Ultra-High-Density Genetic Maps of Jatropha curcas × Jatropha integerrima and Anchoring Jatropha curcas Genome Assembly Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Mapping Population and Plant Material Sampling
2.2. DNA Extraction and Genotyping
2.2.1. AFLP Genotyping
2.2.2. SSR Genotyping
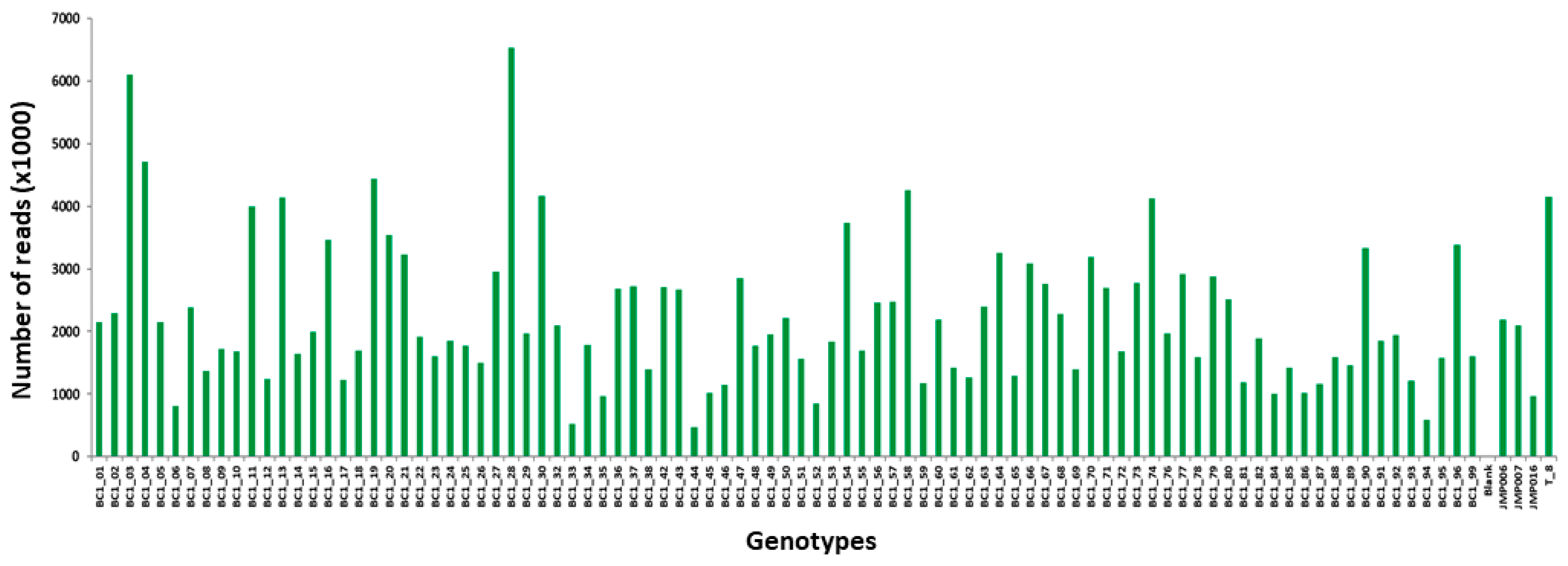
2.2.3. Genotyping-by-Sequencing and SNPs
GBS Library Preparation
Processing of Raw Data Files, SNP Calling
2.3. SNP Annotation
2.4. Linkage Mapping
3. Results
3.1. Molecular Genetic Markers
3.1.1. AFLP
3.1.2. Microsatellite Markers
3.1.3. SNP Markers
3.2. SNP Annotation
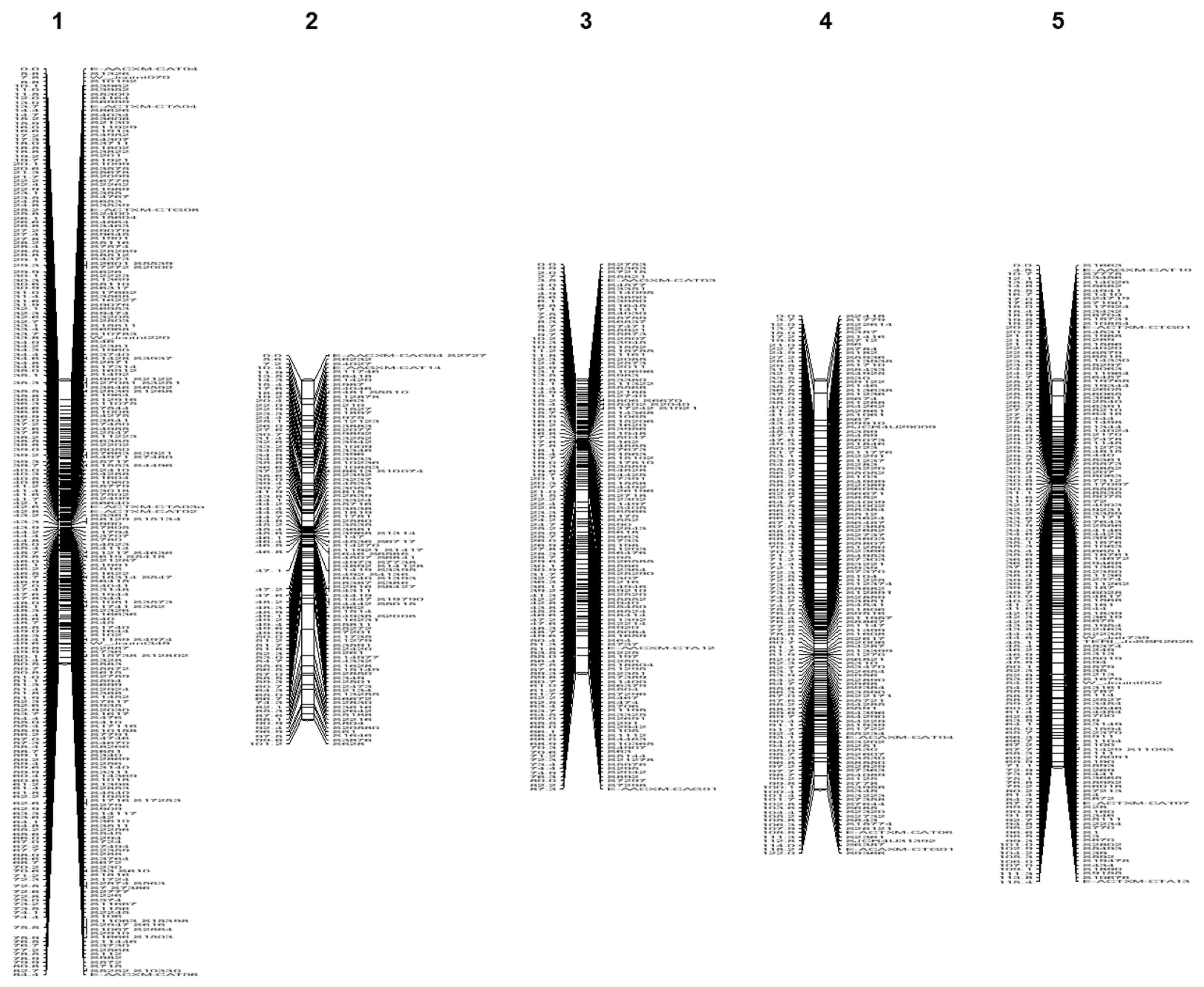
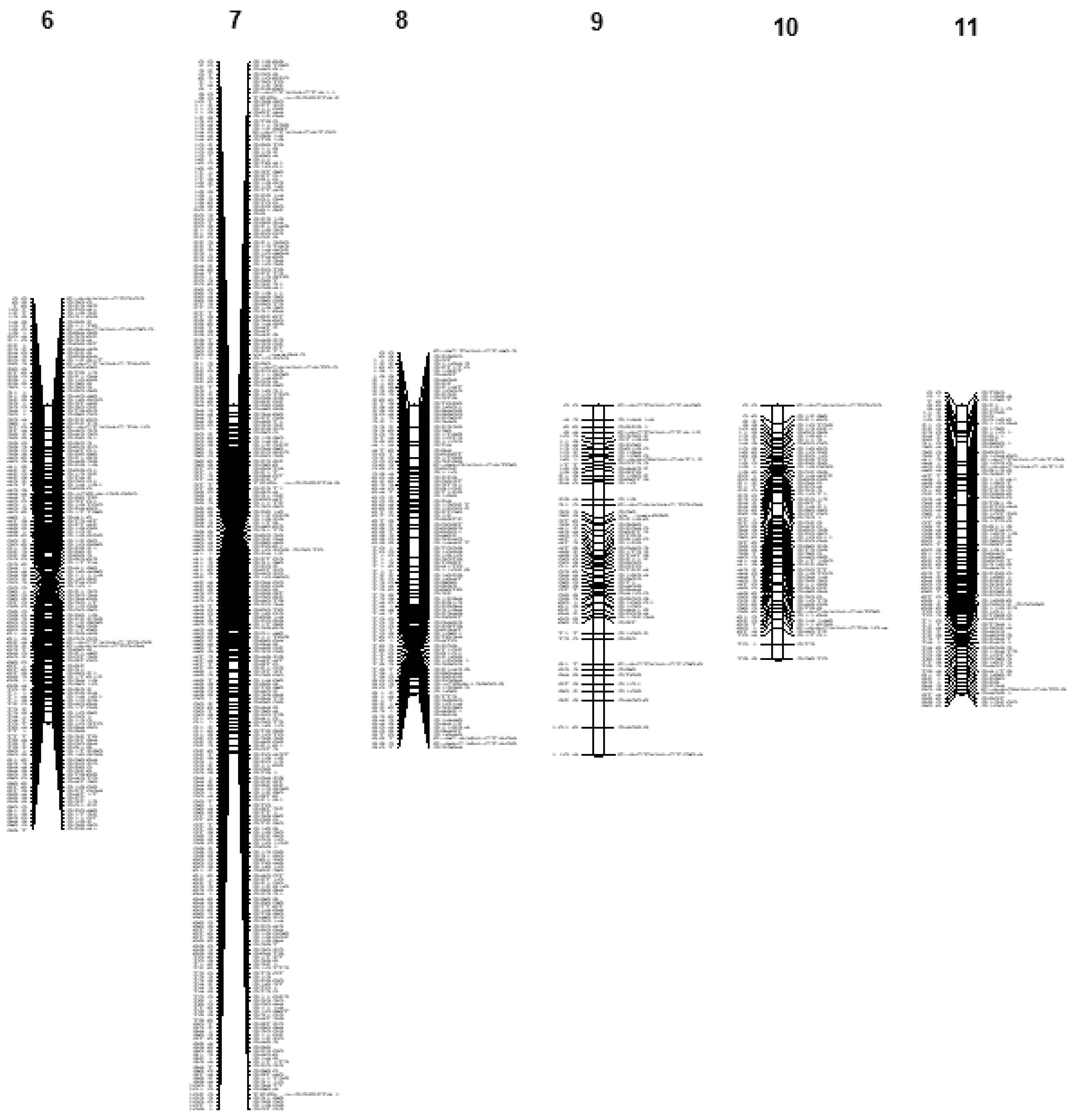
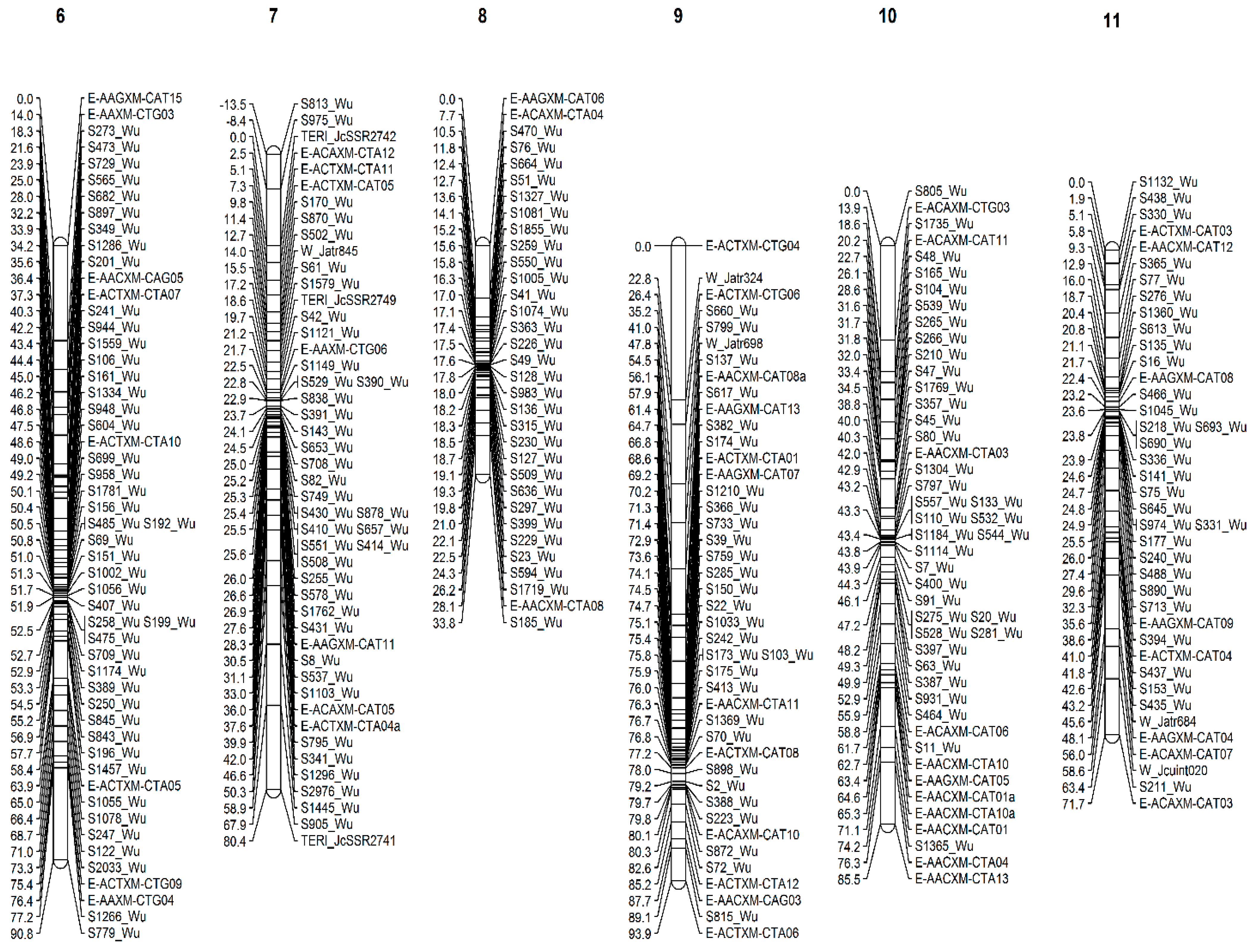
3.3. Linkage Mapping
3.3.1. Jat_r4.5-Based Linkage Map
3.3.2. Wu_JatCur_1.0-Based Linkage Map
3.3.3. Consistency with Published Linkage Groups and between Our Two Linkage Maps
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, B.-Y.; Mann, I.K.; Major, J.E.; Rajora, O.P. Near-saturated and complete genetic linkage map of black spruce (Picea mariana). BMC Genom. 2010, 11, 515. [Google Scholar] [CrossRef]
- Collard, B.C.; Mackill, D.J. Marker-assisted selection: An approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 557–572. [Google Scholar] [CrossRef]
- Martin, G.B.; Brommonshenkel, S.; Chunwongse, J.; Frary, A.; Ganal, M.W.; Spivey, R.; Wu, T.; Earle, E.D.; Tanksley, S.D. Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science 1993, 262, 1432–1435. [Google Scholar] [CrossRef]
- Subramanian, K.A.; Singal, S.K.; Saxena, M.; Singhal, S. Utilization of liquid biofuels in automobile diesel engines: An Indian perspective. Biomass Bioenergy 2005, 29, 65–72. [Google Scholar] [CrossRef]
- Riayatsyah, T.; Sebayang, A.; Silitonga, A.; Padli, Y.; Fattah, I.; Kusumo, F.; Ong, H.; Mahlia, T. Current Progress of Jatropha curcas Commoditisation as Biodiesel Feedstock: A Comprehensive Review. Front. Energy Res. 2022, 9, 815416. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, X.; Liu, B.; Tang, L.; Chen, F. Genetic diversity and genetic relationship of Jatropha curcas between China and Southeast Asian revealed by amplified fragment length polymorphism. Afr. J. Biotechnol. 2011, 10, 2825–2832. [Google Scholar]
