Transcriptomic Analysis Revealed That Low-Density Aphid Infestation Temporarily Changes Photosynthesis and Disease Resistance but Persistently Promotes Insect Resistance in Poplar Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Insects
2.2. Aphid Inoculation
2.3. Determination of Gas Exchange and Photosynthesis
2.4. Transcriptomic Assays
2.5. RT-qPCR Validation of Differentially Expressed Genes (DEGs)
3. Results
3.1. Morphology of Aphid Infestations of Poplar Saplings
3.2. Aphid Inoculation Temporarily Changes Gas Exchange Parameters in Poplar Leaves
3.3. Influence of Aphid Inoculation on Gene Expression in Poplar Leaves
3.3.1. Aphid Inoculation Induces a Transient Significant Change in Gene Expression at the Genome-Wide Level in Poplar Leaves
3.3.2. Functional Analysis of DEGs in Poplar Leaves
3.3.3. Aphid Inoculation Promotes Expression of Genes Involved in Plant–Pathogen Interactions, Plant Hormone Signal Transduction, MAPK Signaling Pathway, and ABC Transporters
3.3.4. Aphid Inoculation Triggers Expression of Genes Involved in Starch and Sucrose Metabolism
3.3.5. Aphid Inoculation Inhibits Expression of Genes Related to Photosynthesis at 8 DAI
3.3.6. Aphid Inoculation Exerts a Lasting Influence on Gene Expression in the Flavonoid Biosynthesis Pathway
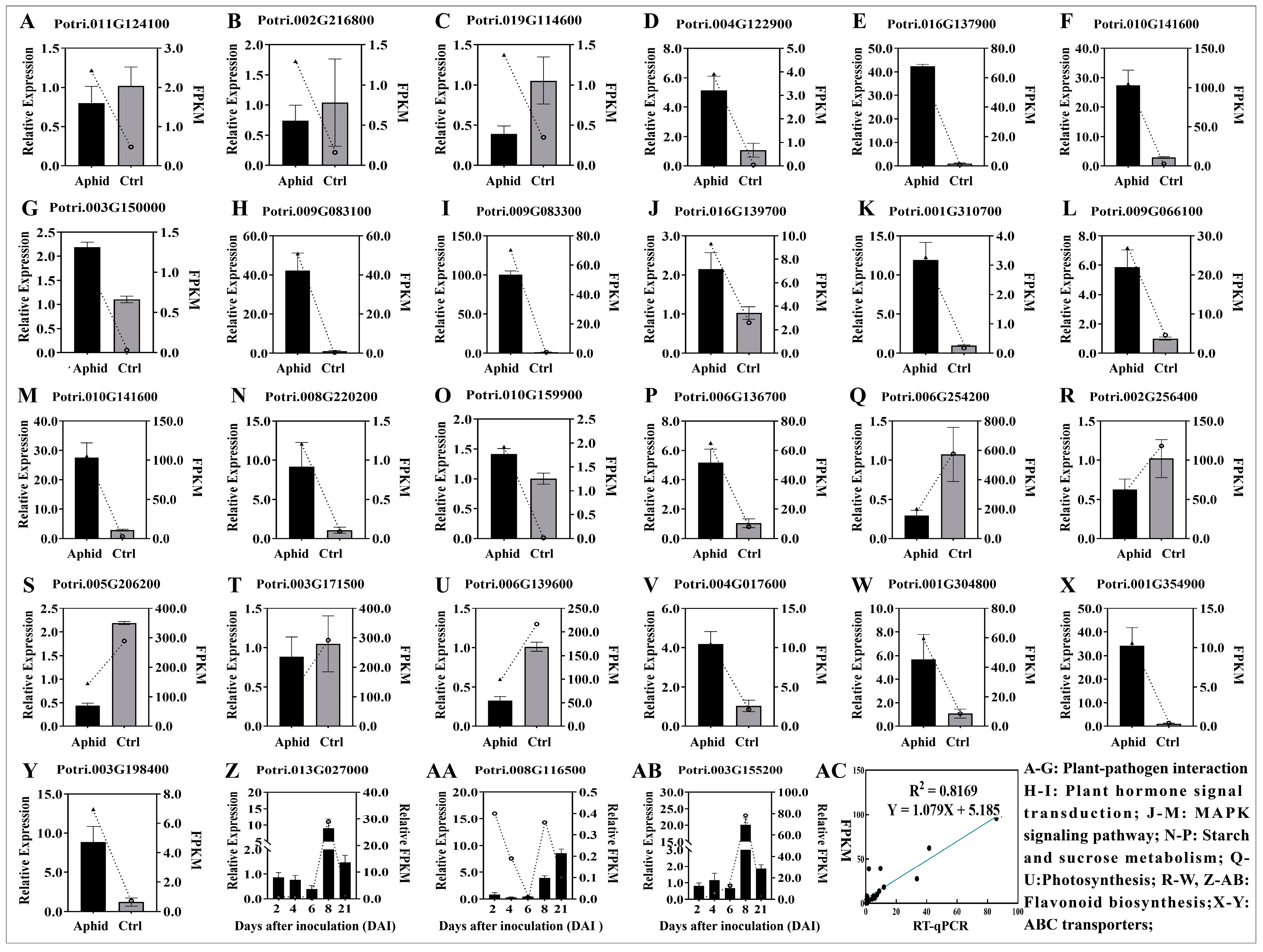
3.4. RT-qPCR Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Foster, A.J.; Pelletier, G.; Tanguay, P.; Seguin, A. Transcriptome analysis of Poplar during leaf spot infection with Sphaerulina spp. PLoS ONE 2015, 10, e0138162. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Rodriguez-Buey, M.; Karlsson, J.; Campbell, M.M. The response of the poplar transcriptome to wounding and subsequent infection by a viral pathogen. New Phytol. 2004, 164, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Duplessis, S.; Major, I.; Martin, F.; Séguin, A. Poplar and pathogen interactions: Insights from Populus genome-wide analyses of resistance and defense gene families and gene expression profiling. Crit. Rev. Plant Sci. 2009, 28, 309–334. [Google Scholar] [CrossRef]
- Veselá, B.; Holub, P.; Urban, O.; Surá, K.; Hodaňová, P.; Oravec, M.; Divinová, R.; Jansen, M.; Klem, K. UV radiation and drought interact differently in grass and forb species of a mountain grassland. Plant Sci. 2022, 325, 111488. [Google Scholar] [CrossRef]
- Zlatkovic, M.; Tenorio-Baigorria, I.; Lakatos, T.; Tóth, T.; Koltay, A.; Pap, P.; Marković, M.; Orlović, S. Bacterial canker disease on Populus× euramericana caused by Lonsdalea populi in Serbia. Forests 2020, 11, 1080. [Google Scholar] [CrossRef]
- Philippe, R.N.; Bohlmann, J. Poplar defense against insect herbivores. Botany 2007, 85, 1111–1126. [Google Scholar]
- Fischer, M.K.; Shingleton, A.W. Host plant and ants influence the honeydew sugar composition of aphids. Funct. Ecol. 2001, 15, 544–550. [Google Scholar] [CrossRef]
- Ni, T. Experiment on aphid control of Populus tomentosa. North. Hortic. 2007, 10, 203–204. (In Chinese) [Google Scholar]
- Felton, G.W.; Eichenseer, H. Herbivore saliva and its effects on plant defense against herbivores and pathogens. In Induced Plant Defenses against Pathogens and Herbivores. Biochemistry, Ecology, and Agriculture; Agrawal, A.A., Tuzun, S., Bent, E., Eds.; APS Press: St. Paul, MN, USA, 1999; pp. 19–36. [Google Scholar]
- Miles, P.W. Specific responses and damage caused by Aphidoidea: Principles. In Aphids: Their Biology, Natural Enemies and Control; Minks, A.K., Harrewijn, P., Eds.; Elsevier: New York, NY, USA, 1989; pp. 23–47. [Google Scholar]
- Cobb, N.S.; Trotter, R.T.; Whitham, T.G. Long-term sexual allocation in herbivore resistant and susceptible pinyon pine (Pinus edulis). Oecologia 2002, 130, 78–87. [Google Scholar] [CrossRef]
