The Relationship between Stand Structure and Tree Growth Form—Investigating the Effects of Selection Cuttings in Mountainous Mixed Beech Forests
Abstract
1. Introduction
2. Materials and Methods
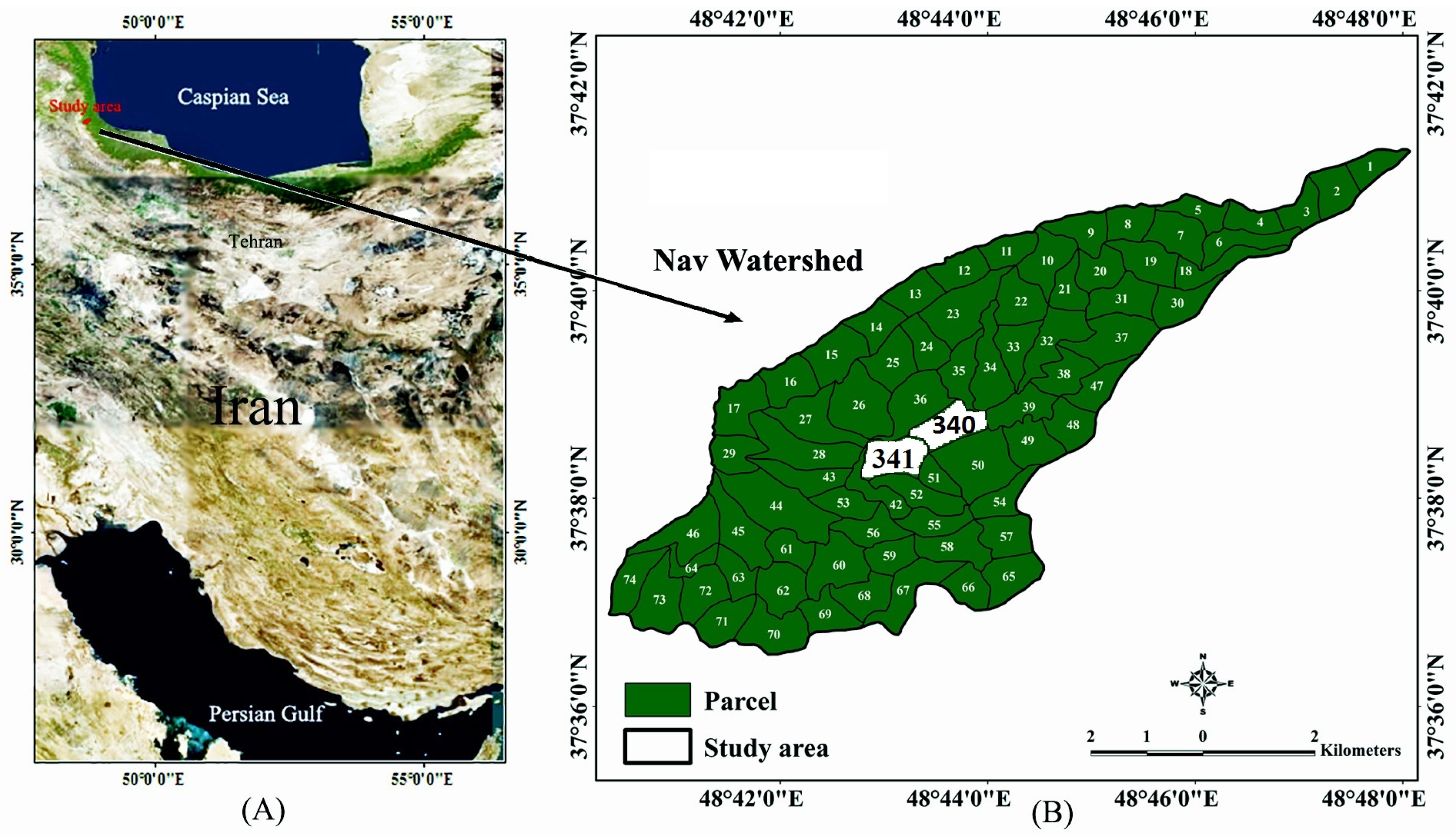
2.1. Study Area
2.2. Data Collection
2.3. Analysis of Data
2.3.1. Height-to-Diameter Ratio (HDR)
2.3.2. Live Crown Ratio (LCR)
2.3.3. Relative Spacing Index (RSI)
2.3.4. Tree Diameter Diversity Index
2.3.5. Tree Height Diversity Index
2.3.6. Canopy Tree Species Richness Index
2.3.7. Stand Structural Complexity Index
2.3.8. Tree Species Diversity Index (TSD)
2.3.9. Tree Species Evenness Index (TSE)
2.3.10. Tree Species Richness Index (TSR)
2.3.11. Species Importance Value (SIV)
2.3.12. Species Composition of Trees
2.4. Statistical Analysis
3. Results
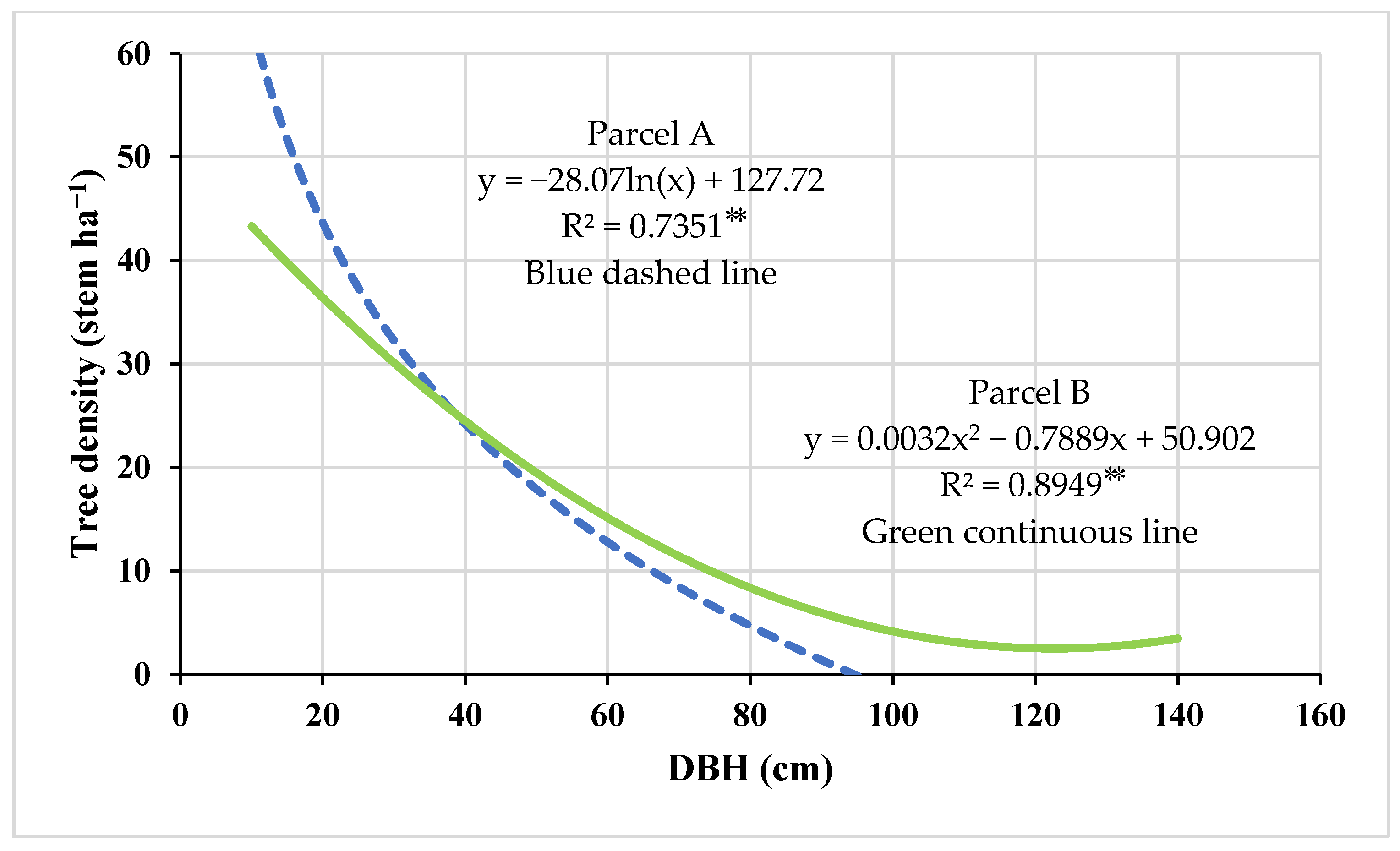
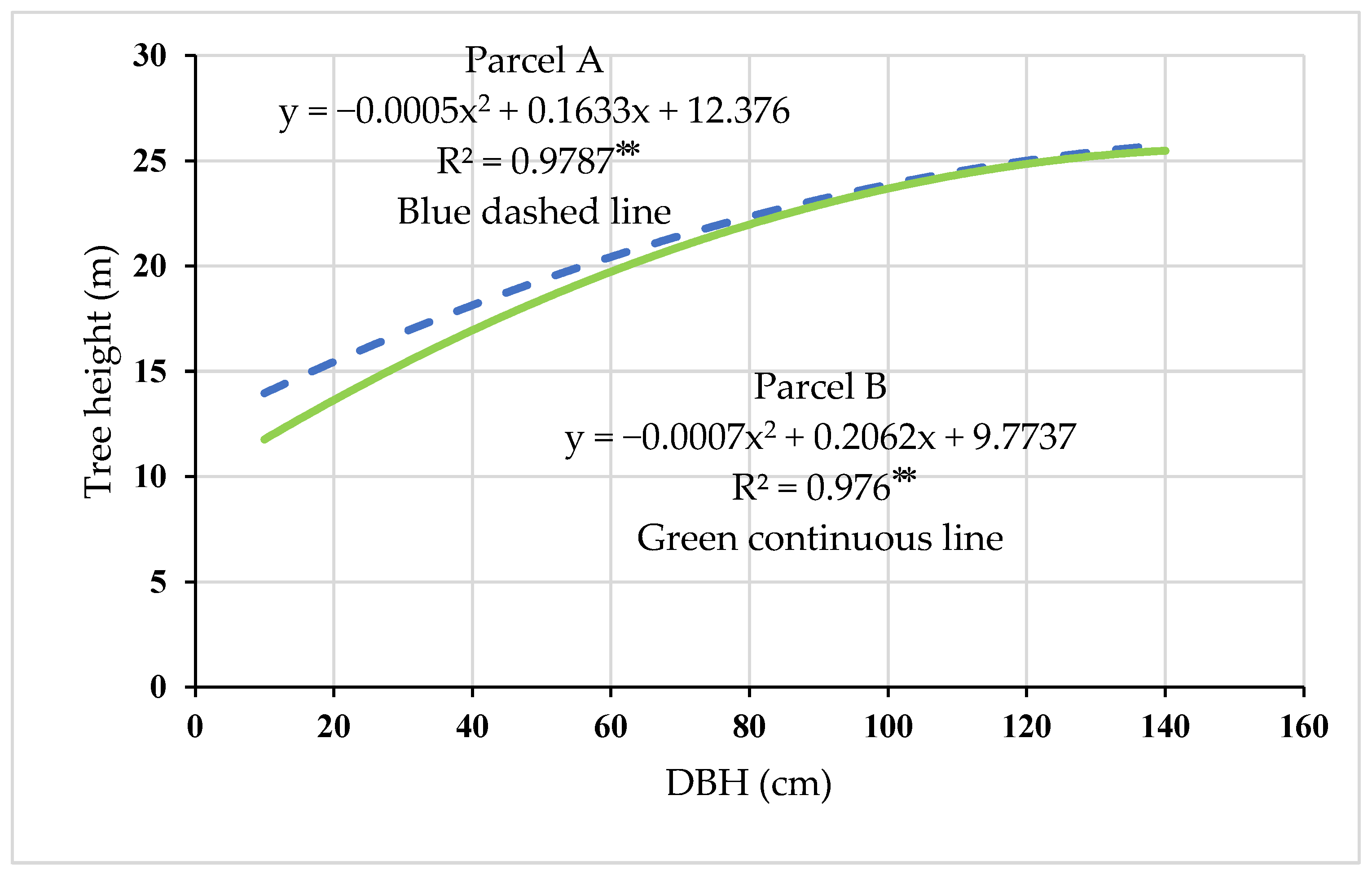
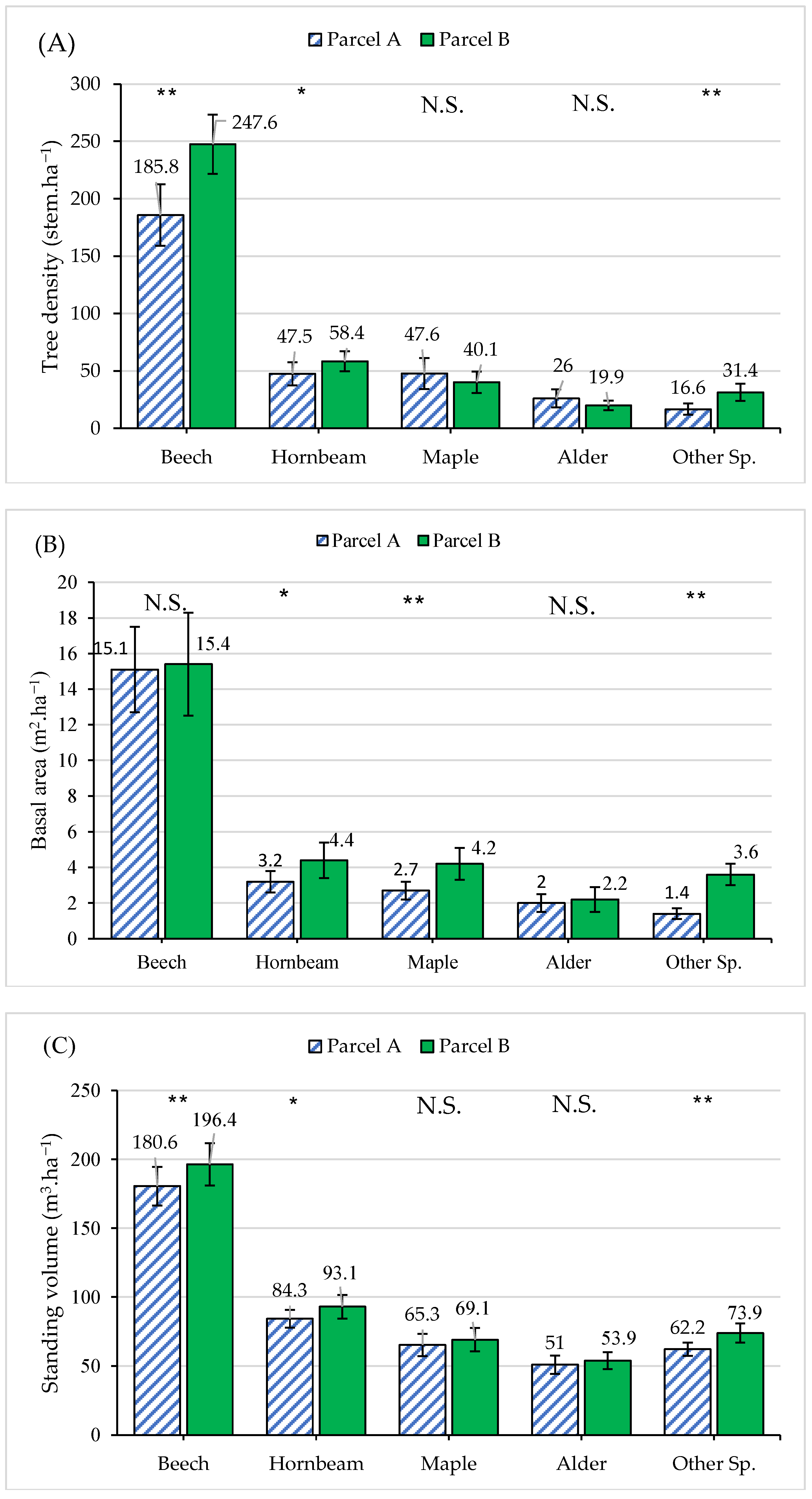
3.1. Stand Characteristics
3.2. Stand Structural Indices
4. Discussion
4.1. Stand Structure
4.2. HDR and LCR of Trees
4.3. Relationship between Stand Structure and HDR and LCR
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gustafsson, L.; Baker, S.C.; Bauhus, J.; Beese, W.J.; Brodie, A.; Kouki, J.; Lindenmayer, D.B.; Lõhmus, A.; Pastur, G.M.; Messier, C.; et al. Retention Forestry to Maintain Multifunctional Forests: A World Perspective. BioScience 2012, 62, 633–645. [Google Scholar] [CrossRef]
- Bauhus, J.; Puettmann, K.J.; Kuhne, C. Close-to-nature forest management in Europe: Does it support complexity and adaptability of forest ecosystems? In Managing Forest as Complex Adaptive Systems-Building Resilience to the Challenge of Global Change; Messier, C., Puettmann, K.J., Coates, K.D., Eds.; Routledge: Oxford, UK, 2013; pp. 187–213. [Google Scholar]
- Castle, M.; Weiskittel, A.; Wagner, R.; Ducey, M.; Frank, J.; Pelletier, G. Variation in stem form and risk of four commercially important hardwood species in the Acadian Forest: Implications for potential sawlog volume and tree classification systems. Can. J. For. Res. 2017, 47, 1457–1467. [Google Scholar] [CrossRef]
- Tavankar, F.; Picchio, R.; Nikooy, M.; Lo Monaco, A.; Venanzi, R.; Iranparast Bodaghi, A. Healing rate of logging wounds on broadleaf trees in hyrcanian forest with some technological implications. Drewno 2017, 60, 65–80. [Google Scholar]
- Tavankar, F.; Nikooy, M.; Lo Monaco, A.; Picchio, R. Long-term impact of selection cutting management on frequency of stem deformity in mixed beech forests of northern Iran. Drewno 2021, 64, 5–27. [Google Scholar] [CrossRef]
- Slik, J.W.F.; Verburg, R.W.; Keßler, P.J.A. Effects of fire and selective logging on the tree species composition of lowland dipterocarp forest in East Kalimantan, Indonesia. Biodivers. Conserv. 2002, 11, 85–98. [Google Scholar] [CrossRef]
- Hall, J.S.; Harris, D.J.; Medjibe, V.; Ashton, P.M.S. The effects of selective logging on forest structure and tree species composition in a Central African forest: Implications for management of conservation areas. For. Ecol. Manag. 2003, 183, 249–264. [Google Scholar] [CrossRef]
- Okuda, T.; Suzuki, M.; Adachi, N.; Quah, E.S.; Hussein, N.A.; Manokaran, N. Effect of selective logging on canopy and stand structure and tree species composition in a lowland dipterocarp forest in peninsular Malaysia. For. Ecol. Manag. 2003, 175, 297–320. [Google Scholar] [CrossRef]
- Bonnell, T.R.; Reyna-Hurtado, R.; Chapman, C.A. Post-logging recovery time is longer than expected in an East African tropical forest. For. Ecol. Manag. 2011, 261, 855–864. [Google Scholar] [CrossRef]
