Aboveground Biomass Productivity and Nutrient Use Dynamics of Clumping Tropical Bamboos in Northern Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Data Collection
2.2.1. Forest Survey
2.2.2. Biomass Sampling
2.2.3. Nutrient Concentration in Plant Components
2.2.4. Soil Sampling and Analysis
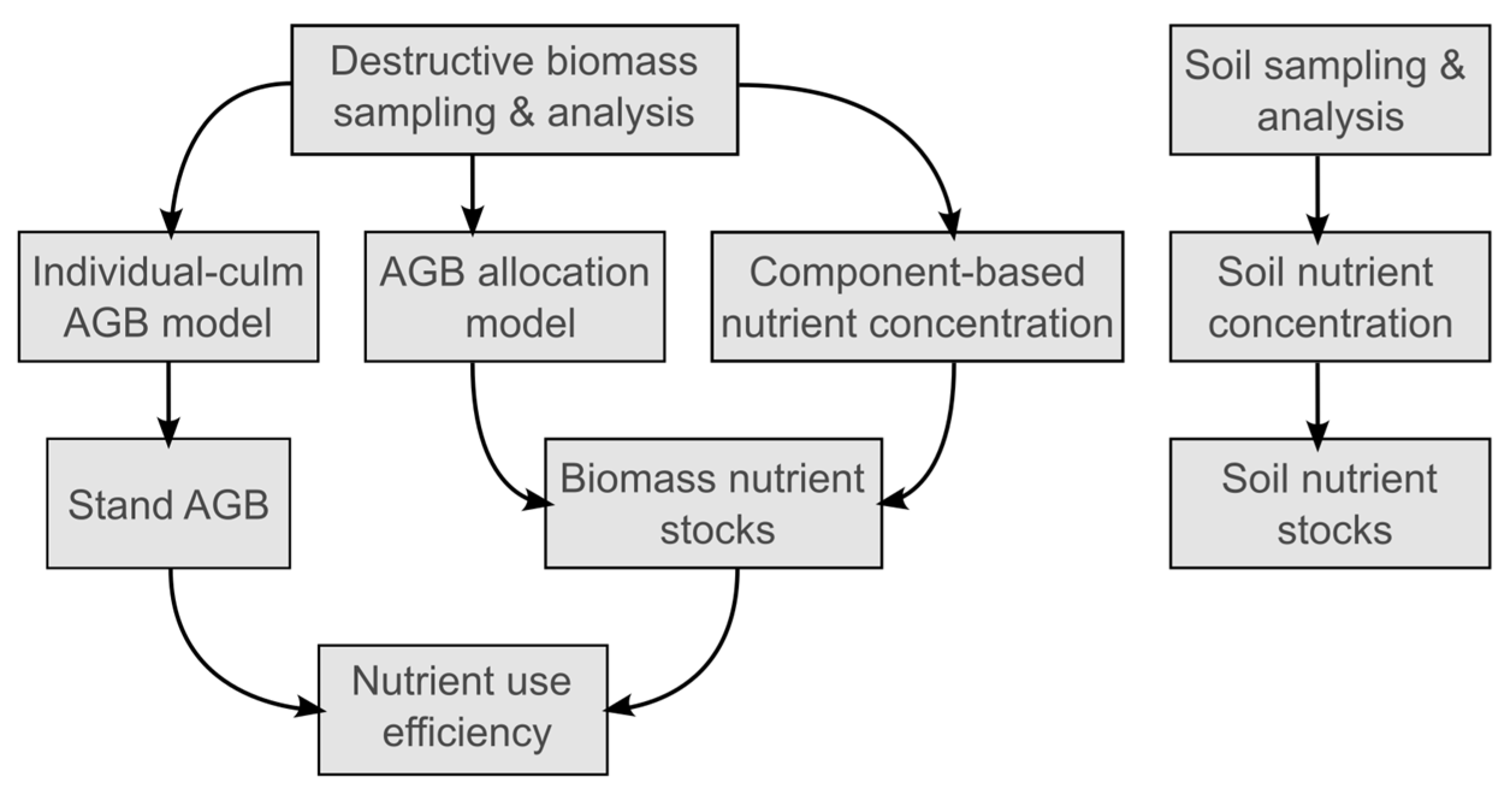
2.3. Data Analyses
2.3.1. AGB and Biomass Allocation
2.3.2. Biomass Nutrient Concentration, Stock, and Use Efficiency
2.3.3. Statistical Analyses
3. Results
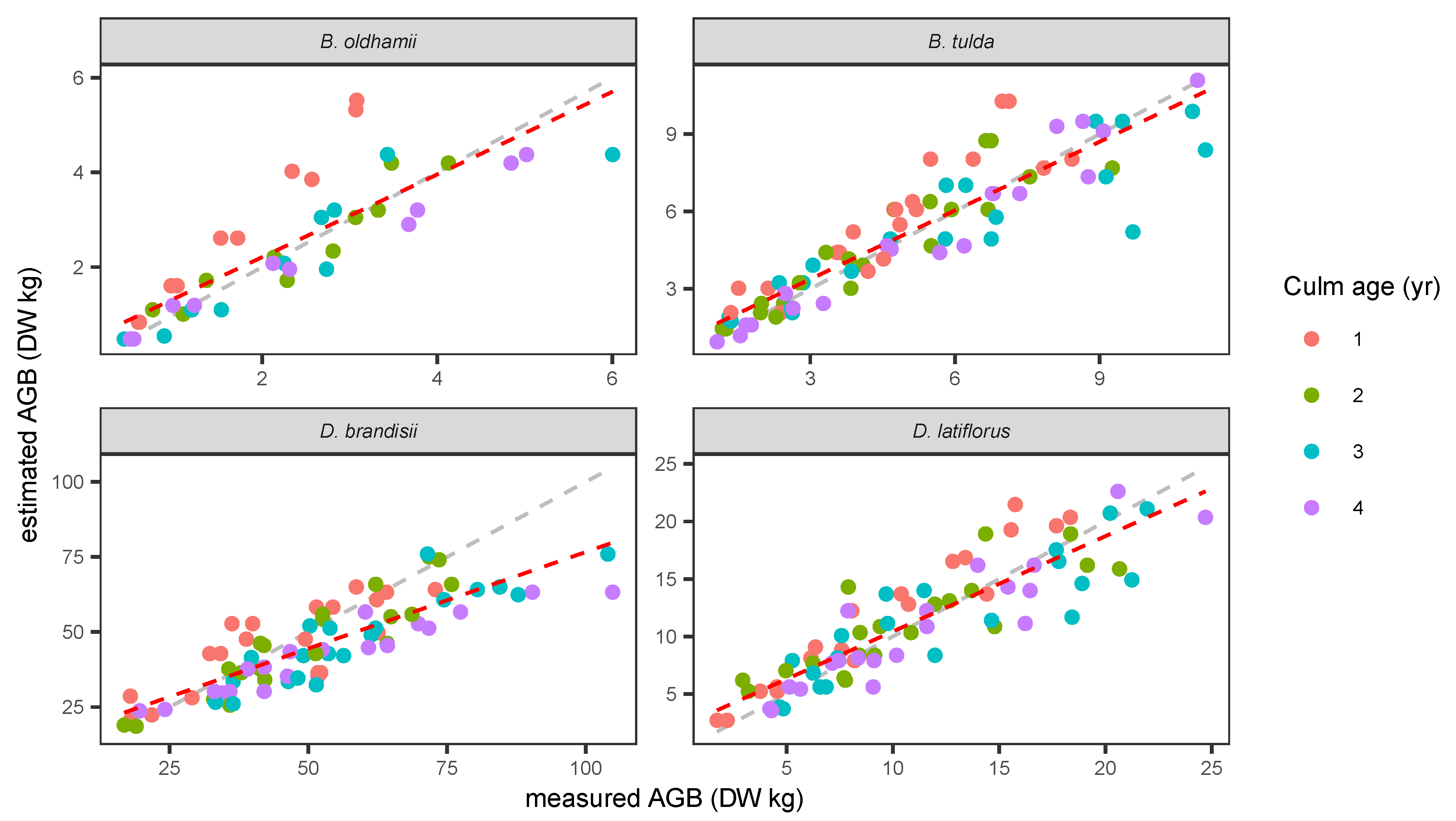
3.1. Aboveground Biomass
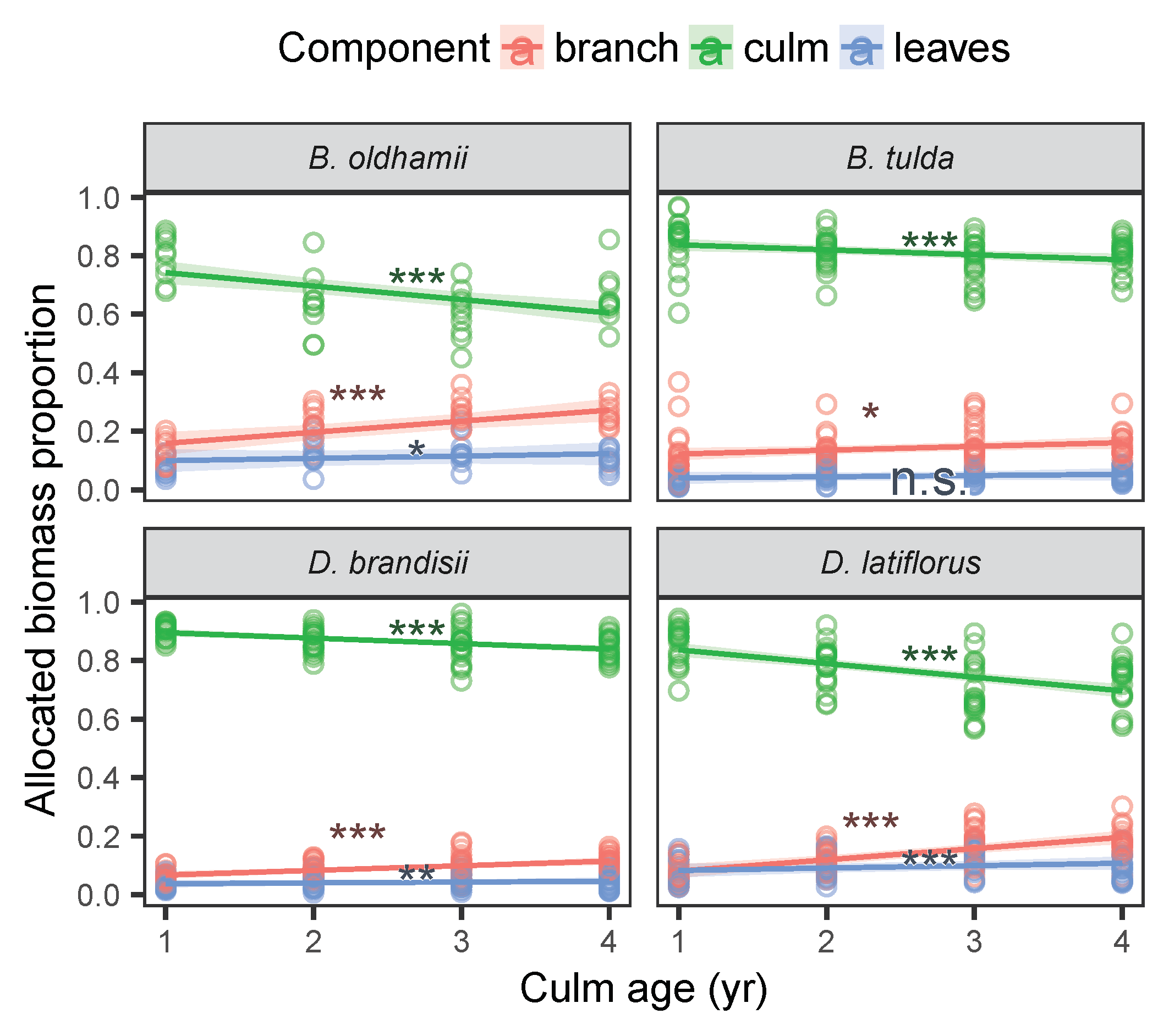
3.2. Biomass Allocation
3.3. Nutrient Stock in Harvestable Biomass and Soil
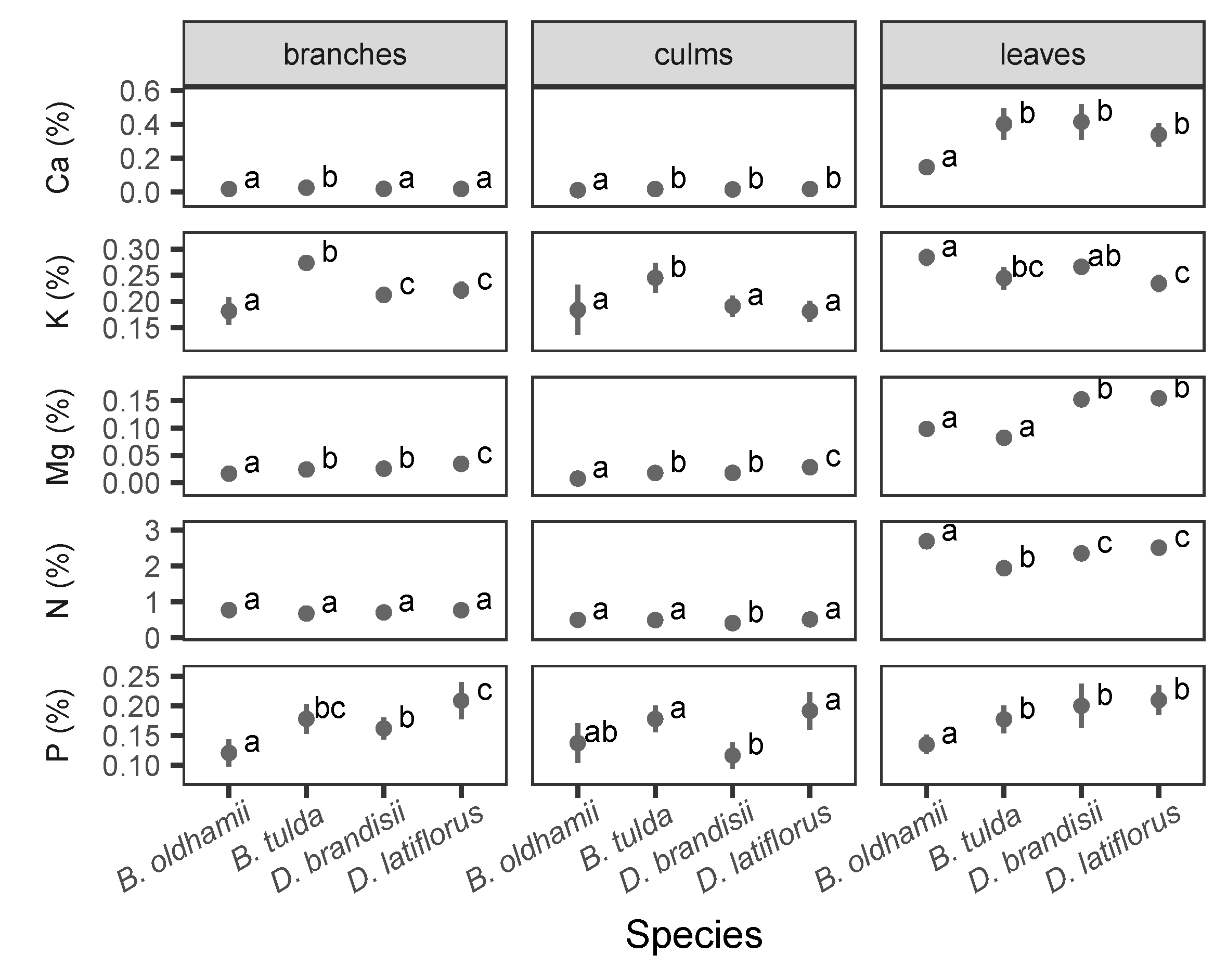
3.4. Nutrient Concentration and NUE
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Model | Predictors | BIC | Adj. R2 | Bias | RMSE | MAPE | Intercept | Coef. (1) | Coef. (2) |
|---|---|---|---|---|---|---|---|---|---|---|
| B. oldhamii | (1) Log-Transformed Linear | logDBH, logAge | 12.590 | 0.914 | 2.623 | 0.476 | 15.667 | −1.877 | 1.936 | 0.358 |
| (2) Power | D2H, Age | 32.840 | 0.913 | 3.910 | 0.392 | 15.332 | 0.076 | 0.690 | 0.436 | |
| (3) Power | DBH, Age | 39.140 | 0.887 | 3.521 | 0.442 | 15.342 | 0.159 | 1.876 | 0.412 | |
| B. tulda | (1) Log-Transformed Linear | logDBH, logAge | −4.242 | 0.910 | 1.839 | 1.022 | 16.207 | −1.515 | 2.133 | 0.209 |
| (2) Log-Transformed Linear | logDBH, Age | −3.807 | 0.911 | 2.542 | 1.053 | 16.516 | −1.584 | 2.131 | 0.096 | |
| (3) Log-Transformed Linear | logDBH | 6.721 | 0.889 | 1.841 | 1.170 | 18.902 | −1.241 | 2.048 | - | |
| D. brandisii | (1) Log-Transformed Power | D2H, Age | −31.080 | 1.000 | 0.974 | 8.244 | 13.242 | 0.608 | 0.215 | 0.050 |