- Guerrieri, E.; Digilio, M.C. Aphid-plant interactions: A review. J. Plant Interact. 2008, 3, 223–232. [Google Scholar] [CrossRef]
- Yin, W.D.; Qiu, G.S.; Yan, W.T.; Sun, L.N.; Zhang, H.J. Host preference and fitness of Aphis citricola (Hemiptera: Aphididae) to mature and young apple leaves. J. Appl. Ecol. 2013, 24, 2000–2006. [Google Scholar]
- Zangerl, A.R.; Hamilton, J.G.; Miller, T.J.; Crofts, A.R.; Oxborough, K.; Berenbaum, M.R.; De Lucia, E.H. Impact of folivory on photosynthesis is greater than the sum of its holes. Proc. Natl. Acad. Sci. USA 2002, 99, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Nykänen, H.; Koricheva, J. Damage-induced changes in woody plants and their effects on insect herbivore performance: A meta-analysis. Oikos 2004, 104, 247–268. [Google Scholar] [CrossRef]
- Nabity, P.D.; Zavala, J.A.; DeLucia, E.H. Indirect suppression of photosynthesis on individual leaves by arthropod herbivory. Ann. Bot. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Haile, F.J.; Higley, L.G. Changes in soybean gas-exchange after moisture stress and spider mite injury. Environ. Entomol. 2003, 32, 433–440. [Google Scholar] [CrossRef]
- Velikova, V.; Salerno, G.; Frati, F.; Peri, E.; Conti, E.; Colazza, S.; Loreto, F. Influence of feeding and oviposition by phytophagous on photosynthesis of herbaceous plants. J. Chem. Ecol. 2010, 36, 629–641. [Google Scholar] [CrossRef]
- Welter, S.C. Arthropod impact on plant gas exchange. In Insect-Plant Interactions; Bernays, E.A., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 135–164. [Google Scholar]
- Macedo, T.B.; Bastos, C.S.; Higley, L.G.; Ostlie, K.R.; Madhavan, S. Photosynthetic responses of soybean to soybean aphid (Homoptera: Aphididae) injury. J. Econ. Entomol. 2003, 96, 188–193. [Google Scholar] [CrossRef]
- Reddall, A.; Sadras, V.O.; Wilson, L.J.; Gregg, P.C. Physiological responses of cotton to two-spotted spider mite damage. Crop Sci. 2004, 44, 835–846. [Google Scholar]
- Wei, C.; Tong, X.; Gao, X.; Jiang, C. Compensatory photosynthesis physiology of tea plant for herbivory by Ectropis oblique. J. Anhui Agric. Univ. 2007, 34, 355–359. [Google Scholar]
- Alieta, E.; David, S.; Pinkard, E.A.; Ian, S.; Ross, C.; Stephen, E.; Chris, B.; Caroline, M. Photosynthetic responses of field-grown Pinus radiata trees to artificial and aphid-induced defoliation. Tree Physiol. 2011, 6, 592–603. [Google Scholar]
- Nowak, R.S.; Caldwell, M.M. A test of compensatory photosynthesis in the field: Implications for herbivory tolerance. Oecologia 1984, 61, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Trumble, J.T.; Kolondy-Hirsch, D.M.; Ting, I.P. Plant compensation for arthropod herbivory. Annu. Rev. Entomol. 1993, 38, 93–119. [Google Scholar] [CrossRef]
- Doescher, P.S.; Svejcar, T.J.; Jaindl, R.G. Gas exchange of Idaho Fescue in response to defoliation and grazing history. J. Range Manag. 1997, 50, 285–289. [Google Scholar] [CrossRef]
- Zou, J.; Rodriguez-Zas, S.; Aldea, M.; Zhu, J.; Gonzalez, D.; Vodkin, L.; DeLucia, E.; Clough, S.J. Expression profiling soybean response to Pseudomonas syringae reveals new defense-related genes and rapid HR-specific downregulation of photosynthesis. Mol. Plant-Microbe Interact. 2005, 18, 1161–1174. [Google Scholar] [CrossRef]
- Berger, S.; Benediktyova, Z.; Matous, K.; Bonfig, K.; Mueller, M.; Nedbal, L.; Roitsch, T. Visualization of dynamics of plant-pathogen interaction by novel combination of chlorophyll fluorescence imaging and statistical analysis: Differential effects of virulent and avirulent strains of P. syringae and of oxylipins on A. thaliana. J. Exp. Bot. 2007, 58, 797–806. [Google Scholar] [CrossRef]