- He, W.; King, A.J.; Khan, M.A.; Cuevas, J.A.; Ramiaramanana, D.; Graham, I.A. Analysis of seed phorbol-ester and curcin content together with genetic diversity in multiple provenances of Jatropha curcas L. from Madagascar and Mexico. Plant Physiol. Biochem. 2011, 49, 1183–1190. [Google Scholar] [CrossRef]
- Basha, S.D.; George, F.; Makkar, H.P.S.; Becker, K.; Sujatha, M. A comparative study of biochemical traits and molecular markers for assessment of genetic relationships between J. curcas (L.) germplasm from different countries. Plant Sci. 2009, 176, 812–823. [Google Scholar] [CrossRef]
- Biabani, A.; Rafii, M.Y.; Saleh, G.B.; Abdul, L.M. Inter- and intra-population genetic variations in Jatropha curcas populations revealed by inter-simple sequence repeat molecular markers. Maydica 2013, 58, 111–118. [Google Scholar]
- Sinha, P.; Md Islam, A.; Negi, M.S.; Tripathi, S.B. First identification of core accessions of Jatropha curcas from India based on molecular genetic diversity. Plant Genet. Resour. 2015, 14, 77–80. [Google Scholar] [CrossRef]
- Trebbi, D.; Papazoglou, E.G.; Saadaoui, E.; Vischi, M.; Baldini, M.; Stevanato, P.; Cettul, E.; Sanzone, A.P.; Gualdi, L.; Fabbri, A. Assessment of genetic diversity in different accessions of Jatropha curcas. Ind. Crop. Prod. 2015, 75, 35–39. [Google Scholar] [CrossRef]
- Shen, J.; Pinyopusarerk, K.; Bush, D.; Chen, X. AFLP-based molecular characterization of 63 populations of Jatropha curcas L. grown in provenance trials in China and Vietnam. Biomass Bioenerg. 2012, 37, 265–274. [Google Scholar] [CrossRef]
- Basha, S.; Sujatha, M. Inter and intra-population variability of Jatropha curcas (L.) characterized by RAPD and ISSR markers and development of population-specific SCAR markers. Euphytica 2007, 156, 375–386. [Google Scholar] [CrossRef]
- Pamidimarri, D.V.; Mastan, S.G.; Rahman, H.; Reddy, M.P. Molecular characterization and genetic diversity analysis of Jatropha curcas L. in India using RAPD and AFLP analysis. Mol. Biol. Rep. 2010, 37, 2249–2257. [Google Scholar] [CrossRef]
- Bhandari, H.; Bhanu, A.; Srivastava, K.; Singh, M.; Shreya, H.A. Assessment of genetic diversity in crop plants—An overview. Adv. Plants Agric. Res. 2017, 7, 279–286. [Google Scholar]
- Blum, E.; Liu, K.; Mazourek, M.; Yoo, E.Y.; Jahn, M.; Paran, I. Molecular mapping of the C locus for presence of pungency in Capsicum. Genome 2002, 45, 702–705. [Google Scholar] [CrossRef]