- Baraloto, C.; Hérault, B.; Paine, C.E.T.; Massot, H.; Blanc, L.; Bonal, D.; Molino, J.F.; Nicolini, E.A.; Sabatier, D. Contrasting taxonomic and functional responses of a tropical tree community to selective logging. J. Appl. Ecol. 2012, 49, 861–870. [Google Scholar] [CrossRef]
- Clark, J.A.; Covey, K.E. Tree species richness and the logging of natural forests: A meta-analysis. For. Ecol. Manag. 2012, 276, 146–153. [Google Scholar] [CrossRef]
- Rutten, G.; Ensslin, A.; Hemp, A.; Fischer, M. Forest structure and composition of previously selectively logged and non-logged montane forests at Mt. Kilimanjaro. For. Ecol. Manag. 2015, 337, 61–66. [Google Scholar] [CrossRef]
- Ding, Y.; Zang, R.; Lu, X.; Huang, J. The impacts of selective logging and clear-cutting on woody plant diversity after 40 years of natural recovery in a tropical montane rain forest, south China. Sci. Total Environ. 2017, 579, 1683–1691. [Google Scholar] [CrossRef]
- Garcia-Florez, L.; Vanclay, J.K.; Glencross, K.; Nichols, J.D. Understanding 48 years of changes in tree diversity, dynamics and species responses since logging disturbance in a subtropical rainforest. For. Ecol. Manag. 2017, 393, 29–39. [Google Scholar] [CrossRef]
- Schnabel, F.; Donoso, P.J.; Winter, C. Short-term effects of single-tree selection cutting on stand structure and tree species composition in Valdivian rainforests of Chile. N. Z. J. For. Sci. 2017, 47, 21. [Google Scholar] [CrossRef]
- Putz, F.E.; Baker, T.; Griscom, B.W.; Gopalakrishna, T.; Roopsind, A.; Umunay, P.M.; Zalman, J.; Ellis, E.A.; Ruslandi Ellis, P.W. Intact Forest in Selective Logging Landscapes in the Tropics. Front. For. Glob. Chang. 2019, 2, 30. [Google Scholar] [CrossRef]
- Latterini, F.; Mederski, P.S.; Jaeger, D.; Venanzi, R.; Tavankar, F.; Picchio, R. The Influence of Various Silvicultural Treatments and Forest Operations on Tree Species Biodiversity. Curr. For. Rep. 2023, 9, 59–71. [Google Scholar] [CrossRef]
- Nyland, R.D. Silviculture. Concepts and Applications; Wavelan Press, Inc.: Long Grove, IL, USA, 2002. [Google Scholar]
- Ali, A. Forest stand structure and functioning: Current knowledge and future challenges. Ecol. Indic. 2019, 98, 665–677. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Tahvonen, O.; Aakala, T. Even-aged and uneven-aged forest management in boreal fennoscandia: A review. Ambio 2012, 41, 720–737. [Google Scholar] [CrossRef]
- Siira-Pietikainen, A.; Haimi, J. Changes in soil fauna 10 years after forest harvestings: Comparison between clear felling and green-tree retention methods. For. Ecol. Manag. 2009, 258, 332–338. [Google Scholar] [CrossRef]
- Lahde, E.; Eskelinen, T.; Vaananen, A. Growth and diversity effects of silvicultural alternatives on an old-growth forest in Finland. Forestry 2002, 75, 395–400. [Google Scholar] [CrossRef]
- Lahde, E.; Laiho, O.; Lin, J. Silvicultural alternatives in an uneven-sized forest dominated by Picea abies. J. For. Res. 2010, 15, 14–20. [Google Scholar] [CrossRef]
- Tahvonen, O.; Pukkala, T.; Laiho, O.; Lähde, E.; Niinimäki, S. Optimal management of uneven-aged Norway spruce stands. For. Ecol. Manag. 2010, 260, 106–115. [Google Scholar] [CrossRef]
- McElhinny, C.; Gibbons, P.; Brack, B.; Bauhus, J. Forest and woodland stand structural complexity: Its definition and meas-urement. For. Ecol. Manag. 2005, 218, 1–24. [Google Scholar] [CrossRef]
