Enhancement of Phytoremediation of Heavy Metal Pollution Using an Intercropping System in Moso Bamboo Forests: Characteristics of Soil Organic Matter and Bacterial Communities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Management
2.2. Soil Sampling and Physicochemical Properties
2.3. High-Throughput Sequencing
2.4. Fourier Transform Infrared (FTIR) Spectroscopic Analysis
2.5. X-ray Diffraction Analysis
2.6. Statistical Analyses
3. Results and Discussion
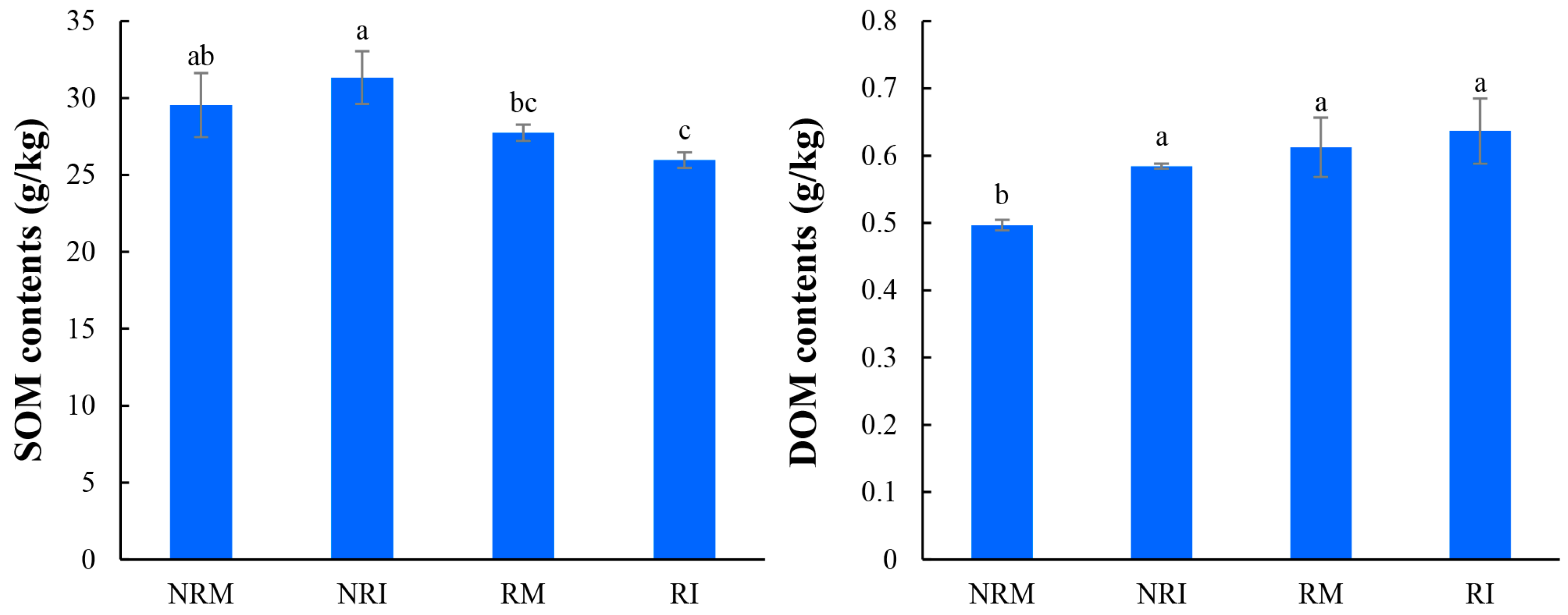
3.1. The Contents of SOM and DOM
3.2. Chemical Components of SOM and Soil Structure
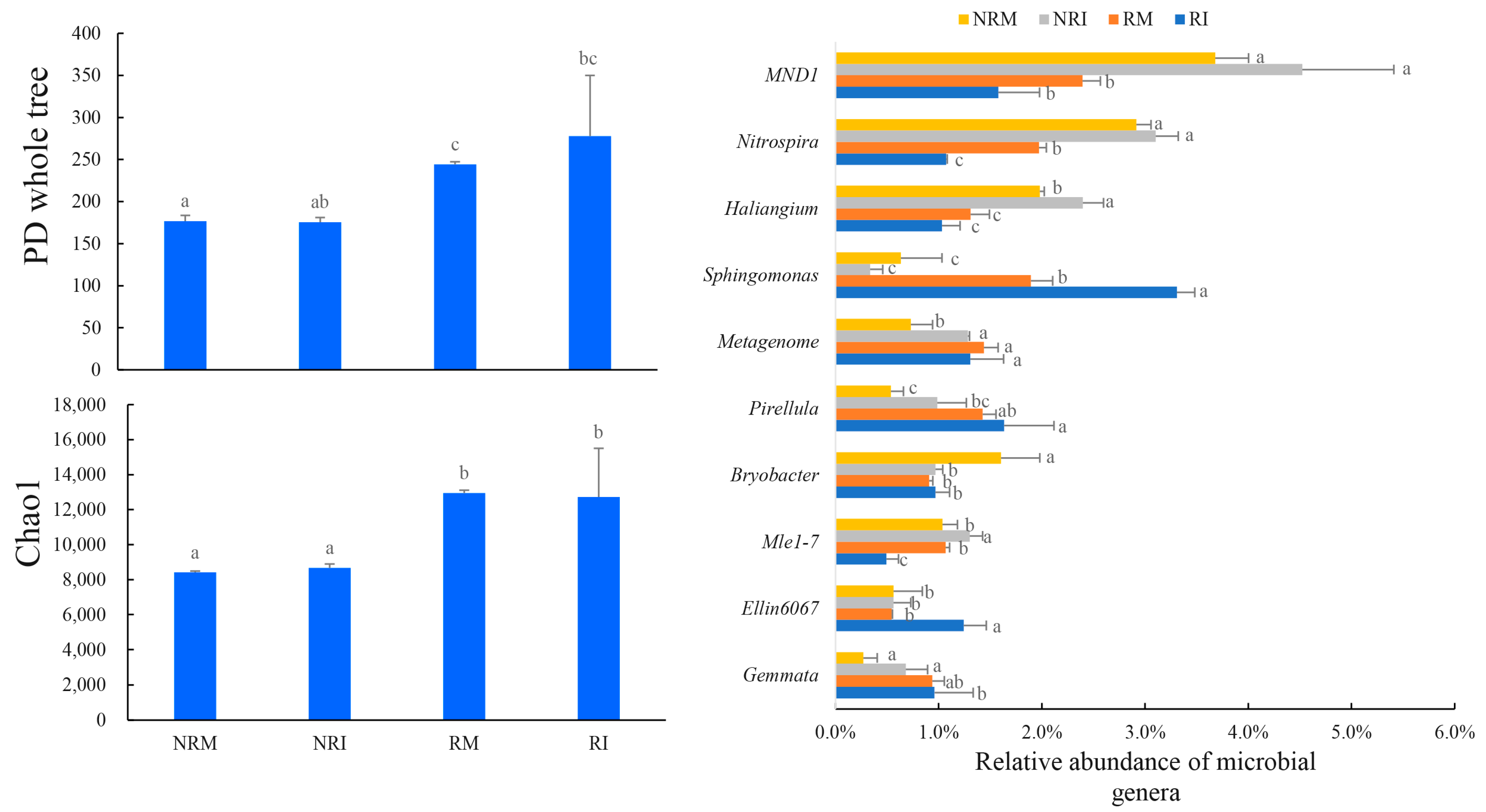
3.3. Soil Bacterial Community
3.4. Relationships among Soil Heavy Metals, Organic Components, and Microorganisms
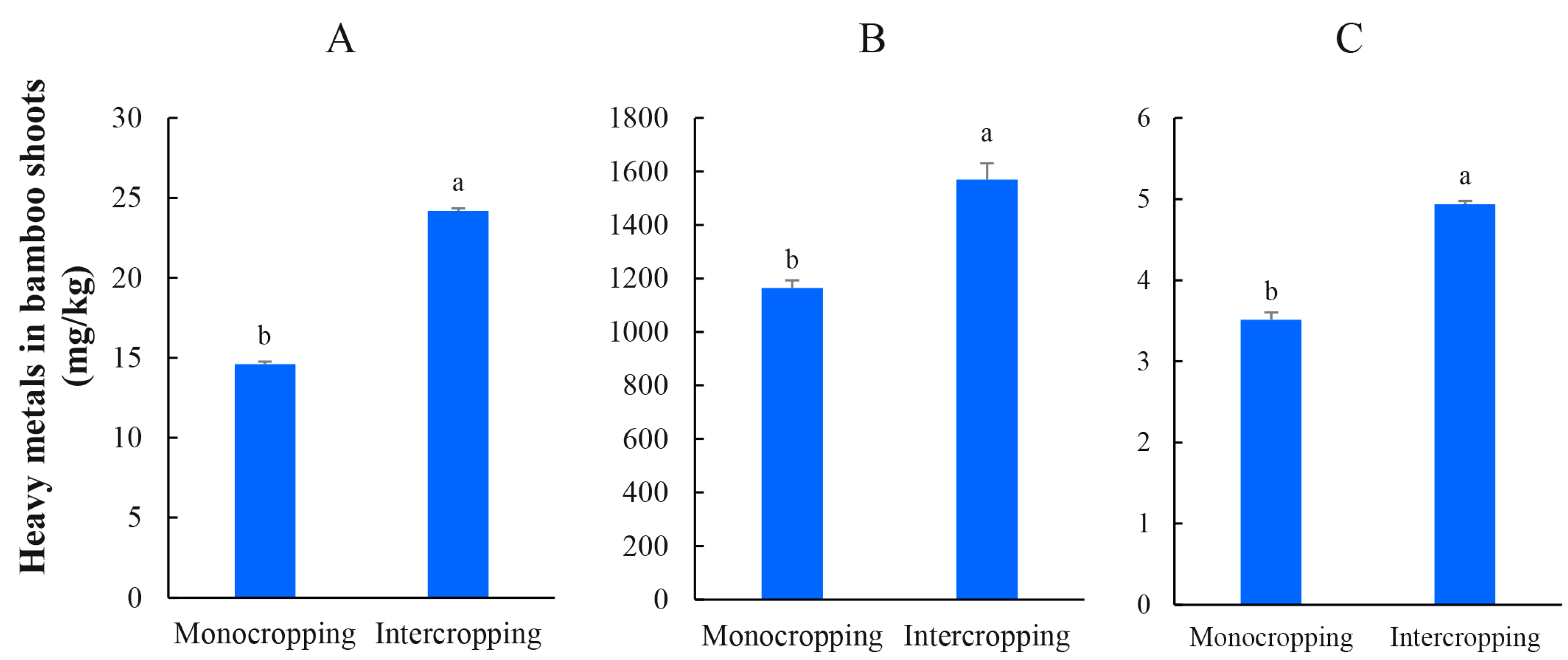
3.5. New Shoot Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Lima, E.C.; Zhang, S.; Shaheen, S.M.; Rinklebe, J. Global soil pollution by toxic elements: Current status and future perspectives on the risk assessment and remediation strategies—A review. J. Hazard. Mater. 2021, 417, 126039. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, F.; Zhong, Z.; Gai, X.; Yang, C. Deciphering the rhizosphere microbiome of a bamboo plant in response to different chromium contamination levels. J. Hazard. Mater. 2020, 399, 123107. [Google Scholar] [CrossRef]
- Chen, W.; Peng, L.; Hu, K.; Zhang, Z.; Peng, C.; Teng, C.; Zhou, K. Spectroscopic response of soil organic matter in mining area to Pb/Cd heavy metal interaction: A mirror of coherent structural variation. J. Hazard. Mater. 2020, 393, 122425. [Google Scholar] [CrossRef]