- Yi, G.; Lee, J.M.; Lee, S.; Choi, D.; Kim, B.D. Exploitation of pepper EST-SSRs and an SSR-based linkage map. Theor. Appl. Genet. 2006, 114, 113–130. [Google Scholar] [CrossRef]
- Parthiban, K.T.; Kumar, R.S.; Thiyagarajan, P.; Subbulakshmi, V.; Vennila, S.; Rao, M.G. Hybrid progenies in Jatropha—A new development. Curr. Sci. 2009, 96, 815–823. [Google Scholar]
- Sinha, P.; Dwivedi, N.; Negi, M.S.; Tripathi, S.B. Genetic variability among Jatropha species as revealed by amplified fragment length polymorphism (AFLP) markers. Indian J. Biotechnol. 2014, 13, 496–501. [Google Scholar]
- Wang, C.M.; Liu, P.; Yi, C.; Gu, K.; Sun, F.; Li, L.; Lo, L.C.; Liu, X.; Feng, F.; Lin, G.; et al. A first generation microsatellite- and SNP-based linkage map of Jatropha. PLoS ONE 2011, 6, e23632. [Google Scholar] [CrossRef]
- King, A.J.; Montes, L.R.; Clarke, J.G.; Affleck, J.; Li, Y.; Witsenboer, H.; van der Vossen, E.; van der Linde, P.; Tripathi, Y.; Tavares, E.; et al. Linkage mapping in the oilseed crop Jatropha curcas L. reveals a locus controlling the biosynthesis of phorbol esters which cause seed toxicity. Plant Biotechnol. J. 2013, 11, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Zhang, S.; Wen, M.; Lu, C.; Sun, Y.; Zou, M.; Wang, W. Construction of an ultrahigh-density genetic linkage map for Jatropha curcas L. and identification of QTL for fruit yield. Biotechnol. Biofuels 2018, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Yepuri, V.; Jalali, S.; Mudunuri, V.; Pothakani, S.; Kancharla, N.; Arockiasamy, S. Genotyping by sequencing-based linkage map construction and identification of quantitative trait loci for yield-related traits and oil content in Jatropha (Jatropha curcas L.). Mol. Biol. Rep. 2022, 49, 4293–4306. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhou, C.; Cheng, S.; Wu, Z.; Lu, W.; Han, J.; Chen, Y.; Chen, Y.; Ni, P.; Wang, Y.; et al. Integrated genome sequence and linkage map of physic nut (Jatropha curcas L.), a biodiesel plant. Plant J. 2015, 81, 810–821. [Google Scholar] [CrossRef]
- Ha, J.; Shim, S.; Lee, T.; Kang, Y.J.; Hwang, W.J.; Jeong, H.; Laosatit, K.; Lee, J.; Kim, S.K.; Satyawan, D.; et al. Genome sequence of Jatropha curcas L., a non-edible biodiesel plant, provides a resource to improve seed-related traits. Plant Biotechnol. J. 2019, 17, 517–530. [Google Scholar] [CrossRef]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef]