- Gibson, L.; Lee, T.M.; Koh, L.P.; Brook, B.W.; Gardne, T.A.; Barlow, J.; Peres, C.A.; Bradshaw, C.J.A.; Laurance, W.F.; Lovejoy, T.E.; et al. Primary Forests Are Irreplaceable for Sustaining Tropical Biodi-Versity. Nature 2011, 478, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Temesgen, H.; LeMay, V.; Mitchell, S.J. Tree crown ratio models for multi-species and multi-layered stands of southeastern British Columbia. For. Chron. 2005, 81, 133–141. [Google Scholar] [CrossRef]
- Brang, P.; Spathelf, P.; Larsen, J.B.; Bauhus, J.; Boncčìna, A.; Chauvin, C.; Drössler, L.; García-Güemes, C.; Heiri, C.; Kerr, G.; et al. Suitability of close-to-nature silviculture for adapting temperate European forests to climate change. Forestry 2014, 87, 492–503. [Google Scholar] [CrossRef]
- Sharma, R.P.; Vacek, Z.; Vacek, S.; Kučera, M. Modelling individual tree height–diameter relationships for multi-layered and multi-species forests in central Europe. Trees 2019, 33, 103–119. [Google Scholar] [CrossRef]
- Crecente-Campo, F.; Tomé, M.; Soares, P.; Dieguez-Aranda, U. A generalized nonlinear mixed-effects height-diameter model for Eucalyptus globulus L. in northwestern Spain. For. Ecol. Manag. 2010, 259, 943–952. [Google Scholar] [CrossRef]
- Schroder, J.; von Gadow, K. Testing a new competition index for Maritime pine in northwestern Spain. Can. J. For. Res. 1999, 29, 280–283. [Google Scholar] [CrossRef]
- Zhao, D.; Kane, M.; Borders, B.E. Crown ratio and relative spacing relationships for loblolly pine plantations. Open J. For. 2012, 2, 101–115. [Google Scholar] [CrossRef]
- Buongiorno, J.; Peyron, J.; Houllier, F.; Bruciamacchie, M. Growth and management of mixed-species, uneven-aged forests in the French Jura: Implications for economic returns and tree diversity. For. Sci. 1995, 41, 397–429. [Google Scholar]
- Shannon, C.E. The mathematical theory of communication. In The Mathematical Theory of Communication; Shannon, C.E., Weaver, W., Eds.; University of Illinois Press: Urbana, IL, USA, 1948; pp. 29–125. [Google Scholar]
- Felipe-Lucia, M.R.; Soliveres, S.; Penone, C. Multiple forest attributes underpin the supply of multiple ecosystem services. Nat. Commun. 2018, 9, 4839. [Google Scholar] [PubMed]
- Guan, S.; Lu, Y.; Liu, X. Evaluation of Multiple Forest Service Based on the Integration of Stand Structural Attributes in Mixed Oak Forests. Sustainability 2022, 14, 8228. [Google Scholar] [CrossRef]
- Kumar, P.; Dobriyal, M.; Kale, A.; Pandey, A.K.; Tomar, R.S.; Thounaojam, E. Calculating forest species diversity with information-theory based indices using sentinel-2A sensor’s of Mahavir Swami Wildlife Sanctuary. PLoS ONE 2022, 17, e0268018. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar]
- Margalef, R. Temporal succession and spatial heterogeneity in phytoplankton. In Perspectives in Marine biology; Buzzati-Traverso, A.A., Ed.; University of California Press: Berkeley, CA, USA, 1958; pp. 323–347. [Google Scholar]
- Tavankar, F.; Latterini, F.; Nikooy, M.; Venanzi, R.; Naghdi, R.; Picchio, R. Influence of Forest Management and Sylvicultural Treatments on Abundance of Snags and Tree Cavities in Mountain Mixed Beech Forests. Environments 2021, 8, 55. [Google Scholar] [CrossRef]
- Sefidi, K.; Etemad, V. The amount and quality of dead trees in a mixed beech forest with different management histories in northern Iran. Biodiversitas 2014, 15, 162–168. [Google Scholar]
- Larrieu, L.; Cabanettes, A.; Delarue, A. Impact of silviculture on dead wood and on the distribution and frequency of tree microhabitats in montane beech-fi r forests of the Pyrenees. Eur. J. For. Res. 2012, 131, 773–786. [Google Scholar]
- Tavankar, F.; Picchio, R.; Lo Monaco, A.; Bonyad, A.E. Forest management and snag characteristics in Northern Iran lowland forests. J. For. Sci. 2014, 60, 431–441. [Google Scholar]