| (2) Log-Transformed Power | logDBH, Age | −27.690 | 1.000 | 1.651 | 8.578 | 14.323 | 0.836 | 1.538 | 0.054 | |
| (3) Log-Transformed Power | DBH, Age | −26.406 | 1.000 | 1.166 | 8.819 | 14.459 | 0.771 | 0.594 | 0.028 | |
| D. latiflorus | (1) Log-Transformed Linear | logDBH, logAge | 6.628 | 0.860 | 3.708 | 2.393 | 18.656 | −2.157 | 2.035 | 0.237 |
| (2) Log-Transformed Power | logDBH, Age | 13.466 | 0.998 | 2.198 | 2.604 | 19.273 | 0.487 | 1.968 | 0.102 | |
| (3) Log-Transformed Linear | logDBH, Age | 7.309 | 0.856 | 3.792 | 2.522 | 18.928 | −2.240 | 2.040 | 0.105 |
References
- Lobovikov, M.; Schoene, D.; Yping, L. Bamboo in climate change and rural livelihoods. Mitig. Adapt. Strateg. Glob. Chang. 2012, 17, 261–276. [Google Scholar] [CrossRef]
- Sileshi, G.W.; Mafongoya, P.L.; Nath, A.J. Agroforestry Systems for Improving Nutrient Recycling and Soil Fertility on Degraded Lands. In Agroforestry for Degraded Landscapes; Dagar, J.C., Gupta, S.R., Teketay, D., Eds.; Springer: Singapore, 2020; pp. 225–253. ISBN 9789811541353. [Google Scholar]
- Hogarth, N.J.; Belcher, B. The contribution of bamboo to household income and rural livelihoods in a poor and mountainous county in Guangxi, China. Int. For. Rev. 2013, 15, 71–81. [Google Scholar] [CrossRef]
- Kleinhenz, V.; Midmore, D.J. Aspects of Bamboo Agronomy. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2001; Volume 74, pp. 99–153. ISBN 978-0-12-000792-9. [Google Scholar]
- Akoto, D.S.; Partey, S.T.; Denich, M.; Kwaku, M.; Borgemeister, C.; Schmitt, C.B. Towards bamboo agroforestry development in Ghana: Evaluation of crop performance, soil properties and economic benefit. Agrofor. Syst. 2020, 94, 1759–1780. [Google Scholar] [CrossRef]