- Fierst, J.L. Using linkage maps to correct and scaffold de novo genome assemblies: Methods, challenges, and computational tools. Front. Genet. 2015, 6, 220. [Google Scholar] [CrossRef]
- Sato, S.; Hirakawa, H.; Isobe, S.; Fukai, E.; Watanabe, A.; Kato, M.; Kawashima, K.; Minami, C.; Muraki, A.; Nakazaki, N.; et al. Sequence analysis of the genome of an oil-bearing tree, Jatropha curcas L. DNA Res. 2010, 18, 65–76. [Google Scholar] [CrossRef]
- Hirakawa, H.; Tsuchimoto, S.; Sakai, H.; Nakayama, S.; Fujishiro, T.; Kishida, Y.; Kohara, M.; Watanabe, A.; Aizu, T.; Toyoda, A.; et al. Upgraded genomic information of Jatropha curcas L. Plant Biotechnol. J. 2012, 29, 123–130. [Google Scholar] [CrossRef]
- Sinha, P.; Md Islam, A.; Negi, M.S.; Tripathi, S.B. Analysis of genetic diversity and fatty acid composition in a prebreeding material of Jatropha. J. Plant Biochem. Biotech. 2015, 25, 111–116. [Google Scholar] [CrossRef]
- Singh, A.; Negi, M.S.; Rajagopal, J.; Bhatia, S.; Tomar, U.K.; Srivastava, P.S.; Lakshmikumaran, M. Assessment of genetic diversity in Azadirachta indica using AFLP markers. Theor. Appl. Genet. 1999, 99, 272–279. [Google Scholar] [CrossRef]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; van de Lee, T.; Hornes, M.; Friters, A.; Pot, J.; Paleman, J.; Kuiper, M.; et al. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Negi, M.S.; Sinha, P.; Kumar, K.; Tripathi, S.B. Assessment of genetic diversity of biodiesel species Pongamia pinnata accessions using AFLP and three endonuclease -AFLP. Plant Mol. Biol. Rep. 2011, 29, 12–18. [Google Scholar] [CrossRef]
- Sinha, P.; Md Islam, A.; Negi, M.S.; Tripathi, S.B. Development of novel microsatellite markers in Jatropha curcas and evaluation of their cross-species transferability. Proc. Natl. Acad. Sci. India Biol. Sci. 2015, 85, 1011–1016. [Google Scholar] [CrossRef]
- Sinha, P.; Md Islam, A.; Negi, M.S.; Tripathi, S.B. Estimation of outcrossing rates in interspecific backcross plants of Jatropha curcas (L.) using AFLP and SSR markers. Physiol. Mol. Biol. Plants 2015, 21, 605–609. [Google Scholar] [CrossRef][Green Version]