- Bilgin, D.D.; Zavala, J.A.; Zhu, J.; Clough, S.J.; Ort, D.R.; Delucia, E.H. Biotic stress globally downregulates photosynthesis genes. Plant Cell Environ. 2010, 33, 1597–1613. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.P.; Wunsche, H.; Mitra, S.; Zavala, J.A.; Muck, A.; Svatoš, A.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, sphingidae) and its natural host Nicotiana attenuata. VII. Changes in the plant’s proteome. Plant Physiol. 2006, 142, 1621–1641. [Google Scholar] [CrossRef]
- Mitra, S.; Baldwin, I.T. Independently silencing two photosynthetic proteins in Nicotiana attenuata has different effects on herbivore resistance. Plant Physiol. 2008, 148, 1128–1138. [Google Scholar] [CrossRef]
- Logemann, E.; Wu, S.C.; Schröder, J.; Schmelzer, E.; Somssich, I.E.; Hahlbrock, K. Gene activation by UV light, fungal elicitor or fungal infection in Petroselinum crispum is correlated with repression of cell cycle-related genes. Plant J. 1995, 8, 865–876. [Google Scholar] [CrossRef]
- Ehness, R.; Ecker, M.; Godt, D.E.; Roitsch, T. Glucose and stress independently regulate source and sink metabolism and defense mechanisms via signal transduction pathways involving protein phosphorylation. Plant Cell 1997, 9, 1825–1841. [Google Scholar] [CrossRef]
- Hermsmeier, D.; Schittko, U.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. I. Large-scale changes in the accumulation of growth- and defense-related plant mRNAs. Plant Physiol. 2001, 125, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Montesano, M.; Scheller, H.V.; Wettstein, R.; Palva, E.T. Down-regulation of photosystem I by Erwinia carotovora-derived elicitors correlates with H2O2 accumulation in chloroplasts of potato. Mol. Plant Pathol. 2004, 5, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Major, I.T.; Constabel, C.P. Molecular analysis of poplar defense against herbivory: Comparison of wound-and insect elicitor-induced gene expression. New Phytol. 2006, 172, 617–635. [Google Scholar] [CrossRef] [PubMed]
- Wool, D.; Hales, D.F. Previous infestation affects recolonization of cotton by Aphis gossypii: Induced resistance or plant damage? Phytoparasitica 1996, 24, 39–48. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Agrawal, A.A. The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol. 1999, 14, 179–185. [Google Scholar] [CrossRef]
- Züst, T.; Agrawal, A.A. Mechanisms and evolution of plant resistance to aphids. Nat. Plants 2016, 2, 15206. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Fyllas, N.M.; Chrysafi, D.; Avtzis, D.N.; Moreira, X. Photosynthetic and defensive responses of two Mediterranean oaks to insect leaf herbivory. Tree Physiol. 2022, 42, 2282–2293. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, Y.; Yang, L.; Li, M.; Fu, M.; Zhang, S. Plagiodera versicolora feeding induces systemic and sexually differential defense responses in poplars. Physiol. Plant. 2022, 174, e13804. [Google Scholar] [CrossRef]
- Li, P.; Liu, W.; Zhang, Y.; Xing, J.; Li, J.; Feng, J.; Su, X.; Zhao, J. Fungal canker pathogens trigger carbon starvation by inhibiting carbon metabolism in poplar stems. Sci. Rep. 2019, 9, 10111. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Li, P.; Zhang, Y.; Li, J.; Liu, Y.; Lachenbruch, B.; Su, X.; Zhao, J. Fungal pathogens of canker disease trigger canopy dieback in poplar saplings by inducing functional failure of the phloem and cambium and carbon starvation in the xylem. Physiol. Mol. Plant Pathol. 2020, 112, 101523. [Google Scholar] [CrossRef]
- Xing, J.; Li, M.; Li, J.; Shen, W.; Li, P.; Zhao, J.; Zhang, Y. Stem canker pathogen Botryosphaeria dothidea inhibits poplar leaf photosynthesis in the early stage of inoculation. Front. Plant Sci. 2022, 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Zhao, J.P.; Jiang, X.L.; Zhang, B.Y.; Su, X.H. Involvement of microRNA-mediated gene expression regulation in the pathological development of stem canker disease in Populus trichocarpa. PLoS ONE 2012, 7, e44968. [Google Scholar] [CrossRef]