- Dev, I.; Ram, A.; Ahlawat, S.P.; Palsaniya, D.R.; Singh, R.; Dhyani, S.K.; Kumar, N.; Tewari, R.K.; Singh, M.; Babanna, S.K.; et al. Bamboo-based agroforestry system (Dendrocalamus strictus + sesame–chickpea) for enhancing productivity in semi-arid tropics of central India. Agrofor. Syst. 2020, 94, 1725–1739. [Google Scholar] [CrossRef]
- Zheng, Y.; Guan, F.; Fan, S.; Yan, X.; Huang, L. Dynamics of Leaf-Litter Biomass, Nutrient Resorption Efficiency and Decomposition in a Moso Bamboo Forest After Strip Clearcutting. Front. Plant Sci. 2022, 12, 799424. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Fan, S.; Guan, F.; Zhang, X.; Zhou, X. Characteristics of the litter dynamics in a Moso bamboo forest after strip clearcutting. Front. Plant Sci. 2022, 13, 1064529. [Google Scholar] [CrossRef]
- Canavan, S.; Richardson, D.M.; Visser, V.; Roux, J.J.L.; Vorontsova, M.S.; Wilson, J.R.U. The global distribution of bamboos: Assessing correlates of introduction and invasion. AoB Plants 2016, 9, plw078. [Google Scholar] [CrossRef]
- Canavan, S.; Kumschick, S.; Le Roux, J.J.; Richardson, D.M.; Wilson, J.R.U. Does origin determine environmental impacts? Not for bamboos. Plants People Planet 2019, 1, 119–128. [Google Scholar] [CrossRef]
- Ni, H.; Su, W.; Fan, S.; Chu, H. Effects of intensive management practices on rhizosphere soil properties, root growth, and nutrient uptake in Moso bamboo plantations in subtropical China. For. Ecol. Manag. 2021, 493, 119083. [Google Scholar] [CrossRef]