- Glaubitz, J.C.; Casstevens, T.M.; Lu, F.; Harriman, J.; Elshire, R.J.; Sun, Q.; Buckler, E.S. TASSEL-GBS: A High Capacity Genotyping by Sequencing Analysis Pipeline. PLoS ONE 2014, 9, e90346. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Van den Beek, M.; Blankenberg, D.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Eberhard, C.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses. Nucl. Acids Res. 2016, 44, W3–W10. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef]
- van Ooijen, J.W.; Voorrips, R.E. JoinMap 4.0: Software for the Calculation of Genetic Maps in Experimental Populations; Kyazma, B.V., Ed.; Plant Research International: Wageningen, The Netherlands, 2006. [Google Scholar]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef]
- Cartwright, D.A.; Troggio, M.; Velasco, R.; Gutin, A. Genetic mapping in the presence of genotyping errors. Genetics 2007, 176, 2521–2527. [Google Scholar] [CrossRef] [PubMed]
- Amkul, K.; Laosatit, K.; Somta, P.; Shim, S.; Lee, S.-H.; Tanya, P.; Srinives, P. Mapping of QTLs for Seed Phorbol Esters, a Toxic Chemical in Jatropha curcas (L.). Genes 2017, 8, 205. [Google Scholar] [CrossRef]
- Kang, B.-Y.; Major, J.E.; Rajora, O.P. A high-density genetic linkage map of a black spruce (Picea mariana) × red spruce (Picea rubens) interspecific hybrid. Genome 2011, 54, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Bresadola, L.; Link, V.; Buerkle, C.A.; Lexer, C.; Wegmann, D. Estimating and accounting for genotyping errors in RAD-seq experiments. Mol. Ecol. Resour. 2020, 20, 856–870. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Yamamoto, T.; Ashikari, M. GBScleanR: Robust genotyping error correction using hidden Markov model with error pattern recognition. bioRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- Luikart, G.; Kardos, M.; Hand, B.; Rajora, O.P.; Aitkin, S.; Hohenlohe, P.A. Population genomics: Advancing understanding of nature. In Population Genomics: Concepts, Approaches and Applications; Rajora, O.P., Ed.; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2019; pp. 3–79. [Google Scholar] [CrossRef]
- Rajora, O.P. Population Genomics: Concepts, Approaches and Applications; Springer Nature Switzerland AG: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]

| Primer Combination | Total | Mapped on Jat_r4.5 | Mapped on Wu_JatCur_1.0 |