- Hu, B.; Shao, S.; Ni, H.; Fu, Z.; Hu, L.; Zhou, Y.; Min, X.; She, S.; Chen, S.; Huang, M. Current status, spatial features, health risks, and potential driving factors of soil heavy metal pollution in China at province level. Environ. Pollut. 2020, 266, 114961. [Google Scholar] [CrossRef]
- Bian, F.; Zhong, Z.; Zhang, X.; Yang, C.; Gai, X. Bamboo—An untapped plant resource for the phytoremediation of heavy metal contaminated soils. Chemosphere 2020, 246, 125750. [Google Scholar] [CrossRef]
- Bian, F.; Zhong, Z.; Li, C.; Zhang, X.; Gu, L.; Huang, Z.; Gai, X.; Huang, Z. Intercropping improves heavy metal phytoremediation efficiency through changing properties of rhizosphere soil in bamboo plantation. J. Hazard. Mater. 2021, 416, 125898. [Google Scholar] [CrossRef]
- Yang, F.; Chang, Y.Z.; Zheng, Y.T.; Pan, X.; Ji, H.; Shao, J.F. Physiological and transcriptomic characterization of cadmium toxicity in Moso bamboo (Phyllostachys edulis), a non-timber forest species. Tree Physiol. 2023, 25, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, E.; D’onghia, G.; Ranieri, F.; Cosanti, B.; Ranieri, A.C. Chromium phytoextraction using Phyllostachys pubescens (Moso Bamboo). Int. J. Phytoremediation 2023, 25, 2097639. [Google Scholar] [CrossRef]
- Yang, C.; Ni, H.; Zhong, Z.; Zhang, X.; Bian, F. Changes in soil carbon pools and components induced by replacing secondary evergreen broadleaf forest with Moso bamboo plantations in subtropical China. CATENA 2019, 180, 309–319. [Google Scholar] [CrossRef]
- Liu, L.; Chang, S.X.; Huang, C.; Zhi, Y.; Jie, Y.; Yu, X.; Jiang, P. Enhancement of phytolith-occluded carbon accumulation of Moso bamboo response to temperatures elevation and different fertilization. Front. Plant Sci. 2023, 14, 1144961. [Google Scholar] [CrossRef]