- Piouceau, J.; Bois, G.; Panfili, F.; Anastase, M.; Dufossé, L.; Arfi, V. Effects of High Nutrient Supply on the Growth of Seven Bamboo Species. Int. J. Phytoremediat. 2014, 16, 1042–1057. [Google Scholar] [CrossRef]
- Franklin, D.C. Wild bamboo stands fail to compensate for a heavy 1-year harvest of culm shoots. For. Ecol. Manag. 2006, 237, 115–118. [Google Scholar] [CrossRef]
- Mao, F.; Zhou, G.; Li, P.; Du, H.; Xu, X.; Shi, Y.; Mo, L.; Zhou, Y.; Tu, G. Optimizing selective cutting strategies for maximum carbon stocks and yield of Moso bamboo forest using BIOME-BGC model. J. Environ. Manag. 2017, 191, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Liese, W. Bamboo as carbon sink—Fact or fiction? J. Bamboo Rattan 2009, 8, 103–114. [Google Scholar]
- Christanty, L.; Kimmins, J.P.; Mailly, D. ‘Without bamboo, the land dies’: A conceptual model of the biogeochemical role of bamboo in an Indonesian agroforestry system. For. Ecol. Manag. 1997, 91, 83–91. [Google Scholar] [CrossRef]
- Kaushal, R.; Islam, S.; Tewari, S.; Tomar, J.M.S.; Thapliyal, S.; Madhu, M.; Trinh, T.L.; Singh, T.; Singh, A.; Durai, J. An allometric model-based approach for estimating biomass in seven Indian bamboo species in western Himalayan foothills, India. Sci. Rep. 2022, 12, 7527. [Google Scholar] [CrossRef]
- Chaiyasart, P.; Poolsiri, R.; Haruthaithanasan, M. Litter Decomposition of Various Bamboo Plantations at Royal Agricultural Station Angkhang, Chiang Mai Province. Thai J. For. 2018, 37, 48–59. [Google Scholar]
- Kim, C.; Baek, G.; Yoo, B.O.; Jung, S.-Y.; Lee, K.S. Regular Fertilization Effects on the Nutrient Distribution of Bamboo Components in a Moso Bamboo (Phyllostachys pubescens (Mazel) Ohwi) Stand in South Korea. Forests 2018, 9, 671. [Google Scholar] [CrossRef]
