.H.C.; Fernandes, G.W. A 15-year post evaluation of the fire effects on ant community in an area of Amazonian forest. Rev. Bras. Entomol. 2008, 52, 82–97. [Google Scholar] [CrossRef][Green Version]
- Arnan, X.; Cerdá, X.; Rodrigo, A.; Retana, J. Response of ant functional composition to fire. Ecography 2013, 36, 1182–1192. [Google Scholar] [CrossRef]
- Véle, A.; Holuša, J.; Trägnerová, J. Ant succession on burned areas in forested landscape: A case study from the Bohemian Switzerland National Park. Zprávy Lesn. Výzk. 2015, 60, 47–52. [Google Scholar]
- Hamilton, N.P.; Burton, P.J. Wildfire disturbance reveals evidence of ecosystem resilience and precariousness in a forest–grassland mosaic. Ecosphere 2023, 14, e4460. [Google Scholar] [CrossRef]
- Vasconcelos, H.L.; Maravalhas, J.B.; Cornelisen, T. Effects of fire disturbance on ant abundance and diversity: A global meta-analysis. Biodivers. Conserv. 2017, 26, 177–188. [Google Scholar] [CrossRef]
- Barlow, J.; Peres, C.A.; Lagan, B.O.; Haugaasen, T. Large tree mortality and the decline of forest biomass following Amazonian wildfires. Ecol. Lett. 2003, 6, 6–8. [Google Scholar] [CrossRef]
- Frouz, J.; Holec, M.; Kalčik, J. The effect of Lasius niger (Hymenoptera, Formicidae) ant nest on selected soil chemical properties. Pedobiologia 2003, 47, 205–212. [Google Scholar] [CrossRef]
- Dostal, P.; Breznova, M.; Kozlickova, V.; Herben, T.; Kovar, P. Ant-induced soil modification and its effect on plant below-ground biomass. Pedobiologia 2005, 49, 127–137. [Google Scholar] [CrossRef]
- Maravalhas, J.; Vasconcelos, H.L. Revisiting the pyrodiversity–biodiversity hypothesis: Long-term fire regimes and the structure of ant communities in a Neotropical savanna hotspot. J. Appl. Ecol. 2014, 51, 1661–1668. [Google Scholar] [CrossRef]
- Hoffmann, B.D.; Andersen, A.N. Patterns of European Ant Communities Reveal a Functionally Coherent Holarctic Fauna. Diversity 2023, 15, 341. [Google Scholar] [CrossRef]
- Vieira, J.; Camarota, F.; Vasconcelos, H.L. Trophic ecology of the arboreal and ground ant communities in forests and savannas of central Brazil. Ecol. Entomol. 2021, 46, 936–945. [Google Scholar] [CrossRef]
- Lienard, J.; Florescu, I.; Strigul, N. An appraisal of the classic forest succession paradigm with the shade tolerance index. PLoS ONE 2015, 10, e0117138. [Google Scholar] [CrossRef]
- Łaska, G. The disturbance and vegetation dynamics: A review and an alternative framework. Plant Ecol. 2001, 157, 77–99. [Google Scholar] [CrossRef]
- Danneyrolles, V.; Dupuis, S.; Fortin, G.; Leroyer, M.; de Römer, A.; Terrail, R.; Vellend, M.; Boucher, Y.; Laflamme, J.; Bergeron, Y.; et al. Stronger influence of anthropogenic disturbance than climate change on century-scale compositional changes in northern forests. Nat. Commun. 2019, 10, 1265. [Google Scholar] [CrossRef]
- Malhi, Y.; Franklin, J.; Seddon, N.; Solan, M.; Turner, M.G.; Field, C.B.; Knowlton, N. Climate change and ecosystems: Threats, opportunities and solutions. Philosophical Transactions of the Royal Society B: Biol. Sci. 2019, 375, 20190104. [Google Scholar] [CrossRef]
- Arnán, X.; Rodrigo, A.; Retana, J. Post-fire regeneration of Mediterranean plant communities at a regional scale is dependent on vegetation type and dryness. J. Veg. Sci. 2007, 18, 111–122. [Google Scholar] [CrossRef]