- Habibul, N.; Chen, W. Structural response of humic acid upon binding with lead: A spectroscopic insight. Sci. Total. Environ. 2018, 643, 479–485. [Google Scholar] [CrossRef]
- Lal, R. Soil organic matter content and crop yield. J. Soil Water Conserv. 2020, 75, 27A–32A. [Google Scholar] [CrossRef]
- Abrar, M.M.; Shah, S.A.A.; Sun, N.; Mehmood, K.; Aziz, T.; Waqas, M.A.; Luo, Y.; Zhou, B.; Ma, X.; Xu, M. Long-term manure application enhances organic carbon and nitrogen stocks in Mollisol subsoil. Land Degrad. Dev. 2023, 34, 815–832. [Google Scholar] [CrossRef]
- Guillaume, T.; Makowski, D.; Libohova, Z.; Bragazza, L.; Sallaku, F.; Sinaj, S. Soil organic carbon saturation in cropland-grassland systems: Storage potential and soil quality. Geoderma 2022, 406, 115529. [Google Scholar] [CrossRef]
- Wen, J.; Li, Z.; Luo, N.; Huang, M.; Yang, R.; Zeng, G. Investigating organic matter properties affecting the binding behavior of heavy metals in the rhizosphere of wetlands. Ecotoxicol. Environ. Saf. 2018, 162, 184–191. [Google Scholar] [CrossRef]
- Muñoz, G.; Orlando, J.; Zuñiga-Feest, A. Plants colonizing volcanic deposits: Root adaptations and effects on rhizosphere microorganisms. Plant Soil 2021, 461, 265–279. [Google Scholar] [CrossRef]
- Stringlis, I.A.; Teixeira, P.J.; Berendsen, R.L.; Pieterse, C.M.; Zamioudis, C. Beneficial Microbiota interacting with the plant immune system. Front. Plant Sci. 2021, 12, 698902. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Terenzi, V. Rhizosphere Microbial Communities and Heavy Metals. Microorganisms 2021, 9, 1462. [Google Scholar] [CrossRef]