- Jaroszewicz, B.; Cholewinska, O.; Checko, E.; Wrzosek, M. Predictors of diversity of deadwood-dwelling macrofungi in a European natural forest. For. Ecol. Manag. 2021, 490, 119123. [Google Scholar]
- Von Oheimb, G.; Westphal, C.; Hardtle, W. Diversity and spatio-temporal dynamics of dead wood in a temperate near-natural beech forest (Fagus sylvatica). Eur. J. For. Res. 2007, 126, 359–370. [Google Scholar] [CrossRef]
- Jonsson, B.G.; Ekström, M.; Esseen, P.A.; Grafstrom, A.; Stahl, G.; Westerlund, B. Dead wood availability in managed Swedish forests—Policy outcomes and implications for biodiversity. For. Ecol. Manag. 2016, 376, 174–182. [Google Scholar] [CrossRef]
- Gray, A.N.; Spies, T.A. Microsite controls on tree seedling establishment in conifer forest canopy gaps. Ecology 1997, 78, 2458–2473. [Google Scholar]
- Sanchez, E.; Gallery, R.; Dalling, J.W. Importance of nurse logs as a substrate for the regeneration of pioneer tree species on Barro Colorado Island, Panama. J. Trop. Ecol. 2009, 25, 429–437. [Google Scholar]
- Fukasawa, Y. Effects of wood decomposer fungi on tree seedling establishment on coarse woody debris. For. Ecol. Manag. 2012, 266, 232–238. [Google Scholar]
- Lutz, J.A.; Struckman, S.; Furniss, T.J.; Birch, J.D.; Yocom, L.L.; McAvoy, D.J. Large-diameter trees, snags, and deadwood in southern Utah, USA. Ecol. Process. 2021, 10, 9. [Google Scholar] [CrossRef]
- Eggleton, P.; Bignell, D.E.; Sands, W.A.; Waite, B.; Wood, T.G.; Lawton, J.H. The species richness of termites (Isoptera) under differing levels of forest disturbance in the Mbalmayo Forest Reserve, southern Cameroon. J. Trop. Ecol. 1995, 11, 85–98. [Google Scholar] [CrossRef]
- Calderón-Cortés, N.; Escalera-Vázquez, L.H.; Oyama, K. Occurrence of termites (Isoptera) on living and standing dead trees in a tropical dry forest in Mexico. PeerJ 2018, 6, e4731. [Google Scholar] [CrossRef]
- Ganey, J.L.; Vojta, S.C. Characteristics of snags containing excavated cavities in northern Arizona mixed-conifer and ponderosa pine forests. For. Ecol. Manag. 2004, 199, 323–332. [Google Scholar]
- Tavankar, F.; Lo Monaco, A.; Nikooy, M.; Venanzi, R.; Bonyad, A.; Picchio, R. Snow damages on trees of an uneven age in mixed broadleaf forests: Effects of topographical conditions and tree characteristics. J. For. Res. 2019, 30, 1383–1394. [Google Scholar] [CrossRef]
- Grove, S.J. Saproxylic insect ecology and the sustainable management of forests. Annu. Rev. Ecol. Syst. 2002, 33, 1–23. [Google Scholar]
- Giles, E.A. Effects of Tree Cavity Availability on Bat Distribution across a Forest Disturbance Gradient in Sabah, Malaysia. Master’s Thesis, University of Kent, Canterbury, UK, 2012. [Google Scholar]
- Lindenmayer, D.B.; Noss, R.E. Salvage logging, ecosystem processes, and biodiversity conservation. Conserv. Biol. 2006, 20, 949–958. [Google Scholar] [CrossRef]
- Brais, S.; Pare, D.; Lierman, C. Tree bole mineralization rates of four species of the Canadian eastern boreal forest: Implications for nutrient dynamics following stand-replacing disturbances. Can. J. For. Res. 2006, 36, 2331–2340. [Google Scholar]
- Kuehne, C.; Donath, C.; Muller-Using, S.I.; Bartsch, N. Nutrient fluxes via leaching from coarse woody debris in a Fagus sylvatica forest in the Solling Mountains, Germany. Can. J. For. Res. 2008, 38, 2405–2413. [Google Scholar]
- Russell, R.E.; Saab, V.A.; Dudley, J.G.; Rotella, J.J. Snag longevity in relation to wildfire and post fire salvage logging. For. Ecol. Manag. 2006, 232, 179–187. [Google Scholar] [CrossRef]