- Zhang, F.; Jin, Q.; Peng, H.; Zhu, T. Soil acidification in moso bamboo (Phyllostachys edulis) forests and changes of soil metal ions (Cu, Pb) concentration. Arch. Agron. Soil Sci. 2021, 67, 1799–1808. [Google Scholar] [CrossRef]
- TEI. Bamboo Value Chain Analysis in Thailand; INBAR, TEI: Bangkok, Thailand, 2021. [Google Scholar]
- Kuehl, Y.; Li, Y.; Henley, G. Impacts of selective harvest on the carbon sequestration potential in Moso bamboo (Phyllostachys pubescens) plantations. For. Trees Livelihoods 2013, 22, 773652. [Google Scholar] [CrossRef]
- Thaiutsa, B. Bamboo Plantation of the Royal Project; Royal Project Foundation: Chiangmai, Thailand, 2000. [Google Scholar]
- Thaiutsa, B. Highland and Reforestation Project: A Forestry Project of the Royal Project Foundation. In Proceedings of the Twentieth Anniversary of Taiwan/Angkhang Forestry Project; Taiwan International Cooperation Development Fund: Chiang Mai, Thailand, 2003; pp. 1–14. [Google Scholar]
- Viswanath, S.; Chethan, K.; Srivastava, A.; Joshi, G.; Sowmya, C.; Joshi, S. Dendrocalamus brandisii—An ideal bamboo species for domestication in humid tropics. IWST Tech Bull 2013, 12, 1–24. [Google Scholar]
- Castaneda-Mendoza, A.; Vargas-Hernández, J.; Gomez-Guerrero, A.; Valdez-Hernandez, J.; Vaquera-Huerta, H. Carbon accumulation in the aboveground biomass of a Bambusa oldhamii plantation. Agrociencia 2005, 39, 107–116. [Google Scholar]
- Yuen, J.Q.; Fung, T.; Ziegler, A.D. Carbon stocks in bamboo ecosystems worldwide: Estimates and uncertainties. For. Ecol. Manag. 2017, 393, 113–138. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Yen, T.-M. Assessing aboveground carbon storage capacity in bamboo plantations with various species related to its affecting factors across Taiwan. For. Ecol. Manag. 2021, 481, 118745. [Google Scholar] [CrossRef]