- Naz, M.; Dai, Z.; Hussain, S.; Tariq, M.; Danish, S.; Khan, I.U.; Qi, S.; Du, D. The soil pH and heavy metals revealed their impact on soil microbial community. J. Environ. Manag. 2022, 321, 115770. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, X.; Guan, D.; Wei, D.; Zhao, B.; Ma, M.; Chen, S.; Li, L.; Cao, F.; Li, J. Long-term fertilization changes bacterial diversity and bacterial communities in the maize rhizosphere of Chinese Mollisols. Appl. Soil Ecol. 2018, 125, 88–96. [Google Scholar] [CrossRef]
- Bian, F.; Zhong, Z.; Zhang, X.; Yang, C. Phytoremediation potential of moso bamboo (Phyllostachys pubescens) intercropped with Sedum plumbizincicola in metal-contaminated soil. Environ. Sci. Pollut. Res. 2017, 24, 27244–27253. [Google Scholar] [CrossRef]
- State Soil Survey Service of China. China Soil; China Agricultural Press: Beijing, China, 1998. [Google Scholar]
- Bolan, N.S.; Baskaran, S.; Thiagarajan, S. An evaluation of the methods of measurement of dissolved organic carbon in soils, manures, sludges, and stream water. Commun. Soil Sci. Plant Anal. 1996, 27, 2723–2737. [Google Scholar] [CrossRef]
- Tüzen, M. Determination of heavy metals in soil, mushroom and plant samples by atomic absorption spectrometry. Microchem. J. 2003, 74, 289–297. [Google Scholar] [CrossRef]
- Laudicina, V.A.; Novara, A.; Barbera, V.; Egli, M.; Badalucco, L. Long-term tillage and cropping system effects on chemical and biochemical characteristics of soil organic matter in a Mediterranean semiarid environment. Land Degrad. Dev. 2015, 26, 45–53. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P. Vegan: Community Ecology Package. R Package Version 2.5-5 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 December 2022).