- Ranius, T.; Kindvall, O.; Kruys, N.; Jonsson, B.G. Modelling dead wood in Norway spruce stands subject to different management regimes. For. Ecol. Manag. 2003, 182, 13–29. [Google Scholar] [CrossRef]
- Verburg, R.; Van Eijk-Bos, C. Effects of selective logging on tree diversity, composition and plant functional type patterns in a Bornean rain forest. J. Veg. Sci. 2003, 14, 99–110. [Google Scholar] [CrossRef]
- Qiu, R.-H.; Chen, H.; Zhuo, L.-X. Effects of selection cutting on the forest structure and species diversity of evergreen broad-leaved forest in northern Fujian, southern China. For. Stud. China 2006, 8, 16–20. [Google Scholar] [CrossRef]
- Chaudhary, A.; Burivalova, Z.; Koh, L.; Hellweg, S. Impact of Forest Management on Species Richness: Global Meta-Analysis and Economic Trade-Offs. Sci. Rep. 2016, 6, 23954. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Leinonen, K.; Nygren, M.; Penttinen, A. Statistical opportunities for comparing stand structural heterogeneity in managed and primeval forests: An example from boreal spruce forest in southern Finland. Silva Fenn. 1996, 30, 5598. [Google Scholar] [CrossRef]
- North, M.P.; Franklin, J.F.; Carey, A.B.; Forsman, E.D.; Hamer, T. Forest stand structure of the northern spotted owl’s foraging habitat. For. Sci. 1999, 45, 520–527. [Google Scholar]
- Nagaike, T.; Hayashi, A. Effects of extending rotation period on plant species diversity in Larix kaempferi plantations in central Japan. Ann. For. Sci. 2004, 61, 197–202. [Google Scholar] [CrossRef]
- Uuttera, J.; Maltamo, M.; Hotanen, J.P. The structure of forest stands in virgin and managed peatlands: A comparison between Finnish and Russian Karelia. For. Ecol. Manag. 1997, 96, 125–138. [Google Scholar] [CrossRef]
- Banda, T.; Schwartz, M.W.; Caro, T. Woody vegetation structure and composition along a protection gradient in a miombo ecosystem of western Tanzania. For. Ecol. Manag. 2006, 230, 179–185. [Google Scholar] [CrossRef]
- Nagaike, T.; Hayashi, A.; Kubo, M.; Takahashi, K.; Abe, M.; Arai, N. Changes in plant species diversity over 5 years in Larix kaempferi plantations and abandoned coppice forests in central Japan. For. Ecol. Manag. 2006, 236, 278–285. [Google Scholar] [CrossRef]
- Liang, J.; Buongiorno, J.; Monserud, R.A.; Kruger, E.L.; Zhou, M. Effects of diversity of tree species and size on forest basal area growth, recruitment, and mortality. For. Ecol. Manag. 2007, 243, 116–127. [Google Scholar] [CrossRef]
- Pretzsch, H. Analysis and modeling of spatial stand structures. Methodological considerations based on mixed beech-larch stands in Lower Saxony. For. Ecol. Manag. 1997, 97, 237–253. [Google Scholar] [CrossRef]
- Boncina, A. Comparison of structure and biodiversity in the Rajhenav virgin forest remnant and managed forest in the Dinaric region of Slovenia. Glob. Ecol. Biogeogr. 2000, 9, 201–211. [Google Scholar] [CrossRef]
- Shimatani, K. On the measurement of species diversity incorporating species differences. Oikos 2001, 93, 135–147. [Google Scholar] [CrossRef]
- Seymour, R.S.; Hunter, M.L. Principles of Ecological Forestry. In Maintaining Biodiversity in Forest Ecosystems; Hunter, M.L., Ed.; Cambridge University Press: New York, NY, USA, 1999; pp. 22–62. [Google Scholar] [CrossRef]
- Sturtevant, B.R.; Bissonette, J.A.; Long, J.N.; Roberts, D.W. Coarse woody debris as a function of age, stand structure, and disturbance in boreal newfoundland. Ecol. Appl. 1997, 7, 702–712. [Google Scholar] [CrossRef]
- Palik, B.; Engstrom, R.T. Species composition. In Maintaining Biodiversity in Forest Ecosystems; Hunter, M.L., Jr., Ed.; Cambridge University Press: New York, NY, USA, 1999; pp. 65–94. [Google Scholar]