|---|---|---|---|
| E-AAC × M-CAG | 8 | 3 | 5 |
| E-AAC × M-CAT | 14 | 3 | 3 |
| E-AAC × M-CTA | 14 | 3 | 3 |
| E-AAG × M-CAT | 15 | 6 | 6 |
| E-AA × M-CTG | 8 | 2 | 3 |
| E-ACA × M-CAT | 11 | 3 | 4 |
| E-ACA × M-CTA | 13 | 1 | 3 |
| E-ACA × M-CTG | 8 | 3 | 3 |
| E-ACT × M-CAT | 8 | 5 | 4 |
| E-ACT × M-CTA | 15 | 8 | 8 |
| E-ACT × M-CTG | 10 | 5 | 6 |
| Total | 124 | 42 | 48 |
| Marker Name | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) | Mapped |
|---|---|---|---|
| W_Jatr698 | AGCAAGTCTAAGAGAGGGAGA | CTCAAGACTCCACACAACTTC | Yes |
| W_Jcuint220 | CATAAAGGCTAAAGCATCTCA | ATTTAGCTTTCCTGCCTAAAA | Yes |
| W_Jatr324 | TGTAGGCTGAATAAGAACAGC | GTCCTTGATCTCTGGCTTTAC | No |
| W_Jatr845 | CTCCTTCCATAGAAGAAAACC | GAGACATGCTTATTCATCCAC | Yes |
| W_Jcuint020 | ATATGGACAGATTAGCCGATT | CACGCAATACCTAACTTGTGT | No |
| W_Jcuint070 | CCTTTCTAGCAAAATAGGAAGA | GTAACAGTTGGAACCACATTC | No |
| W_Jatr739 | TTTTAAGCAAATGAGAAGGTG | CTAGGGCCACCCCACTTTAG | Yes |
| W_Jcuint282 | CCGCATTTCTAACATAATCAG | AGAATTTGAGATGGTTGTTGA | No |
| W_Jcuint152 | CATGCGATCTCTCTCTTTCT | CAAGAAGCTGGTGAGAATAAA | No |
| W_Jcuint002 | AGGAGAAACTACAACACATGC | AAGCACCAAAAACCAATTACT | Yes |
| W_Jcuint349 | CAACAGGTATCTAGTGGTGGT | CAACATTTTATTGAAGTAAGC | Yes |
| W_Jatr684 | TCAACTTCGTATGCTAATGGT | CCTCATGCTCTATTATTGGTG | No |
| TERI_JcSSR2626 | CGCAGCCATCTTGAAGGTTAG | CAAAATTTCAAGCCATGCTC | Yes |
| TERI_JcSSR2741 | CATCAGGAATTGTTTGATGGTC | GGAATTTTCTATGGGACTGAG | Yes |
| TERI_JcSSR2742 | TTGAAACAGACCAAAGGTGTG | ATCGTATGAAGCAGCACACTC | Yes |
| TERI_JcSSR2749 | TGCGATTACCTGGTTTAGGGA | TCGGAAGCCTTGGAGATTTAG | Yes |
| TERI_JcSSR2785 | GAGGTAGCTGAAAAAAACAGC | GTTGAGAAGAATGGTGGCTGC | No |
| Steps | Jat_r4.5 | Wu_JatCur_1.0 |
|---|---|---|
| Total raw reads | 296 million | |
| Good-quality barcoded reads | 212 million | |
| Unique barcoded reads | 340,029 | |
| Reads aligned to single location | 134,402 | 140,455 |
| Reads aligned to multiple locations | 36,114 | 25,716 |
| Nonaligned reads | 169,513 | 173,858 |
| Number of GBS-SNPs | 53,038 | 52,862 |
| Unique GBS-SNPs (after taking one SNP per read) | 22,439 | 21,680 |
| SNPs obtained after removal of missing data (>10%) | 12,996 | 14,768 |
| SNPs obtained after removal of heterozygous sites from parents | 9975 | 11,263 |