- Yaashikaa, P.R.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Li, X.; Shen, J.; Sun, L.; Zaman, S.; Wang, Y.; Ding, Z. Different changes of bacterial diversity and soil metabolites in tea plants-legume intercropping systems. Front. Plant Sci. 2023, 14, 1110623. [Google Scholar] [CrossRef]
- Wang, D.; Yi, W.; Zhou, Y.; He, S.; Tang, L.; Yin, X.; Zhao, P.; Long, G. Intercropping and N application enhance soil dissolved organic carbon concentration with complicated chemical composition. Soil Tillage Res. 2021, 210, 104979. [Google Scholar] [CrossRef]
- Sonsri, K.; Watanabe, A. Insights into the formation and stability of soil aggregates in relation to the structural properties of dissolved organic matter from various organic amendments. Soil Tillage Res. 2023, 232, 105774. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, Z.; Zhang, X.; Bian, F.; Du, X. Responses of soil organic carbon sequestration potential and bacterial community structure in moso bamboo plantations to different management strategies in subtropical China. Forests 2018, 9, 657. [Google Scholar] [CrossRef]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, S.R.; Schellekens, J.; da Silva, W.T.L.; Buurman, P.; Boim, A.G.F.; Vidal-Torrado, P. Selective sorption and desorption of DOM in podzol horizons—FTIR and Py-GC/MS of leachates from a column experiment. Sci. Total. Environ. 2022, 826, 154144. [Google Scholar] [CrossRef]
- Kaplan, D.I.; Xu, C.; Huang, S.; Lin, Y.; Tolić, N.; Roscioli-Johnson, K.M.; Santschi, P.H.; Jaffé, P.R. Unique organic matter and microbial properties in the rhizosphere of a wetland soil. Environ. Sci. Technol. 2016, 50, 4169–4177. [Google Scholar] [CrossRef]
- Risakotta, M.Y.S.; Andayany, H. Mineral identification of rocks from Pohon Batu hot springs in West Seram using FTIR spectroscopy. J. Phys. Conf. Ser. 2020, 1572, 012045. [Google Scholar] [CrossRef]
- Fultz, L.M.; Moore-Kucera, J.; Calderón, F.; Acosta-Martínez, V. Using fourier-transform mid-infrared spectroscopy to distinguish soil organic matter composition dynamics in aggregate fractions of two agroecosystems. Soil Sci. Soc. Am. J. 2014, 78, 1940–1948. [Google Scholar] [CrossRef]
- Rahmanian, O.; Dinari, M.; Neamati, S. Synthesis and characterization of citrate intercalated layered double hydroxide as a green adsorbent for Ni2+ and Pb2+ removal. Environ. Sci. Pollut. Res. 2018, 25, 36267–36277. [Google Scholar] [CrossRef]
- Vergnoux, A.; Guiliano, M.; Di Rocco, R.; Domeizel, M.; Théraulaz, F.; Doumenq, P. Quantitative and mid-infrared changes of humic substances from burned soils. Environ. Res. 2011, 111, 205–214. [Google Scholar] [CrossRef]
- Senneca, O.; Cortese, L.; Di Martino, R.; Fabbricino, M.; Ferraro, A.; Race, M.; Scopino, A. Mechanisms affecting the delayed efficiency of cement based stabilization/solidification processes. J. Clean. Prod. 2020, 261, 121230. [Google Scholar] [CrossRef]
- Butterfield, A.G.; Alameda, L.T.; Schaak, R.E. Emergence and Control of Stacking Fault Formation during Nanoparticle Cation Exchange Reactions. J. Am. Chem. Soc. 2021, 143, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, X.; Zhao, L. Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef]
- Gkarmiri, K.; Mahmood, S.; Ekblad, A.; Alström, S.; Högberg, N.; Finlay, R. Identifying the active microbiome associated with roots and rhizosphere soil of oilseed rape. Appl. Environ. Microbiol. 2017, 83, e01938-17. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Song, J.; Shen, Q.; Wang, L.; Qiu, G.; Shi, J.; Xu, J.; Brookes, P.C.; Liu, X. Effects of Cd, Cu, Zn and their combined action on microbial biomass and bacterial community structure. Environ. Pollut. 2018, 243, 510–518. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Q.; Fan, S.; Zhang, Y.; Zhang, M.; Zhang, J. Distinction between Cr and other heavy–metal–resistant bacteria involved in C/N cycling in contaminated soils of copper producing sites. J. Hazard. Mater. 2021, 402, 123454. [Google Scholar] [CrossRef]