- Ferris, R.; Peace, A.J.; Humphrey, J.W.; Broome, A.C. Relationships between vegetation, site type and stand structure in coniferous plantations in Britain. For. Ecol. Manag. 2000, 136, 35–51. [Google Scholar] [CrossRef]
- Bradshaw, C.J.A.; Warkentin, I.G.; Sodhi, N.S. Urgent preservation of boreal carbon stocks and biodiversity. Trends Ecol. Evol. 2009, 24, 541–548. [Google Scholar] [CrossRef]
- Westfall, J.A. Prediction of standing tree defect proportion using logistic regression and ordered decision thresholds. Can. J. For. Res. 2013, 43, 1085–1091. [Google Scholar] [CrossRef]
- Power, H.; Havreljuk, F. Predicting hardwood quality and its evolution over time in Quebec’s forests. Forestry 2018, 91, 259–270. [Google Scholar] [CrossRef]
- Nykänen, M.L.; Peltola, H.; Quine, C.; Kellomäki, S.; Broadgate, M. Factors affecting snow damage of trees with reference to European conditions. Silva Fenn. 1997, 31, 193–213. [Google Scholar] [CrossRef]
- Valinger, E.; Fridman, J. Factors affecting the probability of windthrow at stand level as a result of Gudrun winter storm in southern Sweden. For. Ecol. Manag. 2011, 262, 398–403. [Google Scholar] [CrossRef]
- Wonn, H.T.; O’Hara, K.L. Height: Diameter ratios and stability relationships for four northern rocky mountain tree species. West. J. Appl. For. 2001, 16, 87–94. [Google Scholar] [CrossRef]
- Rudnicki, M.; Silinus, U.; Lieffers, V.J. Crown cover is correlated with relative density, tree slenderness and tree height in lodgepole pine. For. Sci. 2004, 50, 356–363. [Google Scholar]
- Liu, X.; Silinus, U.; Lieffers, V.J.; Man, R. Stem hydraulic properties and growth in lodgepole pine stands following thinning and sway treatment. Can. J. For. Res. 2003, 33, 1295–1303. [Google Scholar] [CrossRef]
- Moore, J.R. Differences in maximum resistive bending moments of Pinus radiata trees grown on a range of soil types. For. Ecol. Manag. 2000, 135, 63–71. [Google Scholar] [CrossRef]
- Peltola, H.M. Mechanical stability of trees under static loads. Am. J. Bot. 2006, 93, 1501–1511. [Google Scholar] [CrossRef]
- Thompson, I.D.; Mackey, B.; McNulty, S.; Mosseler, A. Forest Resilience, Biodiversity and Climate Change: A Synthesis of the Biodiversity/Resilience/Stability Relationship in Forest Ecosystems; Technical Series Report no. 43; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2009. [Google Scholar]
- Spathelf, P.; Stanturf, J.; Kleine, M.; Jandl, R.; Chiatante, D.; Bolte, A. Adaptive measures: Integrating adaptive forest management and forest landscape restoration. Ann. For. Sci. 2018, 75, 55. [Google Scholar] [CrossRef]
- Jandl, R.; Spathelf, P.; Bolte, A. Forest adaptation to climate change—Is non-management an option? Ann. For. Sci. 2019, 76, 48. [Google Scholar] [CrossRef]
- Wang, Y.; Titus, S.J.; LeMay, V.M. Relationships between tree slenderness coefficients and tree or stand characteristics for major species in boreal mixedwood forests. Can. J. For. Res. 1998, 28, 1171–1183. [Google Scholar] [CrossRef]
- Schelhaas, M.J. The wind stability of different silvicultural systems for Douglas-fir in the Netherlands: A model-based approach. Forestry 2008, 81, 399–414. [Google Scholar] [CrossRef]
- Martín-Alcón, S.; González-Olabarría, J.R.; Coll, L. Wind and snow damage in the Pyrenees pine forests: Effect of stand attributes and location. Silva Fenn. 2010, 44, 399–410. [Google Scholar] [CrossRef]
- Opio, C.; van Diest, K.; Jacob, N. Intra-seasonal changes in height to diameter ratios for lodgepole pine in the central interior of British Columbia. West. J. Appl. For. 2003, 18, 52–59. [Google Scholar] [CrossRef][Green Version]