- Osborne, T.Z.; Kobziar, L.N.; Inglett, P.W. Fire and water: New perspectives on fire’s role in shaping wetland ecosystem. Fire Ecol. 2013, 9, 1–5. [Google Scholar] [CrossRef]
- Dyrcz, A.; Werpachowski, C. Przyroda Biebrzańskiego Parku Narodowego—Monografia; Biebrzański Park Narodowy: Osowiec-Twierdza, Poland, 2005. [Google Scholar]
- Żurek, S. Rzeźba i budowa geologiczna Doliny Biebrzy. In Przyroda Biebrzańskiego Parku Narodowego; Dyrcz, A., Werpachowski, C., Eds.; Biebrzański Park Narodowy: Osowiec-Twierdza, Poland, 2005; pp. 19–32. [Google Scholar]
- Walesiak, M.; Mikusiński, G.; Borowski, Z.; Żmihorski, M. Large fire initially reduces bird diversity in Poland’s largest wetland biodiversity hotspot. Biodivers. Conserv. 2022, 31, 1037–1056. [Google Scholar] [CrossRef]
- Dembek, W.; Oświt, J.; Rycharski, M. Torfowiska i torfy w pradolinie Biebrzy. In Przyroda Biebrzańskiego Parku Narodowego; Dyrcz, A., Werpachowski, C., Eds.; Biebrzański Park Narodowy: Osowiec-Twierdza, Poland, 2005; pp. 33–58. [Google Scholar]
- Underwood, E.C.; Fisher, B.L. The role of ants in conservation monitoring: If, when, and how. Biodivers. Conserv. 2006, 132, 166–182. [Google Scholar] [CrossRef]
- Sienkiewicz-Paderewska, D.; Paderewski, J.; Chodkiewicz, D.; Kwasowski, W.; Suwara, I. Effect of different land-use on occurrence and morphological traits of Carex buxbaumii (study from Biebrza National Park, Poland). Glob. Ecol. Conserv. 2019, 20, e00802. [Google Scholar] [CrossRef]
- Birdlife International. Important Bird Areas Factsheet: Biebrza River Valley. 2021. Available online: http://datazone.birdlife.org/site/factsheet/biebrza-river-valley-iba-poland (accessed on 1 September 2022).
- Wassen, M.J.; Barendregt, A.; Palczynski, A.; de Smidt, J.T.; de Mars, H. Hydro-ecological analysis of the Biebrza mire (Poland). Wetl. Ecol. Manag. 1992, 2, 119–134. [Google Scholar] [CrossRef]
- Górniak, A. Klimat i Termika Wód Powierzchniowych Kotliny Biebrzańskiej. Aktualny Stan, Walory, Zagrożenia i Potrzeby Czynnej Ochrony Środowiska; Banaszuk, H., Ed.; Monografia Przyrodnicza; Wydawnictwo Ekonomia i Środowisko: Białystok, Poland, 2004; pp. 345–362. [Google Scholar]
- Szczygieł, R.; Kwiatkowski, M.; Kołakowski, B. The attempt to assess the fire risk of non-forest terrestrial ecosystems of Biebrza National park—A case study. Folia For. Pol. Ser. A For. 2021, 63, 167–175. [Google Scholar] [CrossRef]
- Durka, M. Wpływ Pożaru w Biebrzańskim Parku Narodowym w Kwietniu 2020 r. Na Zmienność Wskaźnika NDVI; Jagiellonian University: Kraków, Poland, 2020. [Google Scholar]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisk Leśnych Polski; Vademecum Geobotanicum, PWN: Warsaw, Poland, 2001. [Google Scholar]
- Gotelli, N.J.; Ellison, A.M.; Dunn, R.R.; Sanders, N.J. Counting ants (Hymenoptera Formicidae): Biodiversity sampling and statistical analysis for myrmecologists. Myrmecol. News 2011, 15, 13–19. [Google Scholar]
- Grześ, I.M.; Ślipiński, P.; Babik, H.; Moroń, D.; Walter, B.; Trigos Peral, G.; Maak, I.; Witek, M. Colony size and brood investment of Myrmica rubra ant colonies in habitats invaded by goldenrods. Insect. Soc. 2018, 65, 275–280. [Google Scholar] [CrossRef]