- Wang, W.; Liu, A.; Fu, W.; Peng, D.; Wang, G.; Ji, J.; Jin, C.; Guan, C. Tobacco-associated with Methylophilus sp. FP-6 enhances phytoremediation of benzophenone-3 through regulating soil microbial community, increasing photosynthetic capacity and maintaining redox homeostasis of plant. J. Hazard. Mater. 2022, 431, 128588. [Google Scholar] [CrossRef]
- Qi, G.; Chen, S.; Ke, L.; Ma, G.; Zhao, X. Cover crops restore declining soil properties and suppress bacterial wilt by regulating rhizosphere bacterial communities and improving soil nutrient contents. Microbiol. Res. 2020, 238, 126505. [Google Scholar] [CrossRef]
- Li, F.; Guo, Y.; Wang, Z.; Mu, Y. Influence of different phytoremediation on soil microbial diversity and community composition in saline-alkaline land. Int. J. Phytoremediation 2022, 24, 507–517. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Z.; Zhao, J.; Ma, J.; Yu, Q.; Zou, P.; Lin, H.; Ma, J. Fate of heavy metals and bacterial community composition following biogas slurry application in a single rice cropping system. J. Soils Sediments 2022, 22, 968–981. [Google Scholar] [CrossRef]
- Hu, X.; Liu, X.; Qiao, L.; Zhang, S.; Su, K.; Qiu, Z.; Li, X.; Zhao, Q.; Yu, C. Study on the spatial distribution of ureolytic microorganisms in farmland soil around tailings with different heavy metal pollution. Sci. Total Environ. 2021, 775, 144946. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, X.; Liu, Y.; Cui, W.; Sun, Y.; Zhang, S.; Wang, F. Effects of microplastics and carbon nanotubes on soil geochemical properties and bacterial communities. J. Hazard. Mater. 2022, 433, 128826. [Google Scholar] [CrossRef]
- Nagarajan, V.; Tsai, H.-C.; Chen, J.-S.; Hussain, B.; Koner, S.; Hseu, Z.-Y.; Hsu, B.-M. Comparison of bacterial communities and their functional profiling using 16S rRNA gene sequencing between the inherent serpentine-associated sites, hyper-accumulator, downgradient agricultural farmlands, and distal non-serpentine soils. J. Hazard. Mater. 2022, 431, 128557. [Google Scholar] [CrossRef]
- Zhang, M.; Xiong, J.; Zhou, L.; Li, J.; Fan, J.; Li, X.; Zhang, T.; Yin, Z.; Yin, H.; Liu, X. Community ecological study on the reduction of soil antimony bioavailability by SRB-based remediation technologies. J. Hazard. Mater. 2023, 459, 132256. [Google Scholar] [CrossRef]
- Yang, Z.-N.; Liu, Z.-S.; Wang, K.-H.; Liang, Z.-L.; Abdugheni, R.; Huang, Y.; Wang, R.-H.; Ma, H.-L.; Wang, X.-K.; Yang, M.-L. Soil microbiomes divergently respond to heavy metals and polycyclic aromatic hydrocarbons in contaminated industrial sites. Environ. Sci. Ecotechnology 2022, 10, 100169. [Google Scholar] [CrossRef]
- Zhang, C.; Nie, S.; Liang, J.; Zeng, G.; Wu, H.; Hua, S.; Liu, J.; Yuan, Y.; Xiao, H.; Deng, L. Effects of heavy metals and soil physicochemical properties on wetland soil microbial biomass and bacterial community structure. Sci. Total Environ. 2016, 557–558, 785–790. [Google Scholar] [CrossRef]
- Usharani, B. Metagenomics study of the microbes in constructed wetland system treating sewage. Int. Lett. Nat. Sci. 2019, 74, 26–48. [Google Scholar] [CrossRef]
- Dedysh, S.N.; Kulichevskaya, I.S.; Huber, K.J.; Overmann, J. Defining the taxonomic status of described subdivision 3 Acidobacteria: Proposal of Bryobacteraceae fam. nov. Int. J. Syst. Evol. Microbiol. 2017, 67, 498–501. [Google Scholar] [CrossRef]
- Hu, L.; Li, Q.; Yan, J.; Liu, C.; Zhong, J. Vegetation restoration facilitates belowground microbial network complexity and recalcitrant soil organic carbon storage in southwest China karst region. Sci. Total Environ. 2022, 820, 153137. [Google Scholar] [CrossRef]
- Ding, W.; Cong, W.-F.; Lambers, H. Plant phosphorus-acquisition and -use strategies affect soil carbon cycling. Trends Ecol. Evol. 2021, 36, 899–906. [Google Scholar] [CrossRef]
- GB15618-2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land in China. National Standards of the People’s Republic of China: Beijing, China, 2018.