- Harrington, T.B.; Harrington, C.A.; DeBell, D.S. Effects of planting spacing and site quality on 25-year growth and mortality relationships of Douglas-fir (Pseudotsuga menziesii var. menziesii). For. Ecol. Manag. 2009, 258, 18–25. [Google Scholar] [CrossRef]
- Vospernik, S.; Monserud, R.A.; Sterba, H. Do individual-tree growth models correctly represent height:diameter ratios of Norway spruce and Scots pine? For. Ecol. Manag. 2010, 260, 1735–1753. [Google Scholar] [CrossRef]
- Mitchell, S.J. Wind as a natural disturbance agent in forests: A synthesis. Forestry 2013, 86, 147–157. [Google Scholar] [CrossRef]
- Sharma, R.P.; Vacek, Z.; Vacek, S. Modeling individual tree height to diameter ratio for Norway spruce and European beech in Czech Republic. Trees 2016, 30, 1969–1982. [Google Scholar] [CrossRef]
- Põldveer, E.; Potapov, A.; Korjus, H.; Kiviste, A.; Stanturf, J.A. The structural complexity index SCI is useful for quantifying structural diversity of Estonian hemiboreal forests. For. Ecol. Manag. 2021, 490, 119093. [Google Scholar] [CrossRef]
- Valinger, E.; Fridman, J. Modelling probability of snow and wind damage in Scots pine stands using tree characteristics. For. Ecol. Manag. 1997, 97, 215–222. [Google Scholar] [CrossRef]
- Schütz, J.-P.; Götz, M.; Schmid, W.; Mandallaz, D. Vulnerability of spruce (Picea abies) and beech (Fagus sylvatica) forest stands to storms and consequences for silviculture. Eur. J. For. Res. 2006, 125, 291–302. [Google Scholar] [CrossRef]
- Albrecht, A.; Hanewinkel, M.; Bauhus, J.; Kohnle, U. How does silviculture affect storm damage in forests of south-western Germany? Results from empirical modeling based on long-term observations. Eur. J. For. Res. 2012, 131, 229–247. [Google Scholar] [CrossRef]

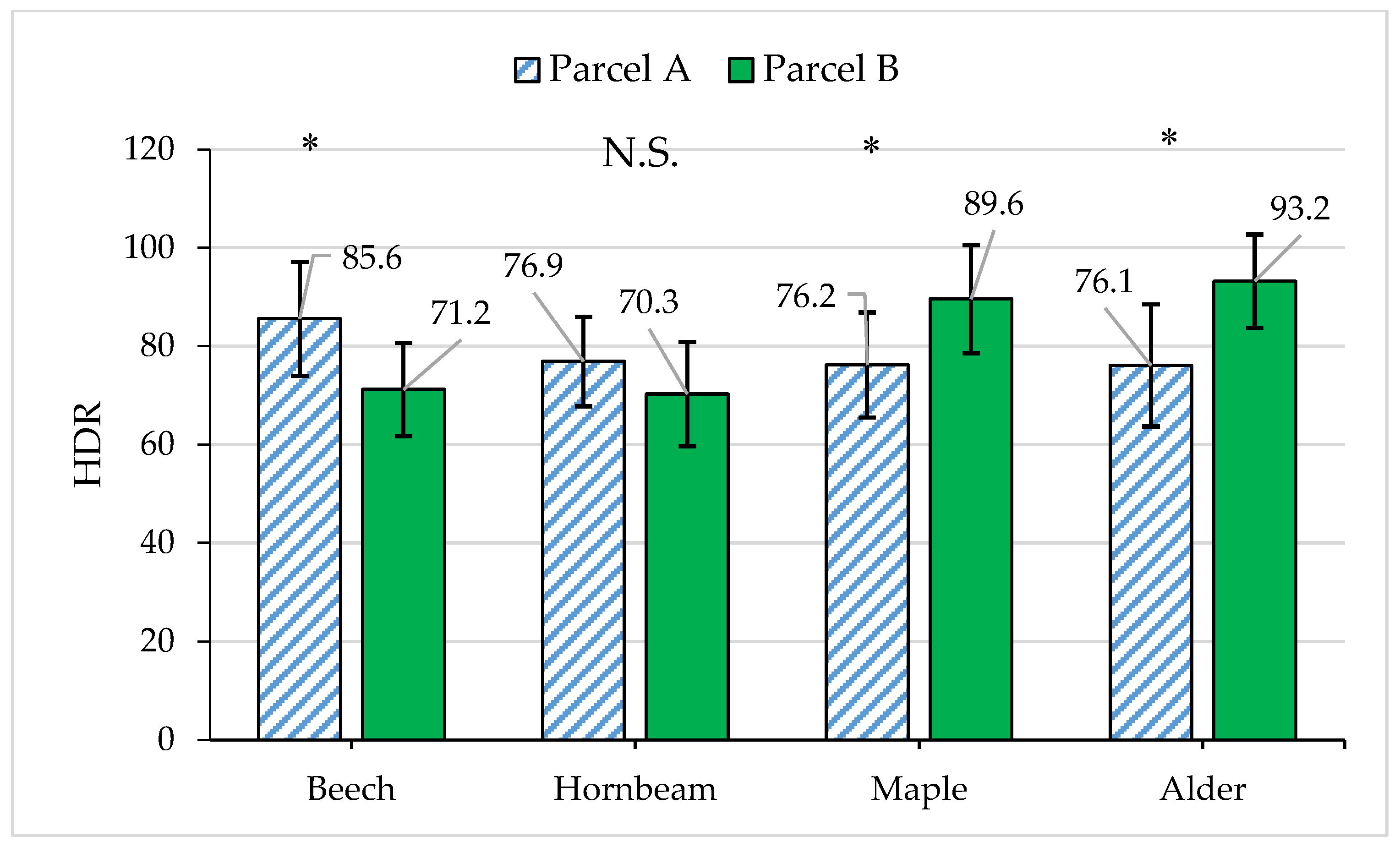
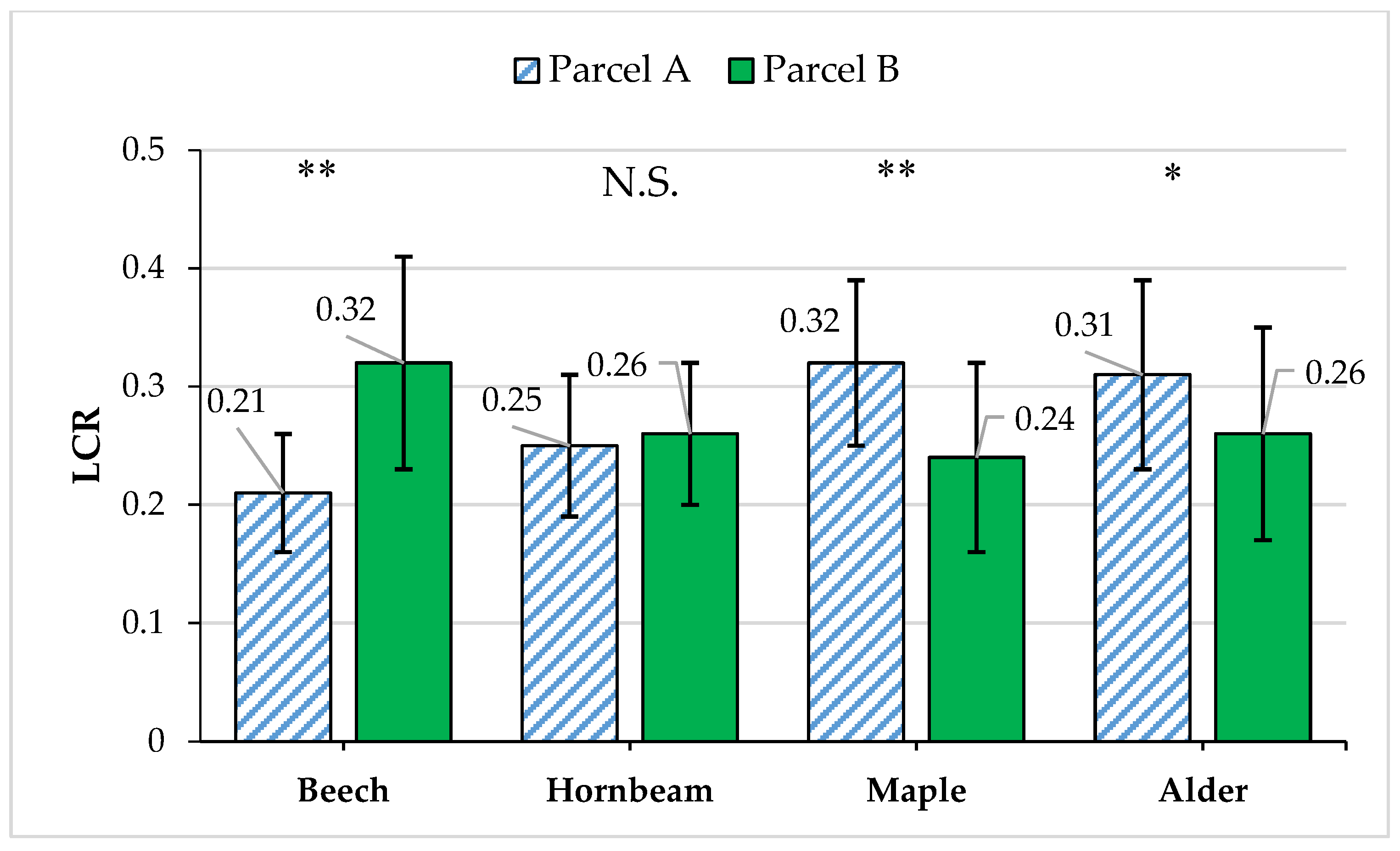
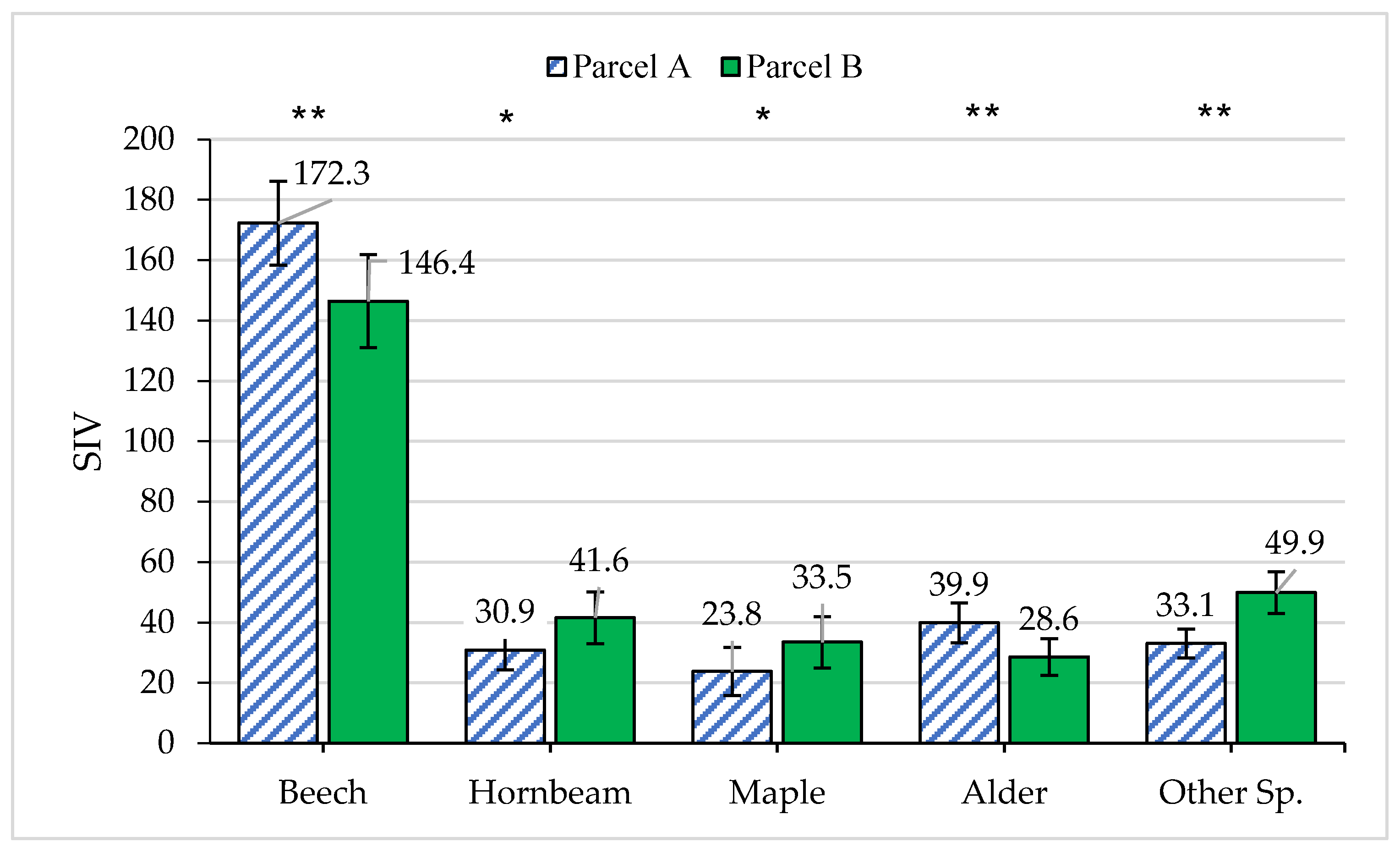
| Year of Operation | Parcel A (Selection Cutting) | Parcel B (Protected) | ||
|---|---|---|---|---|
| Standing Volume (m3/ha) | Harvested Volume (m3/ha) | Standing Volume (m3/ha) | ||
| 2002 | Before cutting | 395.04 | 32.23 | 399.68 |
| After cutting | 362.81 | |||
| 2012 | Before cutting | 434.05 | 25.95 | 442.03 |
| After cutting | 408.10 | |||
| Stand Structural Attributes | Parcel A | Parcel B |
|---|---|---|
| Species composition of trees (% of number) | Be (55.9%) *, H (16.3%) N.S., M (17.4%) *, Al (9.5%) *, Other Sp. (3.6%) * | Be (62.3%), H (14.7%), M (10.1%), Al (5.0%), Other Sp. (7.9%) |
| Crown closure (%) | 84.7 ± 5.2 N.S. | 88.5 ± 4.9 |
| Species number of canopy layer | 3.7 ± 0.9 ** | 4.6 ± 1.1 |
| Density of living trees (stem ha−1) | 323.5 ± 16.0 ** | 397.4 ± 32.5 |
| Average DBH of living trees (cm) | 27.5 ± 16.2 ** | 33.5 ± 16.8 |
| Average height of living trees (m) | 21.2 ± 2.4 N.S. | 23.7 ± 2.6 |
| Average height of dominant trees (m) | 26.4 ± 2.3 ** | 29.5 ± 2.1 |
| Density of dead trees (stem ha−1) | 10.1 ± 2.0 ** | 18.9 ± 1.7 |
| Average DBH of dead trees (cm) | 31.1 ± 5.1 ** | 43.0 ± 5.2 |
| Average height of dead trees (m) | 16.7 ± 1.9 ** | 23.6 ± 2.4 |
| Volume of dead trees (m3 ha−1) | 8.3 ± 109 ** | 15.7 ± 1.8 |
| Volume of fallen boles (m3 ha−1) | 8.4 ± 1.5 ** | 11.8 ± 1.7 |
| Basal area of living trees (m2 ha−1) | 24.5 ± 2.2 ** | 29.8 ± 2.5 |
| Standing volume of living trees (m3 ha−1) | 443.4 ± 15.3 * | 486.4 ± 20.6 |
| Tree Storey (Tree Height, m) | Density (Stem ha−1) | HDR | LCR | |||
|---|---|---|---|---|---|---|
| Parcel A | Parcel B | Parcel A | Parcel B | Parcel A | Parcel B | |