| Number of scaffolds finally represented in the genotype data | 3267 | 484 |
| Linkage Groups | Scaffolds from the Reference Genome Jat_r4.5 | Scaffolds from the Reference Genome WU_JatCur_1.0 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (cM) | Number of Scaffolds Anchored | Number of SNPs | AFLPs and SSRs | Total (SNPs + AFLPs + SSRs) | Markers (Scaffolds + AFLPs + SSRs) /cM | Size (cM) | Number of Scaffolds Anchored | Number of SNPs | AFLPs and SSRs | Total (SNPs + AFLPs + SSRs) | Markers (Scaffolds + AFLPs + SSRs) /cM | |
| LG1 | 84.4 | 238 | 1228 | 9 | 1237 | 2.9 | 76.4 | 40 | 641 | 10 | 651 | 0.7 |
| LG2 | 101.2 | 111 | 493 | 2 | 495 | 1.1 | 77.5 | 43 | 911 | 10 | 921 | 0.7 |
| LG3 | 84.7 | 128 | 665 | 3 | 668 | 1.5 | 68.2 | 27 | 747 | 7 | 754 | 0.5 |
| LG4 | 122.0 | 128 | 621 | 3 | 624 | 1.1 | 90.7 | 28 | 733 | 5 | 738 | 0.4 |
| LG5 | 115.4 | 144 | 847 | 7 | 854 | 1.3 | 101.4 | 37 | 810 | 10 | 820 | 0.5 |
| LG6 | 99.7 | 131 | 624 | 6 | 630 | 1.4 | 90.8 | 46 | 906 | 8 | 914 | 0.6 |
| LG7 | 109.1 | 263 | 1456 | 7 | 1463 | 2.5 | 80.4 | 39 | 1096 | 11 | 1107 | 0.6 |
| LG8 | 90.7 | 98 | 492 | 4 | 496 | 1.1 | 33.8 | 30 | 280 | 3 | 283 | 1.0 |
| LG9 | 110.4 | 51 | 330 | 7 | 337 | 0.6 | 93.9 | 29 | 569 | 14 | 583 | 0.5 |
| LG10 | 79.8 | 57 | 182 | 3 | 185 | 0.8 | 85.5 | 37 | 458 | 11 | 469 | 0.6 |
| LG11 | 90.6 | 79 | 346 | 3 | 349 | 0.9 | 71.7 | 31 | 547 | 10 | 557 | 0.6 |
| Total | 1088 | 1428 | 7284 | 54 | 7338 | 1.4 | 870.3 | 387 | 7698 | 99 | 7797 | 0.6 |
| Type of Population | Types of Crosses | No. of Individuals | No. of Markers | Type of Markers | Total Length of Map (cM) | Average Marker Density (Markers per cM) | Reference |
|---|---|---|---|---|---|---|---|
| BC1F1 | Interspecific | 93 | 506 | Microsatellite and SNP | 1440.90 | 0.35 | [20] |
| F2 | Intraspecific | 974 | 502 | SSR and SNP | 717.00 | 1.50 | [21] |
| BC1F1 | Interspecific | 190 | 1208 | SSR, InDel and SNP | 1655.80 | 0.73 | [24] |
| F1 | Intraspecific | 153 | 3422 | SNP and InDel | 1380.58 | 2.48 | [22] |
| F2 | Intraspecific | 108 | 1186 | SNP | 738.10 | 1.60 | [25] |
| F2 | Intraspecific | 136 | 411 | SNP | 4092.30 | 10.04 | [23] |
| BC1F1 | Interspecific | 91 | 1482 | SNP, SSR and AFLP | 1088.00 | 1.38 | This study (Jat_r4.5 reference genome) |
| BC1F1 | Interspecific | 91 | 486 | SNP, SSR and AFLP | 870.30 | 0.59 | This study (GCF_000696525.1_JatCur_1.0 reference genome) |