- Gavrilescu, M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef]
- Yaacob, N.S.; Ahmad, M.F.; Sivam, A.; Hashim, E.F.; Maniyam, M.N.; Sjahrir, F.; Dzulkafli, N.F.; Wan Mohd Zamri, W.M.I.; Komatsu, K.; Kuwahara, V.S.; et al. The Effectiveness of Soil Extracts from Selangor Peat Swamp and Pristine Forest Soils on the Growth of Green Microalgae sp. Forests 2022, 13, 79. [Google Scholar] [CrossRef]
- Yang, H.; Hu, F.; Yin, W.; Chai, Q.; Zhao, C.; Yu, A.; Fan, Z.; Fan, H.; Ren, X. Integration of tillage and planting density improves crop production and carbon mitigation of maize/pea intercropping in the oasis irrigation area of northwestern China. Field Crop. Res. 2021, 272, 108281. [Google Scholar] [CrossRef]
- GB2762-2022; China National Food Safety Standard Maximum Levels of Contaminants in Food. National Standards of the People’s Republic of China: Beijing, China, 2022.
- Quarshie, S.D.-G.; Xiao, X.; Zhang, L. Enhanced Phytoremediation of soil heavy metal pollution and commercial utilization of harvested plant biomass: A review. Water Air Soil Pollut. 2021, 232, 1–28. [Google Scholar] [CrossRef]
- Yaacob, N.S.; Ahmad, M.F.; Kawasaki, N.; Maniyam, M.N.; Abdullah, H.; Hashim, E.F.; Sjahrir, F.; Wan Mohd Zamri, W.M.I.; Komatsu, K.; Kuwahara, V.S. Kinetics Growth and Recovery of Valuable Nutrients from Selangor Peat Swamp and Pristine Forest Soils Using Different Extraction Methods as Potential Microalgae Growth Enhancers. Molecules 2021, 26, 653. [Google Scholar] [CrossRef]
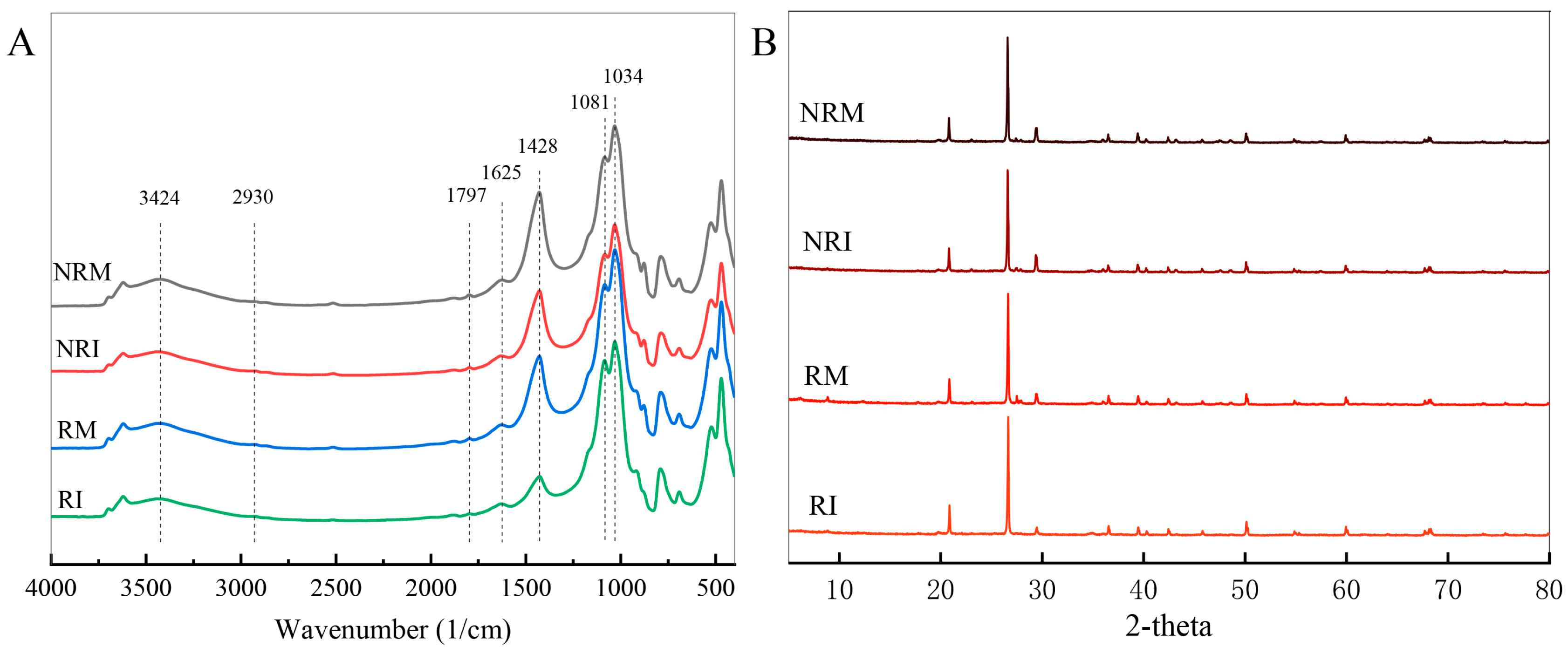

| Peak | Organic Carbon Assignment | References |
|---|---|---|
| 1034 | C-O stretching of polysaccharide in SOM | [10] |
| 1081 | C-O stretch in polysaccharides and fresh plant residues in DOM | [27] |
| 1428 | Symmetric stretching vibrations of the carboxylate group | [28] |
| 1625 | Aromatic C=C stretching of carboxylic acids and amides | [29] |
| 1797 | C-O stretching of carbonate | [30] |
| 2930 | Aliphatic C-H stretching of methyl and methylene groups | [31] |
| 3424 | O-H stretching for intramolecular hydrogen bonds | [32] |
| Soil Types | Peak 1034 | Peak 1081 | Peak 1428 | Peak 1625 | Peak 1797 | Peak 2930 | Peak 3424 |
|---|---|---|---|---|---|---|---|