| Lower (<15, m) | 183.5 ± 20.2 N.S. | 181.5 ± 14.0 | 119.0 ± 11.5 ** | 94.8 ± 8.1 | 0.27 ± 0.03 * | 0.22 ± 0.03 |
| Middle (15–22.5, m) | 112.2 ± 20.2 ** | 170.6 ± 15.3 | 70.3 ± 5.3 * | 88.6 ± 7.4 | 0.28 ± 0.03 N.S. | 0.29 ± 0.04 |
| Upper (>22.5 m) | 27.8 ± 4.0 ** | 45.3 ± 4.5 | 51.9 ± 3.6 N.S. | 50.2 ± 3.5 | 0.33 ± 0.04 * | 0.37 ± 0.04 |
| Stand Structural Attributes | Parcel A | Parcel B |
|---|---|---|
| Relative spacing index (RSI) | 0.21 ± 0.05 * | 0.17 ± 0.03 |
| Stand structural complexity index (SCI) | 0.89 ± 0.09 ** | 1.30 ± 0.10 |
| Tree diameter diversity (TDD) | 1.16 ± 0.20 ** | 1.81 ± 0.34 |
| Tree height diversity (THD) | 0.95 ± 0.11 * | 1.28 ± 0.12 |
| Canopy tree species richness (TSRc) | 0.52 ± 0.10 * | 0.72 ± 0.10 |
| Tree species diversity (TSD) | 1.43 ± 0.11 * | 1.56 ± 0.11 |
| Tree species evenness (TSE) | 0.71 ± 0.08 * | 0.88 ± 0.06 |
| Tree species richness (TSR) | 1.25 ± 0.07 ** | 1.76 ± 0.08 |
| CCT | DLT | DDT | VDT | CWD | BAT | SVT | TDD | THD | TSRc | SCI | TSE | TSD | HDR | LCR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCT | 1.00 | ||||||||||||||
| DLT | 0.65 ** | 1.00 | |||||||||||||
| DDT | 0.34 * | 0.36 * | 1.00 | ||||||||||||
| VDT | 0.36 * | 0.40 * | 0.78 ** | 1.00 | |||||||||||
| CWD | −0.33 * | 0.47 ** | 0.51 ** | 0.55 ** | 1.00 | ||||||||||
| BAT | 0.17 | 0.20 | 0.06 | 0.09 | 0.10 | 1.00 | |||||||||
| SVT | 0.18 | 0.16 | 0.13 | 0.36 * | 0.35 * | 0.76 ** | 1.00 | ||||||||
| TDD | −0.37 * | −0.34 * | 0.08 | 0.14 | 0.34 * | 0.35 * | 0.37 * | 1.00 | |||||||
| THD | −0.37 * | −0.39 * | 0.08 | 0.10 | 0.38 * | 0.40 * | 0.47 * | 0.73 ** | 1.00 | ||||||
| TSRc | 0.59 ** | 0.46 * | 0.13 | 0.45 * | 0.43 * | 0.48 * | 0.51 ** | 0.53 ** | 0.62 ** | 1.00 | |||||
| SCI | 0.47 ** | 0.69 ** | 0.15 | 0.44 * | 0.41 * | 0.53 ** | 0.55 ** | 0.83 ** | 0.65 ** | 0.79 ** | 1.00 | ||||
| TSE | 0.44 * | 0.36 * | 0.03 | 0.03 | 0.04 | −0.09 | −0.07 | −0.08 | 0.11 | −0.04 | −0.08 | 1.00 | |||
| TSD | 0.14 | 0.30 * | 0.19 | 0.31 * | 0.44 * | 0.43 * | 0.36 * | 0.39 * | 0.39 * | 0.52 * | 0.65 ** | 0.70 ** | 1.00 | ||
| HDR | 0.76 ** | 0.69 * | −0.03 | −0.06 | −0.02 | 0.43 * | 0.55 * | 0.35 * | 0.51 * | 0.46 * | 0.40 * | 0.10 | 0.26 | 1.00 | |
| LCR | −0.49 * | −0.63 * | 0.09 | 0.05 | 0.06 | 0.19 | 0.37 * | 0.10 | 0.44 * | 0.38 * | 0.42 * | 0.08 | 0.11 | −0.68 ** | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karamzadeh, S.; Nikooy, M.; Abkenari, K.T.; Tavankar, F.; Lo Monaco, A.; Picchio, R. The Relationship between Stand Structure and Tree Growth Form—Investigating the Effects of Selection Cuttings in Mountainous Mixed Beech Forests. Forests 2023, 14, 1861. https://doi.org/10.3390/f14091861
Karamzadeh S, Nikooy M, Abkenari KT, Tavankar F, Lo Monaco A, Picchio R. The Relationship between Stand Structure and Tree Growth Form—Investigating the Effects of Selection Cuttings in Mountainous Mixed Beech Forests. Forests. 2023; 14(9):1861. https://doi.org/10.3390/f14091861
Chicago/Turabian StyleKaramzadeh, Sarkhosh, Mehrdad Nikooy, Kambiz Taheri Abkenari, Farzam Tavankar, Angela Lo Monaco, and Rodolfo Picchio. 2023. "The Relationship between Stand Structure and Tree Growth Form—Investigating the Effects of Selection Cuttings in Mountainous Mixed Beech Forests" Forests 14, no. 9: 1861. https://doi.org/10.3390/f14091861
APA StyleKaramzadeh, S., Nikooy, M., Abkenari, K. T., Tavankar, F., Lo Monaco, A., & Picchio, R. (2023). The Relationship between Stand Structure and Tree Growth Form—Investigating the Effects of Selection Cuttings in Mountainous Mixed Beech Forests. Forests, 14(9), 1861. https://doi.org/10.3390/f14091861