- Devi, A.S.; Singh, K.S. Carbon storage and sequestration potential in aboveground biomass of bamboos in North East India. Sci. Rep. 2021, 11, 837. [Google Scholar] [CrossRef] [PubMed]
- Sethy, A.K.; Sharma, S.K.; Simon, J. Effect of flowering on physical and mechanical properties of Dendrocalamus brandisii (Munro) Kurz. J. Indian Acad. Wood Sci. 2019, 16, 22–26. [Google Scholar] [CrossRef]
- Maviton, M.E.; Sankar, V.R. Global Priority Species of Economically Important Bamboo; INBAR Technical Report; INBAR: Beijing, China, 2022. [Google Scholar]
- Bhubharuang, B. Soil Characterization and Land Potential Assessment of Ang-Khang Range, Chiangmai; Kasetsart University: Bangkok, Thailand, 1980. [Google Scholar]
- Kaushal, R.; Singh, I.; Thapliyal, S.D.; Gupta, A.K.; Mandal, D.; Tomar, J.M.S.; Kumar, A.; Alam, N.M.; Kadam, D.; Singh, D.V.; et al. Rooting behaviour and soil properties in different bamboo species of Western Himalayan Foothills, India. Sci. Rep. 2020, 10, 4966. [Google Scholar] [CrossRef]
- Page-Dumroese, D.S.; Brown, R.E.; Jurgensen, M.F.; Mroz, G.D. Comparison of Methods for Determining Bulk Densities of Rocky Forest Soils. Soil Sci. Soc. Am. J. 1999, 63, 379–383. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Directions for making mechanical analyses of soils by the hydrometer method. Soil Sci. 1936, 42, 225. [Google Scholar] [CrossRef]
- Burt, R. Soil Survey Investigations Report, no. 45, Version 2.0; United States Department of Agriculture: Washington, DC, USA, 2011.