- Guo, Z.; Yuan, X.; Li, L.; Zeng, M.; Yang, J.; Tang, H.; Duan, C. Genome-wide analysis of the ATP-binding cassette (ABC) transporter family in Zea mays L. and its response to heavy metal stresses. Int. J. Mol. Sci. 2022, 23, 2109. [Google Scholar] [CrossRef]
- Kang, J.; Park, J.; Choi, H. Plant ABC Transporters. Arab. Book 2011, 9, e0153. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, J.; Zhang, W.; Nie, J. Genome-wide characterization, phylogenetic and expression analysis of ABCG gene subfamily in cucumber (Cucumis sativus L.). Front. Plant Sci. 2023, 14, 1178710. [Google Scholar] [CrossRef]
- Roy, B.A.; Alexander, H.M.; Davidson, J.; Campbell, F.T.; Burdon, J.J.; Sniezko, R.; Brasier, C. Increasing forest loss worldwide frominvasive pests requires new trade regulations. Front. Ecol. Environ. 2014, 12, 457–465. [Google Scholar] [CrossRef]
- Retuerto, R.; Fernanez-Lema, B.; Rodrjguez-Roiloa; Obeso, J.R. Increased photosynthetic performance in holly trees infested by scale insects. Funct. Ecol. 2004, 18, 664–669. [Google Scholar] [CrossRef]
- Moustaka, J.; Meyling, N.V.; Hauser, T.P. Induction of a compensatory photosynthetic response mechanism in tomato leaves upon short time feeding by the chewing insect Spodoptera exigua. Insects 2021, 12, 562. [Google Scholar] [CrossRef]
- Aldea, M.; Hamilton, J.; Resti, J.; Zangerl, A.; Berenbaum, M.; Frank, T.; DeLucia, E. Comparison of photosynthetic damage from arthropod herbivory and pathoge-n infection in understory hardwood saplings. Oecologia 2006, 149, 221–232. [Google Scholar] [CrossRef]
- Kerchev, P.I.; Fenton, B.; Foyer, C.H.; Hancock, R.D. Plant responses to insect herbivory: Interactions between photosynthesis, reactive oxygen species and hormonal signaling pathways. Plant Cell Environ. 2012, 35, 441–453. [Google Scholar] [CrossRef]
- Paez, A.; Gonzalez, M.E.O. Water stress and clipping management effects on guineagrass: II. photosynthesis and water relations. Agron. J. 1995, 87, 706–711. [Google Scholar] [CrossRef]
- Damascos, M.A.; Ronquim, C.C.; Prado, C.H.B.A. Gas exchange and plant growth after defoliation on Leandra lacunosa, a cerrado woody species with continuous leaf production. Braz. Arch. Biol. Technol. 2005, 48, 967–974. [Google Scholar] [CrossRef]
- Fernando, S.; Marina, F.; Sonia, M.; Alfonso, E. Effects of simulated herbivory on photosynthesis and N resorption efficiency in Quercus pyrenaica Willd. saplings. Trees 2008, 22, 785–793. [Google Scholar] [CrossRef]
- Kolb, T.E.; Dodds, K.A.; Clancy, K.M. Effect of western spruce budworm defoliation on the physiology and growth of potted Douglas-fir seedlings. For. Sci. 1999, 45, 280–291. [Google Scholar]
- Zhang, Y.; Tian, L.; Yan, D.H.; He, W. Genome-wide transcriptome analysis reveals the comprehensive response of two susceptible poplar sections to Marssonina brunnea infection. Genes 2018, 9, 154. [Google Scholar] [CrossRef]
- Muchero, W.; Sondreli, K.L.; Chen, J.G.; Urbanowicz, B.; Zhang, J.; Singan, V.; Yang, Y.; Brueggeman, R.; Franco-Coronado, J.; Abraham, N.; et al. Association mapping, transcriptomics and transient expression identify candidate genes mediating plant-pathogen interactions in a tree. Proc. Natl. Acad. Sci. USA 2018, 115, 11573–11578. [Google Scholar] [CrossRef]
- Brunt, A.A.; Crabtree, K.; Dallwitz, M.J.; Gibbs, A.J.; Watson, L.; Zurcher, E.J. (Eds.) Plant Viruses Online: Descriptions and Lists from the VIDE Database. Version: 20. 1996. Available online: http://www.agls.uidaho.edu/ebi/vdie/ (accessed on 1 September 2023).