- Radchenko, A.G.; Elmes, G.W. Myrmica Ants (Hymenoptera: Formicidae) of the Old World; Natura Optima Dux Foundation: Warsaw, Poland, 2010. [Google Scholar]
- Czechowski, W.; Radchenko, A.; Czechowska, W.; Vespäläinen, K. The Ants of Poland with Reference to the Myrmecofauna of Europe; Natura Optima Dux Foundation: Warsaw, Poland, 2012. [Google Scholar]
- Seifert, B. The Ants of Central and North Europe; Lutra Verlags- und Vertriebsgesellschaf: Tauer, Germany, 2018. [Google Scholar]
- Philpott, S.M.; Perfecto, I.; Armbrecht, I.; Parr, C.L. Ant diversity and function in disturbed and changing habitats. In Ant Ecology; Lach, L., Parr, C.L., Abbott, K.L., Eds.; Oxford University Press: New York, NY, USA, 2010; pp. 137–156. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org/ (accessed on 10 February 2022).
- Tatsumi, S.; Iritani, R.; Cadotte, M.W. Temporal changes in spatial variation: Partitioning the extinction and colonisation components of beta diversity. Ecol. Lett. 2021, 24, 1063–1072. [Google Scholar] [CrossRef]
- Tatsumi, S.; Iritani, R.; Cadotte, M.W. Partitioning the temporal changes in abundance-based beta diversity into loss and gain components. Methods Ecol. Evol. 2022, 13, 2042–2048. [Google Scholar] [CrossRef]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Stephens, S.S.; Wagner, M.R. Using ground foraging ant (Hymenoptera: Formicidae) functional groups as bioindicators of forest health in northern Arizona ponderosa pine forests. Environ. Entmol. 2006, 35, 937–949. [Google Scholar] [CrossRef]
- Fagundes, R.; Anjos, D.V.; Carvalho, R.; Del-Klaro, K. Availability of food and nesting-sites as regulatory mechanisms for the recovery of ant diversity after fire disturbance. Sociobiology 2015, 62, 1–9. [Google Scholar] [CrossRef]
- Vasconcelos, H.L.; Pacheco, R.; Silva, R.C.; Vasconcelos, P.B.; Lopes, C.T.; Costa, A.N.; Bruna, E.M. Dynamics of the leaf-litter arthropod fauna following fire in a Neotropical woodland savanna. PLoS ONE 2009, 4, e7762. [Google Scholar] [CrossRef] [PubMed]
- Agbeshie, A.A.; Abugre, S.; Atta-Darkwa, T.; Awuah, R. A review of the effects if forest fire on soil properties. J. For. Res. 2022, 33, 1419–1441. [Google Scholar] [CrossRef]
- Trigos-Peral, G.; Casacci, L.P.; Ślipiński, P.; Grześ, I.M.; Moroń, D.; Babik, H.; Witek, M. Ant communities and Solidago plant invasion: Environmental properties and food sources. Entomol. Sci. 2018, 21, 270–278. [Google Scholar] [CrossRef]
- Andersen, A.N. Using ants as bioindicators: Multi-scale issues in ant community ecology. Conserv. Ecol. 1997, 1, 8. [Google Scholar]
- Akhila, A.; Keshamma, E. Recent perspectives on ants as bioindicators: A review. J. Entomol. Zool. Stud. 2022, 10, 11–14. [Google Scholar] [CrossRef]
- Paolucci, L.N.; Schoereder, J.H.; Brando, P.M.; Andersen, A.N. Fire-induced forest transition to derived savannas: Cascading effects on ant communities. Biol. Conserv. 2017, 214, 295–302. [Google Scholar] [CrossRef]
- Andersen, A.N. Immediate and longer-term effects of fire on seed predation by ants in sclerophyllous vegetation in south-eastern Australia. Austral Ecol. 1998, 13, 285–293. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).