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29. [Google Scholar] [CrossRef]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1965; pp. 1149–1178. ISBN 978-0-89118-204-7. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Sumner, M.E.; Miller, W.P. Cation Exchange Capacity and Exchange Coefficients. In Methods of Soil Analysis: Part 3 Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 2018; pp. 1201–1229. ISBN 978-0-89118-866-7. [Google Scholar]
- Huy, B.; Long, T. A Manual for Bamboo Forest Biomass and Carbon Assessment; INBAR Technical Report; INBAR: Beijing, China, 2019. [Google Scholar]
- Huy, B.; Thanh, G.T.; Poudel, K.P.; Temesgen, H. Individual Plant Allometric Equations for Estimating Aboveground Biomass and Its Components for a Common Bamboo Species (Bambusa procera A. Chev. and A. Camus) in Tropical Forests. Forests 2019, 10, 316. [Google Scholar] [CrossRef]
- Wang, D.; Bormann, F.H.; Lugo, A.E.; Bowden, R.D. Comparison of nutrient-use efficiency and biomass production in five tropical tree taxa. For. Ecol. Manag. 1991, 46, 1–21. [Google Scholar] [CrossRef]
- Santana, R.C.; Barros, N.F.; Comerford, N.B. Above-ground biomass, nutrient content, and nutrient use efficiency of eucalypt plantations growing in different sites in Brazil. N. Z. J. For. Sci. 2000, 30, 225–236. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests, R Package Version 0.7.0. 2021. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 10 May 2023).
- Banik, R.L. Some Priority Bamboo Species for South Asian Region. In Silviculture of South Asian Priority Bamboos; Banik, R.L., Ed.; Tropical Forestry; Springer: Singapore, 2016; pp. 15–18. ISBN 978-981-10-0569-5. [Google Scholar]
- Castañeda-Mendoza, A.; Vargas-Hernández, J.J.; Gómez-Guerrero, A. Components of net aerial primary production in a Bambusa oldhamii plantation. Agrociencia 2012, 46, 63–74. [Google Scholar]
- Bahru, T.; Liu, G.; Ding, Y. Effects of standing culm density and fertilizer regimes on Dendrocalamus brandisii (Munro) Kurz shoot production at Simao District, southwestern China. Trees For. People 2021, 4, 100071. [Google Scholar] [CrossRef]
- Hogarth, N.J. The link between smallholder bamboo shoot management, income, and livelihoods: A case study in southern China. For. Trees Livelihoods 2013, 22, 70–85. [Google Scholar] [CrossRef]
- Yen, T.-M.; Sun, P.-K.; Li, L.-E. Predicting Aboveground Biomass and Carbon Storage for Ma Bamboo (Dendrocalamus latiflorus Munro) Plantations. Forests 2023, 14, 854. [Google Scholar] [CrossRef]