- Nault, L.R. Arthropod transmission of plant viruses: A new synthesis. Ann. Entomol. Soc. Am. 1997, 90, 521–541. [Google Scholar] [CrossRef]
- Calla, B. Diverse defenses: O-methylated flavonoids contribute to the maize arsenal against fungal pathogens. Plant Physiol. 2022, 188, 24–25. [Google Scholar] [CrossRef]
- Will, T.; Tjallingii, W.F.; Thönnessen, A.; van Bel, A.J. Molecular sabotage of plant defense by aphid saliva. Proc. Natl. Acad. Sci. USA 2007, 104, 10536–10541. [Google Scholar] [CrossRef]
- Miles, P.W. Aphid saliva. Biol. Rev. 1999, 74, 41–85. [Google Scholar] [CrossRef]
- Cherqui, A.; Tjallingii, W.F. Salivary proteins of aphids, a pilot study on identification, separation, and immunolocalisation. J. Insect Physiol. 2000, 46, 1177–1186. [Google Scholar] [CrossRef]
- Mondal, H.A. Aphid saliva: A powerful recipe for modulating host resistance towards aphid clonal propagation. Arthropod-Plant Interact. 2020, 14, 547–558. [Google Scholar] [CrossRef]
- Cui, N.; Lu, H.; Wang, T.; Zhang, W.; Kang, L.; Cui, F. Armet, an aphid effector protein, induces pathogen resistance in plants by promoting the accumulation of salicylic acid. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180314. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.D.; Oosten, V.R.V.; Poecke, R.M.P.V.; Pelt, J.A.Z.; Pozo, M.J.; Mueller, M.J.; Buchala, A.J.; Métraux, J.P.; Loon, L.C.V.; Dicke, M.; et al. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol. Plant Microbe Interact. 2005, 18, 923–937. [Google Scholar] [CrossRef]
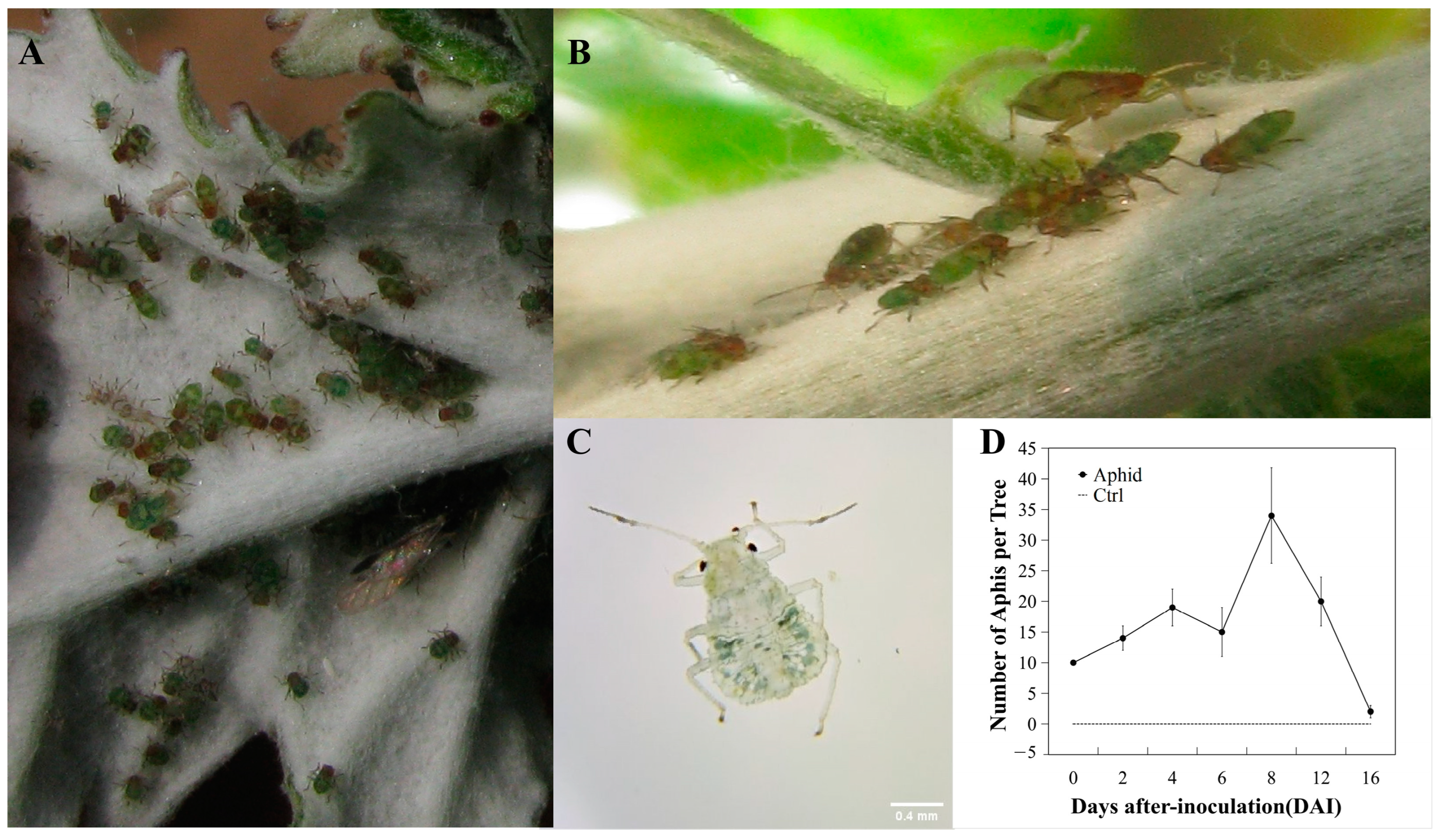
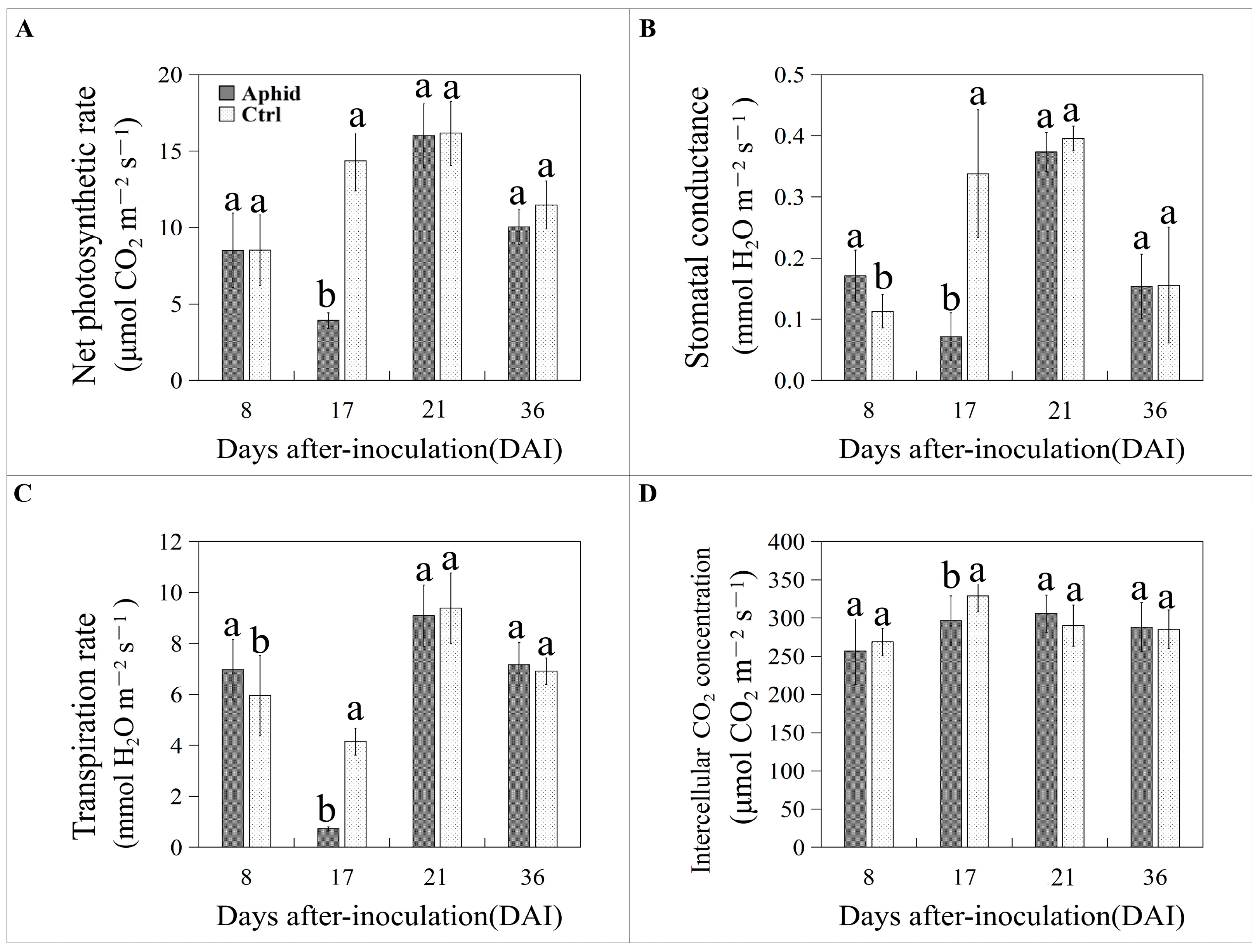
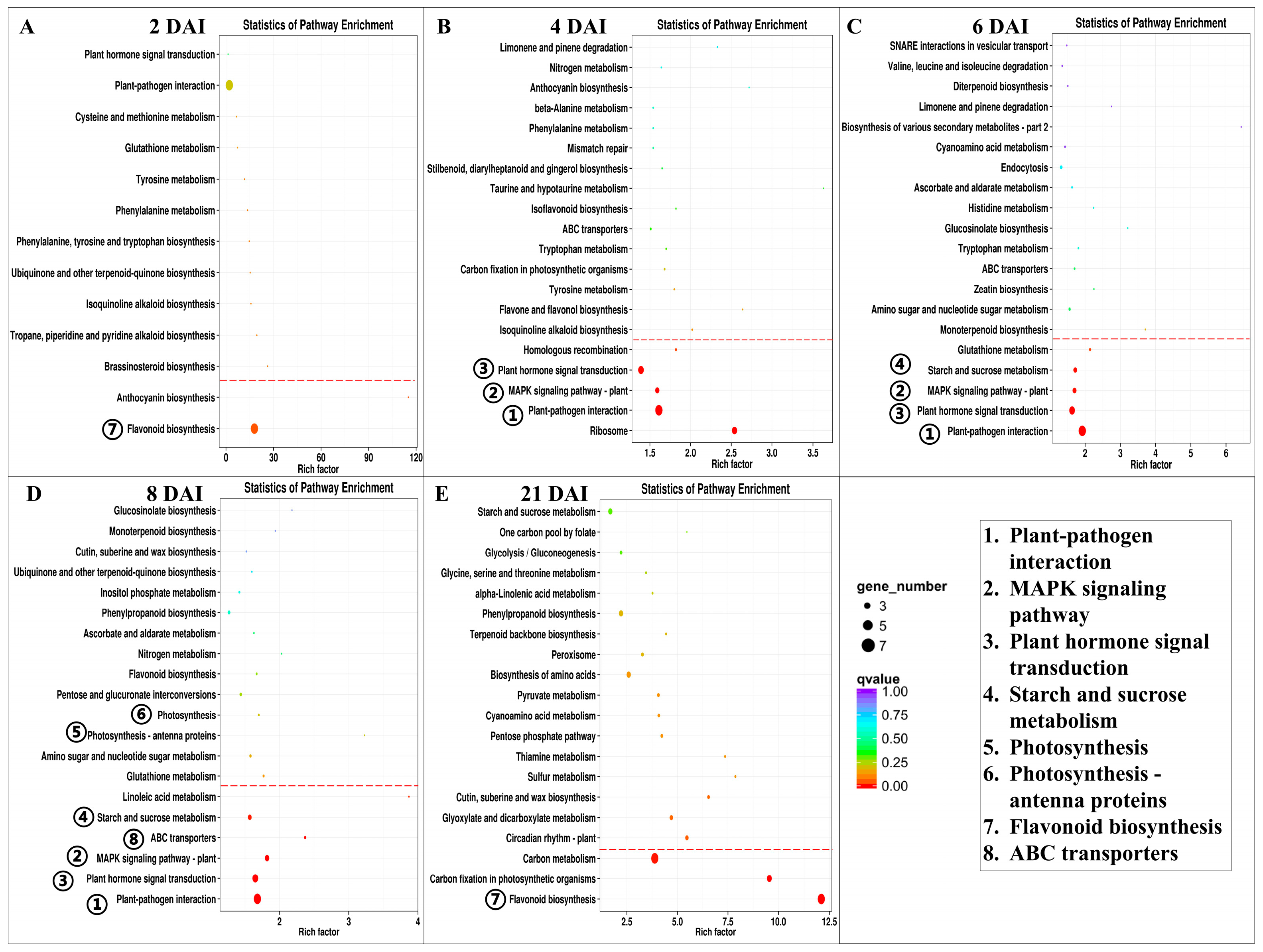

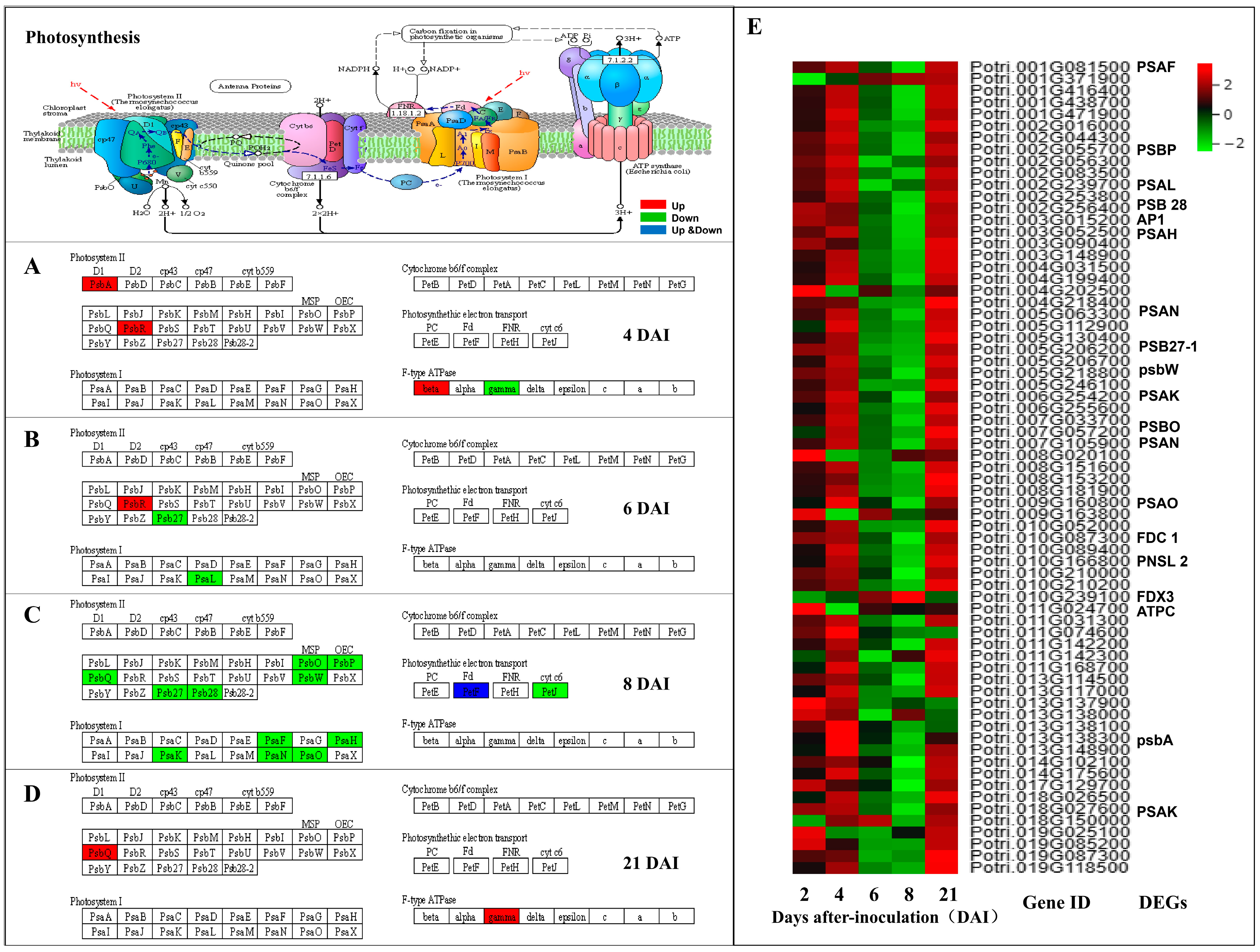