| This Study | Other Studies | |||||||
|---|---|---|---|---|---|---|---|---|
| Linkage Group | Jat_r4.5 Scaffolds | Wu_JatCur_1.0 Scaffolds | Wu et al., 2015 [24] | King et al., 2013 [21] | Wang et al., 2011 [20] | Xia et al., 2018 [22] | Ha et al., 2019 [25] | Yepuri et al., 2022 [23] |
| LG1 | 84.4 | 76.4 | 160.4 | 51.1 | 187.5 | 122.51 | 73.3 | 427.1 |
| LG2 | 101.2 | 77.5 | 86.2 | 67.3 | 164.8 | 109.10 | 60.3 | 397.3 |
| LG3 | 84.7 | 68.2 | 103 | 69.2 | 151.7 | 68.67 | 62.8 | 298.5 |
| LG4 | 122.0 | 90.7 | 148.2 | 62.7 | 171.9 | 170.31 | 62.1 | 449.3 |
| LG5 | 115.4 | 101.4 | 114.7 | 64.4 | 116.7 | 76.20 | 68.8 | 451.1 |
| LG6 | 99.7 | 90.8 | 154.6 | 82.1 | 127.7 | 115.35 | 58.8 | 453.9 |
| LG7 | 109.1 | 80.4 | 131.4 | 76.8 | 82.9 | 153.40 | 63.2 | 280.8 |
| LG8 | 90.7 | 33.8 | 193.9 | 67.1 | 163.5 | 258.60 | 77.3 | 129.9 |
| LG9 | 110.4 | 93.9 | 207.7 | 66.3 | 87.9 | 87.86 | 81.3 | 482.3 |
| LG10 | 79.8 | 85.5 | 221.6 | 53.6 | 101.4 | 92.13 | 72.4 | 241.1 |
| LG11 | 90.6 | 71.7 | 107.1 | 54.9 | 84.9 | 126.45 | 57.8 | 481.1 |
| Total | 1088.0 | 870.3 | 1628.8 | 715.5 | 1440.9 | 1380.6 | 738.1 | 4092.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, A.A.; Sinha, P.; Negi, M.S.; Rajora, O.P.; Tripathi, S.B. Ultra-High-Density Genetic Maps of Jatropha curcas × Jatropha integerrima and Anchoring Jatropha curcas Genome Assembly Scaffolds. Forests 2023, 14, 1907. https://doi.org/10.3390/f14091907
Malik AA, Sinha P, Negi MS, Rajora OP, Tripathi SB. Ultra-High-Density Genetic Maps of Jatropha curcas × Jatropha integerrima and Anchoring Jatropha curcas Genome Assembly Scaffolds. Forests. 2023; 14(9):1907. https://doi.org/10.3390/f14091907
Chicago/Turabian StyleMalik, Anoop Anand, Pratima Sinha, Madan Singh Negi, Om P. Rajora, and Shashi Bhushan Tripathi. 2023. "Ultra-High-Density Genetic Maps of Jatropha curcas × Jatropha integerrima and Anchoring Jatropha curcas Genome Assembly Scaffolds" Forests 14, no. 9: 1907. https://doi.org/10.3390/f14091907
APA StyleMalik, A. A., Sinha, P., Negi, M. S., Rajora, O. P., & Tripathi, S. B. (2023). Ultra-High-Density Genetic Maps of Jatropha curcas × Jatropha integerrima and Anchoring Jatropha curcas Genome Assembly Scaffolds. Forests, 14(9), 1907. https://doi.org/10.3390/f14091907