| NRM | 26.1 ± 1.4 a | 21.4 ± 1.2 c | 25.1 ± 0.2 a | 6.3 ± 0.1 a | 2.3 ± 0.1 b | 3.4 ± 0.1 b | 5.8 ± 0.2 a |
| NRI | 26.2 ± 0.6 a | 21.0 ± 0.4 c | 23.9 ± 0.4 a | 6.5 ± 0.0 a | 2.5 ± 0.0 b | 3.7 ± 0.2 a b | 6.5 ± 1.1 a |
| RM | 26.2 ± 0.7 a | 25.1 ± 0.7 b | 20.8 ± 0.2 b | 6.0 ± 0.1 a | 2.4 ± 0.0 b | 3.4 ± 0.2 b | 6.1 ± 1.2 a |
| RI | 26.4 ± 1.4 a | 33.9 ± 2.2 a | 11.8 ± 1.7 c | 3.5 ± 2.2 b | 3.9 ± 1.2 a | 4.0 ± 0.1 a | 6.3 ± 0.5 a |
| Index | RDA1 | RDA2 | r2 | p | |
|---|---|---|---|---|---|
| SOM | −0.852 | 0.524 | 0.500 | 0.046 | * |
| DOM | 0.989 | 0.149 | 0.704 | 0.005 | ** |
| Cu | −0.985 | 0.174 | 0.977 | 0.001 | *** |
| Zn | −0.912 | 0.409 | 0.838 | 0.002 | ** |
| Cd | −0.810 | 0.586 | 0.858 | 0.001 | *** |
| Polysaccharides C-O | 0.804 | −0.595 | 0.768 | 0.003 | ** |
| Symmetric carboxylate | −0.824 | 0.567 | 0.811 | 0.002 | ** |
| Aromatic C=C | −0.820 | 0.572 | 0.379 | 0.072 | |
| Carbonate C-O | 0.774 | −0.633 | 0.360 | 0.086 | |
| Aliphatic methyl and methylene | 0.779 | −0.627 | 0.261 | 0.239 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, F.; Zhang, X.; Li, Q.; Huang, Z.; Zhong, Z. Enhancement of Phytoremediation of Heavy Metal Pollution Using an Intercropping System in Moso Bamboo Forests: Characteristics of Soil Organic Matter and Bacterial Communities. Forests 2023, 14, 1895. https://doi.org/10.3390/f14091895
Bian F, Zhang X, Li Q, Huang Z, Zhong Z. Enhancement of Phytoremediation of Heavy Metal Pollution Using an Intercropping System in Moso Bamboo Forests: Characteristics of Soil Organic Matter and Bacterial Communities. Forests. 2023; 14(9):1895. https://doi.org/10.3390/f14091895
Chicago/Turabian StyleBian, Fangyuan, Xiaoping Zhang, Qiaoling Li, Zhiyuan Huang, and Zheke Zhong. 2023. "Enhancement of Phytoremediation of Heavy Metal Pollution Using an Intercropping System in Moso Bamboo Forests: Characteristics of Soil Organic Matter and Bacterial Communities" Forests 14, no. 9: 1895. https://doi.org/10.3390/f14091895
APA StyleBian, F., Zhang, X., Li, Q., Huang, Z., & Zhong, Z. (2023). Enhancement of Phytoremediation of Heavy Metal Pollution Using an Intercropping System in Moso Bamboo Forests: Characteristics of Soil Organic Matter and Bacterial Communities. Forests, 14(9), 1895. https://doi.org/10.3390/f14091895