| Days after Inoculation (DAI) | Number of Total DEGs | Number of Upregulated Genes | Number of Downregulated Genes | Differential Regulation of DEGs |
|---|---|---|---|---|
| 2 | 19 | 5 | 14 | −2.80 |
| 4 | 2691 | 1719 | 972 | +1.76 * |
| 6 | 1595 | 1100 | 495 | +2.22 * |
| 8 | 1938 | 1519 | 419 | +3.63 ** |
| 21 | 111 | 107 | 4 | +26.75 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, W.; Fu, Y.; Wang, L.; Yao, Y.; Zhang, Y.; Li, M.; Liu, H.; Su, X.; Zhao, J. Transcriptomic Analysis Revealed That Low-Density Aphid Infestation Temporarily Changes Photosynthesis and Disease Resistance but Persistently Promotes Insect Resistance in Poplar Leaves. Forests 2023, 14, 1866. https://doi.org/10.3390/f14091866
Shen W, Fu Y, Wang L, Yao Y, Zhang Y, Li M, Liu H, Su X, Zhao J. Transcriptomic Analysis Revealed That Low-Density Aphid Infestation Temporarily Changes Photosynthesis and Disease Resistance but Persistently Promotes Insect Resistance in Poplar Leaves. Forests. 2023; 14(9):1866. https://doi.org/10.3390/f14091866
Chicago/Turabian StyleShen, Wanna, Yuchen Fu, Li Wang, Yanxia Yao, Yinan Zhang, Min Li, Huixiang Liu, Xiaohua Su, and Jiaping Zhao. 2023. "Transcriptomic Analysis Revealed That Low-Density Aphid Infestation Temporarily Changes Photosynthesis and Disease Resistance but Persistently Promotes Insect Resistance in Poplar Leaves" Forests 14, no. 9: 1866. https://doi.org/10.3390/f14091866
APA StyleShen, W., Fu, Y., Wang, L., Yao, Y., Zhang, Y., Li, M., Liu, H., Su, X., & Zhao, J. (2023). Transcriptomic Analysis Revealed That Low-Density Aphid Infestation Temporarily Changes Photosynthesis and Disease Resistance but Persistently Promotes Insect Resistance in Poplar Leaves. Forests, 14(9), 1866. https://doi.org/10.3390/f14091866