- Partey, S.T.; Sarfo, D.A.; Frith, O.; Kwaku, M.; Thevathasan, N.V. Potentials of Bamboo-Based Agroforestry for Sustainable Development in Sub-Saharan Africa: A Review. Agric. Res. 2017, 6, 22–32. [Google Scholar] [CrossRef]
- Akoto, D.S.; Partey, S.T.; Abugre, S.; Akoto, S.; Denich, M.; Borgemeister, C.; Schmitt, C.B. Comparative analysis of leaf litter decomposition and nutrient release patterns of bamboo and traditional species in agroforestry system in Ghana. Clean. Mater. 2022, 4, 100068. [Google Scholar] [CrossRef]
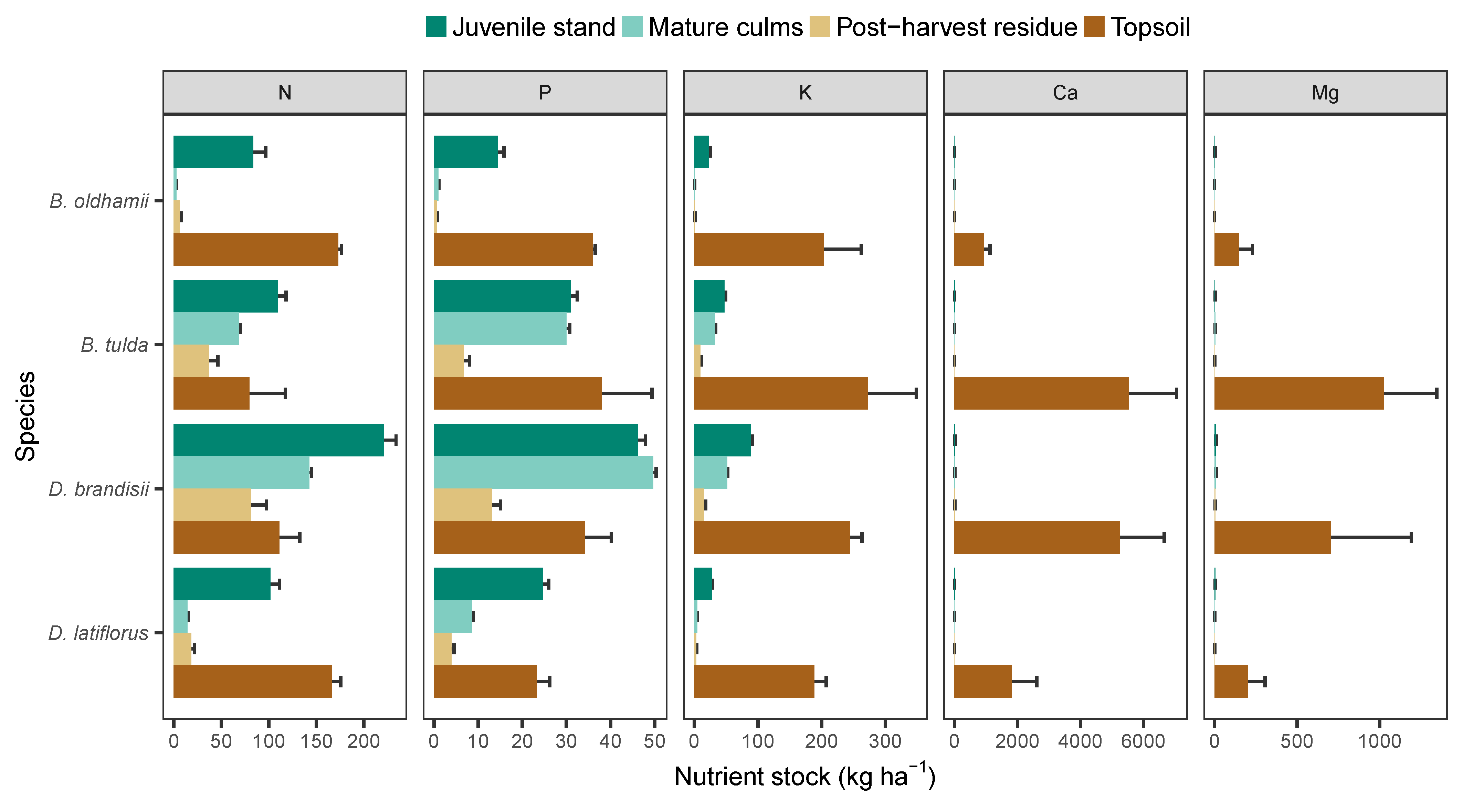

| B. oldhamii | B. tulda | D. brandisii | D. latiflorus | |
|---|---|---|---|---|
| DBH (cm) | 3.36 (0.96) a | 3.75 (0.88) b | 13.92 (2.11) c | 7.71 (2.05) d |
| Culm age distribution (%) | 33.8/23.8/ 27.7/14.7 | 10.8/9.9/ 23.4/55.9 | 10.5/17.1/ 14.9/57.5 | 23.8/18.8/ 24.8/32.6 |
| Culm density (ha−1) | 4512.5 | 7475 | 1968.8 | 1862.5 |
| New culms (culm ha−1 yr−1) | 1283.3 (320) a | 1097.9 (759.2) a | 279.2 (97) b | 418.8 (116.3) b |
| Harvestable culms (Mg ha−1) | 2.48 (0.64) a | 43.48 (16.16) ab | 116.08 (55.35) b | 10.64 (3.55) a |
| MAIAGB (Mg ha−1 yr−1) | 1.83 (1.98) a | 7.17 (3.14) bc | 17.37 (6.75) b | 4.8 (2.94) ac |
| SOM (%) | 5.61 (3.85) a | 2.79 (1.65) b | 4.76 (2.35) ab | 7.73 (2.71) a |
| pH | 4.29 (0.19) a | 5.38 (0.1) b | 5.32 (0.21) b | 4.72 (0.2) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chantarat, P.; Poolsiri, R.; Wannalangka, I.; Kaitpraneet, S.; Puangchit, L.; Jenke, M. Aboveground Biomass Productivity and Nutrient Use Dynamics of Clumping Tropical Bamboos in Northern Thailand. Forests 2023, 14, 1450. https://doi.org/10.3390/f14071450
Chantarat P, Poolsiri R, Wannalangka I, Kaitpraneet S, Puangchit L, Jenke M. Aboveground Biomass Productivity and Nutrient Use Dynamics of Clumping Tropical Bamboos in Northern Thailand. Forests. 2023; 14(7):1450. https://doi.org/10.3390/f14071450
Chicago/Turabian StyleChantarat, Pramena, Roongreang Poolsiri, Ittipong Wannalangka, San Kaitpraneet, Ladawan Puangchit, and Michael Jenke. 2023. "Aboveground Biomass Productivity and Nutrient Use Dynamics of Clumping Tropical Bamboos in Northern Thailand" Forests 14, no. 7: 1450. https://doi.org/10.3390/f14071450
APA StyleChantarat, P., Poolsiri, R., Wannalangka, I., Kaitpraneet, S., Puangchit, L., & Jenke, M. (2023). Aboveground Biomass Productivity and Nutrient Use Dynamics of Clumping Tropical Bamboos in Northern Thailand. Forests, 14(7), 1450. https://doi.org/10.3390/f14071450






